Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10028)
| Target Name | Phospholipid hydroperoxide glutathione peroxidase (GPX4) | ||||
|---|---|---|---|---|---|
| Synonyms |
Glutathione peroxidase 4
Click to Show/Hide
|
||||
| Gene Name | GPX4 | ||||
| Sequence |
MSLGRLCRLLKPALLCGALAAPGLAGTMCASRDDWRCARSMHEFSAKDIDGHMVNLDKYR
GFVCIVTNVASQUGKTEVNYTQLVDLHARYAECGLRILAFPCNQFGKQEPGSNEEIKEFA AGYNVKFDMFSKICVNGDDAHPLWKWMKIQPKGKGILGNAIKWNFTKFLIDKNGCVVKRY GPMEEPLVIEKDLPHYF Click to Show/Hide
|
||||
| Family | Glutathione peroxidase family | ||||
| Function |
Essential antioxidant peroxidase that directly reduces phospholipid hydroperoxide even if they are incorporated in membranes and lipoproteins. Can also reduce fatty acid hydroperoxide, cholesterol hydroperoxide and thymine hydroperoxide. Plays a key role in protecting cells from oxidative damage by preventing membrane lipid peroxidation. Required to prevent cells from ferroptosis, a non-apoptotic cell death resulting from an iron-dependent accumulation of lipid reactive oxygen species. The presence of selenocysteine (Sec) versus Cys at the active site is essential for life: it provides resistance to overoxidation and prevents cells against ferroptosis. The presence of Sec at the active site is also essential for the survival of a specific type of parvalbumin-positive interneurons, thereby preventing against fatal epileptic seizures. May be required to protect cells from the toxicity of ingested lipid hydroperoxides. Required for normal sperm development and male fertility. Essential for maturation and survival of photoreceptor cells. Plays a role in a primary T-cell response to viral and parasitic infection by protecting T-cells from ferroptosis and by supporting T-cell expansion. Plays a role of glutathione peroxidase in platelets in the arachidonic acid metabolism. Reduces hydroperoxy ester lipids formed by a 15-lipoxygenase that may play a role as down- regulator of the cellular 15-lipoxygenase pathway.
Click to Show/Hide
|
||||
| Gene ID | 2879 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
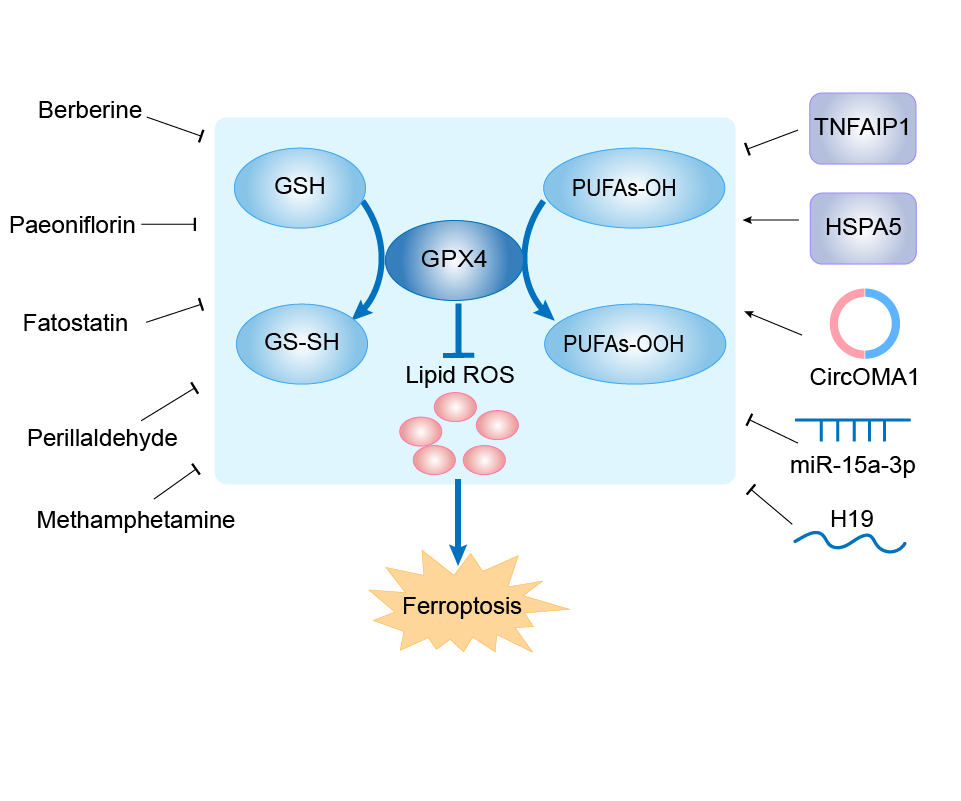
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
GPX4 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Vitamin D3 receptor (VDR)
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Paricalcitol | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
A total of 72 male C57BL/6 mice were purchased from Slyke jingda Biotechnology Company. They were randomly divided into five groups: Control group (n = 8), Cisplatin (20 mg/kg dissolved in saline) only group (n = 16), Cisplatin + paricalcitol (0.2 ug/kg dissolved in sterile water for injection and 20% propylene glycol) group (n = 16), Cisplatin + DMSO group (n = 16), Cisplatin + Fer-1 (5 mg/kg dissolved in DMSO) group (n = 16), were administered intraperitoneally. Cisplatin was injected once to mice, while Fer-1 was injected once an hour before cisplatin, and paricalcitol was injected once daily for five consecutive days before cisplatin. Each eight mice were sacrificed at 48 h and 72 h, respectively after cisplatin injection, and eight mice in the control group were sacrificed together with mice at 72 h.
Click to Show/Hide
|
||||
| Response Description | Pretreatment of paricalcitol could also alleviated Erastin (an inducer of ferroptosis) induced cell death in HK-2 cell. Ferroptosis plays an important role in cisplatin induced acute kidney injury. VDR activation can protect against cisplatin induced renal injury by inhibiting ferroptosis partly via trans-regulation of GPX4. | ||||
Transient receptor potential cation channel subfamily V member 1 (TRPV1)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Capsiate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mSIOs (Mouse small intestinal organoids) | ||||
| In Vivo Model |
Six- to eight-week-old specific pathogen-free male C57BL/6 mice were purchased from the animal center of Nanfang Hospital of Southern Medical University (Guangzhou, China). The mice were anesthetized with isoflurane. A noninvasive microvascular artery clip was placed on the superior mesenteric artery (SMA) for 60 min, and the clip was removed for reperfusion for 2 hours. During the study period, body temperature was maintained at 37 with a heating pad, and liquid resuscitation was performed by subcutaneous injection with 0.5 ml of physiological saline immediately after reperfusion.
Click to Show/Hide
|
||||
| Response Description | The gut microbiota metabolite capsiate enhances Gpx4 expression and inhibits ferroptosis by activating TRPV1 in intestinal ischemia/reperfusion (I/R) injury, providing a potential avenue for the management of intestinal ischemia/reperfusion (I/R) injury. | ||||
Thioredoxin (TXN)
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Cyperquat | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | ||
| In Vivo Model |
Male C57BL/6 mice wild-type (WT), 8 weeks of age, were from Chongqing Medical University, China. Mice were divided into four groups (n = 10-13 per group), control group, MPTP group, h-Trx-1 Tg group, and h-Trx-1 Tg + MPTP group. Control and h-Trx-1 Tg groups were administered saline only. For the Trx-1 knockdown experiment, mice were divided into six groups (n = 10-13 per group), control + saline group, control + MPTP group, AAV9-vehicle + saline group, AAV9-vehicle + MPTP group, AAV9-shRNA-mTrx-1 + saline group, and AAV9-shRNA-mTrx-1 + MPTP.
Click to Show/Hide
|
||||
| Response Description | 1-methyl-4-phenylpyridinium (Cyperquat) decreased cell viability, GPX4, and Trx-1 (TXN). The decreased GPX4 and GSH, and increased ROS were inhibited by Fer-1 and Trx-1 overexpression. Trx-1 reversed the decreases of GPX4 and tyrosine hydroxylase (TH) induced by MPTP in the substantia nigra pars compacta (SNpc). Trx-1 inhibits ferroptosis in parkinson's disease through regulating GPX4 and GSH. | ||||
Sterol regulatory element-binding protein 1 (SREBF1)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Apatinib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | |
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| In Vivo Model |
Female nude mice (BALB/c, nu/nu, 18-22 g, 4-5 weeks old) were obtained from Guangdong Medical Laboratory Animal center, China, and maintained under specific pathogen-free conditions on a 12h/12h light/dark cycle. Each mouse was injected subcutaneously with eight million luciferase-expressing cells resuspended in 50 ul of PBS and 50 ul of Matrigel (BD Biosciences). When a palpable mass had developed, the mice were randomly divided into five groups: apatinib (50 mg/kg/day oral dose for 14 days); RSL3 (100 mg/kg injection of RSL3 twice per week for 2 weeks at the same site); both; apatinib (50 mg/kg/day oral dose for 14 days) plus vitamin E (100 mg/kg/day oral dose for 14 days); and vehicle (DMSO, 100 ul oral dose for 14 days).
Click to Show/Hide
|
||||
| Response Description | Apatinib exerted antitumor effects against gastric cancer cells in vitro and in vivo through the induction of lipid peroxidation mediated by GPX4, then lead to ferroptosis. Furethermore, we found apatinib inhibited transcription of GPX4 via a SREBP1a-mediated pathway. These results indicated that GPX4 may be a potential target for anti-GC efficacy evaluation and treatment of apatinib. | ||||
Sphingosine kinase 1 (SPHK1)
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Dihydromyricetin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Rats were anesthetized by pentobarbital sodium at a dosage of 40 mg/kg by intraperitoneal injection. Rats were first anchored on to an operating table in the supine position. The fur around the incision was shaved and then disinfected. Subsequently, the neck of each rat was incised in the middle to expose the right common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA). The proximal end of the CCA and ECA were ligated and severed using a 0.285 mm nylon suture. The suture was inserted from the ECA stump through the ICA to reach the MCA. The MCA was then occluded for 2 h to create ischemic conditions. Next, the nylon suture was slowly pulled out to restore blood flow and simulate reperfusion condition.
Click to Show/Hide
|
||||
| Response Description | Dihydromyricetin (DHM) repressed ferroptosis by inhibiting the SPHK1/mTOR signaling pathway, thereby alleviating cerebral ischemia reperfusion injury. Moreover, the expression levels of glutathione peroxidase 4 (GPX4) was enhanced while the levels of acyl-CoA synthetase long-chain family member 4 (ACSL4) and phosphatidylethanolamine binding protein 1 (PEBP1) were reduced in OGD/R-treated HT22 cells in the presence of DHM. | ||||
Sphingomyelin phosphodiesterase (SMPD1)
Fibrosarcoma [ICD-11: 2B53]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Erastin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 |
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| Response Description | Erastin (Era) treatment results in the activation of ASM and generation of ceramide, which are required for the Era-induced reactive oxygen species (ROS) generation and LPO in fibrosarcoma. ASM ( SMPD1)-mediated activation of autophagy plays a critical role in ferroptosis inducers (FINs)-induced glutat hione peroxidase 4 (GPX4) degradation and ferroptosis activation. | |||
Signal transducer and activator of transcription 3 (STAT3)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Peoniflorin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| In Vivo Model |
U251 cells (6 x 106) were inoculated into the flanks of 4-to 5-week-old athymic nude mice (Shanghai Laboratory Animal Company, Shanghai, China) subcutaneously to generate a subcutaneous xenograft tumor model. After 2 weeks, the tumor model was successfully constructed, the mice were treated single and combined with 100 mg/kg RSL3 (2 times/week) and 1.0 g/kg/days PF. Tumor volumes were measured every 4 days to draw the growth curve. Mice were sacrificed 4 weeks after cell injection. Tumor xenografts were collected, photographed, and weighed and the tumor apoptosis was analyzed by Tunel staining.
Click to Show/Hide
|
||||
| Response Description | Paeoniflorin (PF) can function as an antitumor agent for glioma treatment by targeting NEDD4L-dependent STAT3 ubiquitination as well as by regulating the Nrf2/GPX4 signaling axis, which might trigger ferroptosis. | ||||
Serine/threonine-protein kinase mTOR (MTOR)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Fatostatin | Investigative | |||
| Pathway Response | Cell adhesion molecules | hsa04514 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
After anesthetizing the nude mice with isoflurane inhalation, we injected 1 x 106 U87 cells that were engineered for the expression of luciferase into the right striatum (3.5 mm from the midline of the brain and 2 mm in front of the coronal suture, injection depth of 3 mm from the brain surface) of the nude mice to establish an intracranial xenograft model. For the detection of pharmacokinetics in mice, RhoB-loaded p28-PLGA NPs were injected into the mice (n = 3) through the tail vein. We collected blood samples at predetermined time points, quantified the RhoB concentrations, and plotted them with time. To characterize NPs for GBM treatment, we randomly divided the tumor-bearing mice into four groups (n = 8) treated with PBS, free fatostatin (25 mg/kg), NPs-FAT (fatostatin equivalent dose at 25 mg/kg), and p28-NPs-FAT (fatostatin equivalent dose at 25 mg/kg). After 7 days of tumor inoculation, the treatment was conducted 3 days per week for 4 weeks. In addition, we performed IVIS imaging of intracranial tumors at 1, 3, and 5 weeks after tumor inoculation to observe tumor progression. IVIS was also used to carry out imaging of IR780-loaded NPs. The mice were monitored regularly and euthanized when they exhibited severe neurological symptoms and/or obvious weight loss (>20% of their body weight). We sacrificed a separate cohort of mice five weeks after tumor inoculation for pathological staining (n = 3).
Click to Show/Hide
|
||||
| Response Description | Fatostatin induces ferroptosis by inhibiting the AKT/ mTORC1/GPX4 signaling pathway in glioblastoma. In addition, fatostatin inhibits cell proliferation and the EMT process through the AKT/mTORC1 signaling pathway. | ||||
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Curcumin | Investigative | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 |
| Response Description | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/Akt/ mTOR signaling pathway. | |||
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Dihydromyricetin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Rats were anesthetized by pentobarbital sodium at a dosage of 40 mg/kg by intraperitoneal injection. Rats were first anchored on to an operating table in the supine position. The fur around the incision was shaved and then disinfected. Subsequently, the neck of each rat was incised in the middle to expose the right common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA). The proximal end of the CCA and ECA were ligated and severed using a 0.285 mm nylon suture. The suture was inserted from the ECA stump through the ICA to reach the MCA. The MCA was then occluded for 2 h to create ischemic conditions. Next, the nylon suture was slowly pulled out to restore blood flow and simulate reperfusion condition.
Click to Show/Hide
|
||||
| Response Description | Dihydromyricetin (DHM) repressed ferroptosis by inhibiting the SPHK1/ mTOR signaling pathway, thereby alleviating cerebral ischemia reperfusion injury. Moreover, the expression levels of glutathione peroxidase 4 (GPX4) was enhanced while the levels of acyl-CoA synthetase long-chain family member 4 (ACSL4) and phosphatidylethanolamine binding protein 1 (PEBP1) were reduced in OGD/R-treated HT22 cells in the presence of DHM. | ||||
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [72] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | ||
| SW1990 cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1723 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 or MIAPaCa2 cells in 100 ul PBS were injected subcutaneously into the right of the dorsal midline in 6- to 8-week-old athymic nude or B6 mice (female). Once the tumors reached 50-70 mm3 at day 7, mice were randomly allocated into groups and treated with rapamycin (20 mg/kg; i.p., once every other day) in the absence or presence of liproxstatin-1 (10 mg/kg; i.p., once every other day) or hydroxychloroquine (50 mg/kg; i.p., once every other day) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | The interplay between the signals of mechanistic target of rapamycin kinase (MTOR) and glutathione peroxidase 4 (GPX4) modulates autophagy-dependent ferroptosis in human pancreatic cancer cells. Both the classical autophagy inducer rapamycin and the classical ferroptosis activator RSL3 can block MTOR activation and cause GPX4 protein degradation in human pancreatic cancer cells. | ||||
RAF proto-oncogene serine/threonine-protein kinase (RAF1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Tetraarsenic tetrasulfide | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| Response Description | On H23 cells treated with realgar, the expression of GPX4, SCL7A11 decreased while ACSL4 expression increased; this effect could also be amplified by Sorafenib. In conclusion, the present study indicated that realgar may induce ferroptosis by regulating the Raf, and hence plays a role in antiKRAS mutant lung cancer. | |||
RAC-alpha serine/threonine-protein kinase (AKT1)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Fatostatin | Investigative | |||
| Pathway Response | Cell adhesion molecules | hsa04514 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
After anesthetizing the nude mice with isoflurane inhalation, we injected 1 x 106 U87 cells that were engineered for the expression of luciferase into the right striatum (3.5 mm from the midline of the brain and 2 mm in front of the coronal suture, injection depth of 3 mm from the brain surface) of the nude mice to establish an intracranial xenograft model. For the detection of pharmacokinetics in mice, RhoB-loaded p28-PLGA NPs were injected into the mice (n = 3) through the tail vein. We collected blood samples at predetermined time points, quantified the RhoB concentrations, and plotted them with time. To characterize NPs for GBM treatment, we randomly divided the tumor-bearing mice into four groups (n = 8) treated with PBS, free fatostatin (25 mg/kg), NPs-FAT (fatostatin equivalent dose at 25 mg/kg), and p28-NPs-FAT (fatostatin equivalent dose at 25 mg/kg). After 7 days of tumor inoculation, the treatment was conducted 3 days per week for 4 weeks. In addition, we performed IVIS imaging of intracranial tumors at 1, 3, and 5 weeks after tumor inoculation to observe tumor progression. IVIS was also used to carry out imaging of IR780-loaded NPs. The mice were monitored regularly and euthanized when they exhibited severe neurological symptoms and/or obvious weight loss (>20% of their body weight). We sacrificed a separate cohort of mice five weeks after tumor inoculation for pathological staining (n = 3).
Click to Show/Hide
|
||||
| Response Description | Fatostatin induces ferroptosis by inhibiting the AKT/mTORC1/GPX4 signaling pathway in glioblastoma. In addition, fatostatin inhibits cell proliferation and the EMT process through the AKT/mTORC1 signaling pathway. | ||||
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Curcumin | Investigative | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 |
| Response Description | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/ Akt/mTOR signaling pathway. | |||
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Lapatinib | Investigative | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | Lapatinib (LAP) inhibited the cell viability and exacerbated cell injury induced by doxorubicin, as well as increased cell apoptosis. LAP aggravated Dox-induced cardiotoxicity by promoting oxidative stress and ferroptosis in cardiomyocytes via PI3K/AKT-mediated mitochondrial dysfunction. Moreover, GPX4 expression was decreased and ASCL4 level was higher following DOX treatment or the combination therapy of LAP and DOX. | |||
PVT1 (IncRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Ketamine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
BALB/c nude mice (age 6 weeks) were brought from the Laboratory Animal Center of Chinese Academy of Sciences (China). HepG2 cell suspension (100 uL, 5 x 105 per site) was hypodermically inoculated into the fat pad of mice. Tumor volume was calculated as follows: tumor volume (mm3) = 0.5 x width (mm)2 x length (mm). When tumor size reached 100 mm3, mice were treated with ketamine (20 mg/kg) or saline intraperitoneally. The mice were succumbed to death when tumor size reached 1000 mm3. Tumors were isolated and weighted. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Click to Show/Hide
|
||||
| Response Description | LncPVT1 directly interacted with miR-214-3p to impede its role as a sponge of GPX4. Depletion of lncPVT1 accelerated the ferroptosis of liver cancer cells, whereas miR-214-3p inhibition and GPX4 overexpression reversed this effect. In this work, we determined that ketamine suppressed viability of liver cancer cells and induced ferroptosis and identified the possible regulatory mechanism of lncPVT1/miR-214-3p/GPX4 axis. | ||||
Protein lifeguard 4 (TMBIM4)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| In Vivo Model |
To generate murine subcutaneous tumours, 1 x 107 control shRNA or S1R-knockdown Huh7 cells in 200 uL of PBS were injected subcutaneously to the right of the dorsal midline. At day seven, the mice were randomly divided into groups and treated with sorafenib (10 mg/kg/intraperitoneal injection (i.p.), once every other day) for 2 weeks. On day 28, tumours were removed.
Click to Show/Hide
|
||||
| Response Description | S1R (TMBIM4) protects hepatocellular carcinoma cells against sorafenib and subsequent ferroptosis. Inhibition of S1R by RNAi and antagonists markedly increased the anticancer activity of sorafenib by modulating the expression of GPX4, iron metabolism and ROS. | ||||
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Curcumin | Investigative | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 |
| Response Description | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/Akt/mTOR signaling pathway. | |||
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Lapatinib | Investigative | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | Lapatinib (LAP) inhibited the cell viability and exacerbated cell injury induced by doxorubicin, as well as increased cell apoptosis. LAP aggravated Dox-induced cardiotoxicity by promoting oxidative stress and ferroptosis in cardiomyocytes via PI3K/AKT-mediated mitochondrial dysfunction. Moreover, GPX4 expression was decreased and ASCL4 level was higher following DOX treatment or the combination therapy of LAP and DOX. | |||
Phosphatidylethanolamine-binding protein 1 (PEBP1)
Depressive disorder [ICD-11: 6A70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Xiaoyaosan | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
The specific-pathogen free (SPF) male C57BL/6 mice (8-week-old, SCXK (Beijing) 2016-0006) were purchased from Beijing Vital River Laboratory Animal Technology Limited Company. A total of 48 mice were randomly assigned to 4 groups (n = 12): a control group (no stress + physiological saline), a CUMS group (CUMS + physiological saline), a Xiaoyaosan group (CUMS + Xiaoyaosan treatment) and a fluoxetine group (CUMS + fluoxetine treatment).
Click to Show/Hide
|
||||
| Response Description | The activation of ferroptosis might exist in the hippocampi of CUMS-induced mice. The PEBP1-GPX4-mediated ferroptosis could be involved in the antidepressant mechanism of Xiaoyaosan. It also implied that ferroptosis could become a new target for research into the depression mechanism and antidepressant drugs. | ||||
Peroxisome proliferator-activated receptor gamma (PPARG)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Pioglitazone | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPNCs (Rat primary nerve cells) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
The rats underwent surgery using an ultraclean table and were fixed in a stereotaxic frame. The scalp was opened to expose the anterior brain region. A dental drill was used to drill a 1-mm-diameter hole in the skull surface. Blood (100 ul) was collected from the rat tail vein and injected into the rat striatum with a microsyringe (stereotaxic coordinates; 2 mm lateral to the midline, 0.2 mm posterior to bregma, and 5.5 mm deep below the skull). First, 60 ul of autogenous blood were injected at a rate of 2 ul/min, and the next 40 ul of blood were injected at 5 ul/min. Finally, the needle was left for 10 min before being removed.
Click to Show/Hide
|
||||
| Response Description | Pioglitazone (PDZ), a PPAR agonist, promotes Gpx4 expression through the interaction between PPAR and the Nrf2 pathway, inhibits ferroptosis of neurons after intracerebral hemorrhage (ICH), and promotes the recovery of neural function. | ||||
Nuclear receptor subfamily 1 group D member 1 (NR1D1)
Aristolochic acid nephropathy [ICD-11: GB55]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Aristololactam | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mRTECs (Mouse renal tubular epithelial cells) | ||||
| M4100-57 (Mouse renal tubular epithelial cells) | |||||
| In Vivo Model |
Wild-type C57BL/6 mice (eight-week-old, male) were obtained from SPF Biotechnology (Beijing, China). Three sets of animal experiments were performed. In the first set of experiments, male wild-type mice (eight-week-old) were randomly assigned to three groups (n = 6 per group): control group, 2.5 mg/kg AAI group, and 5 mg/kg AAI group. The AAI groups of mice were intraperitoneally injected with AAI (2.5 or 5 mg/kg) once daily for 5 days. The control group of mice were treated with vehicle (corn oil). In the second set of experiments, male Rev-erbfl/fl and Rev-erbkKO mice (eight-week-old) were treated with AAI (5 mg/kg) or vehicle once daily for 5 days by intraperitoneal injection. In the third set of experiments, male wild-type mice (eight-week-old) were randomly divided into the following four groups (n = 6 per group): AAI + SR8278, AAI + DFO, AAI, and vehicle.
Click to Show/Hide
|
||||
| Response Description | Renal REV-ERB protein was significantly increased in aristolochic acid I-treated mice. Furthermore, knockdown of Rev-erb by siRNA or SR8278 (a REV-ERB antagonist) treatment attenuated ALI-induced ferroptosis in mRTECs. SR8278 treatment enhanced the cell survival and GPX4 expression in ALI-treated mRTECs. Taken together, small molecule antagonism of REV-ERB alleviates aristolochic acid I-induced renal injury probably through inhibiting ferroptosis in mice. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | SR8278 | Preclinical | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mRTECs (Mouse renal tubular epithelial cells) | ||||
| M4100-57 (Mouse renal tubular epithelial cells) | |||||
| In Vivo Model |
Wild-type C57BL/6 mice (eight-week-old, male) were obtained from SPF Biotechnology (Beijing, China). Three sets of animal experiments were performed. In the first set of experiments, male wild-type mice (eight-week-old) were randomly assigned to three groups (n = 6 per group): control group, 2.5 mg/kg AAI group, and 5 mg/kg AAI group. The AAI groups of mice were intraperitoneally injected with AAI (2.5 or 5 mg/kg) once daily for 5 days. The control group of mice were treated with vehicle (corn oil). In the second set of experiments, male Rev-erbfl/fl and Rev-erbkKO mice (eight-week-old) were treated with AAI (5 mg/kg) or vehicle once daily for 5 days by intraperitoneal injection. In the third set of experiments, male wild-type mice (eight-week-old) were randomly divided into the following four groups (n = 6 per group): AAI + SR8278, AAI + DFO, AAI, and vehicle.
Click to Show/Hide
|
||||
| Response Description | Renal REV-ERB protein was significantly increased in aristolochic acid I-treated mice. Furthermore, knockdown of Rev-erb by siRNA or SR8278 (a REV-ERB antagonist) treatment attenuated ALI-induced ferroptosis in mRTECs. SR8278 treatment enhanced the cell survival and GPX4 expression in ALI-treated mRTECs. Taken together, small molecule antagonism of REV-ERB alleviates aristolochic acid I-induced renal injury probably through inhibiting ferroptosis in mice. | ||||
Nonsense-mediated mRNA decay factor SMG9 (SMG9)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | RSL3 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 cells in 100 ul PBS were injected subcutaneously to the right of the dorsal midline in 6- to 8-week-oldathymic nude mice(n = 5 mice/group). After the tumor reached 60-80 mm3 on day 7, the mice were randomly grouped and then given intratumoral treatment with RSL3 (50 mg/kg, once every other day) at day 7 for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | SMG9, a component of the NMD machinery, is a selective driver for ferroptosis in pancreatic cancer cells. SMG9 is a direct binding protein of GPX4 to promote the degradation of GPX4 in response to RSL3 (a GPX4 inhibitor), but not erastin (a SLC7A11 inhibitor). | ||||
NAD-dependent protein deacylase sirtuin-6 (SIRT6)
Cataract [ICD-11: 9B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Melatonin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
B-3 cells | Normal | Homo sapiens | CVCL_6367 | |
| In Vivo Model |
Six-week-old albino Sprague Dawley (SD) male rats were provided by the Experimental Animal Centre of the Second Affiliated Hospitalof Harbin Medical University. Fifteen minutes before exposure, the rats were anaesthetized by intraperitoneal injection of a mixture of 90 mg/kg ketamine and 15 mg/kg xylazine. Then, tropicamide phenylephrine was dropped in both eyes; at the same time, the rats that received drug treatment were injected subconjunctivally (5 ul/eye) with 500 mM Fer-1, 200 mM MT or the same dose of DMSO used to dissolve the drug using a 28-gauge needle and a Hamilton microinjector. After another 5 min, a single eye of every experimental group rat was exposed to UVB (312 nm) 5 W/m2 for 30 min. Every time, UVB exposure was synchronized with the drug injection, and the frequency was every other day until it was stopped 9 weeks later.
Click to Show/Hide
|
||||
| Response Description | Melatonin inhibited ferroptosis through the SIRT6/p-Nrf2/GPX4 and SIRT6/COA4/FTH1 pathways to neutralize lipid peroxidation toxicity, which protected cells against ferroptotic stress in vitro and delayed cataract formation caused by UVB exposure in rats. | ||||
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [88] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Pathways in cancer | hsa05200 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
NCI-N87 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1603 |
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| Response Description | SIRT6 inhibition led to the inactivation of the Keap1/Nrf2 signalling pathway and downregulation of GPX4. The overexpression of GPX4 or activation of Keap1/Nrf2 reverses the effects of the downregulation of SIRT6 on sorafenib-induced ferroptosis. Thus, targeting the SIRT6/Keap1/Nrf2/GPX4 signalling pathway may be a potential strategy for overcoming sorafenib resistance in gastric cancer. | |||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Quercetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from Gempharmatech Co., Ltd (Changzhou, China). All mice were housed in cages with standard laboratory conditions: a consistent temperature of 24 , a 12 h light/dark cycle, and free access to water and food. The mice were randomized into four groups: 1) the KA group (n = 6), injected intraperitoneally with 20 mg/kg KA, as described in a previous study; while 2) the control group (n = 6), injected intraperitoneally with an equal volume of PBS; 3) the KA + QCT group (n = 6): this group was givenintragastric administrationof 50 mg/kg of QCT once daily for 21 days before KA injection based on the literature; and 4) the KA+ferrostatin1 (Fer-1) group (n = 6), injected intraperitoneally with a well-known ferroptosis inhibitor (3 mg/kg Fer-1) for 21 days before KA administration, as described in a previous study.
Click to Show/Hide
|
||||
| Response Description | The association between the Nrf2-mediated ferroptosis pathway and seizures in a clinical setting. Quercetin effectively protects against seizure-induced neuron death in vivo and in vitro and alleviates cognitive function impairment via the SIRT1/Nrf2/SLC7A11/GPX4 pathway. | ||||
Supraventricular tachycardia [ICD-11: BC81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Icariin | Phase 3 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response Description | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Atherosclerosis [ICD-11: BD40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Ferric ammonium citrate | Investigative | |||
| Pathway Response | Autophagy | hsa04140 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| In Vivo Model |
A total of 20 male Apoe-/-mice (6-8 weeks of age, 18-22 g) were purchased from Charles River (Beijing, China). Mice were randomly assigned to a control group (normal diet: 4% fat, cholesterol free, and sodium cholate) and an AS group (high-fat diet: 20% fat, 1.25% cholesterol, and 0.5% sodium cholate).
Click to Show/Hide
|
||||
| Response Description | Ferric ammonium citrate(FAC) can induce a decrease in foam cell activity rather than macrophage activity, increase lipid ROS levels, decrease GPX4 expression and inhibit SIRT1 expression. Activation of SIRT1 can inhibit the ferroptosis and IL-1 and IL-18 levels of foam cells in excess iron by autophagy, providing a novel therapeutic target for atherosclerosis(AS). | ||||
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Cadmium | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/ Sirt1 axis. | |||
mmu_circRNA_0000309 (circRNA)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Germacrone | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
MPC-5 cells | Normal | Mus musculus | CVCL_AS87 | |
| In Vivo Model |
C57BL/6J mice were purchased from Three Gorges University (Yichang, China), and C57BL/KsJ and male db/db mice were from Changzhou Cavins Laboratory Animal Co. Ltd. (Changzhou, China). All experiments were approved by the Animal Ethics Committee of Zhejiang Provincial People's Hospital, and performed according to specific institutional and national guidelines. The mice were divided into three groups: control C57BL/6J mice, db/db mice, and germacrone-treated db/db mice (db/db + Ger) (n = 10/each group). The db/db + Ger mice received germacrone treatment at a dosage of 10 mg/kg/day, while C57BL/6J mice and db/db mice had been given the same volumes of 0.9% saline simultaneously.
Click to Show/Hide
|
||||
| Response Description | mmu_circRNA_0000309 silence mediates drug resistance to germacrone in Diabetic nephropathy mice. mmu_circRNA_0000309 sponges miR-188-3p, and subsequently upregulates GPX4 expression, inactivating ferroptosis-dependent mitochondrial function and podocyte apoptosis. | ||||
Mitogen-activated protein kinase 8 (MAPK8)
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Seratrodast | Discontinued in Phase 3 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Drugs were dissolved in vehicle (0.1% DMSO + 20% PEG 300 + 0.5% CMC-Na + ddH2O). Mice in Control and PTZ groups were administered for five days with an equivalent volume of vehicle. PTZ-induced seizure model was done for the subsequent 1 h after the last administration of drugs. We performed a preliminary doseresponse trial, the dose of 60 mg/kg was established as being sufficient to trigger seizures with lower mortality and chosen as the optimal dose. One mouse in PTZ group was dead due to a severe seizure. At the end of the experiment, the mice were anesthetized or euthanized. For histopathological studies, the mice were anesthetized and intracardially perfused with 0.9% saline, followed by 0.4% paraformaldehyde for fixation of the brain. For immunoblot analysis, the hippocampus was rapidly isolated.
Click to Show/Hide
|
||||
| Response Description | Seratrodast could reduce lipid ROS production, regulate the system xc-/glutathione (GSH)/glutathione peroxidase 4 (GPX4) axis, and inhibit JNK (MAPK8) phosphorylation and p53 expression. JNK can directly or indirectly modulate the expression and activation of p53, which could regulate ferroptosis through inhibition of SLC7A11 transcription. Seratrodast increased the latency of seizures and reduced seizure duration in pentylenetetrazole-induced seizures. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [25] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | L-F001 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | L-F001 could restore GPX4 and glutamate-cysteine ligase modifier subunit (GCLM) levels, and significantly deceased Cyclooxygenase (COX-2) levels to rescue the lipid peroxidation imbalance. And L-F001 could reduce RSL3-induced c-Jun N-terminal kinase (JNK) activation, which might be a potential drug target for for the therapy of ferroptosis-related diseases, such as cerebral ischemia. | |||
Mitogen-activated protein kinase 14 (MAPK14)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Artesunate | Investigative | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response Description | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 ( MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [27] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Lactate | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H446 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1562 | |
| NCI-H1688 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1487 | |
| Response Description | Lactate derived from metabolic reprogramming increases the expression of glutathione peroxidase 4 (GPX4) to promote ferroptosis resistance in Non-Small Cell Lung Cancer (NSCLC). Mechanistically, Lactate increases mitochondrial ROS generation and drives activation of the p38 (MAPK14)-SGK1 pathway, which attenuates the interaction of NEDD4L with GPX4 and subsequent ubiquitination and degradation of GPX4. | |||
Corpus uteri cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [59] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
KLE cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 |
| Response Description | Silencing of PTPN18 induced ferroptosis in KLE endometrial cancer cells. PTPN18 knockdown increased intracellular ROS level and down-regulated GPX4 and xCT expression. Besides, silencing of PTPN18 also induced the expression of p-p38 (MAPK14). | |||
Mitogen-activated protein kinase 1 (MAPK1)
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [28] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Salidroside | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mLT (Mouse lung tissue) | ||||
| In Vivo Model |
In our study, the 32 mice were randomly divided for four groups (n = 8 per group): (1) room-air-expose (sham), (2) hyperoxia-expose with Sal (Sal + Hyperoxia), (3) hyperoxia-exposed (Hyperoxia), (4) hyperoxia-exposed with Y-320 (an inhibitor of IL-17) (Y-320 + Hyperoxia). The mice exposed to normoxia groups were placed in room air with 21% oxygen, and the mice exposed to hyperoxia were placed in over 90% oxygen for 24 h. The continue exposure to over 90% oxygen was achieved in a self-made airtight box which attached to a medical oxygen cylinder, and the O2 level inside was continuously monitored with O2 analyzer, mice had free access to food and water. In the first three days before exposure to the hyperoxia, mice in the Sal + Hyperoxia group or Y-320 + Hyperoxia group were treated with Sal (100 mg/Kg) or Y-320 (2 mg/Kg) once orally every day, while the rest of groups were given equal isotonic saline. Based on the above experiments, eight 8-week-old KM mice were randomly divided into two groups: Sal + Hyperoxia group and Sal + Hyperoxia + IL-17A group. Sal + Hyperoxia + IL-17A group, mice were i.v. injected with 50 ug/kg of recombinant mouse IL-17A (210-17, Pepro Tech, USA). Animal were sacrificed following reperfusion, and lungs were stored at -80 until further experimental analysis.
Click to Show/Hide
|
||||
| Response Description | When we applied recombinant IL-17A in Sal+hyperoxia group mice, the protein levels of IL-17RA, Act1, TRAF6, p38 MAPK and p-p38 MAPK increased significantly, and the expression level of GPX4 significantly decreased. Therefore, we demonstrated that IL-17A/IL-17RA mediates ferroptosis of AECII, least in part, via Act1/TRAF6/p38 MAPK pathway, which is responsible for the protective effects of salidroside on hyperoxia-induced acute lung injury (HALI). | ||||
LINC01134 (IncRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [29] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Oxaliplatin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response Description | LINC01134 was positively correlated with GPX4 or Nrf2, demonstrating the clinical significance of LINC01134, Nrf2 and GPX4 in OXA resistance of hepatocellular carcinoma (HCC). Silenced LINC01134 enhances Oxaliplatin sensitivity by facilitating ferroptosis through GPX4 in hepatocarcinoma. | |||
Legumain (LGMN)
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [30] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | RR-11a | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
The genetic background of embryonic stem cells and the Flp mice used in this experiment was C57BL/6. Mice were randomly separated into experimental groups and control groups. (1) Bilateral IRI: mice (male, 8-10 weeks old) on the lgmnKO background or littermate control mice were anesthetized by an intraperitoneal (i.p.) injection of chloral hydrate and placed on a warm pad to retain their body temperature. A bilateral flank incision was made, both sides of the renal vessels were occluded with clamps for 40 min followed by removing the clamps to induce blood reperfusion. The same procedure was performed in the control group without vessel clamping. (2) Nephrotoxic folic acid-induced AKI: mice (female, 12-14 weeks old) received a single i.p. injection of folic acid at 250 mg/kg in 0.3 mol/L sodium bicarbonate or the vehicle. For therapeutic experiments, RR-11a was freshly dissolved in saline. Mice were administered an i.p. injection of 20 mg/kg RR-11a or the vehicle before ischemia.
Click to Show/Hide
|
||||
| Response Description | Legumain promotes chaperone-mediated autophagy of GPX4 therefore facilitates tubular ferroptosis in acute kidney injury (AKI). Legumain inhibitor RR-11a attenuates ferroptosis and tubular injury induced by ischemia-reperfusion injury (IRI). | ||||
L-seryl-tRNA(Sec) kinase (PSTK)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [31] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Punicalin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| SNU-387 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0250 | ||
| SNU-182 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0090 | ||
| SNU-398 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0077 | ||
| WRL 68 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0581 | ||
| HUVECs (Human umbilical vein endothelial cells) | |||||
| JHH-2 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_2786 | ||
| JHH-7 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_2805 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| Li-7 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_3840 | ||
| In Vivo Model |
Female Nod-SCID mice of 6-8 weeks old were purchased from HFK BIOSCIENCE (Beijing). Hep3B-vehicle/Hep3B-PSTK-KO cells were harvested and injected subcutaneously (1 x 107 cells in 200 uL PBS) into Nod-SCID mice (upper flank). Treatments were started when tumor volumes reached around 50 mm3. Included mice were randomly divided into four groups and injected intraperitoneally with Abemaciclib (50 mg/kg, every other day) or vehicle. Mice were sacrificed when the tumor volume exceeded 2000 mm3. PSTK-KO or vehicle Hep3B cells were implanted and treated with Sorafenib (50 mg/kg, every other day) or Erastin (50 mg/kg, every other day) for 42 days. Tumor volumes were monitored and quantified by the modified ellipsoidal formula, tumor volume = (length x width2)/2. To check the efficacities and appraisal the side effects of PSTK inhibitors, Hep3B cells were harvested and in injected subcutaneously (5 x 106 cells in 200 uL PBS) into Nod-SCID mice (upper flank). Treatments were started when tumor volumes reached around 50 mm3. Included mice were randomly divided into six groups and intragastrically treated with Punicalin (100 mg/kg, every day), Geraniin (100 mg/kg, every day), Sorafenib (50 mg/kg, every day) with or without PSTK inhibitors (Punicalin/Geraniin) for 30 days. Tumor volumes and mice weights were measured every three days.
Click to Show/Hide
|
||||
| Response Description | The depletion of PSTK resulted in the inactivation of glutathione peroxidative 4 (GPX4) and the disruption of glutathione (GSH) metabolism owing to the inhibition of selenocysteine and cysteine synthesis, thus enhancing the induction of ferroptosis upon targeted chemotherapeutic treatment. Punicalin, an agent used to treat hepatitis B virus (HBV), was identified as a possible PSTK inhibitor that exhibited synergistic efficacy when applied together with Sorafenib to treat Hepatocellular carcinoma in vitro and in vivo. | ||||
Krueppel-like factor 15 (KLF15)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [32] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Elabela | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
rAFs (Rat adventitial fibroblasts) | |||
| Response Description | KLF15 siRNA impeded the beneficial roles of elabela (ELA) in DOX-pretreated rat aortic AFs by suppressing the Nrf2/SLC7A11/GPX4 signaling. In conclusion, ELA prevents DOX-triggered promotion of cytotoxicity, and exerts anti-oxidative and anti-ferroptotic effects in rat aortic AFs via activation of the KLF15/GPX4 signaling, indicating a promising therapeutic value of ELA in antagonizing DOX-mediated cardiovascular abnormality and disorders. | |||
hsa-miR-744-5p (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Propofol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
BALB/c nude mice (5 weeks) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (license no: SYXK (Beijing) 20170033). For tumor formation, 8 x 106 A549/Cis cells were subcutaneously injected into the right axilla of each mouse. On the 7th d, Cis (4.0 mg/kg) was intraperitoneally injected into each mouse every 4 days. Then, mice were allocated into 3 groups: Control group (no additional injection); SO group (intraperitoneal injection of soybean oil); and Propofol group [intraperitoneal injection of soybean oil-dissolved propofol (35 mg/kg)]. The volume of the tumor was measured by a caliper every 7 days. Tumor volume was measured according to the formula: V (mm3) = 1/2 ab2 (a: the longest axis of tumor; b: the shortest axis of tumor). Then 35 d after transplantation, mice were euthanatized to measure tumor weight using an electronic balance. A part of transplanted tumors was immediately conserved at liquid nitrogen and -80 . The rest was used for paraffin-embedding and immunohistochemical staining.
Click to Show/Hide
|
||||
| Response Description | In summary, propofol inhibited GPX4-mediated ferroptosis and reduces CR of non-small cell lung cancer (NSCLC) cells to Cis through the miR-744-5p/miR-615-3p axis. | ||||
hsa-miR-615-3p (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Propofol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
BALB/c nude mice (5 weeks) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (license no: SYXK (Beijing) 20170033). For tumor formation, 8 x 106 A549/Cis cells were subcutaneously injected into the right axilla of each mouse. On the 7th d, Cis (4.0 mg/kg) was intraperitoneally injected into each mouse every 4 days. Then, mice were allocated into 3 groups: Control group (no additional injection); SO group (intraperitoneal injection of soybean oil); and Propofol group [intraperitoneal injection of soybean oil-dissolved propofol (35 mg/kg)]. The volume of the tumor was measured by a caliper every 7 days. Tumor volume was measured according to the formula: V (mm3) = 1/2 ab2 (a: the longest axis of tumor; b: the shortest axis of tumor). Then 35 d after transplantation, mice were euthanatized to measure tumor weight using an electronic balance. A part of transplanted tumors was immediately conserved at liquid nitrogen and -80 . The rest was used for paraffin-embedding and immunohistochemical staining.
Click to Show/Hide
|
||||
| Response Description | In summary, propofol inhibited GPX4-mediated ferroptosis and reduces CR of non-small cell lung cancer (NSCLC) cells to Cis through the miR-744-5p/miR-615-3p axis. | ||||
hsa-miR-34a-5p (miRNA)
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Cadmium | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/Sirt1 axis. | |||
hsa-miR-324-3p (miRNA)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [34] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Metformin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| In Vivo Model |
Six-week-old athymic nude mice were obtained from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Mice were divided into five groups: sham group, metformin group, metformin + NC group, metformin + miR-324-3p overexpression group, and metformin + miR-324-3p knockdown group (n = 6 in each group). Mice were injected with 3 x 106 MDA-MB-231 cells subcutaneously into the right flank. For the miR-324-3p overexpression or knockdown in the mice, two groups of mice were treated with miR-324-3p overexpression or knockdown lentivirus (GenePharma), respectively, by intratumoral injection of 50 ul of lentivirus (4 x 107 IU/ml) after the tumor cell injection. One day after tumor cell inoculation, the sham-treated group was treated with PBS and metformin-treated groups were treated with 200 mg/kg metformin every 2 days through intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Metformin promotes ferroptosis of breast cancer by targeting the miR-324-3p/GPX4 axis. The effect of miR-324-3p was mediated by directly targeting glutathione peroxidase 4 (GPX4). Metformin could act as a potential anti-cancer agent through the induction of ferroptosis. | ||||
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [35] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Icariside II | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
ACHN cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_1067 | |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
A total of 30 male BALB/c nude mice (4-6 weeks old; 18-23 g) were randomized into four groups (7-8 mice per group): i) control group; ii) treated with 15 mg/kg ICS II; iii) treated with 25 mg/kg ICS II; and, iv) treated with 35 mg/kg ICS II. ACHN and Caki-1 cells (1 x 107) were suspended in 50 ul MEM media mixed with 50 ul Matrigel (BD Biosciences) and injected subcutaneously into the right flank of mice with 1.5%pentobarbital sodium (60 mg/kg body weight; intraperitoneal injection) under anesthesia. Weight lossof more than 20% was considered a humane endpoint.
Click to Show/Hide
|
||||
| Response Description | Icariside II (ICS II) treatment triggered ferroptosis in renal cell carcinoma (RCC) cells by downregulating GPX4 in a p53-independent manner. Furthermore, ICS II treatment resulted in upregulation of miR-324-3p, which negatively regulated the expression of GPX4. | ||||
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [110] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| A549-CR cells | Lung adenocarcinoma | Homo sapiens | CVCL_IP03 | |
| Response Description | MiR-324-3p was able to reduce the viability and increase death of cisplatin-resistant A549 cells. Its function may be exerted through its direct binding to GPX4, a key regulator of ferroptosis. MiR-324-3p could serve as a potential target in the treatment of non small cell lung cancer (NSCLC). | |||
hsa-miR-214-3p (miRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Ketamine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
BALB/c nude mice (age 6 weeks) were brought from the Laboratory Animal Center of Chinese Academy of Sciences (China). HepG2 cell suspension (100 uL, 5 x 105 per site) was hypodermically inoculated into the fat pad of mice. Tumor volume was calculated as follows: tumor volume (mm3) = 0.5 x width (mm)2 x length (mm). When tumor size reached 100 mm3, mice were treated with ketamine (20 mg/kg) or saline intraperitoneally. The mice were succumbed to death when tumor size reached 1000 mm3. Tumors were isolated and weighted. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Click to Show/Hide
|
||||
| Response Description | LncPVT1 directly interacted with miR-214-3p to impede its role as a sponge of GPX4. Depletion of lncPVT1 accelerated the ferroptosis of liver cancer cells, whereas miR-214-3p inhibition and GPX4 overexpression reversed this effect. In this work, we determined that ketamine suppressed viability of liver cancer cells and induced ferroptosis and identified the possible regulatory mechanism of lncPVT1/miR-214-3p/GPX4 axis. | ||||
hsa-miR-188-3p (miRNA)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Germacrone | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
MPC-5 cells | Normal | Mus musculus | CVCL_AS87 | |
| In Vivo Model |
C57BL/6J mice were purchased from Three Gorges University (Yichang, China), and C57BL/KsJ and male db/db mice were from Changzhou Cavins Laboratory Animal Co. Ltd. (Changzhou, China). All experiments were approved by the Animal Ethics Committee of Zhejiang Provincial People's Hospital, and performed according to specific institutional and national guidelines. The mice were divided into three groups: control C57BL/6J mice, db/db mice, and germacrone-treated db/db mice (db/db + Ger) (n = 10/each group). The db/db + Ger mice received germacrone treatment at a dosage of 10 mg/kg/day, while C57BL/6J mice and db/db mice had been given the same volumes of 0.9% saline simultaneously.
Click to Show/Hide
|
||||
| Response Description | mmu_circRNA_0000309 silence mediates drug resistance to germacrone in Diabetic nephropathy mice. mmu_circRNA_0000309 sponges miR-188-3p, and subsequently upregulates GPX4 expression, inactivating ferroptosis-dependent mitochondrial function and podocyte apoptosis. | ||||
Histone acetyltransferase KAT5 (KAT5)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [36] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Ketamine | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | |
| Response Description | The treatment of Ketamine induced the levels of MDA, lipid ROS, and Fe2+, while KAT5 or GPX4 overexpression could reverse this effect in breast cancer cells. Ketamine suppresses proliferation and induces ferroptosis and apoptosis of breast cancer cells by targeting KAT5/GPX4 axis. Ketamine may serve as a potential therapeutic strategy for breast cancer. | |||
High mobility group protein B1 (HMGB1)
Hypoxic ischemic brain injury [ICD-11: 8B24]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [37] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Glycyrrhizin | Phase 3 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPCNs (Rat primary cortical neurons) | ||||
| In Vivo Model |
Male and female neonatal SpragueDawley rats on postpartum day 7 (P7) were provided by SPF Biotechnology (Beijing, China). Each animal was anesthetized with isoflurane (4% for induction, 2% for maintenance), the skin was incised, and the left common carotid artery was exposed. This artery was ligated with a 5-0 suture and cut, and the skin was sutured closed. Next, the pups recovered for 1 h with their mother. Subsequently, the pups were placed in a hypoxia chamber (8% O2 + 92% N2 mixture) for 2 h. After 2 h of hypoxia, the animals were placed back with their dam.
Click to Show/Hide
|
||||
| Response Description | Glycyrrhizin (GL) not only inhibited ferroptosis induced by RSL3 and oxygen-glucose deprivation in vitro but also inhibited ferroptosis induced by hypoxic-ischemic brain damage (HIBD) in vivo. GL could suppress the occurrence of neuronal ferroptosis and reduce neuronal loss in HIBD via the HMGB1/GPX4 pathway. | ||||
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [38] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Isoliquiritigenin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (aged 6-8 weeks and weighing 22-25g) were obtained from the Experimental Animal Center, Sichuan Provincial Peoples Hospital, and were fed a standard laboratory diet. LPS and ISL were dissolved in normal saline and 0.5% Tween-20/saline, respectively. AKI mice were developed by intraperitoneal (i.p.) LPS injection. A total of 30 mice were randomly divided into six groups (n = 5): control, ISL, Fer, LPS, LPS plus ISL, and LPS plus Fer. An intraperitoneal injection of LPS (10 mg/kg) was made to induce septic AKI. ISL was administered via gavage at 50 mg/kg 30 min before LPS injection. Mice were dosed intraperitoneally with Fer (Ferrostatin-1, SML0583, Sigma-Aldrich, St. Louis, MO) at 5 mg/kg. Mice were sacrificed by cervical dislocation 8 h after LPS injection. Kidney tissue and serum samples were collected concurrently.
Click to Show/Hide
|
||||
| Response Description | Isoliquiritigenin (ISL) attenuates septic acute kidney injury by regulating ferritinophagy-mediated ferroptosis. ISL inhibited Fe2+ and lipid peroxidation accumulation in LPS-stimulated HK2 cells. It also increased the expression of GPX4 and xCT, reduced the expression of HMGB1 and NCOA4 then attenuated mitochondria injury in renal tubular following LPS stimulation. | ||||
HCG18 (IncRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [39] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
BALB/c nude mice (4-6 weeks old) from Beijing Vital River Laboratory Animal Technology (Beijing, China) were reared in a standard laboratory with free access to food and water. Lentivirus LV-sh-NC and LV-sh-HCG18 were from GenePharma (Shanghai, China). In order to establish subcutaneous xenograft tumor models, Huh7-SR cells were infected with lentivirus LV-sh-NC or LV-sh-HCG18 and then resuspended in PBS at 5 x 105/mL. Totally 100 uL cells were subcutaneously injected into the right dorsal area of each nude mouse. When the tumor volume reached 150 mm3, sorafenib (10 mg/kg) was orally administered to nude mice once a day to the end. Tumor volume (V) was calculated: V = 0.5 x L x W2, where L and W were defined as tumor length (L) and width (W). After 28 days of cell injection, the nude mice were euthanized by intraperitoneal injection of excessive pentobarbital sodium (100 mg/kg).
Click to Show/Hide
|
||||
| Response Description | HCG18 sponged miR-450b-5p to regulate GPX4. Collectively, Silencing HCG18 inhibits GPX4 by binding to miR-450b-5p, promotes GPX4-inhibited ferroptosis, and averts sorafenib resistance in hepatocellular carcinoma (HCC). Silencing HCG18 inhibited cell proliferation, promoted apoptosis, and impaired sorafenib resistance. | ||||
Glutathione hydrolase 1 proenzyme (GGT1)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [40] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Oridonin | Investigative | ||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 |
| Response Description | The levels of intracellular iron, malondialdehyde, and reactive oxygen species after oridonin (Ori) treatment, while interfering with the effects of Ori with ferroptosis inhibitor, demonstrating that Ori's inhibition of TE1( esophageal cancer cell) cell proliferation is associated with ferroptosis. Ori can inhibit the gamma-glutamyl cycle by inhibiting the activity of GGT1 and binding to cysteine, thereby inducing ferroptosis to exert anti-cancer activity. Eventually, the value of intracellular GSH/GSSG was reduced, and the enzymatic activity of the glutathione peroxidase 4 (GPX4) was significantly decreased. | |||
Ferritin, mitochondrial (FTMT)
Osteoporosis [ICD-11: FB83]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [41] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Carbonyl cyanide-m-chlorophenyl-hydrazine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hFOB 1.19 cells | Normal | Homo sapiens | CVCL_3708 | |
| In Vivo Model |
Forty-five SD rats (3 months old, 200 ± 20 g) were obtained from the Department of Experimental Animals in China Medical University (Animal Certificate Number: SCXK (Liaoning) 2008-0005). Fifteen rats grew as control while other thirty rats were established T2DOP model. The model rats were given a high-fat feed and 12 h/day water for 2 months. Then streptozotocin was intraperitoneally injected at 30 mg/kg. Seventy-two hours later, the model was successfully established when insulin sensitivity index decreased and fasting plasma glucose exceeded 7.8 mmol/L. Then all rats continue grew 3 months to cause osteoporosis. Thirty model rats were divided into two groups. One was fifteen T2DOP rats only, and other was fifteen T2DOP rats with deferoxamine (DFO) treatment (60 mg/kg/day, intraperitoneally inject, last for the last 1 month).
Click to Show/Hide
|
||||
| Response Description | Carbonyl cyanide-m-chlorophenyl-hydrazine (CCCP) is a mitophagy agonist. Through adding mitophagy agonist CCCP to osteoblasts, we found the increase of ROS and lipid peroxidation while GPX4 decreased. FtMt inhibited the occurrence of ferroptosis in osteoblasts by reducing oxidative stress caused by excess ferrous ions, and FtMt deficiency induced mitophagy in the pathogenesis of type 2 diabetic osteoporosis (T2DOP). | ||||
Endoplasmic reticulum chaperone BiP (HSPA5)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [42] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Dihydroartemisinin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| In Vivo Model |
Specific pathogen-free athymic nude BALB/c mice (4-6 weeks old) were obtained from Guangdong Experimental Animal Centre (Guangzhou, China). To generate murine subcutaneous tumors, cells (for U251: 2 x 106 cells; for U373: 2 x 106 cells) were suspended in 0.2 ml PBS and injected into the flanks of mice (n = 6/group). Tumor volume was measured once every 3 days using calipers.
Click to Show/Hide
|
||||
| Response Description | HSPA5 upregulation increased the expression and activity of glutathione peroxidase 4 (GPX4), which neutralized Dihydroartemisinin-induced lipid peroxidation and thus protected glioma cells from ferroptosis. Ferroptosis might be a novel anticancer mechanism of DHA in glioma and HSPA5 may serve as a negative regulator of DHA-induced ferroptosis. | ||||
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [131] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Male BALB/c nude mice (4-6 weeks) were purchased from the Air Force Medical University Laboratory Animal Center. The mice were kept in the SPF environment and had free access to food and water. 3 x 106 SW480 cells were injected subcutaneously into nude mice (n = 4 or 5). Erastin was dissolved in 5% DMSO/corn oil and intraperitoneally injected into nude mice at a dose of 15 mg/kg three times. Three weeks later, mice were anesthetized by intraperitoneal injection of 10% chloral hydrate (35 mg/kg). When mice were successfully anesthetized five minutes later, mice were sacrificed and the tumors were resected and weighed. The tumors were divided into two parts. One sample was lysed and used for protein analysis. The other part was used to test for Ki67 expression.
Click to Show/Hide
|
||||
| Response Description | HSPA5 restrained ferroptosis to promote colorectal cancer development by maintaining GPX4 stability. HSPA5 was demonstrated to play a diagnostic role and correlated to the immune microenvironment in CRC patients. | ||||
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [132] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| CFPAC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_1119 | ||
| MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | ||
| Panc 02.03 cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1633 | ||
| Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 2 x 106 PANC1 cells were injected subcutaneously to the right of the dorsal midline in nude mice. Once the tumors reached ~50 mm3 at day seven, mice were randomly allocated into groups and treated with chemotherapy for two weeks (n = 5 mice/group). To generate orthotopic tumors, B6 mice were surgically implanted with 1 x 106 Panc02 into the tail of the pancreas. Two weeks after implantation, mice were randomly allocated into groups and treated with chemotherapy for three weeks (n = 6 mice/group).
Click to Show/Hide
|
||||
| Response Description | The HSPA5-GPX4 pathway mediated ferroptosis resistance, limiting the anticancer activity of gemcitabine. Genetic or pharmacologic inhibition of the HSPA5-GPX4 pathway enhanced gemcitabine sensitivity by disinhibiting ferroptosisin vitroand in both subcutaneous and orthotopic animal models of pancreatic ductal adenocarcinoma. | ||||
Degenerative arthritis [ICD-11: FA05]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [68] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
A rat model of OA with destabilization of the medial meniscus was established . After anesthetization with 3% pentobarbital sodium (Tocris, Avonmouth, UK), the hair on the right knee was clipped. Right knee was subsequently exposed before an incision was made in the medial aspect of the joint capsule, then the anterior cruciate ligament was transected, and the medial meniscus was completely resected in a manner that did not injure the articular cartilage. Subsequently, the joint was irrigated with normal saline, the capsule was sutured with 4-0 chromic catgut, and the skin was closed with 4-0 nylon mattress sutures. And the rats were allowed to move, eat, and drink freely after surgery. Experimental groups are as follows: sham group (A medial incision was made to expose the knee joint cavity, and sutured), OA model group (Destabilization of the medial meniscus), sh-NC group (OA rats were injected with sh-NC), and sh-SND1 group (OA rats were injected with sh-SND1).
Click to Show/Hide
|
||||
| Response Description | The RNA-binding protein SND1 promotes the degradation of GPX4 by destabilizing the HSPA5 mRNA and suppressing HSPA5 expression, promoting ferroptosis in osteoarthritis chondrocytes. | ||||
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP2)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [43] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Doxorubicin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | ENPP2 was transcriptionally regulated by FoxO4 to protect cardiomyocytes from doxorubicin-induced cardiotoxicity by inhibiting ferroptosis. In addition, the inhibitory effects of ENPP2 on Dox-induced ferroptosis were significantly reduced by FoxO4 overexpression, as demonstrated by increased Fe2+ and lipid ROS activity levels, decreased SLC7A11, GPX4 and FPN1 expression, and increased NOX4 expression, which were observed following FoxO4 overexpression. | |||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [269] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | ENPP2 overexpression causes upregulation of GPX4 in H9c2 cells. In erastin-induced ferroptosis of H9c2 cells, both NRF2 and ACSL4 are increased, whereas ENPP2 overexpression reduces their expression in erastin-treated H9c2 cells. | |||
E3 ubiquitin-protein ligase TRIM21 (TRIM21)
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [44] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Fedratinib | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Mice were fasted for 12 h and anesthetized (1% pentobarbital sodium, i.p.) before surgery. Bilateral renal pedicles were clamped for 30 min, then remove the arterial clamps. The sham groups were treated in the same way, except for the clamping of the renal pedicle. Blood samples were collected 24 h after reperfusion, mice were killed, and kidney were collected for follow-up experiments. Fedratinib (5 mg/kg body weight) was injected (i.p.) into mice 24 h once in advance before surgery.
Click to Show/Hide
|
||||
| Response Description | A JAK2 inhibitor Fedratinib downregulated TRIM21 expression and reduced damage both in vivo and in vitro, which is correlated with the upregulation of GPX4. Our study showed that loss of TRIM21 could alleviate ferroptosis induced by I/R, revealed the mechanism of ubiquitination degradation of GPX4 by TRIM21 and suggested TRIM21 is a potential target for the treatment of acute kidney injury (AKI). | ||||
E3 ubiquitin-protein ligase NEDD4-like (NEDD4L)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Peoniflorin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| In Vivo Model |
U251 cells (6 x 106) were inoculated into the flanks of 4-to 5-week-old athymic nude mice (Shanghai Laboratory Animal Company, Shanghai, China) subcutaneously to generate a subcutaneous xenograft tumor model. After 2 weeks, the tumor model was successfully constructed, the mice were treated single and combined with 100 mg/kg RSL3 (2 times/week) and 1.0 g/kg/days PF. Tumor volumes were measured every 4 days to draw the growth curve. Mice were sacrificed 4 weeks after cell injection. Tumor xenografts were collected, photographed, and weighed and the tumor apoptosis was analyzed by Tunel staining.
Click to Show/Hide
|
||||
| Response Description | Paeoniflorin (PF) can function as an antitumor agent for glioma treatment by targeting NEDD4L-dependent STAT3 ubiquitination as well as by regulating the Nrf2/GPX4 signaling axis, which might trigger ferroptosis. | ||||
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [27] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Lactate | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H446 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1562 | |
| NCI-H1688 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1487 | |
| Response Description | Lactate derived from metabolic reprogramming increases the expression of glutathione peroxidase 4 (GPX4) to promote ferroptosis resistance in Non-Small Cell Lung Cancer (NSCLC). Mechanistically, Lactate increases mitochondrial ROS generation and drives activation of the p38 (MAPK14)-SGK1 pathway, which attenuates the interaction of NEDD4L with GPX4 and subsequent ubiquitination and degradation of GPX4. | |||
Ovarian dysfunction [ICD-11: 5A80]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [134] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
KGN cells | Ovarian granulosa cell tumor | Homo sapiens | CVCL_0375 | |
| In Vivo Model |
Adult female C57BL/6J mice were purchased from Cyagen Bioscience (Santa Clara, CA, USA). Prior to the experiment, the animals were allowed to adapt to the environment for 1 week. For this study, all procedures were approved by the Ethics Committee of Shanghai Seventh Peoples Hospital (item number: 2021-AR-059). Adult female C57BL/6J mice were housed withaccess to food and waterad libitum. The PCOS mouse model was established as described previously. In brief, female C57BL/6J mice (4 weeks old) were subcutaneously injected with DHEA (6 mg/0.1kg body weight) daily for 20 days.
Click to Show/Hide
|
||||
| Response Description | NEDD4L facilitates GC ferroptosis by promoting GPX4 ubiquitination and degradation and contributes to the development of polycystic ovary syndrome. | ||||
E3 ubiquitin-protein ligase MIB2 (MIB2)
Cognition disorder [ICD-11: MB21]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [45] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Sevoflurane | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
mPRs (Mouse primary neurons) | ||||
| In Vivo Model |
Male C57BL/6 mice were obtained from Beijing HFK Bioscience Co., Ltd., China. The mice were then randomly separated into sham and sevoflurane administrated (SEV) groups, with each group containing 20 animals. In SEV groups, mice were placed in an anesthetizing chamber and exposed to 2.5% sevoflurane (CAS No. 28523-86-6, no. S2464, Selleck, Shanghai, China) with complete oxygen for 2 h, and sham group mice were conducted with the same procedure without sevoflurane exposure.
Click to Show/Hide
|
||||
| Response Description | Postoperative cognitive dysfunction (POCD) is a complication of the central nervous system (CNS) often occurred after surgery or anesthesia in the elder patients. Downregulation of MIB2 could alleviate the sevoflurane-anesthesia-induced cognitive dysfunction and neuron injury through reducing ferroptosis via GPX4. | ||||
Cystathionine beta-synthase (CBS)
Depressive disorder [ICD-11: 6A70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [46] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Sodium hydrosulfide | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
BV-2 cells | Normal | Mus musculus | CVCL_0182 | |
| In Vivo Model |
Adult male 22-24 g C57BL/6J mice were purchased from the Vital River Laboratory Animal Technology Co., Ltd. Mice were randomly divided into four groups, the control group (CON group, n = 8), diabetes mellitus group (DM group, n = 8), DM + sodium hydrosulfide (DM + 5.6 mg/kg NaHS, n = 8) group, and CON + sodium hydrosulfide (CON + 5.6 mg/kg NaHS, n = 8) group. In this experiment, mice have received daily intraperitoneally injection of NaHS during the last 4 weeks. Then, all mice were tested by the open field test (OFT), elevated plus maze test (EPM test), forced swimming test (FST), and tail suspension test (TST).
Click to Show/Hide
|
||||
| Response Description | Sodium hydrosulfide (NaHS) ameliorated the ferroptosis via increasing the protein expressions of SLC7A11, glutathione peroxidase 4 (GPX4), and cystathionine -synthase (CBS), reducing the pro-inflammatory cytokines, decreasing the levels of Fe2+, MDA, ROS, and lipid ROS. In conclusion, NaHS did alleviate anxiety and depression. | ||||
CircOMA1 (circRNA)
Prolactinoma [ICD-11: 2F37]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [47] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Cabergoline | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
Cellular tumor antigen p53 (TP53)
Oral squamous cell carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [48] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Quisinostat | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
| Cell pyroptosis | |||||
In Vitro Model |
CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | |
| Tca8113 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6851 | ||
| In Vivo Model |
Adult male athymic BALB/c nude mice (20-22 g of 5-week-old mice) were housed in a controlled environment at 23 ± 2 and 40%-70% humidity under a 12 h dark/light cycle with free access to irradiated food and sterile water. A suspension of 6 x 106/100 uL TCA-8113 cells was inoculated subcutaneously into the hind flank region of each nude mouse. The average tumor volume in nude mice reached 100 mm3, and mice were randomly divided into three groups. Quisinostat was formulated in normal saline and administered at 3 and 10 mg/kg/day byintraperitoneal injection. Control mice were given equal volume saline intraperitoneally. The tumor volume and the bodyweight of mice were monitored every three days.
Click to Show/Hide
|
||||
| Response Description | Quisinostat could increase the apoptosis rate in the tumor tissues of nude mice. Up-regulation of the expression of p53 and down-regulated expression of GPX4 in cell lines were observed by immunofluorescent staining, and the expression locations of p53 and GPX4 proteins in TSCC cells were observed. Quisinostat may be a potential drug for the treatment of tongue squamous cell carcinoma. | ||||
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [49] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Apigenin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
5-weeks-old kainate (KA)-induced BALB/c nude mice, a widely used epilepsy mouse model, were performed with intraperitoneal (i.p.) injection of KA (6 mg/kg). Pre-treatment 21 with antioxidant apigenin (60 mg/Kg, 2 days) or post-treatment with apigenin (60 mg/Kg, 1 day), mice were injected with KA (6 mg/kg) via intraperitoneal (i.p.) injection, and then HCP (0.5 mg/Kg) were injected by intravenous (i.v.) injection. In vivo and Ex vivo fluorescence images of relative ClO levels in mice brains 5, 15, 30, 45, and 60 min post injection of HCP were further performed by using the IVIS Spectrum imaging system (Nanjing University) with an excitation filter of 430 nm and the collection wavelength range is from 500-600 nm.
Click to Show/Hide
|
||||
| Response Description | Apigenin can efficiently reduce the expression of intracellular MPO and increase the levels of GPX4 and SIRT1, thereby conferring neuroprotection through regulation of kainic acid (KA)-induced ferroptosis. And the level of Ac-p53 inside the brains treated with apigenin was down-regulated, suggesting that the p53-mediated ferroptosis pathway might be blocked. Overall, apigenin was screened and confirmed as an efficient lead compound for epilepsy prevention and treatment. | ||||
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [77] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| HIEC-6 cells | Normal | Homo sapiens | CVCL_6C21 | |
| Response Description | RRM1 increases the instability of p53 by regulating the physical interaction of p53 with the ubiquitinating enzyme MDM2 and the deubiquitinating enzyme USP11, subsequently suppressing p21 (CDKN1A) and GPX4 in colon carcinoma cells, thereby promoting the accumulation of lipid peroxidation and occurrence of radiation-induced ferroptosis. | |||
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [108] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Six 4-week-old male BALB/c nude mice were ordered from the Shanghai Laboratory Animal Center (Shanghai SLAC Laboratory Animal Co., Ltd., China). A total of 5 x 106 TIPE+/+ SW480 cells were suspended in 100 uL of PBS and subcutaneously injected into the right axilla flank of each nude mouse, and the same amount of vector SW480 cells was into the left. At 2 weeks after inoculation, the xenograft tumor size was measured using Vernier calipers every 2 days.
Click to Show/Hide
|
||||
| Response Description | MiR-539 can bind to and regulate the expression of TIPE, and miR-539 activates SAPK/JNK to downregulate the expression of glutathione peroxidase 4 (GPX4) and promote ferroptosis. In addition, SAPK/JNK is the upstream molecule of p53. MiR-539 is a new therapeutic target for colorectal cancer (CRC) patients. | ||||
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [141] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Response Description | Cold induction promotes the process of ferroptosis by inducing the expression of CIRBP and then regulating key factors such as p53 and GPX4[. In addition, cold induction significantly inhibited the proliferation of pancreatic cancer cells and induced cell apoptosis, but after the addition of ferroptosis inhibitor, cell proliferation and apoptosis did not change significantly. | |||
Bromodomain-containing protein 4 (BRD4)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [50] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | JQ1 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| In Vivo Model |
Female athymic BALB/c nude mice (4-6-week old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Approximately 1 x 107 cells (A549) in 200 uL of serum-free medium and Matrigel solution were injected directly into the right axilla of each mouse. Tumor growth was measured with calipers every 3 days.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis was induced under (+)-JQ1 treatment and BRD4 knockdown, indicating that (+)-JQ1 induces ferroptosis via BRD4 inhibition in breast adenocarcinoma. In addition, expression of the ferroptosis-associated genes GPX4, SLC7A11, and SLC3A2 was downregulated under (+)-JQ1 treatment. Moreover, JQ1 treatment and BRD4 knockdown led to decreased FTH1 expression. | ||||
Androgen receptor (AR)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [51] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | ALZ003 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 | |
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| In Vivo Model |
NOD-SCID male mice (8-week-old) were purchased from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan). For glioblastoma and TMZ-resistant glioblastoma transplantation, luciferase-expressed U87MG cells (2 x 105) and U87MG-R cells (2 x 105) were injected into the cortex, respectively, at the depth of 3 mm using stereotactic guidance and microprocessor single syringe (Harvard Apparatus, Holliston, MA, USA). After 10 days of transplantation, TMZ (15 mg/kg) and ALZ003 were orally and intravenously administrated three times per week, respectively.
Click to Show/Hide
|
||||
| Response Description | ALZ003 targeting AR for degradation strongly exhibits the therapeutic effect on glioblastoma, including TMZ-resistant tumor,in vitroandin vivo. Particularly, GPX4 was positively regulated by AR, and overexpression of AR also prevented lipid peroxidation. | ||||
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [52] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Furosine dihydrochloride | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mPKCs (Mouse primary kidney cells) | ||||
| In Vivo Model |
A total of 60 ICR female mice (20 ± 2g, 5 mice/group) were divided into 12 groups (control and 10 furosine treatment groups). Furosine was dissolved in distilled water and a dose of 0.24 g/kg b.w. was administered by gavage or tail vein injection (0.2 mL volume per mouse) once at the beginning. This dose was chosen based on the median lethal dose (LD50) determined in previous acute toxicity experiments, in which the LD50 of furosine was 1.6 g/kg b.w. Mice were fasted for 4 h prior to dosing; animals were sacrificed at 0 (controls), 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10, 12 h after administration, and kidney tissue was dissected and blood samples were collected.
Click to Show/Hide
|
||||
| Response Description | Furosine might decrease the activity of GPX4 via AR, thereby disrupting the conversion of peroxides into non-toxic reduced forms. Once GPX4 loses its reduction activity, excessivelipid peroxidationin kidney cells can lead to cell death by ferroptosis. To conclude, the study demonstrated for the first time the toxicity of furosine toward kidney injury. | ||||
5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [53] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Artesunate | Investigative | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BV-2 cells | Normal | Mus musculus | CVCL_0182 | |
| In Vivo Model |
Rats were anaesthetised through intraperitoneal injection of pentobarbital (40 mg/kg) and placed onto a stereotaxic instrument (RWD Life Science Co., Ltd.). A 1-cm midline incision was performed in the rat scalp to expose the intersection point. Then, a hole 3.2 mm lateral and 1.4 mm anterior to the right bregma was produced. Next, 1.0 ul collagenase type IV (0.25 IU/ul; C5138; Sigma-Aldrich, USA) was injected into the basal ganglia via a microinjection pump (4.2 mm depth below the endocranium) at a rate of 0.2 ul/min. The needle was maintained for 5 min after injection to prevent backflow. Thereafter, the skin incision was closed using sutures. Rats in the sham group received 1.0 ul saline instead of collagenase type IV.
Click to Show/Hide
|
||||
| Response Description | Artesunate alleviates intracerebral haemorrhage secondary injury by inducing ferroptosis in M1-polarized microglia and suppressing inflammation through AMPK/mTORC1/GPX4 pathway | ||||
3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Simvastatin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| In Vivo Model |
MDA-MB-231 cells were injected to subcutaneous of mice to build a tumor model. When the tumor volume reaches about 60 mm3, all mice were randomly divided into five groups (n = 5) for various treatments. Then, mice were treated with PBS, Fe3O4@PCBMA, SIM, Fe3O4-SIM and Fe3O4@PCBMA-SIM through injected intravenously. The injected doses of SIM were 4 mg/kg body weight in each mouse on days 0, 3, 6, and 9.
Click to Show/Hide
|
||||
| Response Description | The study presented the ferroptosis nanomedicine by loading simvastatin (SIM), a ferroptosis drugs, into zwitterionic polymer coated of magnetic nanoparticles (Fe3O4@PCBMA), thereby improving the therapeutic effect of triple negative breast cancer. SIM could inhibit the expression of HMGCR to downregulate the mevalonate (MVA) pathway and glutathione peroxidase 4 (GPX4), thereby inducing cancer cell ferroptosis. | ||||
Zinc finger E-box-binding homeobox 1 (ZEB1)
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [55] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | |
| SW1353 cells | Bone chondrosarcoma | Homo sapiens | CVCL_0543 | ||
| MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | ||
| SAOS-2 cells | Osteosarcoma | Homo sapiens | CVCL_0548 | ||
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | ||
| HOB (Human normal osteoblastic cells) | |||||
| In Vivo Model |
The OS model of nude mice was constructed using the CDTX model. After transfection, the h143B cells were prepared into a single-cell suspension and subcutaneously injected into the left proximal tibia of 36 (3 mice per group) 4-weeks-old nude mice (1 x 107 cells per mouse).
Click to Show/Hide
|
||||
| Response Description | MiR-144-3p can induce the occurrence of ferroptosis by negatively regulating the expression of ZEB1, thereby inhibiting the proliferation, migration, and invasion of osteosarcoma (OS) cells. The overexpression of ZEB1 caused the lower expression level of ACSL4 and higher expression level of xCT and GPX4. | ||||
Fibrosarcoma [ICD-11: 2B53]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [56] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| LOX-IMVI cells | Melanoma | Homo sapiens | CVCL_1381 | ||
| WI-38 cells | Normal | Homo sapiens | CVCL_0579 | ||
| RKN cells | Ovarian leiomyosarcoma | Homo sapiens | CVCL_3156 | ||
| KP-4 cells | Pancreatic carcinoma | Homo sapiens | CVCL_1338 | ||
| HUVECs (Human umbilical vein endothelial cells) | |||||
| BJeH (Human foreskin fibroblasts) | |||||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| NCI-H358 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1559 | ||
| HCC4006 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1269 | ||
| M000921 cells | Melanoma | Homo sapiens | CVCL_S808 | ||
| M980513 cells | Melanoma | Homo sapiens | CVCL_S675 | ||
| AA01 (Pancreatic cancer cells) | |||||
| AA02 (Pancreatic cancer cells) | |||||
| In Vivo Model |
Xenografts for LOXIMVI sgEGFP (WT) and LOXIMVI sgGPX4 (KO) cells were established by injecting 10 million cells in a 1:1 PBS:Matrigel mixture containing 2.5 uM ferrostatin-1 into the flanks of athymic mice (NRC Nude, Taconic). Animals were dosed daily with 2 mg kg-1 ferrostatin-1 by intraperitoneal injections. Tumour volume was measured twice a week.
Click to Show/Hide
|
||||
| Response Description | The resulting dependency of ZEB1-high cells on lipid-peroxidase activity is most effectively exploited by direct inhibition of GPX4 in Fibrosarcoma. | ||||
ZFAS1 (IncRNA)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [57] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
To simulate the animal model of diabetic cardiomyopathy, male db/+ mice and db/db mice (age, 7 weeks, weight, 24 g) were fed a normal diet for 4 weeks and kept at 24 under a 14-h light/8-h dark cycle. The animals were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). Diabetic mice were intracoronarily administered equal volumes (80 ul) of adenoviruses Ad-ZFAS1, Ad-sh-ZFAS1, Ad-CCND2, Ad-sh-CCND2 or Ad-NC.33 miR-150-5p mimics and mimic control (NC) were injected into the tail vein of mice (50 ug/kg) every 15 days for 12 weeks. Db/db mice were treated with or without ferrostatin-1 (Fer-1, ferroptosis inhibitor; Sigma-Aldrich, 5 mg/kg) for an additional 12 weeks.
Click to Show/Hide
|
||||
| Response Description | lncRNA-ZFAS1 acted as a ceRNA to sponge miR-150-5p and downregulate CCND2 to promote cardiomyocyte ferroptosis and Diabetic cardiomyopathy development. Inhibition of ZFAS1 restored the expression of FTH1, reduced the expression of 4HNE, rescued the expression of GPX4 and inhibited the expression of apoptosisrelated genes. | ||||
WD repeat domain phosphoinositide-interacting protein 2 (WIPI2)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [58] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| Response Description | The expression level of ACSL4 decreased and that of GPX4 increased when WIPI2 was knocked down, suggesting that WIPI2 can potentially positively regulate colorectal cancer ferroptosis. | |||
Tyrosine-protein phosphatase non-receptor type 18 (PTPN18)
Corpus uteri cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [59] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
KLE cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 |
| Response Description | Silencing of PTPN18 induced ferroptosis in KLE endometrial cancer cells. PTPN18 knockdown increased intracellular ROS level and down-regulated GPX4 and xCT expression. Besides, silencing of PTPN18 also induced the expression of p-p38 (MAPK14). | |||
Tumor protein 63 (TP63)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [60] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| OVCAR5 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_1628 | ||
| COV362 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_2420 | ||
| FT190 cells | Normal | Homo sapiens | CVCL_UH57 | ||
| PEO1 cells | Metastasis of ovarian carcinoma | Homo sapiens | CVCL_2686 | ||
| PEO4 cells | Ovarian cystadenocarcinoma | Homo sapiens | CVCL_2690 | ||
| In Vivo Model |
To develop platinum resistant OC cells in vivo, female (6-8 weeks old) athymic nude mice (Foxn1nu, Envigo) were injected subcutaneously (s.c.) with 2 million SKOV3 or OVCAR3 cells, or intraperitoneally (i.p.) with 2 million OVCAR5 cells to induce tumors.
Click to Show/Hide
|
||||
| Response Description | Overexpression of FZD7 activated the oncogenic factor Tp63, driving upregulation of glutathione metabolism pathways, including glutathione peroxidase 4 (GPX4), which protected cells from chemotherapy-induced oxidative stress. FZD7 platinum-tolerant ovarian cancer cells were more sensitive and underwent ferroptosis after treatment with GPX4 inhibitors. | ||||
Tripartite motif-containing protein 46 (TRIM46)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [61] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HRCECs (Human retinal capillary endothelial cells) | |||
| Response Description | TRIM46 interacted with GPX4, an important enzyme that suppresses ferroptosis, and promoted GPX4 ubiquitination. The role of TRIM46 in GPX4 ubiquitination should inspire the future development of new therapies against diabetic retinopathy (DR). | |||
Transportin-1 (TNPO1)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [62] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
Eca-109 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | |
| In Vivo Model |
Nude mice of both sexes (age: 6-8 weeks, weight: 22-25 g) were purchased from HUNAN SJA LABRATORY ANIMAL CO., LTD (Hunan, China). The EC109 cells stably expressing sh-circBCAR3 or sh-nc were established by infection with corresponding lentivirus vectors. 1 x 106 mL-1 (100 uL) cells were subcutaneously inoculated into the nude mice. The tumor volumes had been measured from day 5 to day 25. On day 25, the xenograft tumors were removed surgically, and the tumor weight was detected.
Click to Show/Hide
|
||||
| Response Description | CircBCAR3 binds with miR-27a-3p to promote TNPO1 expression. GPX4 protein levels were increased by silencing of circBCAR3. And circBCAR3 promoted the proliferation, migration, invasion, and ferroptosis of esophageal cancer cells by miR-27a-3p. | ||||
Transcriptional activator Myb (MYB)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [63] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| In Vivo Model |
1 x 106 BGC823 control or CDO1 short hairpin (sh)RNA treated cells in 150 ul PBS were injected subcutaneously right of the dorsal midline in athymic nude mice. Once the tumors reached 80 to 100 mm3 at day 10, mice were allocated randomly into groups of five and treated with erastin (30 mg/kg intraperitoneally, twice every other day).
Click to Show/Hide
|
||||
| Response Description | Silencing CDO1 inhibited erastin-induced ferroptosis in gastric cancer cells both in vitro and in vivo. Mechanistically, c-Myb (MYB) transcriptionally regulated CDO1, and inhibition of CDO1 expression upregulated GPX4 expression. | ||||
Transcription factor JunD (JUND)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [64] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hIBECs (Human intrahepatic biliary epithelial cells) | ||||
| HuCC-T1 cells | Intrahepatic cholangiocarcinoma | Homo sapiens | CVCL_0324 | ||
| HCCC-9810 cells | Intrahepatic cholangiocarcinoma | Homo sapiens | CVCL_6908 | ||
| QBC939 cells | Cholangiocarcinoma | Homo sapiens | CVCL_6942 | ||
| HuH-28 cells | Cholangiocarcinoma | Homo sapiens | CVCL_2955 | ||
| RBE cells | Intrahepatic cholangiocarcinoma | Homo sapiens | CVCL_4896 | ||
| In Vivo Model |
For the proliferation assays, HuCCT1 cells with linc00976 knockdown, linc00976 overexpression, and negative control were subcutaneously injected into BALB/c nude mice. The mice were weighed every week and euthanized 5 weeks after injection. Finally, tumors were dissected and weighed. For the proliferation assays, HuCCT1 cells with linc00976 knockdown, linc00976 overexpression, and negative control were subcutaneously injected into BALB/c nude mice. The mice were weighed every week and euthanized 5 weeks after injection. Finally, tumors were dissected and weighed.
Click to Show/Hide
|
||||
| Response Description | JUND promotes linc00976 transcription, and linc00976 plays a crucial role in accelerating Cholangiocarcinoma tumorigenesis and metastasis and inhibiting ferroptosis by modulating the miR-3202/GPX4 axis. | ||||
Thymosin beta-4, Y-chromosomal (TMSB4Y)
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [65] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
The 42 Specified Pathogen Free (SPF)-grade Sprague Dawley (SD) male rats with weighing (180 ± 20) g were purchased from Changsha Tianqin Experimental Animal Center. All rat were randomly divided into seven groups (6 rat per group) using a random number table. Rats were deeply anesthetized with chloral hydrate (0.5 ml/kg) and killed at the end of the experiment, after 8 weeks of modeling and 4 weeks of drug treatment.
Click to Show/Hide
|
||||
| Response Description | T4 (TMSB4X and TMSB4Y) protects hepatocytes by inhibiting the GPX4-mediated ferroptosis pathway, which provides a new strategy and target for the treatment of non-alcoholic fatty liver disease (NAFLD). | ||||
Thymosin beta-4 (TMSB4X)
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [65] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
The 42 Specified Pathogen Free (SPF)-grade Sprague Dawley (SD) male rats with weighing (180 ± 20) g were purchased from Changsha Tianqin Experimental Animal Center. All rat were randomly divided into seven groups (6 rat per group) using a random number table. Rats were deeply anesthetized with chloral hydrate (0.5 ml/kg) and killed at the end of the experiment, after 8 weeks of modeling and 4 weeks of drug treatment.
Click to Show/Hide
|
||||
| Response Description | T4 ( TMSB4X and TMSB4Y) protects hepatocytes by inhibiting the GPX4-mediated ferroptosis pathway, which provides a new strategy and target for the treatment of non-alcoholic fatty liver disease (NAFLD). | ||||
Target of rapamycin complex subunit LST8 (MLST8)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [66] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
UM-RC-6 cells | Renal cell carcinoma | Homo sapiens | CVCL_2741 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| ACHN cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_1067 | ||
| NCI-H226 cells | Pleural epithelioid mesothelioma | Homo sapiens | CVCL_1544 | ||
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
PDX tumor derived from lung cancer patient rinsed in cold DMEM media were minced into fragments 1-2 mm3 in volume. Then tumor fragment was subcutaneously inoculated into the dorsal flank of NSG mice. The tumor growth in mice was monitored by bi-dimensional tumor measurements. When tumors grew to a volume of 200 mm3, the mice were divided randomly into four groups (n = 5/group) and treated with vehicle, 10 mg/kg AZD8055, 30 mg/kg IKE, or both (10% dimethyl sulfoxide/90% corn oil) by daily intraperitoneal administration. Body weights of mice in each group during treatment were also recorded accordingly.
Click to Show/Hide
|
||||
| Response Description | Pharmacologic inhibition of mTORC1 ( mTOR associated protein, MLST8) decreases GPX4 protein levels, sensitizes renal cell carcinoma cells to ferroptosis, and synergizes with ferroptosis inducers to suppress patient-derived xenograft tumor growth in vivo. | ||||
Sterol carrier protein 2 (SCP2)
Degenerative arthritis [ICD-11: FA05]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [67] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
A total of 16 Sprague-Dawley rats (6 weeks old, female) were purchased from Guangdong Medical Laboratory Animal Center (Foshan, China) and randomly divided into four groups (n = 4): Sham, Hulth, Hulth + ScpI2 (0.1 mg/kg) (Vitas-M, Apeldoorn, Netherlands), Hulth + ScpI2 (0.5 mg/kg). One week after surgery, the rats were intraarticularly injected with vehicle or ScpI2 twice a week for 4 consecutive weeks.
Click to Show/Hide
|
||||
| Response Description | Inhibition of SCP2 markedly protects mitochondria and reduces LPO levels, attenuating chondrocyte ferroptosis in vitro and alleviating the progression of osteoarthritis (OA) in rats. In OA cartilage, the positive cells for SCP2 and iron level were markedly elevated while the expression of GPX4 (a major anti-peroxidant enzyme) was markedly decreased. | ||||
Staphylococcal nuclease domain-containing protein 1 (SND1)
Degenerative arthritis [ICD-11: FA05]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [68] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
A rat model of OA with destabilization of the medial meniscus was established . After anesthetization with 3% pentobarbital sodium (Tocris, Avonmouth, UK), the hair on the right knee was clipped. Right knee was subsequently exposed before an incision was made in the medial aspect of the joint capsule, then the anterior cruciate ligament was transected, and the medial meniscus was completely resected in a manner that did not injure the articular cartilage. Subsequently, the joint was irrigated with normal saline, the capsule was sutured with 4-0 chromic catgut, and the skin was closed with 4-0 nylon mattress sutures. And the rats were allowed to move, eat, and drink freely after surgery. Experimental groups are as follows: sham group (A medial incision was made to expose the knee joint cavity, and sutured), OA model group (Destabilization of the medial meniscus), sh-NC group (OA rats were injected with sh-NC), and sh-SND1 group (OA rats were injected with sh-SND1).
Click to Show/Hide
|
||||
| Response Description | The RNA-binding protein SND1 promotes the degradation of GPX4 by destabilizing the HSPA5 mRNA and suppressing HSPA5 expression, promoting ferroptosis in osteoarthritis chondrocytes. | ||||
Sorting nexin-5 (SNX5)
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In Vivo Model |
All animal maintenance and experiments were approved by The Ethical Committee of Guangzhou University of Chinese Medicine. A total of 36 SD rats (Sprague-Dawley; male; 230-260 g; 8-9-weeks old) were divided randomly into two study groups (n = 18 rats/group), i.e., the sham group (unilateral injection of the equal volume of saline) and model group (unilateral injection of 20 g of 6-OHDA). All surgical procedures were performed under (10 mg/kgxylazine, and 100 mg/kg ketamine, intraperitoneal administration) anesthesia using stereotactic apparatus (RWD, Shenzhen, China). The rats received either unilateral injections of 5 ul of 6-OHDA (Sigma-Aldrich, Germany; 4 ug/ul, dissolved in PBS) or 5 ul of saline into MFB (left medial forebrain bundle) at the rate of 1 ul/min. Injection coordinates were as follows (with reference to bregma): anteroposterior (A/P) = - 2.2 mm, lateral (LAT) = 1.5 mm, and dorsoventral (D/V) = 8.0 mm. The rats were placed in animal holding room (humidity: 50 ± 5%; temperature: 21 ± 1 ; 12-h dark/light cycle).
Click to Show/Hide
|
||||
| Response Description | This study investigated the mechanism by which PD-specific SE driven SNX5 promoted the ferroptosis level in Parkinson's disease models. The results showed that the GPX4 expression level significantly reduced. | ||||
Short/branched chain specific acyl-CoA dehydrogenase, mitochondrial (ACADSB)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [70] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 |
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | |
| Response Description | Overexpression of ACADSB inhibits colorectal cancer cell migration, invasion, and proliferation, while ACADSB knockdown has the opposite effect. More importantly, ACADSB negatively regulates expression of glutathione reductase and glutathione peroxidase 4 (GPX4), the two main enzymes responsible for clearing glutathione (GSH) in CRC cells. | |||
SERTA domain-containing protein 4 (SERTAD4)
Cardiovascular diseases [ICD-11: BE2Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [71] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hAAFs (Human aortic adventitial fibroblasts) | |||
| Response Description | UII inhibited miR-124 expression through up-regulating circ0004372 expression, thereby promoting SERTAD4 expression. UII significantly promoted the generation of ROS, MDA and 4-HNE, reduced the activities of SOD, GST and GR, increased Fe2+ concentration and inhibited GPX4 expression through circ0004372/miR-124/ SERTAD4 for cardiovascular diseases. | |||
Serine/arginine-rich splicing factor 9 (SRSF9)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [73] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
LOVO cells were stably transfected with SRSF9-shRNA1 or NC-shRNA. Caco-2 cells were stably transfected with SRSF9-OE or empty vector. Then the transfected CRC cells (2*105 cells/100 uL) were injected into the right armpit of mouse 6-8-week-old male athymic nude mice. When the tumors reached 50 mm3 at day 7, erastin (40 mg/kg) was administrated to mice by intraperitoneal injection twice every other day.
Click to Show/Hide
|
||||
| Response Description | SRSF9's regulation of GPX4 as an essential mechanism driving colorectal cancer (CRC) tumorigenesis and resistance of erastin-induced ferroptosis. This molecular mechanism may provide a novel method for improving the sensitivity of CRC to erastin. | ||||
RUNX1-IT1 (IncRNA)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [74] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| In Vivo Model |
MDA-MB-231 cells with stable RUNX1-IT1 knockdown were injected into NOD/SCID mice, which were randomly divided into three groups, five in each group. The protocol of establishment of breast cancer orthotopic transplantation model was described in our previous study.
Click to Show/Hide
|
||||
| Response Description | RUNX1-IT1 promotes breast cancer carcinogenesis through blocking ferroptosis via elevating GPX4, targeting of the previously unappreciated regulatory axis of RUNX1-IT1/IGF2BP1/GPX4 may be a promising treatment for patient with breast cancer. | ||||
rno-miR-375-3p (miRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [75] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
rMTs (Rat myocardial tissues) | ||||
| hCFs (Cardiac Fibroblasts) | |||||
| In Vivo Model |
Forty-two SD rats were randomly divided into sham operation group (n = 6) and I/R model group (n = 36). In the model group, rats were ligated with left anterior descending coronary artery to simulate MI. Specifically, after anesthetizing the animals in the I/R model group, an oblique incision was made in the third and fourth intercostal spaces of the left chest to expose the heart. Under a stereomicroscope, the junction of the pulmonary artery cone and the left atrial appendage was ligated with 6/0 noninvasive suture needle silk threads at 1-2 mm below the starting point of the coronary artery. Successful ischemia was indicated by ST segment elevation or T wave height and peaks of MI performance on electrocardiogram (ECG). The ligation was stopped after 45 minutes of ischemia, and the rats were maintained for 24 hours after reperfusion. As a drug treatment, 6 I/R model rats were treated with 20 nmol miRNA NC inhibitor (Thermo Fisher Scientific, Waltham, MA), 20 nmol miR-375-3p antagomir (Thermo Fisher Scientific, Waltham, MA) and 2 mg/kg Ferrostatin-1 (Fer-1; MCE, USA) for 28 days. The myocardial tissues of rats in the sham operation and I/R model groups as well as I/R model drug treatment group were then used for subsequent testing.
Click to Show/Hide
|
||||
| Response Description | MiR-375-3p is an important factor inducing myocardial fibrosis after Myocardial infarction, which accelerates the ferroptosis of cardiomyocytes and promotes fibrosis by down-regulating GPX4. | ||||
RNA-binding motif, single-stranded-interacting protein 1 (RBMS1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [76] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
C57BL/6 mice (4- to 6-week-old male) were fed in a pathogen-free vivarium under standard conditions at the animal care facility at Sun Yat-sen University. Hepa 1-6 cells transduced with RBMS1 or GPX4 or circIDE overexpression lentiviral vectors were subcutaneously injected into the right flank of mice in 100 ul of sterile PBS. IVIS images were taken.
Click to Show/Hide
|
||||
| Response Description | RBMS1 overexpression inhibited hepatocellular Carcinoma (HCC) cell growth by attenuating the expression of glutathione peroxidase 4 (GPX4)and further facilitated ferroptosis in vitro and in vivo. More importantly, a novel circIDE (hsa_circ_0000251) was identified to elevate RBMS1 expression via sponging miR-19b-3p in HCC cells. | ||||
Ribonucleoside-diphosphate reductase large subunit (RRM1)
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [77] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| HIEC-6 cells | Normal | Homo sapiens | CVCL_6C21 | |
| Response Description | RRM1 increases the instability of p53 by regulating the physical interaction of p53 with the ubiquitinating enzyme MDM2 and the deubiquitinating enzyme USP11, subsequently suppressing p21 (CDKN1A) and GPX4, thereby promoting the accumulation of lipid peroxidation and occurrence of radiation-induced ferroptosis in Colon carcinoma. | |||
Rapamycin-insensitive companion of mTOR (RICTOR)
Thymoma [ICD-11: 2C27]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [78] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
mBMCs (Mouse blood mononuclear cells) | ||||
| EL4 cells (BCMA expression) cells | Thymoma | Mus musculus | CVCL_0255 | ||
| In Vivo Model |
A total of 1 x 106 naive SMARTA (CD45.1) cells were adoptively transferred into naive WT congenic recipient mice (CD45.2). On the following day, the recipients were intraperitoneally infected with 1 x 10++6PFU of LCMV Arm. BM cells were collected from WT C57BL/6J mice and KO mice.
Click to Show/Hide
|
||||
| Response Description | The mTOR kinase can be assembled into two structurally and functionally distinct complexes, mTORC1 and mTORC2, both of which share the same catalytic subunit mTOR but differ in two scaffold subunits, Raptor for mTORC1 and Rictor for mTORC2, respectively. mTORC2 inactivation resulted in the impaired phosphorylation of downstream AKT and GSK3B kinases, which induced aberrant mitochondrial reactive oxygen species (ROS) accumulation and ensuing ferroptosis-causative lipid peroxidation in virus-specific memory CD4T cells; furthermore, the disruption of this signaling cascade also inhibited glutathione peroxidase 4 (GPX4) in thymoma cells. | ||||
Pumilio homolog 2 (PUM2)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [79] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| hBMSCs (Bone marrow stromal cells) | |||||
| In Vivo Model |
A total of 96 C57BL/6 male mice (20-25 g) aged 11-12 weeks were purchased from experimental animal center of experimental animal center of Guangdong Medical University. 96 mice were randomly divided into four groups (24 mice per group): Sham group (200 ul of PBS), Sham + BMSCs-Exo group (200 ul of BMSCs-Exo), I/R group (200 ul of PBS) and I/R + BMSCs-Exo group (200 ul of BMSCs-Exo). After 10 days of adaptive feeding, all mice were injected intraperitoneally with 0.4-0.5 mL/100 g 1%Pentobarbital Sodium. I/R and I/R + BMSCs-Exo group mice were subjected to cardiac I/R injury induced by ligation of the left anterior descending artery (LAD) for 30 min followed by 24 h reperfusion. Sham and Sham + BMSCs-Exo mice were sham treated and subjected to the same surgical procedures as I/R mice except that they did not receive ligation of the left anterior descending coronary artery.
Click to Show/Hide
|
||||
| Response Description | Cellular ferroptosis is involved in the pathogenesis of Ischemia-Reperfusion Injury. BMSCs-Exo lncRNA Mir9-3hg can inhibit ferroptosis by modulating the Pum2/PRDX6 axis to exhibit cardioprotective effectsinvivoandinvitro. Silence of PRDX6 markedly decreased cell proliferation, GSH content and Gpx4 protein level, as well as prominently increased iron ion concentration and levels of ROS content and ACSL4 protein in H/R-treated HL-1 cells. | ||||
Protein LYRIC (MTDH)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [80] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1975 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | ||
| DMS53 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1177 | ||
| DMS 273 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1176 | ||
| KLE cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 | ||
| AN3CA cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_0028 | ||
| RL95-2 cells | Endometrial adenosquamous carcinoma | Homo sapiens | CVCL_0505 | ||
| HEC-1-A cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_0293 | ||
| Ishikawa cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| Hec50 cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2929 | ||
| In Vivo Model |
To generate tumor xenograft models, 5 x 106 MTDH WT and KO MDA-MB-231 cells were injected into the second and fifth mammary fat pads (both sides, total four sites) of the NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG, Jackson Laboratories, Bar Harbor, ME) immunodeficient female mice. To study the metastasis from this orthotopic mouse model, tumor volumes were allowed to grow to ~1000 mm3, after which livers were resected to examine incidence as well as tumor burden of liver metastasis.
Click to Show/Hide
|
||||
| Response Description | Metadherin (MTDH) confers a therapy-resistant mesenchymal-high cell state and enhanced sensitivity to inducers of ferroptosis. Mechanistically, MTDH inhibited GPx4, as well as the solute carrier family 3 member 2 (SLC3A2, a system Xc-heterodimerization partner), at both the messenger RNA and protein levels in Lung adenocarcinoma. | ||||
Plasmanylethanolamine desaturase (PEDS1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [81] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| In Vivo Model |
Twenty BALB/c nude female mice (4- to 6-week-old) were purchased from Charles River Laboratories (Beijing, China) to establish tumorigenesis (5 mice in each). We injected MCF-7 or MDA-MB-231 cells (4 x 106) transfected with the stable knockdown and over-expressing TMEM189 into the flanks of mice to construct tumorigenesis models, respectively. Meanwhile, the sh-Con and empty vector were served as the control groups for each model.
Click to Show/Hide
|
||||
| Response Description | TMEM189 (PEDS1) could inhibit autophagy to mediate ferroptosis in breast cancer cells. Moreover, TMEM189 ablation strongly up-regulated LC3BII and transferrin receptor 1 (TfR1) expression levels in breast cancer cells, whereas down-regulated p62 and GPX4. | ||||
Piezo-type mechanosensitive ion channel component 1 (PIEZO1)
Degenerative arthritis [ICD-11: FA05]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [82] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
Mice were orally administered 0.1 g/kg Co-Q10 daily for 8 weeks after the DMM model was established to investigate the therapeutic effect of Co-Q10 in GPX4-CKO mice with osteoarthritis. Mice and rats were anaesthetized with pentobarbital. Destabilization of the medial meniscus (DMM) surgery was performed under a microscope. The incision was sutured and disinfected daily until it healed.
Click to Show/Hide
|
||||
| Response Description | In mouse osteoarthritis model and chondrocyte experiments, inhibition of Piezo1 channel activity increased GPX4 expression, attenuated ferroptosis phenotype and reduced the severity of osteoarthritis. | ||||
Peroxisome proliferator-activated receptor alpha (PPARA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [83] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hepa 1-6 cells | Hepatocellular carcinoma | Mus musculus | CVCL_0327 | |
| In Vivo Model |
C57BL/6J SPF mice were purchased from Huazhong Agricultural University Experimental Animal Center. Mice were given tertian intraperitoneal injections of either PBS (control) or dextriferron (500 mg/kg body weight) for 2 weeks and then sacrificed. Mice were given a daily intraperitoneal injection of either vehicle or ferrostatin-1 (Fer1, 1 mg/kg body weight) for 3 weeks before sacrificed.
Click to Show/Hide
|
||||
| Response Description | PPARa activation alleviates iron overload-induced ferroptosis in mouse livers through Gpx4 and TRF, suggesting that PPAR may be a promising therapeutic target for drug discovery in ferroptosis-related tissue injuries in Hepatocellular carcinoma. | ||||
Immunoglobulin A nephropathy [ICD-11: MF8Y]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [84] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hMCs (Human mesangial cells) | |||
| Response Description | In PPAR lentivirus-transfected HMCs stimulated by Gd-IgA1, ROS, MDA, and ACSL4 were decreased; glutathione and GPX4, and immunofluorescence colocalization of PPAR and GPX4, increased; and damaged mitochondria reduced. Hence, PPAR pathway downregulation can reduce FABP1 expression, affecting GPX4 and ACSL4 levels, causing HMC ferroptosis, and contributing to immunoglobulin A nephropathy (IgAN) pathogenesis. | |||
Peroxiredoxin-6 (PRDX6)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [79] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| hBMSCs (Bone marrow stromal cells) | |||||
| In Vivo Model |
A total of 96 C57BL/6 male mice (20-25 g) aged 11-12 weeks were purchased from experimental animal center of experimental animal center of Guangdong Medical University. 96 mice were randomly divided into four groups (24 mice per group): Sham group (200 ul of PBS), Sham + BMSCs-Exo group (200 ul of BMSCs-Exo), I/R group (200 ul of PBS) and I/R + BMSCs-Exo group (200 ul of BMSCs-Exo). After 10 days of adaptive feeding, all mice were injected intraperitoneally with 0.4-0.5 mL/100 g 1%Pentobarbital Sodium. I/R and I/R + BMSCs-Exo group mice were subjected to cardiac I/R injury induced by ligation of the left anterior descending artery (LAD) for 30 min followed by 24 h reperfusion. Sham and Sham + BMSCs-Exo mice were sham treated and subjected to the same surgical procedures as I/R mice except that they did not receive ligation of the left anterior descending coronary artery.
Click to Show/Hide
|
||||
| Response Description | Cellular ferroptosis is involved in the pathogenesis of Ischemia-Reperfusion Injury. BMSCs-Exo lncRNA Mir9-3hg can inhibit ferroptosis by modulating the Pum2/ PRDX6 axis to exhibit cardioprotective effectsinvivoandinvitro. Silence of PRDX6 markedly decreased cell proliferation, GSH content and Gpx4 protein level, as well as prominently increased iron ion concentration and levels of ROS content and ACSL4 protein in H/R-treated HL-1 cells. | ||||
OIP5-AS1 (IncRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [85] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
Eca-109 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 |
| TE-13 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_4463 | |
| TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| T.Tn cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_3175 | |
| hOECs (Normal oesophageal epithelial cells) | ||||
| Response Description | OIP5-AS1 inhibition significantly inhibited Oesophageal cancer (EC) cell viability and proliferation, induced ferroptosis, and downregulated GPX4 levels, while GPX4 reversed these effects. | |||
Nitric oxide synthase, inducible (NOS2)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [86] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| NF-kappa B signaling pathway | hsa04064 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| In Vivo Model |
All nude mice were purchased from Guangdong Medical Laboratory Animal Center. NOS2-overexpressing and control cell lines were transplanted subcutaneously into the bilateral flanks, and appropriate care was given to these animals.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis-related genes (FRGs) have potential prognostic value in colorectal cancer patients and that NOS2 suppresses tumor progression, providing a novel therapeutic target for CRC treatment based on ferroptosis. And NOS2 overexpression in CACO2 cells decreased the expression of GPX4. | ||||
NADPH--cytochrome P450 reductase (POR)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [87] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
WRL 68 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0581 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| SNU-387 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0250 | ||
| In Vivo Model |
Animal experiments were conducted under the guidance of Animal Management Regulations in Chongqing University. The tumor volume was calculated as follows: (length x width2)/2. After 24 days, the mice were killed and their tumors were collected, fixed and sectioned, stained by hematoxylin and eosin, and examined by a light microscopy for histological changes.
Click to Show/Hide
|
||||
| Response Description | G6PD (glucose-6-phosphate dehydrogenase) was highly expressed in hepatocellular carcinoma and was associated with poor prognosis. G6PD promoted the proliferation, migration and invasion, as well as inhibited ferroptosis in HCC cells. G6PD inhibited ferroptosis inin HCC cells through POR. GPX4 was positively regulated by G6PD. | ||||
NAD-dependent protein deacetylase sirtuin-3, mitochondrial (SIRT3)
Gestational diabetes [ICD-11: JA63]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [89] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Autophagy | hsa04140 | |||
| mTOR signaling pathway | hsa04150 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
HTR-8/SVneo cells | Normal | Homo sapiens | CVCL_7162 |
| pTr2 cells | Normal | Sus scrofa | CVCL_YB18 | |
| Response Description | Upregulated SIRT3-enhanced autophagy activation by promoting AMPK-mTOR pathway and decreasing GPX4 level to induce ferroptosis in trophoblastic cells. SIRT3 deficiency was resistant to high glucose- and erastin-induced autophagy-dependent ferroptosis and is, therefore, a potential therapeutic approach for treating gestational diabetes mellitus (GDM). | |||
Mucin-1 (MUC1)
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [90] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6J male mice (6-8 weeks) were purchased from Slac Lab Animals (Shanghai, China). The basic principle of the CLP method was to find the caecum through anatomy and puncture at the blind end and extrude the contents into the abdominal cavity. Diffuse peritonitis was formed, and systemic infection appeared in mice. Mice in the control group were only treated with laparotomy.
Click to Show/Hide
|
||||
| Response Description | Inhibition of MUC1 dimerization could increase the expression level of Keap1, reduce the phosphorylation level of GSK3, inhibit the entry of Nrf2 into the nucleus, further inhibit the expression level of GPX4, enhance the lipid peroxidation level of lung tissues, trigger ferroptosis, and aggravate lung injury. And inhibiting MUC1 reversed the alleviating effect of vitamin E on acute lung injury caused by sepsis. | ||||
mmu-miR-214-3p (miRNA)
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [91] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
TCMK-1 cells | Normal | Mus musculus | CVCL_2772 | |
| In Vivo Model |
The C57BL/6 male mice (8-week-old, weighing approximately 20-25 g) were procured from Beijing Huafukang Bioscience Co. Inc. The mice were raised under the SPF condition. Cisplatin was injected into the mice intraperitoneally and only once at a dose of 30 mg/kg to induce AKI, while the control mice were injected with PBS. Intravenous administration of 10 mg/kg of agomir negative control (agomir NC) or agomir miR-214-3p (GenePharma Co. Ltd, Shanghai, China) was performed for the control group mice and model group mice, respectively. The ferroptosis inhibitor named Fer-1 (#S7243, Selleck Chemicals, Houston, TX, USA) was dissolved in DMSO and then diluted in 0.9% NaCl to prepare separate Fer-1 solutions each with a concentration of 0.2 mg/mL. Fer-1 was injected into the mice intraperitoneally, 1 h prior to injecting cisplatin, while the control mice received the injection of only 0.9% NaCl in 0.1% DMSO. Both experimental and control group mice were sacrificed at Day 1, 2, and 3, separately, post-cisplatin injection.
Click to Show/Hide
|
||||
| Response Description | GPX4 was predicted as a target of miR-214-3p. Moreover, inhibiting miR-214-3p enhanced the expressions of GPX4 and SLC7A11 while decreasing the ACSL4 expression. Furthermore, miR-214-3p down-regulation protected against TEC death and renal tubule damage both in vitro and in vivo. According to these findings, inhibiting miR-214-3p would alleviate TEC ferroptosis in acute kidney injury (AKI) induced by cisplatin (cis-AKI) via GPX4. | ||||
mmu-miR-182-5p (miRNA)
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [92] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| TCMK-1 cells | Normal | Mus musculus | CVCL_2772 | ||
| In Vivo Model |
Male Sprague-Dawley (SD) rats (5 weeks old, weighting 180-220 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. SD rats were anesthetized with an intraperitoneal (i.p.) injection of pentobarbital sodium (25 mg/kg) and placed on a surgical thermostator. Then the rats were subjected to an abdominal incision, and the right kidney was carefully liberated from surrounding tissue, and nephrectomy was performed. The left kidney was exposed after a midline incision, and the renal artery was clamped with non-traumatic clamps for 45 min, followed by restoring of the renal blood flow. The kidneys were harvested and the serum was collected 48 h after the surgery. The rats in sham group were subjected to an abdominal incision without clamping the renal artery.
Click to Show/Hide
|
||||
| Response Description | MiR-182-5p and miR-378a-3p induced ferroptosis in cells. And miR-182-5p and miR-378a-3p regulated the expression of GPX4 and SLC7A11 negatively by directly binding to the 3'UTR of GPX4 and SLC7A11 mRNA. In vivo study showed that silencing miR-182-5p and miR-378a-3p alleviated the I/R-induced kidney injury in rats. | ||||
mmu-miR-15a-5p (miRNA)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [93] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
The male C57BL/6 mice (20-25 g, 10-week-old) were purchased from the Experimental Animal Center of Harbin Medical University (Harbin, China). Mice were anaesthetized with pentobarbital sodium (30 mg/kg, Sigma-Aldrich, St. Louis, USA) by intraperitoneal injection. The animals were fixed on the operating table in supine position, and the chest were sterilized and opened by blunt separation at the left 4th intercosal space. In the model group, the left anterior descending artery (LAD) was ligated with 7/0 silk suture for 3 days.
Click to Show/Hide
|
||||
| Response Description | GPX4 was the direct target of miR-15a-5p by luciferase reporter assay. Mechanistically, silencing transcription factor early growth response-1 (Egr-1) inhibited the level of miR-15a-5p, increased the protein expression of GPX4, accompanied by reduced ferroptosis and alleviated myocardial injury. These results provide a novel signaling pathway during the progression of acute myocardial infarction, namely Egr-1/miR-15a-5p/GPX4/ferroptosis. | ||||
MIR9-3HG (IncRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [79] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| hBMSCs (Bone marrow stromal cells) | |||||
| In Vivo Model |
A total of 96 C57BL/6 male mice (20-25 g) aged 11-12 weeks were purchased from experimental animal center of experimental animal center of Guangdong Medical University. 96 mice were randomly divided into four groups (24 mice per group): Sham group (200 ul of PBS), Sham + BMSCs-Exo group (200 ul of BMSCs-Exo), I/R group (200 ul of PBS) and I/R + BMSCs-Exo group (200 ul of BMSCs-Exo). After 10 days of adaptive feeding, all mice were injected intraperitoneally with 0.4-0.5 mL/100 g 1%Pentobarbital Sodium. I/R and I/R + BMSCs-Exo group mice were subjected to cardiac I/R injury induced by ligation of the left anterior descending artery (LAD) for 30 min followed by 24 h reperfusion. Sham and Sham + BMSCs-Exo mice were sham treated and subjected to the same surgical procedures as I/R mice except that they did not receive ligation of the left anterior descending coronary artery.
Click to Show/Hide
|
||||
| Response Description | Cellular ferroptosis is involved in the pathogenesis of Ischemia-Reperfusion Injury. BMSCs-Exo lncRNA Mir9-3hg can inhibit ferroptosis by modulating the Pum2/PRDX6 axis to exhibit cardioprotective effectsinvivoandinvitro. Silence of PRDX6 markedly decreased cell proliferation, GSH content and Gpx4 protein level, as well as prominently increased iron ion concentration and levels of ROS content and ACSL4 protein in H/R-treated HL-1 cells. | ||||
Metalloproteinase inhibitor 1 (TIMP1)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [94] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 |
| Response Description | TIMP1 depletion in colorectal cancer cells enhances sorafenib-triggered ferroptosis by reducing PI3K/Akt axis signal transduction. TIMP1 knockdown repressed the activation of the PI3K/Akt pathway and reduced levels of glutathione peroxidase 4 (GPX4), enhancing sorafenib-induced ferroptosis. | |||
Maleylacetoacetate isomerase (GSTZ1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [95] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| SNU-449 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
Mice were divided into five groups as follows: WT + DMSO (control), WT + Sora, Gstz1-/-+DMSO, Gstz1-/-+Sora, and Gstz1-/-+Sora + RSL3. Each group included three male and three female mice. At 2 weeks of age, all mice were administered an intraperitoneal injection of diethylnitrosamine (DEN; Sigma, St. Louis, MO, USA) at a dose of 75 mg/kg. At the third week, the mice were intraperitoneally administered carbon tetrachloride (CCl4; Macklin, Shanghai, China) at 2 ml/kg twice a week for 12 weeks. In the WT + Sora and Gstz1-/-+Sora group, the mice at 22 weeks were administered intraperitoneally sorafenib (30 mg/kg) every 2 days for 4 weeks until euthanasia. In the Gstz1-/-+Sora + RSL3 group, in addition to sorafenib administration as described above, the mice were injected intraperitoneally with RSL3 (10 mg/kg) every 2 days for 4 weeks at the same weeks.
Click to Show/Hide
|
||||
| Response Description | GSTZ1 enhanced sorafenib-induced ferroptosis by inhibiting the NRF2/GPX4 axis in hepatocellular carcinoma (HCC) cells. Combination therapy of sorafenib and GPX4 inhibitor RSL3 may be a promising strategy in HCC treatment. | ||||
Lysosome-associated membrane glycoprotein 2 (LAMP2)
Danon disease [ICD-11: 5C51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [96] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| HfRPE (Human retinal pigment epithelial cells) | ||||
| Response Description | Mutations of LAMP2 cause the classic triad of myopathy, cardiomyopathy and encephalopathy of Danon disease (DD). LAMP2-KD reduced the concentration of cytosolic cysteine, resulting in low glutathione (GSH), inferior antioxidant capability and mitochondrial lipid peroxidation. ROS induced RPE cell death through ferroptosis. Inhibition of glutathione peroxidase 4 (GPx4) increased lethality in LAMP2-KD cells compared to controls. | |||
Lon protease homolog, mitochondrial (LONP1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [97] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Pathways in cancer | hsa05200 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 |
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | |
| Response Description | Elevation of mitochondrial LONP1 in erastin-induced ferroptosis of pancreatic cancer cell lines. Inhibition of LONP1 activates the Nrf2/Keap1 signal pathway and up-regulates the expression of GPX4, a key peroxidase in regulating ferroptosis. | |||
Lnc-TC (IncRNA)
Hematotoxicity [ICD-11: 3A70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [98] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
AHH-1 cells | Normal | Homo sapiens | CVCL_3640 |
| Response Description | Lnc-TC/miR-142-5p/CUL4B signaling axis promoted cell ferroptosis to participate in benzene hematotoxicity, and was a potential biomarker for risk screening and health surveillance of benzene-exposed workers. The expression of GPX4 was negatively correlated with both Lnc-TC and CUL4B. | |||
LINC00976 (IncRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [64] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hIBECs (Human intrahepatic biliary epithelial cells) | ||||
| HuCC-T1 cells | Intrahepatic cholangiocarcinoma | Homo sapiens | CVCL_0324 | ||
| HCCC-9810 cells | Intrahepatic cholangiocarcinoma | Homo sapiens | CVCL_6908 | ||
| QBC939 cells | Cholangiocarcinoma | Homo sapiens | CVCL_6942 | ||
| HuH-28 cells | Cholangiocarcinoma | Homo sapiens | CVCL_2955 | ||
| RBE cells | Intrahepatic cholangiocarcinoma | Homo sapiens | CVCL_4896 | ||
| In Vivo Model |
For the proliferation assays, HuCCT1 cells with linc00976 knockdown, linc00976 overexpression, and negative control were subcutaneously injected into BALB/c nude mice. The mice were weighed every week and euthanized 5 weeks after injection. Finally, tumors were dissected and weighed. For the proliferation assays, HuCCT1 cells with linc00976 knockdown, linc00976 overexpression, and negative control were subcutaneously injected into BALB/c nude mice. The mice were weighed every week and euthanized 5 weeks after injection. Finally, tumors were dissected and weighed.
Click to Show/Hide
|
||||
| Response Description | JUND promotes linc00976 transcription, and linc00976 plays a crucial role in accelerating Cholangiocarcinoma tumorigenesis and metastasis and inhibiting ferroptosis by modulating the miR-3202/GPX4 axis. | ||||
LINC00239 (IncRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [99] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| FHC cells | Normal | Homo sapiens | CVCL_3688 | ||
| In Vivo Model |
To clarify the role of LINC00239 in vivo, we used 4-week-old male BALB/c nude mice provided by the Experimental Animal Center of the Air Force Military Medical University. HCT116 or SW620 cells (1 x 107 cells) were injected subcutaneously into the right flanks of these mice to establish a CRC xenograft model. One week after the injection of cells, the volume of xenografts was continuously monitored (once a week). Four weeks later, the xenografts were removed, and the weights were measured.
Click to Show/Hide
|
||||
| Response Description | LINC00239 plays a novel and indispensable role in ferroptosis by nucleotides 1-315 of LINC00239 to interact with the Kelch domain (Nrf2-binding site) of Keap1, inhibiting Nrf2 ubiquitination and increasing Nrf2 protein stability. And LINC00239 expression has a positive correlation with Nrf2 and GPX4 expression in colorectal cancer tissues. LINC00239 inhibition in combination with ferroptosis induction might be a promising therapeutic strategy for CRC patients. | ||||
Krueppel-like factor 2 (KLF2)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [100] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| HK-2 cells | Normal | Homo sapiens | CVCL_0302 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| 769-P cells | Renal cell carcinom | Homo sapiens | CVCL_1050 | ||
| ACHN cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_1067 | ||
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | ||
| In Vivo Model |
BALB/c mice were purchased from the Animal Core Facility of Nanjing Medical University. Injection into the tail vein of 6-week-old male mice with Renca-luci (luciferase) cells (1 x 105 cells) was adopted to build the model oflung metastasis. Before lungs were harvested after 1 month to assess pulmonary metastasis, lung metastatic nodules were tracked with IVIS spectrum imaging system in vivo or not (n = 5/group). For survival analysis, the time of death was recorded in each group (n = 10/group) after cells were injected. For assessing the effect of liproxstatin-1 (Lipro, Sigma), one week after injection of cells in the tail vein, mice were tail intravenous administrated with 2.5 mg/kg Lipro three times on a weekly basis for two weeks, then lungs were harvested ( n= 5/group).
Click to Show/Hide
|
||||
| Response Description | Analysis of clinical specimens revealed that there is a close correlation between KLF2 and GPX4 in clear cell renal cell carcinoma (ccRCC). Mechanistically, KLF2 deficiency is sufficient to inhibit ferroptosis on account of the impairment of transcriptional repression of GPX4 and thus promotes the migration and invasion of RCC cells. | ||||
Junctional adhesion molecule C (JAM3)
Meningiomas [ICD-11: 2A01]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [101] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
In Vitro Model |
hMCCs (Human meningeal cells) | ||||
| hPMCs (Human Primary Meningeal Cells) | |||||
| hMCCs (Human meningeal cells) | |||||
| IOMM-Lee cells | Intracranial meningioma | Homo sapiens | CVCL_5779 | ||
| CTCC-400-0154 (Human malignant meningioma cells) | |||||
| In Vivo Model |
Meningioma model mice were constructed as follows: after abdominal skin disinfection, 1% pentobarbital sodium (135 pL) was intraperitoneally injected into the nude mice. The head of the nude mice was fixed with a stereotaxometer, and two ear needles were symmetrically fixed in the bony parts slightly in front of each ear of the nude mice. The skin of the head of nude mice was cut approximately 0.6 cm lengthwise at 5 mm after the intersection of the inner canthal line and sagittal midline. The skin and fascia on both sides of the incision were bluntly separated with forceps to fully expose the skull. The location of the drill hole was determined according to the stereotactic anatomical map of the head: 0.5 mm behind the intersection of the coronal and sagittal suture and 2.5 mm to the right of the midline. The electric drill drilled a hole of approximately 1 mm at this position. The cells of the third generation of the logarithmic growth stage were taken. The cell suspension was absorbed with a 10 uL microsyringe, and the needle was slowly injected vertically to a depth of approximately 1.5 mm. The cell suspension (5 x 105 cells/100 uL) was slowly injected, and the needle remained for 2 min after injection before being slowly withdrawn.
Click to Show/Hide
|
||||
| Response Description | miR-127-5p Targets JAM3 to Regulate Ferroptosis, Proliferation, and Metastasis in Malignant Meningioma Cells. Upregulation of miR-127-5p increased LDH, MDA, and ROS levels and Fe2+ content and inhibited the expression of GPX4 protein. | ||||
Isocitrate dehydrogenase [NADP], mitochondrial (IDH2)
Fibrosarcoma [ICD-11: 2B53]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [102] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| Hepa 1-6 cells | Hepatocellular carcinoma | Mus musculus | CVCL_0327 | ||
| In Vivo Model |
Eight week-old male nude mice weighing approximately 21-23 g were divided into two groups (DMSO group and erastin group) with 5 mice per group. Each mouse was injected with 5 x 106 cells transfected non-targeting shRNA and idh2-targeting shRNA on the left and right hind legs, respectively. Erastin was administered intraperitoneally at 5 mg/kg for 15 consecutive days starting on the same day as tumor injection.
Click to Show/Hide
|
||||
| Response Description | IDH2 is major enzyme that produces NADPH, which is essential for GSH turnover. The data suggest that decreased growth of tumors with IDH2-knockdown is due to inhibition of Gpx4 followed by a shortage of GSH, resulting in ferroptotic cell death in Fibrosarcoma. | ||||
Interferon alpha-8 (IFNA8)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-7 (IFNA7)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-6 (IFNA6)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-5 (IFNA5)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-4 (IFNA4)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-21 (IFNA21)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-2 (IFNA2)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-17 (IFNA17)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-16 (IFNA16)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-14 (IFNA14)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-10 (IFNA10)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- (IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Interferon alpha-1/13 (IFNA1; IFNA13)
Systemic lupus erythematosus [ICD-11: 4A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hPBMCs (Human peripheral blood mononuclear cells) | ||||
| In Vivo Model |
In ferroptosis rescue experiments, LPX-1 (10 mg/kg), CTX (20 mg/kg) or 0.1 ml DMSO (10%) was administered intraperitoneally to female MRL/Mpj and MRL/lpr mice every other day for six weeks beginning at 12 weeks of age. Urine was collected in the morning once a week. Mice were sacrificed at 18 weeks of age, and spleen, kidneys, lymph nodes, and peripheral blood were collected for analysis. For in vivo treatment, MRL/lprmice were administered 0.1 ml DMSO (10%), Cl-amidine (20 mg/kg), LPX-1 (10 mg/kg), or Cl-amidine (20 mg/kg) combined with LPX-1 (10 mg/kg) intraperitoneally every other day for 3 weeks starting at 12 weeks of age.
Click to Show/Hide
|
||||
| Response Description | IFN- ( IFNA1, IFNA2, IFNA4, IFNA5, IFNA6, IFNA7, IFNA8, IFNA10, IFNA13, IFNA14, IFNA16, IFNA17, IFNA21) and SLE IgG suppresses the transcription of GPX4 by promoting binding of CREM to the Gpx4 promoter. Together, neutrophil ferroptosis is an important driver of neutropenia in systemic lupus erythematosus (SLE) and heavily contributes to disease manifestations. | ||||
Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [74] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| In Vivo Model |
MDA-MB-231 cells with stable RUNX1-IT1 knockdown were injected into NOD/SCID mice, which were randomly divided into three groups, five in each group. The protocol of establishment of breast cancer orthotopic transplantation model was described in our previous study.
Click to Show/Hide
|
||||
| Response Description | RUNX1-IT1 promotes breast cancer carcinogenesis through blocking ferroptosis via elevating GPX4, targeting of the previously unappreciated regulatory axis of RUNX1-IT1/IGF2BP1/GPX4 may be a promising treatment for patient with breast cancer. | ||||
HULC (IncRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [104] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 |
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | |
| Response Description | The expression of ferroptosis-related protein GPX4 decreased after HULC knockdown, and the GPX4 expression level was reversed when the inhibitor miR-3200-5p was added simultaneously. HULC was found to function as a ceRNA of miR-3200-5p, and miR-3200-5p regulates ferroptosis by targeting ATF4, resulting in the inhibition of proliferation and metastasis within hepatocellular carcinoma cells. | |||
hsa-miR-93-5p (miRNA)
Ovarian dysfunction [ICD-11: 5A80]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [105] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| NF-kappa B signaling pathway | hsa04064 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
KGN cells | Ovarian granulosa cell tumor | Homo sapiens | CVCL_0375 | |
| In Vivo Model |
C57BL/6 J (3-week-old) female mice, weighing 15-20 g, were purchased from Beijing Vitalriver Laboratory Animal Technology Co Ltd., China. Experimental animals were randomly divided into control (glycerol treatment) and experimental groups (PCOS, DHEA treatment). The modeling method was as described previously. The PCOS model and control was verified successfully as our previous results, and ovarian tissue was extracted for RT-qPCR detection.
Click to Show/Hide
|
||||
| Response Description | The overexpression of miR-93-5p can promote apoptosis by reducing the expression of Bcl2 and increasing ferroptosis by downregulating GPX4, SLC7A11 and Nrf2 expression in the KGN cell line. miR-93-5p promotes the apoptosis and ferroptosis in GC by regulating the NF-kB signaling pathway. Our study identified miR-93-5p as a new molecular target for improving the function of GCs in polycystic ovary syndrome patients. | ||||
hsa-miR-6516-5p (miRNA)
Endometriosis [ICD-11: GA10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [106] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
In Vitro Model |
hEECs (Human esophageal epithelial cells) | ||||
| mESCs (Mouse endometrial stromal cells) | |||||
| mEESCs (Mouse ectopic endometrial stromal cells) | |||||
| In Vivo Model |
Female BALB/c mice (4-6 weeks old, 18-20 g) were obtained from Shanghai Regan Biotechnology Co., Ltd. (Shanghai, China) and were reared in a specific, pathogen-free facility. After 1 week of acclimatization, mice were randomly divided into two groups: the donor group (n = 10) and recipient groups (n = 10). Ovariosteresis and estradiol valerate injection (0.5 ug/mouse/week; Aladdin, Shanghai, China) was carried out to avoid differences in the estrous cycle. Mice were anesthetized by 2% isoflurane, and then the ovaries on both sides were exposed through flank incisions and removed. Donor mice were sacrificed under isoflurane anesthesia, and each uterine horn of the donor mice was concentrated and peeled in warm PBS to remove uterine muscle. Endometrial tissues were weighed and cut into small fragments with scissors and resuspended in sterile PBS with 1 x ampicillin (Beyotime, Shanghai, China). After that, endometrium preparation was intraperitoneally injected into two recipient mice (50 mg/mouse). Two weeks after EM transplantation, endometriosis lesions and eutopic endometrial tissues were removed from the peritoneal cavities and uteri.
Click to Show/Hide
|
||||
| Response Description | ADAMTS9-AS1 functioned as a competing endogenous RNA (ceRNA) by sponging miR-6516-5p to derepress the expression of GPX4, the critical repressor of ferroptosis. Taken together, these results demonstrate that upregulated ADAMTS9-AS1 accelerates ESC proliferation and migration by regulating miR-6516-5p/GPX4-dependent ferroptosis and may be a potential target for the treatment of Endometriosis. | ||||
hsa-miR-541-3p (miRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [107] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
THLE-2 cells | Normal | Homo sapiens | CVCL_3803 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| In Vivo Model |
The 5-week-old BALB/c male nude mice (n = 10) were purchased from the Animal Center of the Chinese Academy of Medical Sciences (Beijing, China). A number of 2 x 106 HuH-7 cells were transfected with lentiviral vectors containing sh-circIL4R or sh-NC to establish the stably expressed cell lines. Ten mice were subcutaneously injected with HuH-7 cells with sh-circIL4R or sh-NC (five mice per group), constructing the xenograft model of HCC in vivo. Every 5 days after injection, tumor size was measured by a vernier caliper and tumor volume (length x width2 x 0.5) was calculated.
Click to Show/Hide
|
||||
| Response Description | CircIL4R acted as a miR-541-3p sponge to regulate its target glutathione peroxidase 4 (GPX4). GPX4 upregulation relieved the miR-541-3p-induced tumor inhibition and ferroptosis aggravation. CircIL4R played an oncogenic role in hepatocellular carcinoma via the miR-541-3p/GPX4 axis in vivo. | ||||
hsa-mir-539 (Precursor RNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [108] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Six 4-week-old male BALB/c nude mice were ordered from the Shanghai Laboratory Animal Center (Shanghai SLAC Laboratory Animal Co., Ltd., China). A total of 5 x 106 TIPE+/+ SW480 cells were suspended in 100 uL of PBS and subcutaneously injected into the right axilla flank of each nude mouse, and the same amount of vector SW480 cells was into the left. At 2 weeks after inoculation, the xenograft tumor size was measured using Vernier calipers every 2 days.
Click to Show/Hide
|
||||
| Response Description | MiR-539 can bind to and regulate the expression of TIPE, and miR-539 activates SAPK/JNK to downregulate the expression of glutathione peroxidase 4 (GPX4) and promote ferroptosis. In addition, SAPK/JNK is the upstream molecule of p53. MiR-539 is a new therapeutic target for colorectal cancer (CRC) patients. | ||||
hsa-miR-4715-3p (miRNA)
Gastrointestinal cancer [ICD-11: 2B5B]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [109] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
OE33 cells | Barrett adenocarcinoma | Homo sapiens | CVCL_0471 |
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| STKM-2 cells | Gastric carcinoma | Homo sapiens | CVCL_M570 | |
| Response Description | Upper gastrointestinal adenocarcinoma (UGC) tissue samples and cell models demonstrated significant overexpression of AURKA with downregulation of miR-4715-3p. Inhibition of AURKA or reconstitution of miR-4715-3p inhibited GPX4 and induced cell death, suggesting a link between AURKA and ferroptosis. | |||
hsa-miR-3202 (miRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [64] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hIBECs (Human intrahepatic biliary epithelial cells) | ||||
| HuCC-T1 cells | Intrahepatic cholangiocarcinoma | Homo sapiens | CVCL_0324 | ||
| HCCC-9810 cells | Intrahepatic cholangiocarcinoma | Homo sapiens | CVCL_6908 | ||
| QBC939 cells | Cholangiocarcinoma | Homo sapiens | CVCL_6942 | ||
| HuH-28 cells | Cholangiocarcinoma | Homo sapiens | CVCL_2955 | ||
| RBE cells | Intrahepatic cholangiocarcinoma | Homo sapiens | CVCL_4896 | ||
| In Vivo Model |
For the proliferation assays, HuCCT1 cells with linc00976 knockdown, linc00976 overexpression, and negative control were subcutaneously injected into BALB/c nude mice. The mice were weighed every week and euthanized 5 weeks after injection. Finally, tumors were dissected and weighed. For the proliferation assays, HuCCT1 cells with linc00976 knockdown, linc00976 overexpression, and negative control were subcutaneously injected into BALB/c nude mice. The mice were weighed every week and euthanized 5 weeks after injection. Finally, tumors were dissected and weighed.
Click to Show/Hide
|
||||
| Response Description | JUND promotes linc00976 transcription, and linc00976 plays a crucial role in accelerating Cholangiocarcinoma tumorigenesis and metastasis and inhibiting ferroptosis by modulating the miR-3202/GPX4 axis. | ||||
hsa-miR-3200-5p (miRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [104] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 |
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | |
| Response Description | The expression of ferroptosis-related protein GPX4 decreased after HULC knockdown, and the GPX4 expression level was reversed when the inhibitor miR-3200-5p was added simultaneously. HULC was found to function as a ceRNA of miR-3200-5p, and miR-3200-5p regulates ferroptosis by targeting ATF4, resulting in the inhibition of proliferation and metastasis within hepatocellular carcinoma cells. | |||
hsa-miR-30a-5p (miRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [111] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 | |
| KYSE-70 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1356 | ||
| hEECs (Human esophageal epithelial cells) | |||||
| In Vivo Model |
BALB/c nude male mice of 4 weeks old were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). After one week of adaptive feeding, EC9706 cells (3 x 106) stably expressing sh-NC and sh-circPVT1, sh-NC + 5-FU and sh-circPVT1 + 5-FU were subcutaneously were injected into the right flank of the nude mice in a serum-free DMEM medium.
Click to Show/Hide
|
||||
| Response Description | CircPVT1 regulated the chemosensitivity of esophageal squamous cell carcinoma cells through ROS and Wnt/-catenin pathwaysvia miR-30a-5p/FZD3. Knockdown of circPVT1 promoted chemosensitivity in ESCC by increasing ferroptosis via downregulating GPX4 and SLC7A11. | ||||
hsa-miR-27a-3p (miRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [62] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
Eca-109 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | |
| In Vivo Model |
Nude mice of both sexes (age: 6-8 weeks, weight: 22-25 g) were purchased from HUNAN SJA LABRATORY ANIMAL CO., LTD (Hunan, China). The EC109 cells stably expressing sh-circBCAR3 or sh-nc were established by infection with corresponding lentivirus vectors. 1 x 106 mL-1 (100 uL) cells were subcutaneously inoculated into the nude mice. The tumor volumes had been measured from day 5 to day 25. On day 25, the xenograft tumors were removed surgically, and the tumor weight was detected.
Click to Show/Hide
|
||||
| Response Description | CircBCAR3 binds with miR-27a-3p to promote TNPO1 expression. GPX4 protein levels were increased by silencing of circBCAR3. And circBCAR3 promoted the proliferation, migration, invasion, and ferroptosis of esophageal cancer cells by miR-27a-3p. | ||||
hsa-miR-200b-3p (miRNA)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [112] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Downregulation of circ-PSEN1 ameliorates HG-induced ferroptosis in ARPE19 cells via miR-200b-3p/CFL2 and may be a novel therapeutic target for diabetic retinopathy. Enhancement of CFL2 suppressed the mRNA and protein expression of GPX4 and SLC7A11. However, TFR1 expression was promoted. | |||
hsa-miR-19b-3p (miRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [76] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
C57BL/6 mice (4- to 6-week-old male) were fed in a pathogen-free vivarium under standard conditions at the animal care facility at Sun Yat-sen University. Hepa 1-6 cells transduced with RBMS1 or GPX4 or circIDE overexpression lentiviral vectors were subcutaneously injected into the right flank of mice in 100 ul of sterile PBS. IVIS images were taken.
Click to Show/Hide
|
||||
| Response Description | RBMS1 overexpression inhibited hepatocellular Carcinoma (HCC) cell growth by attenuating the expression of glutathione peroxidase 4 (GPX4)and further facilitated ferroptosis in vitro and in vivo. More importantly, a novel circIDE (hsa_circ_0000251) was identified to elevate RBMS1 expression via sponging miR-19b-3p in HCC cells. | ||||
hsa-miR-193a-5p (miRNA)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [113] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SiHa cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| Response Description | miR-193a-5p was able to target GPX4 and circACAP2 promoted GPX4 expression by sponging miR-193a-5p in cervical cancer cells.Therefore, we concluded that circular RNA circACAP2 repressed ferroptosis of cervical cancer during malignant progression by miR-193a-5p/GPX4. | |||
hsa-miR-15a-3p (miRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [114] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| KM12 cells | Colon carcinoma | Homo sapiens | CVCL_1331 | ||
| NCM460 cells | Normal | Homo sapiens | CVCL_0460 | ||
| In Vivo Model |
We obtained the 6-week-old nude mice (BALB/c) from Beijing HFK Bioscience Co., Ltd. and randomly divided into eight groups (n = 5/group): (a) NC + dimethyl sulfoxide (DMSO) group; (b) NC + erastin group; (c) mimic + DMSO group; (d) mimic + erastin group; (e) iNC + DMSO group; (f) iNC + erastin group; (g) inhibitor + DMSO group; (h) inhibitor + erastin group. Control and transfected cells (7 x 106) were subcutaneously injected into the nude mice. After the tumor sizes reached roughly >50 mm3, mice in Groups B, D, F, and H were treated with 15 mg/kg erastin twice every day for about 20 days. Meanwhile, mice in Groups A, C, E, and G were treated with an equal volume DMSO. Besides, tumor formation and mass were monitored and the size of tumor was counted using the formula V = 0.5 x L x W2 (L, length and W, width). At last, after killing the nude mice and then we isolated the mice tumor tissues.
Click to Show/Hide
|
||||
| Response Description | Overexpression of miR-15a-3p repressed GPX4 through binding to the 3'-untranslated region of GPX4, resulting in increased reactive oxygen species level, intracellular Fe2+ level, and malondialdehyde accumulation in vitro and in vivo. MiR-15a-3p suppressed colorectal cancer cell growth and enhanced cell ferroptosis by inactivating GPX4. | ||||
hsa-mir-15a (Precursor RNA)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [115] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
LNCaP cells | Prostate carcinoma | Homo sapiens | CVCL_0395 |
| Response Description | MiR-15a induces ferroptosis by regulating GPX4 in prostate cancer cells, which provides evidence for investigating the therapeutic strategies of prostate cancer. | |||
hsa-miR-150-5p (miRNA)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [57] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
To simulate the animal model of diabetic cardiomyopathy, male db/+ mice and db/db mice (age, 7 weeks, weight, 24 g) were fed a normal diet for 4 weeks and kept at 24 under a 14-h light/8-h dark cycle. The animals were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). Diabetic mice were intracoronarily administered equal volumes (80 ul) of adenoviruses Ad-ZFAS1, Ad-sh-ZFAS1, Ad-CCND2, Ad-sh-CCND2 or Ad-NC.33 miR-150-5p mimics and mimic control (NC) were injected into the tail vein of mice (50 ug/kg) every 15 days for 12 weeks. Db/db mice were treated with or without ferrostatin-1 (Fer-1, ferroptosis inhibitor; Sigma-Aldrich, 5 mg/kg) for an additional 12 weeks.
Click to Show/Hide
|
||||
| Response Description | lncRNA-ZFAS1 acted as a ceRNA to sponge miR-150-5p and downregulate CCND2 to promote cardiomyocyte ferroptosis and Diabetic cardiomyopathy development. Inhibition of ZFAS1 restored the expression of FTH1, reduced the expression of 4HNE, rescued the expression of GPX4 and inhibited the expression of apoptosisrelated genes. | ||||
hsa-miR-144-3p (miRNA)
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [55] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | |
| SW1353 cells | Bone chondrosarcoma | Homo sapiens | CVCL_0543 | ||
| MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | ||
| SAOS-2 cells | Osteosarcoma | Homo sapiens | CVCL_0548 | ||
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | ||
| HOB (Human normal osteoblastic cells) | |||||
| In Vivo Model |
The OS model of nude mice was constructed using the CDTX model. After transfection, the h143B cells were prepared into a single-cell suspension and subcutaneously injected into the left proximal tibia of 36 (3 mice per group) 4-weeks-old nude mice (1 x 107 cells per mouse).
Click to Show/Hide
|
||||
| Response Description | MiR-144-3p can induce the occurrence of ferroptosis by negatively regulating the expression of ZEB1, thereby inhibiting the proliferation, migration, and invasion of osteosarcoma (OS) cells. The overexpression of ZEB1 caused the lower expression level of ACSL4 and higher expression level of xCT and GPX4. | ||||
hsa-miR-142-5p (miRNA)
Hematotoxicity [ICD-11: 3A70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [98] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
AHH-1 cells | Normal | Homo sapiens | CVCL_3640 |
| Response Description | Lnc-TC/ miR-142-5p/CUL4B signaling axis promoted cell ferroptosis to participate in benzene hematotoxicity, and was a potential biomarker for risk screening and health surveillance of benzene-exposed workers. The expression of GPX4 was negatively correlated with both Lnc-TC and CUL4B. | |||
hsa-miR-140-5p (miRNA)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [116] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Wnt signaling pathway | hsa04310 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPNs (Rat primary neurons) | ||||
| In Vivo Model |
C57BL/6 J pregnant mice (E15 ~ E16 day) were disinfected with 75% alcohol and then decapitated. The brain was collected and washed in pre-cold D-Hanks solution with the midbrain and hippocampus being removed. The cortex was collected and the meninx was removed. Then the brain tissues were made into 1 mm3 blocks for digestion with 1 ~ 2 mL lysis at 37 for 15 min. The tissues were cultured in high glucose DMEM to terminate the digestion and made into single-cell suspension. The suspension was filtered through a 70 um mesh screen for centrifugation for 5 min with the supernatant being removed. High glucose DMEM was used to re-suspended cells and the concentration of cells was adjusted for cells were seeded into the plate. About 4 h later, the high glucose DMEM was replaced with Neurobasal medium (containing 2% B27). The culture medium was half refreshed every 2 ~ 3 d. The cells were cultured for 8 ~ 10 d before following experiments.
Click to Show/Hide
|
||||
| Response Description | Treatment of miR-1405p mimics led to decreased COX-2 and ACSL4 expressions, and elevated GPX-4 expressions. circAFF1 can bind miR-1405p to up-regulate GSK-3 expression, thus inhibiting the Wnt/-catenin signaling pathway. And circAFF1 knockdown can suppress ferroptosis of neurons in vitro and therefore attenuate intracerebral hemorrhage (ICH). | ||||
hsa-miR-135b-3p (miRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [117] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague-Dawley rats aged 8-10 weeks and weighing 220 g were obtained from the Nanjing Biomedical Research Institute of Nanjing University. Following acclimatization for 1 week, the rats were divided into five groups of six rats each before the experiment. The establishment of the myocardial I/R model was based on previous studies . Sodium pentobarbital (45 mg/kg, i.p.) was used to anesthetize the rats, and the left coronary artery (LCA) was exposed using left thoracotomy at the fifth intercostal space. Following the LCA ligation with 7-0 silk sutures, a smooth catheter was applied to the artery to achieve ischemia for 30 min. The rats were then sacrificed 120 min after reperfusion. Rats in the sham group (without the LCA I/R) underwent surgery and were treated with saline. The miR-135b-3p group rats were injected with miR-135b-3p overexpression virus or knockdown lentivirus (1 x 108 U/ml, 0.2 ml), respectively, for five consecutive days before surgery.
Click to Show/Hide
|
||||
| Response Description | MiR-135b-3p was found to promote the myocardial I/R injury by downregulating GPX4 expression. The results of this study elucidate a novel function of miR-135b-3p in exacerbating cardiomyocyte ferroptosis, providing a new therapeutic target for improving myocardial ischemia/reperfusion injury. | ||||
hsa-mir-132 (Precursor RNA)
Atherosclerosis [ICD-11: BD40]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [118] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | MiR-132 promotes atherosclerosis by inducing mitochondrial oxidative stress-mediated ferroptosis, which may serve as a promising therapeutic target for atherosclerosis. The key iron death protein GPX4 was significantly down-regulated and the oxidized protein NOX4 was significantly increased in miR-132-overexpressing HUVECs (P < 0.001). | |||
hsa-miR-1287-5p (miRNA)
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [119] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hFOB 1.19 cells | Normal | Homo sapiens | CVCL_3708 |
| SAOS-2 cells | Osteosarcoma | Homo sapiens | CVCL_0548 | |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| Response Description | MiR-1287-5p directly bound to the 3'-untranslated region of glutathione peroxidase 4 (GPX4) to inhibit its protein level and activity, and that GPX4 overexpression completely abolished the miR-1287-5p mimic-mediated ferroptotic induction and tumor suppression. The findings prove that miR-1287-5p promotes ferroptosis of osteosarcoma cells through inhibiting GPX4. | |||
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [120] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 | ||
| NCI-H522 cells | Non-small cell lung carcinoma | Homo sapiens | CVCL_1567 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| In Vivo Model |
The Shanghai SLAC Animal Center (Shanghai, China) provided 4-6-week-old BALB/c male nude mice, which were kept according to the standards for the use and care of laboratory animals. A total of 1 x 107 NSCLC cells infected with shRNA were injected subcutaneously into the left flank of nude mice (3 per group). Every 3 days, the tumor volume was measured.
Click to Show/Hide
|
||||
| Response Description | It was identified that circDTL exerts its oncogenic effects via the circDTL/miR-1287-5p/GPX4 axis and GPX4 inhibits both ferroptosis and apoptosis. Finally, silencing of circDTL promoted the sensitivity of non-small cell lung cancer cells to chemotherapeutic agents and inhibited the growth of tumors in vivo. | ||||
hsa-miR-127-5p (miRNA)
Meningiomas [ICD-11: 2A01]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [101] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
In Vitro Model |
hMCCs (Human meningeal cells) | ||||
| hPMCs (Human Primary Meningeal Cells) | |||||
| hMCCs (Human meningeal cells) | |||||
| IOMM-Lee cells | Intracranial meningioma | Homo sapiens | CVCL_5779 | ||
| CTCC-400-0154 (Human malignant meningioma cells) | |||||
| In Vivo Model |
Meningioma model mice were constructed as follows: after abdominal skin disinfection, 1% pentobarbital sodium (135 pL) was intraperitoneally injected into the nude mice. The head of the nude mice was fixed with a stereotaxometer, and two ear needles were symmetrically fixed in the bony parts slightly in front of each ear of the nude mice. The skin of the head of nude mice was cut approximately 0.6 cm lengthwise at 5 mm after the intersection of the inner canthal line and sagittal midline. The skin and fascia on both sides of the incision were bluntly separated with forceps to fully expose the skull. The location of the drill hole was determined according to the stereotactic anatomical map of the head: 0.5 mm behind the intersection of the coronal and sagittal suture and 2.5 mm to the right of the midline. The electric drill drilled a hole of approximately 1 mm at this position. The cells of the third generation of the logarithmic growth stage were taken. The cell suspension was absorbed with a 10 uL microsyringe, and the needle was slowly injected vertically to a depth of approximately 1.5 mm. The cell suspension (5 x 105 cells/100 uL) was slowly injected, and the needle remained for 2 min after injection before being slowly withdrawn.
Click to Show/Hide
|
||||
| Response Description | miR-127-5p Targets JAM3 to Regulate Ferroptosis, Proliferation, and Metastasis in Malignant Meningioma Cells. Upregulation of miR-127-5p increased LDH, MDA, and ROS levels and Fe2+ content and inhibited the expression of GPX4 protein. | ||||
hsa-miR-124-3p (miRNA)
Cardiovascular diseases [ICD-11: BE2Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [71] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hAAFs (Human aortic adventitial fibroblasts) | |||
| Response Description | UII inhibited miR-124 expression through up-regulating circ0004372 expression, thereby promoting SERTAD4 expression. UII significantly promoted the generation of ROS, MDA and 4-HNE, reduced the activities of SOD, GST and GR, increased Fe2+ concentration and inhibited GPX4 expression through circ0004372/ miR-124/SERTAD4 for cardiovascular diseases. | |||
hsa-miR-1231 (miRNA)
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [121] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
KAT-5 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0370 | |
| TPC-1 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | ||
| In Vivo Model |
KAT-5 and TPC-1 cells (2 x 107) were subcutaneously injected into nude mice (four mice/group, 4-week-old) and treated with intratumoral injection (50 uL si-circCON, si-circKIF4A) every four days. The volume of tumors was estimated every four days according to the formula 0.5 x width2 x length. After 28 days, the tumors were weighed. Forin vivolung metastasis assay, cells (1 x 105) were injected through tail veins (four mice/group).
Click to Show/Hide
|
||||
| Response Description | circKIF4A facilitated the malignant progress of papillary thyroid tumor by sponging miR-1231 and upregulating GPX4 expression. And circKIF4A- miR-1231-GPX4 axis played a vital role in cancer proliferation and ferroptosis by competing endogenous RNAs. Therefore, targeting circKIF4A is very likely to be a potential method for treatment of papillary thyroid cancer in the future. | ||||
hsa-miR-10a-5p (miRNA)
Intervertebral disc degeneration [ICD-11: FA80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [122] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hCDs (Chondrocytes) | |||
| Response Description | Inflammatory cytokine IL-6 appeared in Intervertebral disc degeneration (IDD) aggravates its degeneration by inducing cartilage cell ferroptosis. This is caused partially by inhibiting miR-10a-5p and subsequently derepressing IL-6R signaling pathway. The ferroptosis-inhibitory effect exhibited by overexpressing miR-10a-5p was achieved by promoting GPX4 and ferroportin-1 (FPN1) but suppressing divalent metal transporter 1 (DMT1) expression in IL-6-treated cartilage cells. | |||
hsa-miR-1-3p (miRNA)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [123] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HOSE 96-9-98 cells | Normal | Homo sapiens | CVCL_UW70 |
| HO8910 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6868 | |
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| Response Description | FZD7 was a direct target of miR-1-3p, which inhibited the expression of FZD7 by binding to the 3'-untranslated region (3'UTR) site of FZD7. In ovarian cancer tissues, overexpression of FZD7 reduced the sensitivity of platinum-resistant ovarian cancer cells to ferroptosis by up-regulating GPX4 expression. | |||
Histone-lysine N-methyltransferase EZH2 (EZH2)
Diffuse large B-cell lymphoma [ICD-11: 2A81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [124] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Karpas-422 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1325 | |
| U-2932 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1896 | ||
| WILL-2 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1901 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal experiments were approved by the Institutional Animal Care and Use Committee of Shanghai Institute of Materia Medica and performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care. Tumors were measured twice weekly and volumes were calculated using the formula TV=length x width2 x 1/2.
Click to Show/Hide
|
||||
| Response Description | EZH2 inhibition upregulated-TfR-1 dysregulation leads to drug resistance in EZH2 WT diffuse large B-cell lymphoma (DLBCL). On the other hand, EZH2i impaired the occurrence of ferroptosis by upregulating the heat shock protein family A (Hsp70) member 5 (HSPA5) and stabilizing glutathione peroxidase 4 (GPX4), a ferroptosis suppressor. | ||||
HEPFAL (IncRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [125] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | |
| Response Description | LncRNA HEPFAL promotes the ubiquitination of SLC7A11, resulting in a decrease in GSH production, which in turn affects the activity of GPX4 and ultimately leads to the occurrence of ferroptosis. And LncRNA HEPFAL has the potential as a target for the diagnosis and treatment of hepatocellular carcinoma. | |||
Heat shock factor protein 1 (HSF1)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [126] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Palmitic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Hsf1 and Hsf1+/+-/- mice were kindly given as a present by Dr. Ivor J. Benjamin (Froedtert & Medical College of Wisconsin, Milwaukee, WI, USA). Sex-matched Hsf1-/- mice and Hsf1 littermates were used at 16-20 weeks old. Each mouse was injected intraperitoneally with 2.5 umol PA (dissolved in 0.5 mL 10% BSA) or an equal volume of BSA twice daily for 7 days.
Click to Show/Hide
|
||||
| Response Description | Palmitic acid (PA) decreased the protein expression levels of both heat shock factor 1 (HSF1) and glutathione peroxidase 4 (GPX4) in a dose- and time-dependent manner, which were restored by different ferroptosis inhibitors. Altogether, HSF1 may function as a key defender against PA-induced ferroptosis in cardiomyocytes by maintaining cellular iron homeostasis and GPX4 expression. | ||||
H19 (IncRNA)
Spontaneous abortion [ICD-11: JA00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [127] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
HTR-8/SVneo cells | Normal | Homo sapiens | CVCL_7162 |
| Response Description | Silencing H19 downregulated Bcl-2 and GPX4 expression and upregulated Bax expression at both the mRNA and protein levels in HTR-8/SVneo trophoblast cells. In conclusion, the present findings suggested that H19 has important roles in spontaneous abortion (SA) by promoting apoptosis and ferroptosis. | |||
Glycogen synthase kinase-3 beta (GSK3B)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [116] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Wnt signaling pathway | hsa04310 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPNs (Rat primary neurons) | ||||
| In Vivo Model |
C57BL/6 J pregnant mice (E15 ~ E16 day) were disinfected with 75% alcohol and then decapitated. The brain was collected and washed in pre-cold D-Hanks solution with the midbrain and hippocampus being removed. The cortex was collected and the meninx was removed. Then the brain tissues were made into 1 mm3 blocks for digestion with 1 ~ 2 mL lysis at 37 for 15 min. The tissues were cultured in high glucose DMEM to terminate the digestion and made into single-cell suspension. The suspension was filtered through a 70 um mesh screen for centrifugation for 5 min with the supernatant being removed. High glucose DMEM was used to re-suspended cells and the concentration of cells was adjusted for cells were seeded into the plate. About 4 h later, the high glucose DMEM was replaced with Neurobasal medium (containing 2% B27). The culture medium was half refreshed every 2 ~ 3 d. The cells were cultured for 8 ~ 10 d before following experiments.
Click to Show/Hide
|
||||
| Response Description | Treatment of miR-1405p mimics led to decreased COX-2 and ACSL4 expressions, and elevated GPX-4 expressions. circAFF1 can bind miR-1405p to up-regulate GSK-3 expression, thus inhibiting the Wnt/-catenin signaling pathway. And circAFF1 knockdown can suppress ferroptosis of neurons in vitro and therefore attenuate intracerebral hemorrhage (ICH). | ||||
Glucose-6-phosphate 1-dehydrogenase (G6PD)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [87] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
WRL 68 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0581 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| SNU-387 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0250 | ||
| In Vivo Model |
Animal experiments were conducted under the guidance of Animal Management Regulations in Chongqing University. The tumor volume was calculated as follows: (length x width2)/2. After 24 days, the mice were killed and their tumors were collected, fixed and sectioned, stained by hematoxylin and eosin, and examined by a light microscopy for histological changes.
Click to Show/Hide
|
||||
| Response Description | G6PD (glucose-6-phosphate dehydrogenase) was highly expressed in hepatocellular carcinoma and was associated with poor prognosis. G6PD promoted the proliferation, migration and invasion, as well as inhibited ferroptosis in HCC cells. G6PD inhibited ferroptosis inin HCC cells through POR. GPX4 was positively regulated by G6PD. | ||||
G1/S-specific cyclin-D2 (CCND2)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [57] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
To simulate the animal model of diabetic cardiomyopathy, male db/+ mice and db/db mice (age, 7 weeks, weight, 24 g) were fed a normal diet for 4 weeks and kept at 24 under a 14-h light/8-h dark cycle. The animals were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). Diabetic mice were intracoronarily administered equal volumes (80 ul) of adenoviruses Ad-ZFAS1, Ad-sh-ZFAS1, Ad-CCND2, Ad-sh-CCND2 or Ad-NC.33 miR-150-5p mimics and mimic control (NC) were injected into the tail vein of mice (50 ug/kg) every 15 days for 12 weeks. Db/db mice were treated with or without ferrostatin-1 (Fer-1, ferroptosis inhibitor; Sigma-Aldrich, 5 mg/kg) for an additional 12 weeks.
Click to Show/Hide
|
||||
| Response Description | lncRNA-ZFAS1 acted as a ceRNA to sponge miR-150-5p and downregulate CCND2 to promote cardiomyocyte ferroptosis and Diabetic cardiomyopathy development. Inhibition of ZFAS1 restored the expression of FTH1, reduced the expression of 4HNE, rescued the expression of GPX4 and inhibited the expression of apoptosisrelated genes. | ||||
Furin (FURIN)
Ulcerative colitis [ICD-11: DD71]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [128] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
NCM460 cells | Normal | Homo sapiens | CVCL_0460 | |
| In Vivo Model |
Male C57BL/6 mice wild-type (WT), 8 weeks of age, were from Chongqing Medical University, China. Mice were divided into four groups (n = 10-13 per group), control group, MPTP group, h-Trx-1 Tg group, and h-Trx-1 Tg + MPTP group. Control and h-Trx-1 Tg groups were administered saline only. For the Trx-1 knockdown experiment, mice were divided into six groups (n = 10-13 per group), control + saline group, control + MPTP group, AAV9-vehicle + saline group, AAV9-vehicle + MPTP group, AAV9-shRNA-mTrx-1 + saline group, and AAV9-shRNA-mTrx-1 + MPTP.
Click to Show/Hide
|
||||
| Response Description | Furin protects epithelial cells from DSS-induced ferroptosis-like cell injury and alleviates experimental ulcerative colitis by activating the Nrf2-Gpx4 signaling pathway. | ||||
Fumarate hydratase, mitochondrial (FH)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [129] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
UOK262 cells | Hereditary leiomyomatosis | Homo sapiens | CVCL_1D72 |
| HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| Response Description | Hereditary leiomyomatosis and renal cell cancer (HLRCC) is a hereditary cancer syndrome characterized by inactivation of the Krebs cycle enzyme fumarate hydratase (FH). Mechanistically, the FH sensitivity to ferroptosis is attributed to dysfunctional GPX4, the primary cellular defender against ferroptosis. | |||
Frizzled-7 (FZD7)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [123] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HOSE 96-9-98 cells | Normal | Homo sapiens | CVCL_UW70 |
| HO8910 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6868 | |
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| Response Description | FZD7 was a direct target of miR-1-3p, which inhibited the expression of FZD7 by binding to the 3'-untranslated region (3'UTR) site of FZD7. In ovarian cancer tissues, overexpression of FZD7 reduced the sensitivity of platinum-resistant ovarian cancer cells to ferroptosis by up-regulating GPX4 expression. | |||
Frizzled-3 (FZD3)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [111] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 | |
| KYSE-70 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1356 | ||
| hEECs (Human esophageal epithelial cells) | |||||
| In Vivo Model |
BALB/c nude male mice of 4 weeks old were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). After one week of adaptive feeding, EC9706 cells (3 x 106) stably expressing sh-NC and sh-circPVT1, sh-NC + 5-FU and sh-circPVT1 + 5-FU were subcutaneously were injected into the right flank of the nude mice in a serum-free DMEM medium.
Click to Show/Hide
|
||||
| Response Description | CircPVT1 regulated the chemosensitivity of esophageal squamous cell carcinoma cells through ROS and Wnt/-catenin pathwaysviamiR-30a-5p/FZD3. Knockdown of circPVT1 promoted chemosensitivity in ESCC by increasing ferroptosis via downregulating GPX4 and SLC7A11. | ||||
Fatty acid-binding protein, liver (FABP1)
Immunoglobulin A nephropathy [ICD-11: MF8Y]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [84] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hMCs (Human mesangial cells) | |||
| Response Description | In PPAR lentivirus-transfected HMCs stimulated by Gd-IgA1, ROS, MDA, and ACSL4 were decreased; glutathione and GPX4, and immunofluorescence colocalization of PPAR and GPX4, increased; and damaged mitochondria reduced. Hence, PPAR pathway downregulation can reduce FABP1 expression, affecting GPX4 and ACSL4 levels, causing HMC ferroptosis, and contributing to immunoglobulin A nephropathy (IgAN) pathogenesis. | |||
Epidermal growth factor receptor (EGFR)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [130] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hTERT-HME1 cells | Normal | Homo sapiens | CVCL_3383 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | ||
| In Vivo Model |
2.5 x 105 NCI-H1650 cells were inoculated 1:1 in Matrigel: PBS (100 mL) by subcutaneous injection into eight non-obese diabetic (NOD) severe combined immunodeficiency (SCID) gamma male mice. Tumors were allowed to engraft and grow for 30 days (tumor volume averaged ~200 mm3) and mice treated by intraperitoneal (i.p.) injection with 100 mg/kg cyst(e)inase or 100 mg/kg heat-inactivated cyst(e)inase (n = 4 ea.) on day 30, with a second dose given on day 33. Mice were necropsied 24 hr after the second dose.
Click to Show/Hide
|
||||
| Response Description | In non-small-cell lung cancer (NSCLC) cells, active MAPK signaling downstream of active EGFR can sensitize cells to ferroptosis upon cystine depletion. Sensitization involves both impaired detoxification of lipid peroxides, due to reduced expression of GPX4, and generation of hydrogen peroxide, via NOX4. | ||||
ELAV-like protein 1 (ELAVL1)
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [133] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In Vivo Model |
Rats were placed on a heating panel after anesthetized with pentobarbital sodium (30 mg/kg). We operated the intraluminal middle cerebral artery occlusion (MCAO) to establish the focal cerebral ischemia. Then 2 h later, we established the reperfusion. In brief, the left internal carotid artery of the rats was isolated. Then the ligation of middle cerebral artery was performed by a 4/0 surgical nylon monofilament to occlude the blood flow. 2 h later, we removed the filament to restore the blood reperfusion for 24 h.
Click to Show/Hide
|
||||
| Response Description | ELAVL1 silencing observably facilitated cell viability, GSH content, GPX4 and SLC7A11 expression. ELAVL1 plays a critical role in protecting against ferroptosis-induced cerebral I/R and subsequent brain damage via DNMT3B/PINK1 axis, thus providing a new potential target for ischemic stroke treatment. | ||||
Early growth response protein 1 (EGR1)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [93] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
The male C57BL/6 mice (20-25 g, 10-week-old) were purchased from the Experimental Animal Center of Harbin Medical University (Harbin, China). Mice were anaesthetized with pentobarbital sodium (30 mg/kg, Sigma-Aldrich, St. Louis, USA) by intraperitoneal injection. The animals were fixed on the operating table in supine position, and the chest were sterilized and opened by blunt separation at the left 4th intercosal space. In the model group, the left anterior descending artery (LAD) was ligated with 7/0 silk suture for 3 days.
Click to Show/Hide
|
||||
| Response Description | GPX4 was the direct target of miR-15a-5p by luciferase reporter assay. Mechanistically, silencing transcription factor early growth response-1 ( Egr-1) inhibited the level of miR-15a-5p, increased the protein expression of GPX4, accompanied by reduced ferroptosis and alleviated myocardial injury. These results provide a novel signaling pathway during the progression of acute myocardial infarction, namely Egr-1/miR-15a-5p/GPX4/ferroptosis. | ||||
E3 ubiquitin-protein ligase MARCHF5 (MARCHF5)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [135] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
In all experiments using KO mice (male, 8-12 weeks), we used age-matched, tamoxifen-treated MerCreMer single-genotype animals as controls. For inducing cardiac-specific knockout of MITOL, an ethanol-corn oil emulsion of tamoxifen (TAX; H5648, Sigma-Aldrich) was injected intraperitoneally per day of 30 mg tamoxifen/kg body weight (150 mg/kg total) for 5 days.
Click to Show/Hide
|
||||
| Response Description | MITOL/ MARCH5 is an E3 ubiquitin ligase that plays a crucial role in the control of mitochondrial quality and function. The mitochondrial ubiquitin ligase MITOL is identified as a novel regulator of DOX-induced cardiomyopathy. A knockdown of MITOL in cardiomyocytes reduced GPX4 to induce the accumulation of lipid peroxide, resulting in ferroptosis. | ||||
DnaJ homolog subfamily B member 6 (DNAJB6)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [136] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 | ||
| Eca-109 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | ||
| KYSE150 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1348 | ||
| KYSE-450 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1353 | ||
| In Vivo Model |
Female BALB/c athymic nude mice (4 weeks of age) were obtained from the HFK Bioscience Co, Beijing. To generate murine subcutaneous tumors, 2 x 106 Eca109 cells and KYSE 150 cells in 100 ul PBS were injected subcutaneously on the left of the nude mices dorsal midline. The xenografts were measured every 4 days.
Click to Show/Hide
|
||||
| Response Description | The correlation between DNAJB6 level and lymph node metastasis in esophageal squamous cell carcinoma (ESCC) patient was negative. Overexpressing DNAJB6a shows tumor-suppressive effects in vitro and in vivo. In addition, DNAJB6a overexpression was accompanied together with a remarkable reduction in the protein levels of GPX4 and phosphorylated AKT (p-AKT). | ||||
Dihydroorotate dehydrogenase (quinone), mitochondrial (DHODH)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [137] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
UM-RC-2 cells | Clear cell renal carcinoma | Homo sapiens | CVCL_2739 | |
| UM-RC-6 cells | Renal cell carcinoma | Homo sapiens | CVCL_2741 | ||
| RCC4 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0498 | ||
| TK-10 cells | Renal carcinoma | Homo sapiens | CVCL_1773 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| NCI-H226 cells | Pleural epithelioid mesothelioma | Homo sapiens | CVCL_1544 | ||
| In Vivo Model |
5 x 106 HT-1080 or 1 x 107 NCI-H226 cells were injected into mice subcutaneously. When the tumor reached 50-100 mm3, the mice were assigned randomly into different treatment groups. Brequinar or sulfasalazine was dissolved in dimethyl sulfoxide (DMSO) and diluted in PBS. Brequinar was intraperitoneally injected into mice at a dose of 30 mg/kg every three days. Sulfasalazine was intraperitoneally injected daily at a dose of 100 mg/kg. Liproxstatin-1 diluted in PBS was intraperitoneally injected daily at a dose of 10 mg/kg. The daily injection of brequinar, sulfasalazine, or liproxstatin-1 was continued until the endpoint as indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | DHODH operates in parallel to mitochondrial GPX4 (but independently of cytosolic GPX4 or FSP1) to inhibit ferroptosis in the mitochondrial inner membrane by reducing ubiquinone to ubiquinol (a radical-trapping antioxidant with anti-ferroptosis activity) in Clear cell renal carcinoma. | ||||
Delta(3,5)-Delta(2,4)-dienoyl-CoA isomerase, mitochondrial (ECH1)
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [138] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Six-week-old male C57BL/6 mice were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All of the mice were fed either a standard chow diet (SCD) (containing 62.2% carbohydrate, 24.6% protein, and 13.2% fat) or only a methionine-choline deficient diet (MCD) (containing 20% carbohydrate, 20% protein, and 60% fat) for 8 wk.
Click to Show/Hide
|
||||
| Response Description | GPX4, a crucial regulator of ferroptosis, were upregulated in the livers of the ECH1-overexpressing mice. ECH1 knockdown exacerbated nonalcoholic steatohepatitis (NASH) progression, but this phenomenon was reversed through ferroptosis inhibition. | ||||
Cysteine--tRNA ligase, cytoplasmic (CARS1)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [139] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
KYSE30 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1351 |
| KYSE-410 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1352 | |
| Response Description | CARS1 significantly inhibited cell proliferation, and the ability of migration and invasion promoted the relative level of MDA and ROS and decreased GPX4 expression level in two esophageal squamous cell carcinoma(ESCC) cell lines. | |||
Cysteine dioxygenase type 1 (CDO1)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [63] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| In Vivo Model |
1 x 106 BGC823 control or CDO1 short hairpin (sh)RNA treated cells in 150 ul PBS were injected subcutaneously right of the dorsal midline in athymic nude mice. Once the tumors reached 80 to 100 mm3 at day 10, mice were allocated randomly into groups of five and treated with erastin (30 mg/kg intraperitoneally, twice every other day).
Click to Show/Hide
|
||||
| Response Description | Silencing CDO1 inhibited erastin-induced ferroptosis in gastric cancer cells both in vitro and in vivo. Mechanistically, c-Myb transcriptionally regulated CDO1, and inhibition of CDO1 expression upregulated GPX4 expression. | ||||
Cyclin-dependent kinase inhibitor 1 (CDKN1A)
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [77] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| HIEC-6 cells | Normal | Homo sapiens | CVCL_6C21 | |
| Response Description | RRM1 increases the instability of p53 by regulating the physical interaction of p53 with the ubiquitinating enzyme MDM2 and the deubiquitinating enzyme USP11, subsequently suppressing p21 (CDKN1A) and GPX4, thereby promoting the accumulation of lipid peroxidation and occurrence of radiation-induced ferroptosis in Colon carcinoma. | |||
Cyclic AMP-responsive element-binding protein 5 (CREB5)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [140] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | |
| WI-38 cells | Normal | Homo sapiens | CVCL_0579 | |
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| NCI-H226 cells | Pleural epithelioid mesothelioma | Homo sapiens | CVCL_1544 | |
| NCI-H358 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1559 | |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| Response Description | It was observed that CREB (CREB1, CREB3 and CREB5) suppressed lipid peroxidation by binding the promoter region of glutathione peroxidase 4 (GPX4), and this binding could be enhanced by E1A binding protein P300 (EP300). Therefore, targeting this CREB/EP300/GPX4 axis may provide new strategies for treating lung adenocarcinoma. | |||
Cyclic AMP-responsive element-binding protein 3 (CREB3)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [140] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | |
| WI-38 cells | Normal | Homo sapiens | CVCL_0579 | |
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| NCI-H226 cells | Pleural epithelioid mesothelioma | Homo sapiens | CVCL_1544 | |
| NCI-H358 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1559 | |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| Response Description | It was observed that CREB (CREB1, CREB3 and CREB5) suppressed lipid peroxidation by binding the promoter region of glutathione peroxidase 4 (GPX4), and this binding could be enhanced by E1A binding protein P300 (EP300). Therefore, targeting this CREB/EP300/GPX4 axis may provide new strategies for treating lung adenocarcinoma. | |||
Cyclic AMP-responsive element-binding protein 1 (CREB1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [140] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | |
| WI-38 cells | Normal | Homo sapiens | CVCL_0579 | |
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| NCI-H226 cells | Pleural epithelioid mesothelioma | Homo sapiens | CVCL_1544 | |
| NCI-H358 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1559 | |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| Response Description | It was observed that CREB (CREB1, CREB3 and CREB5) suppressed lipid peroxidation by binding the promoter region of glutathione peroxidase 4 (GPX4), and this binding could be enhanced by E1A binding protein P300 (EP300). Therefore, targeting this CREB/EP300/GPX4 axis may provide new strategies for treating lung adenocarcinoma. | |||
Cyclic AMP-dependent transcription factor ATF-4 (ATF4)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [104] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 |
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | |
| Response Description | The expression of ferroptosis-related protein GPX4 decreased after HULC knockdown, and the GPX4 expression level was reversed when the inhibitor miR-3200-5p was added simultaneously. HULC was found to function as a ceRNA of miR-3200-5p, and miR-3200-5p regulates ferroptosis by targeting ATF4, resulting in the inhibition of proliferation and metastasis within hepatocellular carcinoma cells. | |||
Cullin-4B (CUL4B)
Hematotoxicity [ICD-11: 3A70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [98] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
AHH-1 cells | Normal | Homo sapiens | CVCL_3640 |
| Response Description | Lnc-TC/miR-142-5p/ CUL4B signaling axis promoted cell ferroptosis to participate in benzene hematotoxicity, and was a potential biomarker for risk screening and health surveillance of benzene-exposed workers. The expression of GPX4 was negatively correlated with both Lnc-TC and CUL4B. | |||
Cold-inducible RNA-binding protein (CIRBP)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [141] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Response Description | Cold induction promotes the process of ferroptosis by inducing the expression of CIRBP and then regulating key factors such as p53 and GPX4. In addition, cold induction significantly inhibited the proliferation of pancreatic cancer cells and induced cell apoptosis, but after the addition of ferroptosis inhibitor, cell proliferation and apoptosis did not change significantly. | |||
Cofilin-2 (CFL2)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [112] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Downregulation of circ-PSEN1 ameliorates HG-induced ferroptosis in ARPE19 cells via miR-200b-3p/ CFL2 and may be a novel therapeutic target for diabetic retinopathy. Enhancement of CFL2 suppressed the mRNA and protein expression of GPX4 and SLC7A11. However, TFR1 expression was promoted. | |||
CircPVT1 (circRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [111] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 | |
| KYSE-70 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1356 | ||
| hEECs (Human esophageal epithelial cells) | |||||
| In Vivo Model |
BALB/c nude male mice of 4 weeks old were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). After one week of adaptive feeding, EC9706 cells (3 x 106) stably expressing sh-NC and sh-circPVT1, sh-NC + 5-FU and sh-circPVT1 + 5-FU were subcutaneously were injected into the right flank of the nude mice in a serum-free DMEM medium.
Click to Show/Hide
|
||||
| Response Description | CircPVT1 regulated the chemosensitivity of esophageal squamous cell carcinoma cells through ROS and Wnt/-catenin pathwaysviamiR-30a-5p/FZD3. Knockdown of circPVT1 promoted chemosensitivity in ESCC by increasing ferroptosis via downregulating GPX4 and SLC7A11. | ||||
CircKIF4A (circRNA)
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [121] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
KAT-5 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0370 | |
| TPC-1 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | ||
| In Vivo Model |
KAT-5 and TPC-1 cells (2 x 107) were subcutaneously injected into nude mice (four mice/group, 4-week-old) and treated with intratumoral injection (50 uL si-circCON, si-circKIF4A) every four days. The volume of tumors was estimated every four days according to the formula 0.5 x width2 x length. After 28 days, the tumors were weighed. Forin vivolung metastasis assay, cells (1 x 105) were injected through tail veins (four mice/group).
Click to Show/Hide
|
||||
| Response Description | circKIF4A facilitated the malignant progress of papillary thyroid tumor by sponging miR-1231 and upregulating GPX4 expression. And circKIF4A-miR-1231-GPX4 axis played a vital role in cancer proliferation and ferroptosis by competing endogenous RNAs. Therefore, targeting circKIF4A is very likely to be a potential method for treatment of papillary thyroid cancer in the future. | ||||
CircIL4R (circRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [107] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
THLE-2 cells | Normal | Homo sapiens | CVCL_3803 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| In Vivo Model |
The 5-week-old BALB/c male nude mice (n = 10) were purchased from the Animal Center of the Chinese Academy of Medical Sciences (Beijing, China). A number of 2 x 106 HuH-7 cells were transfected with lentiviral vectors containing sh-circIL4R or sh-NC to establish the stably expressed cell lines. Ten mice were subcutaneously injected with HuH-7 cells with sh-circIL4R or sh-NC (five mice per group), constructing the xenograft model of HCC in vivo. Every 5 days after injection, tumor size was measured by a vernier caliper and tumor volume (length x width2 x 0.5) was calculated.
Click to Show/Hide
|
||||
| Response Description | CircIL4R acted as a miR-541-3p sponge to regulate its target glutathione peroxidase 4 (GPX4). GPX4 upregulation relieved the miR-541-3p-induced tumor inhibition and ferroptosis aggravation. CircIL4R played an oncogenic role in hepatocellular carcinoma via the miR-541-3p/GPX4 axis in vivo. | ||||
CircIDE (circRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [76] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
C57BL/6 mice (4- to 6-week-old male) were fed in a pathogen-free vivarium under standard conditions at the animal care facility at Sun Yat-sen University. Hepa 1-6 cells transduced with RBMS1 or GPX4 or circIDE overexpression lentiviral vectors were subcutaneously injected into the right flank of mice in 100 ul of sterile PBS. IVIS images were taken.
Click to Show/Hide
|
||||
| Response Description | RBMS1 overexpression inhibited Hepatocellular Carcinoma (HCC) cell growth by attenuating the expression of glutathione peroxidase 4 (GPX4)and further facilitated ferroptosis in vitro and in vivo. More importantly, a novel circIDE (hsa_circ_0000251) was identified to elevate RBMS1 expression via sponging miR-19b-3p in HCC cells. | ||||
CircDTL (circRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [120] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 | ||
| NCI-H522 cells | Non-small cell lung carcinoma | Homo sapiens | CVCL_1567 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| In Vivo Model |
The Shanghai SLAC Animal Center (Shanghai, China) provided 4-6-week-old BALB/c male nude mice, which were kept according to the standards for the use and care of laboratory animals. A total of 1 x 107 NSCLC cells infected with shRNA were injected subcutaneously into the left flank of nude mice (3 per group). Every 3 days, the tumor volume was measured.
Click to Show/Hide
|
||||
| Response Description | It was identified that circDTL exerts its oncogenic effects via the circDTL/miR-1287-5p/GPX4 axis and GPX4 inhibits both ferroptosis and apoptosis. Finally, silencing of circDTL promoted the sensitivity of non-small cell lung cancer cells to chemotherapeutic agents and inhibited the growth of tumors in vivo. | ||||
CircBCAR3 (circRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [62] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
Eca-109 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | |
| In Vivo Model |
Nude mice of both sexes (age: 6-8 weeks, weight: 22-25 g) were purchased from HUNAN SJA LABRATORY ANIMAL CO., LTD (Hunan, China). The EC109 cells stably expressing sh-circBCAR3 or sh-nc were established by infection with corresponding lentivirus vectors. 1 x 106 mL-1 (100 uL) cells were subcutaneously inoculated into the nude mice. The tumor volumes had been measured from day 5 to day 25. On day 25, the xenograft tumors were removed surgically, and the tumor weight was detected.
Click to Show/Hide
|
||||
| Response Description | CircBCAR3 binds with miR-27a-3p to promote TNPO1 expression. GPX4 protein levels were increased by silencing of circBCAR3. And circBCAR3 promoted the proliferation, migration, invasion, and ferroptosis of esophageal cancer cells by miR-27a-3p. | ||||
CircAFF1 (circRNA)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [116] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Wnt signaling pathway | hsa04310 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPNs (Rat primary neurons) | ||||
| In Vivo Model |
C57BL/6 J pregnant mice (E15 ~ E16 day) were disinfected with 75% alcohol and then decapitated. The brain was collected and washed in pre-cold D-Hanks solution with the midbrain and hippocampus being removed. The cortex was collected and the meninx was removed. Then the brain tissues were made into 1 mm3 blocks for digestion with 1 ~ 2 mL lysis at 37 for 15 min. The tissues were cultured in high glucose DMEM to terminate the digestion and made into single-cell suspension. The suspension was filtered through a 70 um mesh screen for centrifugation for 5 min with the supernatant being removed. High glucose DMEM was used to re-suspended cells and the concentration of cells was adjusted for cells were seeded into the plate. About 4 h later, the high glucose DMEM was replaced with Neurobasal medium (containing 2% B27). The culture medium was half refreshed every 2 ~ 3 d. The cells were cultured for 8 ~ 10 d before following experiments.
Click to Show/Hide
|
||||
| Response Description | Treatment of miR-1405p mimics led to decreased COX-2 and ACSL4 expressions, and elevated GPX-4 expressions. circAFF1 can bind miR-1405p to up-regulate GSK-3 expression, thus inhibiting the Wnt/-catenin signaling pathway. And circAFF1 knockdown can suppress ferroptosis of neurons in vitro and therefore attenuate intracerebral hemorrhage (ICH). | ||||
CircACAP2 (circRNA)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [113] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SiHa cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| Response Description | miR-193a-5p was able to target GPX4 and circACAP2 promoted GPX4 expression by sponging miR-193a-5p in cervical cancer cells.Therefore, we concluded that circular RNA circACAP2 repressed ferroptosis of cervical cancer during malignant progression by miR-193a-5p/GPX4. | |||
Circ0004372 (circRNA)
Cardiovascular diseases [ICD-11: BE2Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [71] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hAAFs (Human aortic adventitial fibroblasts) | |||
| Response Description | UII inhibited miR-124 expression through up-regulating circ0004372 expression, thereby promoting SERTAD4 expression. UII significantly promoted the generation of ROS, MDA and 4-HNE, reduced the activities of SOD, GST and GR, increased Fe2+ concentration and inhibited GPX4 expression through circ0004372/miR-124/SERTAD4 for cardiovascular diseases. | |||
Circ-PSEN1 (circRNA)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [112] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Downregulation of circ-PSEN1 ameliorates HG-induced ferroptosis in ARPE19 cells via miR-200b-3p/CFL2 and may be a novel therapeutic target for diabetic retinopathy. Enhancement of CFL2 suppressed the mRNA and protein expression of GPX4 and SLC7A11. However, TFR1 expression was promoted. | |||
CD82 antigen (CD82)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [142] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 |
| Response Description | High expression of the KAI1 (CD82) gene promoted the occurrence of ferroptosis in pancreatic cancer cells through its extensive effect on FPN and GPX4. KAI1induced ferroptosis did not significantly inhibit the proliferation of PC cells. | |||
Caveolin-1 (CAV1)
Head neck squamous cell carcinoma [ICD-11: 2D60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [143] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
HN6 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_8129 |
| HN30 cells | Pharyngeal squamous cell carcinoma | Homo sapiens | CVCL_5525 | |
| CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | |
| SCC-9 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1685 | |
| SCC-25 cells | Squamous carcinoma | Homo sapiens | CVCL_1682 | |
| Response Description | Overexpression of CAV1 in head and neck squamous cell carcinoma (HNSCC) inhibited the process of ferroptosis, leading to aggressive phenotypes, as well as worse prognosis. And the knockdown of CAV1 could reduce the expression of GPX4. | |||
BTB/POZ domain-containing adapter for CUL3-mediated RhoA degradation protein 2 (TNFAIP1)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [144] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Downregulation of TNFAIP1 alleviates OGD/Rinduced neuronal damage by suppressing Nrf2/GPX4 mediated ferroptosis, which might lay the foundation for the investigation of targeted-therapy for cerebral ischemia-reperfusion injury in clinic. | |||
Beta-enolase (ENO3)
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [145] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Eight-week-old C57BL/6 mice, body weight about 22-24 g, male (n = 24) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd China. The mice were randomly divided into four groups and were maintained on a MCD diet (Medicience, Yangzhou, China) for 4, 8, and 12 weeks to induce NASH. Liver tissue and blood samples (from the eyeballs of the mice) were harvested for further analyses. Mice on a normal diet were used as the control.
Click to Show/Hide
|
||||
| Response Description | ENO3 promoted the progression of NASH by negatively regulating ferroptosis via elevating GPX4 expression and lipid accumulation. These findings provided solid foundation for the mechanism of ferroptosis on the progression of NASH regulated by ENO3, suggesting that ENO3 may be a potential therapeutic target for non-alcoholic fatty liver disease. | ||||
Aurora kinase A (AURKA)
Gastrointestinal cancer [ICD-11: 2B5B]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [109] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
OE33 cells | Barrett adenocarcinoma | Homo sapiens | CVCL_0471 |
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| STKM-2 cells | Gastric carcinoma | Homo sapiens | CVCL_M570 | |
| Response Description | Upper gastrointestinal adenocarcinoma (UGC) tissue samples and cell models demonstrated significant overexpression of AURKA with downregulation of miR-4715-3p. Inhibition of AURKA or reconstitution of miR-4715-3p inhibited GPX4 and induced cell death, suggesting a link between AURKA and ferroptosis. | |||
Aldo-keto reductase family 1 member C2 (AKR1C2)
Essential hypertension [ICD-11: BA00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [146] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | |
| In Vivo Model |
Aortic smooth muscle cells were isolated from wild-type male 6-8-week and CSE-knockout mice. Mice were sacrificed by decapitation, followed by separating the aorta via removing the fatty tissue and vascular adventitia. Then, the mouse aorta was cut into pieces and allowed for digestion for 8 h in the DMEM containing collagenase type 1. The obtained VSMCs were centrifuged at 300 xg for 5 min, and the pellet was resuspended in the DMEM supplemented with 10% FBS, 2 mM l-glutamine, and 100 U/mL penicillin/streptomycin. The medium was replaced every 2 days.
Click to Show/Hide
|
||||
| Response Description | Aortic GPX4 (a core regulator of ferroptosis) significantly downregulated association with VSMC novel phenotype elevation in SHR rats and hypertension patients. The ferroptosis inhibitor ferrostatin-1 (Fer-1) administration blocked HHP-induced VSMC inflammatory (CXCL2 expression) and endothelial function inhibitory ( AKR1C2 expression) phenotyping switch association with elevation in the GPX4 expression, reduction in the reactive oxygen species (ROS), and lipid peroxidation production. | ||||
Aldehyde dehydrogenase family 3 member A2 (ALDH3A2)
Acute myeloid leukaemia [ICD-11: 2A60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [147] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| MOLM-14 cells | Leukemia | Homo sapiens | CVCL_7916 | ||
| Mono-Mac-6 cells | Acute monocytic leukemia | Homo sapiens | CVCL_1426 | ||
| NB4 cells | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | ||
| NOMO-1 cells | Acute monocytic leukemia | Homo sapiens | CVCL_1609 | ||
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| In Vivo Model |
Six- to 12-week-old Aldh-mut and Aldh-Ctrl mice were used to generate MLL-AF9 leukemia through retroviral transduction. This was transplanted into lethally irradiated (9 Gy) primary leukemic C57BL/6J mice and then into sublethally irradiated (4.5 Gy) secondary leukemic C57BL/6J mice. Forty-eight hours after injection into secondary recipients, these mice received 3 doses of polyinosinic-polycytidylic acid (GE Healthcare) on alternate days.
Click to Show/Hide
|
||||
| Response Description | Aldh3a2 inhibition was synthetically lethal with glutathione peroxidase-4 (GPX4) inhibition; GPX4 inhibition is a known trigger of ferroptosis that by itself minimally affects acute myeloid leukemia cells. Inhibiting Aldh3a2 provides a therapeutic opportunity and a unique synthetic lethality to exploit the distinctive metabolic state of malignant cells. | ||||
ADAMTS9-AS1 (IncRNA)
Endometriosis [ICD-11: GA10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [106] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
In Vitro Model |
hEECs (Human esophageal epithelial cells) | ||||
| mESCs (Mouse endometrial stromal cells) | |||||
| mEESCs (Mouse ectopic endometrial stromal cells) | |||||
| In Vivo Model |
Female BALB/c mice (4-6 weeks old, 18-20 g) were obtained from Shanghai Regan Biotechnology Co., Ltd. (Shanghai, China) and were reared in a specific, pathogen-free facility. After 1 week of acclimatization, mice were randomly divided into two groups: the donor group (n = 10) and recipient groups (n = 10). Ovariosteresis and estradiol valerate injection (0.5 ug/mouse/week; Aladdin, Shanghai, China) was carried out to avoid differences in the estrous cycle. Mice were anesthetized by 2% isoflurane, and then the ovaries on both sides were exposed through flank incisions and removed. Donor mice were sacrificed under isoflurane anesthesia, and each uterine horn of the donor mice was concentrated and peeled in warm PBS to remove uterine muscle. Endometrial tissues were weighed and cut into small fragments with scissors and resuspended in sterile PBS with 1 x ampicillin (Beyotime, Shanghai, China). After that, endometrium preparation was intraperitoneally injected into two recipient mice (50 mg/mouse). Two weeks after EM transplantation, endometriosis lesions and eutopic endometrial tissues were removed from the peritoneal cavities and uteri.
Click to Show/Hide
|
||||
| Response Description | ADAMTS9-AS1 functioned as a competing endogenous RNA (ceRNA) by sponging miR-6516-5p to derepress the expression of GPX4, the critical repressor of ferroptosis. Taken together, these results demonstrate that upregulated ADAMTS9-AS1 accelerates ESC proliferation and migration by regulating miR-6516-5p/GPX4-dependent ferroptosis and may be a potential target for the treatment of Endometriosis. | ||||
26S proteasome non-ATPase regulatory subunit 14 (PSMD14)
Bladder cancer [ICD-11: 2C94]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [148] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
T24 cells | Bladder carcinoma | Homo sapiens | CVCL_0554 | |
| 5637 cells | Bladder carcinoma | Homo sapiens | CVCL_0126 | ||
| J82 cells | Bladder carcinoma | Homo sapiens | CVCL_0359 | ||
| UM-UC-3 cells | Bladder carcinoma | Homo sapiens | CVCL_1783 | ||
| In Vivo Model |
Twenty female BALB/c nude mice (4-6-weeks old, 15 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). All mice were housed under specific pathogen-free conditions in 12/12 cycle of light at room temperature (24-26 ). Mice were fed a full fat diet and autoclaved water. The number of mice did not exceed five per cage. A total of 1 x 107 infected 5637 cells were suspended in 100 uL PBS and injected into the shoulder of the mice. Tumor length (L) and width (W) were observed for 4 weeks. Tumor volume (V) was monitored by measuring the length and width of the tumor using the following equation: V = (L x W2) x 0.5. The mice were euthanized by cervical dislocation after inhalational of CO2 when the maximum diameter of any tumor was near 1.5 cm. Tumor tissues were excised and embedded in paraffin for ematoxylin and eosin (HE) or IHC staining.
Click to Show/Hide
|
||||
| Response Description | PSMD14 is highly expressed in bladder cancer tissues, and that PSMD14 expression correlated with poor disease-free survival. Depletion of PSMD14 could inhibit the proliferation and induce ferroptosis of bladder cancer cells through the downregulation of GPX4. | ||||
Unspecific Regulator
HIV Infection [ICD-11: 1C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [149] | |||
| Responsed Drug | Methamphetamine | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
BV-2 cells | Normal | Mus musculus | CVCL_0182 |
| Response Description | Methamphetamine (METH) and HIV-1 lead to oxidative stress and their combined effect increases the risk of HIV-associated neurocognitive disorder (HAND), which may be related to the synergistic ferroptotic impairment in microglia. We found that METH and HIV-1 Tat reduced the expression of ferroptotic protein GPX4 and the cell viability and enhanced the expression of P53 and the level of ferrous iron, while the above indices were significantly improved with pretreatment of ferrostatin-1. | |||
Sepsis [ICD-11: 1G40]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [150] | ||||
| Responsed Drug | Acetaminophen | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hHCs (Hippocampal cells) | ||||
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| In Vivo Model |
Healthy male C57BL/6J mice, weighing 22-24 g, 6 weeks old, were purchased from Tianyao Biotechnology Company (Tianjin, China) and housed in an environment free of specific pathogenic bacteria: temperature 22-24 , relative humidity 50%-70%, alternating day and night every 12 h, and free access to water. The cecal ligation and puncture (CLP) approach was used to establish septic mouse models. The survival rates for 7 days were determined. The Morris water maze (MWM) was utilized to assess cognitive function. Hematoxylin and eosin (HE) staining identified histopathologic alterations in hippocampal tissue.
Click to Show/Hide
|
||||
| Response Description | In both animal and cell studies, Acetaminophen reduced iron content, ROS, glutamate antiporter (xCT), 4-hydroxy-2-nonenal (4-HNE) levels but increased GPX4 expression. Our findings suggest that APAP reduces sepsis-induced cognitive impairment by reducing ferroptosis, which is mediated by the GPX4 signaling pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [151] | ||||
| Responsed Drug | Iridin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Eight-week-old wild-type (WT) and Nrf2-knockout (Nrf2-/-) littermate male mice on a C57BL/6J background were purchased from Cyagen (Suzhou, China.) and maintained at the Centre for Animals of Wuhan University (Wuhan, China). Before the experiment, the mice were separated and given light and dark cycles for 12 h, 22 ± 0.5 temperature, 60 ± 10% humidity, and free accessed to food and water for at least 1 week. Mice were randomly distributed into sham, CLP, CLP + Irisin (Ir group) and CLP + Irisin + Era (Ir + Era group) groups.
Click to Show/Hide
|
||||
| Response Description | In conclusion, irisin could ameliorate inflammatory microenvironment in Sepsis-associated encephalopathy by suppressing hippocampus ferroptosis via the Nrf2/GPX4 signaling pathway. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [152] | ||||
| Responsed Drug | Dexmedetomidine | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mVTs (Mouse ventricular tissues) | ||||
| In Vivo Model |
A total of 32 male C57BL/6 mice (25 g, 8 weeks old) were obtained from the Guangdong Medical Lab Animal Center and housed in the Laboratory Animal Service Center (Jinan University, Guangdong, China). Mice were anesthetized with isoflurane (RWD Life Science) inhalation at the concentration of 2.5% for anesthetic induction and then at 1% for anesthetic maintenance until the end of the CLP. During the experiment, the body temperature was kept at 36-38 with a heating pad. Anesthetized mice were subjected to midline laparotomy. The cecum was carefully separated to avoid blood vessels damage and the cecum was identified and punctured twice with a 22-gauge needle. Then, the abdominal cavity was closed with two epithelium layers, followed by a normal saline injection subcutaneously for resuscitation before mice were returned to the cage.
Click to Show/Hide
|
||||
| Response Description | The attenuation of sepsisinduced HO1 overexpression and iron concentration, and the reduction of ferroptosis via enhancing GPX4, may be the major mechanisms via which Dexmedetomidine alleviates sepsis induced myocardial cellular injury. | ||||
Glioblastoma [ICD-11: 2A00]
| In total 4 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [153] | ||||
| Responsed Drug | Sodium Selenite | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | ||
| HT-29 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0320 | ||
| SVG p12 cells | Normal | Homo sapiens | CVCL_3797 | ||
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| Response Description | Sodium selenite (SS) down-regulates ferroptosis regulators; solute carrier family 7 member 11 (SLC7A11), glutathione (GSH), and glutathione peroxidase 4 (GPx4), while it up-regulates iron accumulation and lipid peroxidation (LPO) in Glioblastoma. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [154] | ||||
| Responsed Drug | RSL3 | Investigative | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female B-NDG mice (4-6 weeks old, 16-20 g) were purchased from Biocytogen (Biocytogen Jiangsu Co., Ltd., Jiangsu, China) and housed under specific pathogen-free conditions. 5 x 106 U87 cells were resuspended in 200 uL PBS buffer and then inoculated into the left hind limb of each mouse. Once tumor volumes reached >=50 mm3, the mice were randomly divided into four groups (n = 5): the control, RSL3-only, BAY-only, and RSL3 plus BAY groups. Chemicals were administered through intratumor injection (100 mg/kg for RSL3 and 1 mg/kg for BAY 11-7082) biweekly for two weeks.
Click to Show/Hide
|
||||
| Response Description | NF-kB pathway activation is vital for RSL3-induced ferroptosis in glioblastoma cells both in vitro and in vivo. Furthermore, RNAi-mediated GPX4 silencing cannot trigger ferroptosis in glioblastoma cells unless the NF-kB pathway is activated simultaneously. Finally, NF-kB pathway activation promotes ferroptosis by downregulating the expression of ATF4 and SLC7A11. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [155] | ||||
| Responsed Drug | Dihydroartemisinin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| Response Description | Dihydroartemisinin (DHA) had a selective killing effect on glioblastoma, which was associated with over-expression of transferrin receptors. The primary mechanism by which DHA caused ferroptosis was down-regulation of GPX4 and the following lipid ROS accumulation. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [157] | ||||
| Responsed Drug | Dihydrotanshinone I | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HEB (Human glial cells) | ||||
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| Response Description | Dihydrotanshinone I (DHI) inhibited the proliferation of human glioma cells. Following treatment of the U251 and U87 cells with DHI, changes in the expression levels of ferroptosis-associated proteins were observed; the expression level of GPX4 decreased and that of ACSL-4 increased. DHI also increased the levels of LDH and MDA in the human glioma cells and reduced the GSH/GSSG ratio. | ||||
Myelodysplastic syndrome [ICD-11: 2A3Z]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [158] | ||||
| Responsed Drug | Decitabine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell necroptosis | |||||
In Vitro Model |
SKM-1 cells | Acute myeloid leukemia | Homo sapiens | CVCL_0098 | |
| MUTZ-1 cells | Burkitt lymphoma | Homo sapiens | CVCL_1431 | ||
| In Vivo Model |
C57BL/6 mice were purchased from Vital River (Beijing, China) at 6 to 8 weeks of age. Twenty mice were housed with five individuals per cage and used at a weight of approximately 20.0-22.0 g. They were randomly divided into four groups, five in each group, namely control group, low-dose group, middle-dose group, and high-dose group. The low-, middle-, and high-dose group mice were administered an intraperitoneal injection of 0.2-ml iron dextran at a concentration of 6.25, 12.5, and 25 mg/ml, respectively, every 3 days for 10 weeks to establish iron overload model. At the same time, normal saline was given to the control group.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis may account for the main mechanisms of how decitabine induced death of myelodysplastic syndrome (MDS) cells. Decitabine-induced ROS raise leads to ferroptosis in MDS cells by decreasing GSH level and GPX4 activity. | ||||
Acute myeloid leukaemia [ICD-11: 2A60]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [159] | ||||
| Responsed Drug | Sulforaphane | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
In Vitro Model |
U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | |
| MV4-11 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0064 | ||
| Response Description | Sulforaphane triggers different types of PCD in a concentration-dependent manner on the two tested acute myeloid leukemia cell lines. Deepening the molecular mechanisms on U-937 cells, we discovered that at lower concentrations, SFN induces apoptosis; at higher concentrations, SFN elicits ferroptosis, characterized by the depletion of intracellular GSH, the downregulation of GPX4 protein expression, and lipid peroxidation. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [160] | ||||
| Responsed Drug | Perillaldehyde | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Jurkat cells | T acute lymphoblastic leukemia | Homo sapiens | CVCL_0065 | |
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | ||
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | ||
| Response Description | We investigated and characterized its antileukemic potential in vitro, disclosing its ability to trigger ferroptosis. Specifically, perillaldehyde induced lipid peroxidation, decreased glutathione peroxidase 4 protein expression, and depleted intracellular glutathione on HL-60 promyelocytic leukemia cells. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [161] | ||||
| Responsed Drug | APR-246 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| MOLM-14 cells | Leukemia | Homo sapiens | CVCL_7916 | ||
| SET-2 cells | Acute megakaryoblastic leukemia | Homo sapiens | CVCL_2187 | ||
| MV4-11 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0064 | ||
| OCI-AML-2 cells | Acute myeloid leukemia | Homo sapiens | CVCL_1619 | ||
| OCI-AML3 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_1844 | ||
| K-562 cells | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | ||
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| UT-7/Epo cells | Acute megakaryoblastic leukemia | Homo sapiens | CVCL_5202 | ||
| SKM-1 cells | Acute myeloid leukemia | Homo sapiens | CVCL_0098 | ||
| NB4 cells | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | ||
| Kasumi-1 cells | Acute myeloid leukemia | Homo sapiens | CVCL_0589 | ||
| In Vivo Model |
Xenograft tumors were generated by randomly injecting 1 x 106 MOLM14 shCTRL or shSLC7A11 cells into the tail veins of NOD/SCID IL-2 receptor g-chain-null mice (NSG) aged 6-9 weeks. Fourteen days after injection, doxycycline (200 mg/mL) and sucrose (1% weight:volume) were added to the drinking water of these animals. After 3 days, the mice were randomly treated with a daily intraperitoneal injection of APR-246 (100 mg/kg) or vehicle (phosphate-buffered saline [PBS]) for 4 days.
Click to Show/Hide
|
||||
| Response Description | APR-246 is a promising new therapeutic agent that targets p53 mutated proteins in myelodysplastic syndromes and in acute myeloid leukemia (AML). The association of APR-246 with induction of ferroptosis (either by pharmacological compounds, or genetic inactivation of SLC7A11 or GPX4) had a synergistic effect on the promotion of cell death, both in vivo and ex vivo. | ||||
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [162] | ||||
| Responsed Drug | Baicalin | Terminated | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| 143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | ||
| hBMMSCs (Human bone marrow mesenchymal stem cells) | |||||
| In Vivo Model |
A total of 24 BALB/c-nude mice (4-5 weeks old) were purchased and MG63 cells were injected into the right tibial bone marrow cavity of mice in a volume of 1 x 106/100 ul. When the tumor volume was visible, all animals were randomly divided into four groups (n = 6): the control (10% DMSO + 40% PEG300 + 5% Tween-80 + 45% Saline) group, the baicalin (200 mg/kg/day) group, the Fer-1 (0.8 mg/kg/day) group and Fer-1 + baicalin group. The baicalin and Fer-1 were intraperitoneally administered every day for two consecutive weeks and tumor sizes were measured every two days.
Click to Show/Hide
|
||||
| Response Description | By promoting the Fe accumulation, ROS formation, MDA production and suppressing the ratio of GSH/GSSG, baicalin was found to trigger ferroptosis in Osteosarcoma and ferroptosis inhibitor ferrostatin-1 (Fer-1) successfully reversed these suppressive effects, indicating that ferroptosis participated in the baicalin mediated anti-OS activity. Mechanistically, baicalin physically interacted with Nrf2, a critical regulator of ferroptosis, and influenced its stability via inducing ubiquitin degradation, which suppressed the Nrf2 downstream targets GPX4 and xCT expression, and led to stimulating ferroptosis. | ||||
Fibrosarcoma [ICD-11: 2B53]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [163] | |||
| Responsed Drug | 1,2-Dioxolane | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 |
| BJ1-hTERT cells | Normal | Homo sapiens | CVCL_6573 | |
| BJ-eLR (Human fibroblast cancer cells) | ||||
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| Response Description | FINO2 is an endoperoxide-containing 1,2-dioxolane that can initiate ferroptosis selectively in engineered cancer cells. FINO2 both indirectly inhibits GPX4 enzymatic function and directly oxidizes iron, ultimately causing widespread lipid peroxidation in Fibrosarcoma. | |||
Gastrointestinal cancer [ICD-11: 2B5B]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [164] | ||||
| Responsed Drug | Berberine | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| TMK-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4384 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
Five-week-old male BALB/c mice were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained in a pathogen-free animal facility under a 12 h light/dark cycle in a temperature (22 )- and humidity-controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following the current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval no. 12924, 5 November 2020). Animals were acclimated to their housing for seven days before the start of the experiment. For the peritoneal dissemination tumor model, CT26 cancer cells (1 x 107 in 0.2 mL per mouse) were injected into the mouse peritoneal cavity. To measure tumor weight, mice were euthanized on Day 12 and the tumors were excised, while the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, with gross removal of non-tumor tissues. The largest tumor was formed on the diaphragm, and paraffin-embedded sections of the excised diaphragmatic tumor were prepared and stained with hematoxylin-eosin. BBR was diluted with distilled water to produce a final concentration of 48 mg/mL. The solutions were ultrasonically treated for 1 h, and fully vortexed for 30 min. BBR solution was administered by free drinking. The intake calculated from the amount of water consumed was 15.2 mg/kg body weight/day.
Click to Show/Hide
|
||||
| Response Description | Berberine induces apoptosis and ferroptosis by inhibiting mitochondrial complex I and promoting autophagy, leading to combined cell death in the GIC and suppressing stemness. BBR induces cell death in gastrointestinal cancer cells accompanied by increased mitochondrial superoxide and ACSL4 levels, decreased SLC7A11, and impaired antioxidant mechanisms, indicated by decreased GPX4 expression and decreased GSH. | ||||
Nasopharyngeal cancer [ICD-11: 2B6B]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [165] | ||||
| Responsed Drug | Cucurbitacin B | Phase 3 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| CNE1 cells | Nasopharyngeal carcinoma | Homo sapiens | CVCL_6888 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| H157 cells | Oral cavity Squamous cell carcinoma | Homo sapiens | CVCL_2458 | ||
| HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | ||
| In Vivo Model |
The animal experiment was performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University. BALB/c nude mice (5 weeks old, female, Guangdong Medical Laboratory Animal Centre, China) were used for animal experiments. Approximately 4 million CNE1 cells were injected subcutaneously into the right flank of each mouse. Palpable solid tumours developed within a month after tumour cell inoculation, and mice were randomly allocated to four different groups (five mice /group) as follows: control (PBS) group, CuB treatment groups [0.5 mg/kg (low-dose) group and 1 mg/kg (high-dose) group] and gemcitabine (GEM, 25 mg/kg) group. Mice received intraperitoneal injections of PBS, CuB, and gemcitabine 3 times weekly.
Click to Show/Hide
|
||||
| Response Description | Cucurbitacin B caused intracellular accumulation of iron ions and depletion of glutathione. Detailed molecular mechanism investigation confirmed that CuB both induced widespread lipid peroxidation and downregulated the expression of GPX4, ultimately initiating a multipronged mechanism of ferroptosis. The study highlighted the therapeutic potential of CuB as a ferroptosis-inducing agent for nasopharyngeal cancer. | ||||
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [166] | ||||
| Responsed Drug | 5-aminolevulinic acid | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
KYSE30 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1351 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
KYSE30 cells were subcutaneously inoculated with 5 x 106 cells per site into both flanks on day 0. At 1 week after transplantation, tumor-bearing mice were randomly assigned to one of the following three groups: (1) saline as a control, (2) 10 mg/kg/day of 5-ALA, or (3) 30 mg/kg/day of 5-ALA. The treatment groups were orally administered 5-ALA once daily for 4 weeks, and the control group was orally administered saline during the same period.
Click to Show/Hide
|
||||
| Response Description | Modulation of GPX4 and HMOX1 by 5-aminolevulinic acid (5-ALA) induced ferroptosis in esophageal squamous cell carcinoma (ESCC). Furthermore, 5-ALA led to an increase in lipid peroxidation and exerted an antitumor effect in various cancer cell lines, which was inhibited by ferrostatin-1. Thus, 5-ALA could be a promising new therapeutic agent for ESCC. | ||||
Gastric cancer [ICD-11: 2B72]
| In total 6 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [167] | ||||
| Responsed Drug | Atranorin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hGCCs (Gastric cancer cells) | ||||
| In Vivo Model |
NOD-scid mice (NOD.CB17-Prkdcscid/NcrCrl) aged 6-7 weeks and weighing 20-22 g were used in the experiment. The animal study was performed at the Shanghai University of Traditional Chinese Medicine with approval from the Institutional Animal Care and Use Committee in accordance with the institutional guidelines. All mice were randomly divided into two groups, and each group consisted of four mice. In experimental group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul Atranorin@SPION (10 mg/kg) every 2 days. In control group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul SPION (10 mg/kg) alone every 2 days. After 2 months, the mice were sacrificed, and their tumors were excised.
Click to Show/Hide
|
||||
| Response Description | Atranorin@SPIONs significantly reduced the 5-hydroxymethylcytidine modification level of GPX4 and SLC7A11 mRNA 3' untranslated region in gastric cancer cells. This study revealed the molecular biological mechanism by which Atranorin@SPION inhibit the in vitro and in vivo activity of GCSCs, that is, Atranorin@SPION reduced the expression of members of the Xc-/GPX4 axis and reduced their mRNA 5-hydroxymethylcytidine modification, finally induced ferroptosis of GCSCs. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [168] | ||||
| Responsed Drug | Thioguanine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| In Vivo Model |
Female BALB/c nude mice (6-7 weeks, 17-18 g) were purchased from Hunan Slack Scene of Laboratory Animal Co., Ltd. and used to establish the xenograft mouse model with 5 x 106 exponentially growing MGC-803 cells inoculated subcutaneously into the right forelimb for each mouse. Once the volume of tumors reached 100 mm3, the mice were divided into 3 groups: solvent control; 6-TG (10 mg/kg/day); 6-TG (10 mg/kg/day) + Fer-1(50 mg/kg/day).
Click to Show/Hide
|
||||
| Response Description | 6-Thioguanine was identified as a potential ferroptosis inducer in gastric cancer cells for the first time. It could inactivate system xc, block the generation of GSH, down-regulate the expression of GPX4, increase the level of Lipid ROS, and finally trigger the Fe-2+-mediated ferroptosis in MGC-803 and AGS cell lines. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [169] | ||||
| Responsed Drug | XN4 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | ||
| Response Description | The pro-ferroptotic role of XN4 in gastric cancer (GC) might enable it to become a promising drug for GC treatment in the future despite the need for extensive research. Moreover, GPX4 levels decreased, but NOX4 and ferroptosis-related protein PTGS2 levels increased in GC cells following XN4 treatment, which was nullified by NOX4 knockdown. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [170] | ||||
| Responsed Drug | (6R,6aR,9S,11bS,14R)-4,4-Dimethyl-8-methylene-7,11,12-trioxododecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-14-yl valinate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | ||
| In Vivo Model |
Male nude mice (ages 6-8 weeks) used in the studies were purchased from Hunan SJA Laboratory Animal Co. (Changsha, China). Male nude mice were subcutaneously injected with MGC-803cells into the right flank of mouse. Once the tumor volume reached 100-200 mm3, mice were randomly divided into 5 groups (6 mice/group) and administered with saline,a2(5, 10, and 20 mg/kg), or 5-fluorouracil (5-FU, 15mg/kg) once a day for 21 daysviatail vein injection.
Click to Show/Hide
|
||||
| Response Description | (6R,6aR,9S,11bS,14R)-4,4-Dimethyl-8-methylene-7,11,12-trioxododecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-14-yl valinate (a2), a new JDA derivative, inhibited the growth of gastric cancer cells. Importantly, compounda2decreased GPX4 expression and overexpressing GPX4 antagonized the anti-proliferative activity ofa2. Furthermore, a2caused ferrous iron accumulation through the autophagy pathway, prevention of which rescueda2induced ferrous iron elevation and cell growth inhibition. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [171] | ||||
| Responsed Drug | Actinidia chinensis Planch | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
In Vitro Model |
HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| In Vivo Model |
Wild type AB strain of zebrafish (Danio rerio) was obtained from Southern Medical University. The HGC-27 cells labeled with EGFP were resuspended in PBS in the concentration of 5*107/ml. 10 nl cell suspension containing approximately 300 cells were loaded into capillary needles and injected into the abdominal perivitelline space of zebrafish embryos by a nanoliter injector (Narishige, Tokyo, Japan). After injection, the tumor-bearing embryos were transferred into a 24-well plate and acclimated in embryo water at 35 for 24 h and then incubated at 0, 90, 180 mg/ml ACP decoction for 48 h.
Click to Show/Hide
|
||||
| Response Description | Actinidia chinensis Planch (ACP) increased the accumulation of ROS via inhibited the glutathione peroxidase 4 (GPx4) and xCT (SLC7A11) proteins, while were inhibited by Ferrostatin-1 (Fer-1) significantly. In conclusion, ACP was a promising antineoplastic agent for the treatment of gastric cancer by regulating apoptosis, ferroptosis and mesenchymal phenotype. | ||||
| Experiment 6 Reporting the Ferroptosis-centered Disease Response of This Regulator | [172] | ||||
| Responsed Drug | Polyphyllin B | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
NUGC-3 cells | Gastric carcinoma | Homo sapiens | CVCL_1612 | |
| MKN-1 cells | Gastric carcinoma | Homo sapiens | CVCL_1415 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| In Vivo Model |
The nude mice were raised in our laboratory for a week before the experiment. Then, 5 x 106 MKN-1 cells were subcutaneously injected to establish the subcutaneous xenograft tumour model in nude mice. When the maximum diameter of the xenograft tumours grew steadily to 1 cm, they were dissected completely and cut into 1 mm3 tissue fragments. Then, the tissue fragment was inserted into the surface of the serosa on the greater curvature of the stomach. Different doses of PB (2.5 mg/kg or 5.0 mg/kg) were given by intraperitoneal injection once a day for 3 weeks. The control group was given the same volume of vehicle. The positive control group was given 5-Fu at the dose of 10 mg/kg. The body weight and tumour size of nude mice were recorded. Mice were administered fluorescein substrate (150 mg/kg) intraperitoneally for in vivo imaging twice a week on a Xenogen IVIS 200 imaging system (Caliper Life Sciences, USA). The tumour inhibition rate was analysed using LT Living Image 4.3 Software.
Click to Show/Hide
|
||||
| Response Description | We identified a novel GPx4 inhibitor, polyphyllin B (PB), which can induce ferroptosis by down-regulating GPx4 expression in gastric cancer (GC) cells. It has also been shown to inhibit cell proliferation, suppress invasion and migration, induce apoptosis, and block the cell cycle progression in GC cellsin vitro. Then, immunofluorescence and western blotting assay confirmed that PB can regulate the expression of LC3B, TFR1, NOCA4 and FTH1in vitro, which suggested that suggest that PB may increase the level of Fe2+by transporting Fe3+into the cell by TFR1 and promoting NCOA4-dependent iron autophagy. | ||||
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [173] | ||||
| Responsed Drug | Honokiol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SW48 cells | Colon adenocarcinoma | Homo sapiens | CVCL_1724 | |
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| LS174T cells | Colon adenocarcinoma | Homo sapiens | CVCL_1384 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | ||
| RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Ten BALB/c nude mice (male, 4 weeks old, 18.0 ± 2.0 g) were randomly divided into two groups for the in vivo xenograft assay. Mice were injected with 5 x 106 RKO cells with stable overexpression GPX4 (described Lv-GPX4 group), control vector (described Lv-NC group). Cells were subcutaneously injected into the right anterior axilla of mice in both groups. Mice then received HNK (0.5 mg/kg/w) by intraperitoneal injection for 4 weeks. The subcutaneous tumor volumes in the nude mice in the two groups were recorded every two days.
Click to Show/Hide
|
||||
| Response Description | Honokiol reduced the viability of Colon cancer (CC) cell lines by increasing ROS and Fe2+levels. HNK decreased the activity of Glutathione Peroxidase 4 (GPX4) but did not affect system Xc-. Thus, HNK can induce ferroptosis in CC cells by reducing the activity of GPX4. | ||||
Colorectal cancer [ICD-11: 2B91]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [174] | ||||
| Responsed Drug | Curcumin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response Description | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [175] | ||||
| Responsed Drug | RSL3 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| Response Description | RSL3 triggered ferroptotic cell death by promoting the accumulation of cellular ROS and increasing the cellular LIP level. Mechanismly, we found transferrin expression were elevated in colorectal cancer cells treated with RSL3 accompanied by a decrease in the expression of GPX4, indicating an iron-dependent cell death. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [174] | ||||
| Responsed Drug | Andrographis | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response Description | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
Pancreatic cancer [ICD-11: 2C10]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [176] | ||||
| Responsed Drug | Wogonin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| AsPC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0152 | ||
| HPDE6-C7 cells | Normal | Homo sapiens | CVCL_0P38 | ||
| In Vivo Model |
Female BALB/c nude mice (5 weeks old) were procured from Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd (Zhejiang, China) and given 5 days to acclimate to their surroundings. PANC-1 cells (1 x 107) in 100 uL PBS at the logarithmic growth phase were administered to mice subcutaneously in the left flank. The mice were treated with indicated treatments after nearly 10 days when the tumour size was approximately 1,000 mm3. In the control group, mice (n = 5) received intraperitoneal injections of the vehicle. In the treatment group, the mice (n = 5) were administered 50 uL of 60 mg/kg body weight of wogonin once a day for 12 days. A slide calliper size was used to measure the tumour size. The equation for calculating tumour volume is as follows: tumour volume = AB2/2, wherein A is the length, and B is the width of the tumour. The mice were sacrificed the next day after the treatment procedure was complete by cervical dislocation. The tumour tissues were harvested and snap-frozen using liquid nitrogen for subsequent analyses.
Click to Show/Hide
|
||||
| Response Description | Wogonin could significantly reduces pancreatic cancer cell proliferation and induce ferroptosisviathe Nrf2/GPX4 axis. Therefore, wogonin could be potentially used for treating patients with pancreatic cancer. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [177] | ||||
| Responsed Drug | QD394-Me | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| In Vivo Model |
Female Balb/c mice were purchased from Envigo. At the time of implantation, all mice were aged 5-6 weeks. Mice were implanted subcutaneously in the right flank with 1 x 106 CT-26 cells in 100 uL DPBS. Seven days after implantation, mice were randomized into groups (n = 5) with mean tumor volumes ranging from 97 to 117 mm3. The negative control group was dosed daily in the intraperitoneal cavity (IP) with the same vehicle used for QD394. QD394 was dosed at 10 mg/kg IP, and QD394-Me was dosed 3 times weekly intravenously (IV) at 20 mg/kg.
Click to Show/Hide
|
||||
| Response Description | QD394 causes an iron- and ROS-dependent, GPX4 mediated cell death, suggesting ferroptosis as a major mechanism. Importantly, QD394 decreases the expression of LRPPRC and PNPT1. Pharmacokinetics-guided lead optimization resulted in the derivative QD394-Me, which showed improved plasma stability and reduced toxicity in mice compared to QD394. Overall, QD394 and QD394-Me represent novel ROS-inducing drug-like compounds warranting further development for the treatment of pancreatic cancer. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [177] | ||||
| Responsed Drug | QD394 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| In Vivo Model |
Female Balb/c mice were purchased from Envigo. At the time of implantation, all mice were aged 5-6 weeks. Mice were implanted subcutaneously in the right flank with 1 x 106 CT-26 cells in 100 uL DPBS. Seven days after implantation, mice were randomized into groups (n = 5) with mean tumor volumes ranging from 97 to 117 mm3. The negative control group was dosed daily in the intraperitoneal cavity (IP) with the same vehicle used for QD394. QD394 was dosed at 10 mg/kg IP, and QD394-Me was dosed 3 times weekly intravenously (IV) at 20 mg/kg.
Click to Show/Hide
|
||||
| Response Description | QD394 causes an iron- and ROS-dependent, GPX4 mediated cell death, suggesting ferroptosis as a major mechanism. Importantly, QD394 decreases the expression of LRPPRC and PNPT1. Pharmacokinetics-guided lead optimization resulted in the derivative QD394-Me, which showed improved plasma stability and reduced toxicity in mice compared to QD394. Overall, QD394 and QD394-Me represent novel ROS-inducing drug-like compounds warranting further development for the treatment of pancreatic cancer. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 7 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [178] | ||||
| Responsed Drug | Solasonine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| HepaRG cells | Hepatocellular carcinoma | Homo sapiens | CVCL_9720 | ||
| In Vivo Model |
BALB/c nude mice aged 4-6 weeks, weighing 15~20 g, were purchased from Shanghai SLAC Laboratory Animal Co.,Ltd (Shanghai, China). Following acclimation, the right flank of each experimental mouse was subcutaneously injected with HepG2 cells (2 x 106) suspended in PBS (200 uL) and then randomly assigned to: (i) the control group and received no further treatment or (ii) the intervention group and received solasonine (50 mg/kg body weight) in an equal volume of PBS. Tumor volumes were measured every 5 days. After 30 days, the mice were sacrificed and the tumors were resected, weighed, and processed for histological analysis.
Click to Show/Hide
|
||||
| Response Description | Solasonine increased lipid ROS levels in HepG2 cells by suppression of GPX4 and GSS. However, the use of a ferroptosis inhibitor reversed solasonine-induced ROS production and cell apoptosis. Taken together, solasonine promotes ferroptosis of hepatocellular carcinoma cells via GPX4-induced destruction of the glutathione redox system. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [179] | ||||
| Responsed Drug | l-Buthionine sulfoximine | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| Response Description | Auranofin/buthionine sulfoxime (BSO) and Erastin/BSO cotreatment alters redox homeostasis by increasing levels of Nrf2 and HO-1 and decreasing GPX4 levels. Targeting these two main ferroptotic pathways simultaneously can overcome chemotherapy resistance in hepatocellular carcinoma (HCC). | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [180] | ||||
| Responsed Drug | Atractylodin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hccm (Human hepatocellular carcinoma cells) | |||||
| Response Description | Atractylodin can inhibit the proliferation, migration, and invasion of Huh7 and Hccm liver cancer cells, and induce cell apoptosis and cell cycle arrest. In addition, atractylodin may induce ferroptosis in hepatocellular carcinoma cells by inhibiting the expression of GPX4 and FTL proteins, and up-regulating the expression of ACSL4 and TFR1 proteins. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [179] | ||||
| Responsed Drug | Auranofin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| Response Description | Auranofin/buthionine sulfoxime (BSO) and Erastin/BSO cotreatment alters redox homeostasis by increasing levels of Nrf2 and HO-1 and decreasing GPX4 levels. Targeting these two main ferroptotic pathways simultaneously can overcome chemotherapy resistance in hepatocellular carcinoma (HCC). | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [179] | ||||
| Responsed Drug | Erastin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| Response Description | Auranofin/buthionine sulfoxime (BSO) and Erastin/BSO cotreatment alters redox homeostasis by increasing levels of Nrf2 and HO-1 and decreasing GPX4 levels. Targeting these two main ferroptotic pathways simultaneously can overcome chemotherapy resistance in hepatocellular carcinoma (HCC). | ||||
| Experiment 6 Reporting the Ferroptosis-centered Disease Response of This Regulator | [181] | ||||
| Responsed Drug | Seco-Lupane Triterpene Derivatives | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response Description | A new seco-lupane triterpene derivative, compound21, was found to regulate cell growth through the cell cycle and ferroptosis, which in turn inhibited the proliferation, migration, and invasion of HepG2 cells. And it was found that compound 21 significantly upregulated ACSL4 protein expression and downregulated GPX4 protein expression. It has the potential to become an effective new drug for the treatment of hepatocellular carcinoma. | ||||
| Experiment 7 Reporting the Ferroptosis-centered Disease Response of This Regulator | [182] | ||||
| Responsed Drug | 2-pyridylhydrazone dithiocarbamate s-acetic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response Description | 2-pyridylhydrazone dithiocarbamate s-acetic acid (PdtaA) induced both apoptosis and cell cycle arrest. Notably, PdtaA also induced ferroptosis via downregulation of GPx4 and xCT in liver cancer cells. Autophagy inhibitor 3-methyladenin or genetic knockdown of NCOA4 was employed to inhibit ferritinophagy, which significantly neutralized the action of PdtaA in both apoptosis and ferroptosis. | ||||
Lung cancer [ICD-11: 2C25]
| In total 6 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [183] | ||||
| Responsed Drug | Dihydroartemisinin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
In Vitro Model |
NCI-H292 cells | Lung mucoepidermoid carcinoma | Homo sapiens | CVCL_0455 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| MDA-MB-453 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| In Vivo Model |
GPX4 iKO H292 cells were inoculated by injecting 3 x 106 cells in 0.1 mL PBS subcutaneously in the right flank of six- to eight-week-old female athymic nude Foxn1nu/Foxn1 mice (Envigo, East Millstone, NJ, USA). Following inoculation, the mice were monitored until they have fully recovered and are moving. Mice were randomly allocated into their respective groups (non-blinded). Tumor growth was monitored regularly via external caliper measurements.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin (DAT) can augment GPX4 inhibition-induced ferroptosis in a cohort of cancer cells that are otherwise highly resistant to ferroptosis. Collectively, artemisinin compounds can sensitize cells to ferroptosis by regulating cellular iron homeostasis in Lung mucoepidermoid carcinoma. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [184] | ||||
| Responsed Drug | Ginkgetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SPC-A1 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | ||
| In Vivo Model |
Briefly, when tumours on transplanted nude mice reached around 100 mm3, the mice were randomized divided into eight groups: control, ginkgetin, DDP, ginkgetin + DDP, UAMC 3203, ginkgetin + UAMC 3203, DDP + UAMC 3203, ginkgetin + DDP + UAMC 3203. Both DDP (3 mg/kg) and ginkgetin (30 mg/kg) were administered by intraperitoneal injection, with 2 - 3 times per week and once per day, respectively. UAMC 3203 (10 mg/kg) was administered 5 days/week by intraperitoneally injection. Tumour size and body weight were measured 3 times per week. After dosing 31 days, the nude mice were sacrificed, and tumours were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | The induction of ferroptosis mediated by ginkgetin was further confirmed by the decreased expression of SLC7A11 and GPX4, and a decreased GSH/GSSG ratio. Simultaneously, ginkgetin disrupted redox hemostasis in DDP-treated cells, as demonstrated by the enhanced ROS formation and inactivation of the Nrf2/HO-1 axis. Ginkgetin also enhanced DDP-induced mitochondrial membrane potential (MMP) loss and apoptosis in cultured non-small cell lung cancer (NSCLC) cells. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [185] | ||||
| Responsed Drug | Gefitinib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRASmutant non-small-cell lung cancer (NSCLC) cells. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [185] | ||||
| Responsed Drug | Betulin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRASmutant non-small-cell lung cancer (NSCLC) cells. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [186] | ||||
| Responsed Drug | Capsaicin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 | ||
| Response Description | Capsaicin inhibited the proliferation of A549 and NCI-H23 cells and induced ferroptosis by inactivating SLC7A11/GPX4 signaling. Capsaicin could be used as a potential anticancer agent in the treatment of non-small cell lung cancer (NSCLC). | ||||
| Experiment 6 Reporting the Ferroptosis-centered Disease Response of This Regulator | [188] | ||||
| Responsed Drug | Orlistat | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| AMPK signaling pathway | hsa04152 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| LL/2 (LLC1) cells | Lung cancer | Mus musculus | CVCL_4358 | ||
| In Vivo Model |
C57BL/6 mice were anesthetized, and 5 x 105 LLC cells were implanted subcutaneously into the right flank. Five days post-implant, mice were randomized and assigned into two groups and treated with orlistat (10 mg/kg, intraperitoneal injection) or PBS daily for 14 days. The tumor volume was measured twice a week with a caliper, and the tumor volume was calculated according to the formula ((length x width2 )/2).
Click to Show/Hide
|
||||
| Response Description | Orlistat, as a single agent, inhibited the proliferation and viabilities of lung cancer cells and induced ferroptosis-like cell death in vitro. Mechanistically, we found that orlistat reduced the expression of GPX4, a central ferroptosis regulator, and induced lipid peroxidation. | ||||
Melanoma [ICD-11: 2C30]
| In total 3 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [189] | |||
| Responsed Drug | [4-[Bis(4-chlorophenyl)methyl]piperazin-1-yl]-(5-methyl-4-nitro-1,2-oxazol-3-yl)methanone | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
LOX-IMVI cells | Melanoma | Homo sapiens | CVCL_1381 |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| HEK293-EBNA1-6E cells | Normal | Homo sapiens | CVCL_HF20 | |
| CJM cells | Melanoma | Homo sapiens | CVCL_U797 | |
| WM88 cells | Melanoma | Homo sapiens | CVCL_6805 | |
| KP-4 cells | Pancreatic carcinoma | Homo sapiens | CVCL_1338 | |
| HCC4006 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1269 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | |
| Caki-2 cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_0235 | |
| Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | |
| MC-38 cells | Colon adenocarcinoma | Homo sapiens | CVCL_B288 | |
| Response Description | ML210 is a prodrug that is converted in cells into a nitrile-oxide electrophile that covalently inhibits GPX4 with remarkable proteome-wide selectivity in melanoma. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [190] | |||
| Responsed Drug | DETD-35 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HaCaT cells | Normal | Homo sapiens | CVCL_0038 |
| CCD-966Sk cells | Normal | Homo sapiens | CVCL_U267 | |
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | |
| A2058 cells | Amelanotic melanoma | Homo sapiens | CVCL_1059 | |
| SK-MEL-2 cells (MEK inhibitor-resistant) cells | Melanoma | Homo sapiens | CVCL_0069 | |
| Response Description | Sesquiterpene lactones DET and DETD-35 significantly reprogram this metabolic adaptation and inhibit GPX4 activity to disturb glutathione metabolism and induce ferroptosis. Targeting ferroptosis and GPX4 could be a novel approach to cope with drug resistance in melanoma cancers. | |||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [190] | |||
| Responsed Drug | DET | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HaCaT cells | Normal | Homo sapiens | CVCL_0038 |
| CCD-966Sk cells | Normal | Homo sapiens | CVCL_U267 | |
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | |
| A2058 cells | Amelanotic melanoma | Homo sapiens | CVCL_1059 | |
| SK-MEL-2 cells (MEK inhibitor-resistant) cells | Melanoma | Homo sapiens | CVCL_0069 | |
| Response Description | Sesquiterpene lactones DET and DETD-35 significantly reprogram this metabolic adaptation and inhibit GPX4 activity to disturb glutathione metabolism and induce ferroptosis. Targeting ferroptosis and GPX4 could be a novel approach to cope with drug resistance in melanoma cancers. | |||
Breast cancer [ICD-11: 2C60]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [191] | ||||
| Responsed Drug | Lycium barbarumpolysaccharide | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| Response Description | Lycium barbarum polysaccharide (LBP) effectively inhibited proliferation of breast cancer cells and promoted ferroptosis by modulation of the xCT/GPX4 pathway. GPX4 inactivity and repression of SLC7A11 (the gene for xCT) result in ROS accumulation, thereby modulating ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [192] | ||||
| Responsed Drug | Etoposide | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| Response Description | The combined treatment of etoposide and erastin synergistically induced oxidative stress and lipid peroxidation, while suppressing glutathione peroxidase activity in breast cancer cells. More importantly, the combination treatment synergistically increased iron accumulation, which was associated with altered expression of IREB2/FPN1. Additionally, ferroptosis-regulating proteins ACSF2 and GPX4 were altered more potently by the combination treatment, compared to untreated cells and erastin treatment alone (p<0.05). | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [193] | ||||
| Responsed Drug | Tubastatin A | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| In Vivo Model |
Female 5-week-old athymic nude mice were obtained from Sun Yat-sen University. All mice were kept under specific-pathogen free conditions in the animal facility of Sun Yat-sen University Cancer Centre. Cancer cells were suspended and counted in 1 x DMEM, and 2 x 106 MDA-MB-231 cells were injected into mice subcutaneously. When the tumours reached 50-100 mm3, the mice were randomly assigned to different treatment groups. Tumours were irradiated with a JL Shepherd Mark I-68A irradiator at a dose of 10 Gy. Tub was dissolved in solvent containing 1% DMSO, 30% polyethylene glycol, 1% Tween 80 and 68% H2O and then subcutaneously administered to mice at a dose of 2.5 mg/kg once a day. Lipro-1 diluted in PBS was intraperitoneally injected daily at a dose of 10 mg/kg. Tub or Lipro-1 was administered three times before irradiation followed by continued daily administration until the endpoint, as indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Tubastatin A (Tub) as a novel GPX4 inhibitor that induced ferroptosis through large-scale drug screening. We showed that IR-mediated GPX4 expression restrained ferroptosis to drive radioresistance in breast cancer. | ||||
Ovarian cancer [ICD-11: 2C73]
| In total 4 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [194] | ||||
| Responsed Drug | Apatinib | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| Response Description | Apatinib combined with olaparib-induced ferroptosis via a p53-dependent manner in ovarian cancer. Further studies showed that apatinib combined with olaparib-induced ferroptosis by inhibiting the expression of Nrf2 and autophagy, thereby inhibiting the expression of GPX4. The Nrf2 activator RTA408 and the autophagy activator rapamycin rescued the combination drug-induced ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [195] | ||||
| Responsed Drug | Triptolide | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A2780/DDP cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_D619 | |
| In Vivo Model |
All female BALB/cnude mice(4-6 weeks old, 15-20 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). They were raised in specific pathogen-free conditions and allowed to access sterile water and food freely. A2780/DDP cell suspension (100 uL) with a density of 1 x 107 cells/mL was injected subcutaneously into the axilla of the mice. After observing the nude mice for a week, it was confirmed that subcutaneous A2780/DDP cells were inoculated successfully. Sterile saline (100 uL) was injected into the abdominal cavity of the nude mice in the control group for 14 days. The mice in the DDP treatment group were given DDP (4 mg/kg/day) intraperitoneally on the first and eighth days. TG (100 uL, 1 mg/kg) diluted with sterile physiological saline were injected into the abdominal cavity of the nude mice in the TG treatment group for 14 days. In addition, the nude mice in the TG + DDP treatment group were given TG (100 uL, 1 mg/kg) for 14 days and DDP (4 mg/kg/day) intraperitoneally on the first and eighth days.
Click to Show/Hide
|
||||
| Response Description | Tripterygium (TG) can effectively inhibit the proliferation of drug-resistant ovarian tumor cells A2780/DDP and increase the sensitivity to cisplatin chemotherapy both invitro and invivo. In terms of mechanism, TG induces ferroptosis by targeting the NRF2/GPX4 signal axis to weaken the antioxidant capacity of cancer cells. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [194] | ||||
| Responsed Drug | Olaparib | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| Response Description | Apatinib combined with olaparib-induced ferroptosis via a p53-dependent manner in ovarian cancer. Further studies showed that apatinib combined with olaparib-induced ferroptosis by inhibiting the expression of Nrf2 and autophagy, thereby inhibiting the expression of GPX4. The Nrf2 activator RTA408 and the autophagy activator rapamycin rescued the combination drug-induced ferroptosis. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [196] | ||||
| Responsed Drug | Norcantharidin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Athymic nu/nu female mice aged 6-8 weeks (n = 9; mean weight, 20.21 ± 1.54 g) were purchased from the specific pathogen SPF (Beijing) Lab Animals Technology Co. Ltd. Mice were housed in a temperature- and humidity-controlled environment (20-24 , 45-55% humidity), with free access to food and water and in groups of three. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC ID: 17-3256) at Nantong University and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Nuclear factor erythroid 2-related factor 2 (NRF2), heme oxygenase 1 (HO-1), glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (xCT) expression levels were significantly decreased following norcantharidin (NCTD) treatment. Collectively, NCTD may represent a potent anticancer agent in ovarian cancer cells, and NCTD-induced ferroptotic cell death may be achieved by inhibiting the NRF2/HO-1/GPX4/xCT axis. | ||||
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [197] | |||
| Responsed Drug | Dihydroartemisinin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SiHa cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | |
| Response Description | Dihydroartemisinin (DHA) treatment initiated ferroptosis, as evidenced by the accumulation of reactive oxygen species (ROS), malondialdehyde (MDA) and liquid peroxidation (LPO) levels and simultaneously depletion of glutathione peroxidase 4 (GPX4) and glutathione (GSH). Moreover, nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy was also induced by DHA leading to subsequent increases of intracellular labile iron pool (LIP), exacerbated the Fenton reaction resulting in excessive ROS production, and enhanced cervical cancer ferroptosis. | |||
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [198] | |||
| Responsed Drug | Buthionine sulfoximine | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
VCaP cells | Prostate carcinoma | Homo sapiens | CVCL_2235 |
| LNCaP cells | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| LNCaP C4-2 cells | Prostate carcinoma | Homo sapiens | CVCL_4782 | |
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | |
| RWPE-1 cells | Normal | Homo sapiens | CVCL_3791 | |
| MDA-kb2 cells | Breast adenocarcinoma | Homo sapiens | CVCL_6421 | |
| Response Description | ITC-ARi 13 and buthionine sulfoximine (BSO) cooperatively downregulate AR and induce ferroptosis likely through increasing the accessibility of 13/12b to cellular targets, escalating free intracellular ferrous iron and attenuating GSH-centered cellular defense and adaptation. Further studies on the combination of ITC-ARi and GSH synthesis inhibitor could result in a new modality against castration-resistant prostate cancer (CRPC). Collectively, the combination of ITC-ARi 13 and BSO reveals a pro-ferroptotic role of Nrf2 through upregulating HO-1 under GSH-deficient conditions. | |||
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [199] | |||
| Responsed Drug | Lycorine | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | |
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| Response Description | Lycorine could inhibit the proliferation in human renal cell carcinoma (RCC) cells. The anti-tumor effect of lycorine was associated with the induction of ferroptosis. After lycorine treatment, the expression levels of GPX4 in RCC cells decreased, whereas those of ACSL4 increased. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [200] | |||
| Responsed Drug | Tetrachlorobenzoquinone | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Tetrachlorobenzoquinone (TCBQ)-induced ferroptosis occurred as a result of iron accumulation and inhibition of GPX4 expression. Mechanistically, TCBQ promotes the iron import into cells by improving the expression of TF and TFR1, and the complex of TF and TFR1 is internalized by endocytosis in Adrenal gland pheochromocytoma. | |||
Bladder cancer [ICD-11: 2C94]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [201] | |||
| Responsed Drug | Quinazolinyl-arylurea derivatives 7J | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell autophagy | ||||
| Cell proliferation | ||||
In Vitro Model |
BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| HCC827 cells | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | |
| T24 cells | Bladder carcinoma | Homo sapiens | CVCL_0554 | |
| RT4 cells | Bladder carcinoma | Homo sapiens | CVCL_0036 | |
| Response Description | Compound 7j treatment could trigger three different cell death forms including apoptosis, ferroptosis, and autophagy; which form would occur depended on the concentrations and incubation time of 7j. Ferroptosis and autophagy occurred in the case of higher concentrations combining with extended incubation time through effectively regulating the Sxc-/GPx4/ROS and PI3K/Akt/mTOR/ULK1 pathways, respectively. Compound 7j could be a promising lead for molecular-targeted anti- bladder cancer agents' discovery. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [202] | |||
| Responsed Drug | FIN56 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
J82 cells | Bladder carcinoma | Homo sapiens | CVCL_0359 |
| 253J cells | Bladder carcinoma | Homo sapiens | CVCL_7935 | |
| T24 cells | Bladder carcinoma | Homo sapiens | CVCL_0554 | |
| RT-112 cells | Bladder carcinoma | Homo sapiens | CVCL_1670 | |
| mEFs (Mouse embryonic fibroblasts) | ||||
| Response Description | Fin56, a type 3 inducer, leads to ferroptosis mainly by promoting GPX4 degradation. Fin56 induces ferroptosis and autophagy in bladder cancer cells and that Fin56-triggered ferroptosis mechanistically depends on the autophagic machinery. | |||
Thyroid cancer [ICD-11: 2D10]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [203] | |||
| Responsed Drug | Ascorbic Acid | Approved | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| Cell proliferation | ||||
In Vitro Model |
8505C cells | Thyroid gland anaplastic carcinoma | Homo sapiens | CVCL_1054 |
| C643 cells | Thyroid gland anaplastic carcinoma | Homo sapiens | CVCL_5969 | |
| Response Description | Vitamin C could significantly inhibit anaplastic thyroid cancer (ATC) cells growth through ferroptosis activation, evidenced by the GPX4 inactivation, ROS accumulation and iron-dependent lipid peroxidation. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [204] | |||
| Responsed Drug | Curcumin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 |
| Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | |
| FTC 238 cells | Thyroid gland follicular carcinoma | Homo sapiens | CVCL_2447 | |
| Response Description | Knockdown of HO-1 inhibits ferroptosis by upregulating the GPX4 expression in follicular thyroid cancer cells. We conclude that curcumin inhibits the tumorigenesis of follicular thyroid cancer via HO-1-induced activation of the ferroptosis signalling pathway. | |||
Diabetes mellitus [ICD-11: 5A10]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [205] | ||||
| Responsed Drug | Berberine | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Response Description | Berberine (BBR) stimulated GPX4 expression to reduce the content of Fe2+ and ROS, thereby repressing the ferroptosis of islet cells in diabetes mellitus, which functioned similarly as ferroptosis inhibitor Fer-1. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [206] | ||||
| Responsed Drug | Empagliflozin | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| HUVECs (Human umbilical vein endothelial cells) | |||||
| MOVAS-1 cells | Normal | Homo sapiens | CVCL_0F08 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
For diabetes induction, C57BL/6 mice were fed with high fat diet (HFD) for 3 weeks (20% kcal protein, 20% kcal carbohydrate, and 60% kcal fat). Intraperitoneal administration of 60 mg/kg body weight streptozotocin (STZ, Sigma-Aldrich, St Louis, MO, USA) diluted in sodium citrate buffer was then performed for the following six days. Mice were fasted overnight prior to each STZ injection and blood glucose level measurement. Blood glucose level was evaluated using Accu-Check Integra (Roche Diagnostics, Shanghai, China). Mice with blood glucose level above 16.6 mM were assumed as diabetic mice, and were used for establishing diabetic HLI model as described previously.
Click to Show/Hide
|
||||
| Response Description | Empagliflozin, a clinical hypoglycemic gliflozin drug, can inhibit ferroptosis and enhance skeletal muscle cell survival and paracrine function under hyperglycemic condition via restoring the expression of GPX4. This study highlights the potential of intramuscular injection of empagliflozin for treating diabetic hindlimb ischemia. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [207] | ||||
| Responsed Drug | Salidroside | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| HUVECs (Human umbilical vein endothelial cells) | |||||
| MOVAS-1 cells | Normal | Homo sapiens | CVCL_0F08 | ||
| In Vivo Model |
For diabetes induction, C57BL/6 mice were given a high-fat diet for three weeks that contained 20% protein, 20% carbohydrate, and 60% fat. Sodium citratebuffer-diluted 60 mg/kg body weight streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO) were administered intraperitoneally for the next constitutive six days. Prior to each STZ injection and blood glucose testusing Accu-Check Integra (Roche Diagnostics, Shanghai, China), mice were fasted overnight. Mice with blood glucose levels higher than 16.6 mM were considered diabetic and were utilized to establish the diabetic HLI model.
Click to Show/Hide
|
||||
| Response Description | Salidroside/GPX4-mediated ferroptosis inhibition is crucial for promoting angiogenesis and blood perfusion recovery in diabetic hindlimb ischemia mice. | ||||
Ovarian dysfunction [ICD-11: 5A80]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [208] | ||||
| Responsed Drug | Metformin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
n = 6 blank control group was created. The mice in the control group were fed regular food and gavaged with normal saline daily. The mice in the control group were given a high-fat diet and 1 mg/kg of letrozole via gavage for 21 days to establish a PCOS model of insulin resistance and hyperandrogenism. The mice, after successful modeling, were randomly divided into PCOS group and metformin group (n = 6). During the treatment period, the control group continued to be fed with normal feed and given normal saline; the PCOS group was fed with continuous high-fat feed and given letrozole (1 mg/kg/day) by intragastric administration, and the metformin group was given metformin by intragastric administration (200 mg/kg/day). After 30 days of treatment, the experimental mice were euthanized, serum was collected, one mouse ovary was collected for histological examination, and the other was stored in a -80 refrigerator for molecular biology experimental research.
Click to Show/Hide
|
||||
| Response Description | Morphological results showed that after metformin treatment, polycystic lesions in ovaries were reduced, the ovarian function was restored, and the expressions of SIRT3 and GPX4 were elevated. Metformin could regulate ferroptosis to improve polycystic ovary syndrome via the SIRT3/AMPK/mTOR pathway. | ||||
Obesity [ICD-11: 5B81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [209] | ||||
| Responsed Drug | Atorvastatin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell senescence | |||||
| In Vivo Model |
Mice were sacrificed by cervical dislocation. As described previously, epidydimal adipose tissues (EAT) were isolated and minced into ~5-mg pieces in DMEM containing 10% FBS. After 2 h of incubation, 50 mg of small pieces were placed in serum-free DMEM and exposed to 1 umol/L atorvastatin for 18 h, and 0.1% DMSO served as a control. In specific experiments, EAT explants were also treated with GGPP (50 uM; GlpBio), or ferrostatin-1 (Fer-1, 8 uM), and added to the culture medium at the same time as was atorvastatin. Group animal size was n = 6-8 per group. The exact group size is specially described in the Figure legends.
Click to Show/Hide
|
||||
| Response Description | Atorvastatin decreased the level of GPX4 and depleted GGPP production, but not Fer-1. Atorvastatin was able to induce ferroptosis in adipose tissue, which was due to increased ROS and an increase in cellular senescence. Moreover, this effect could be reversed by the supplement of GGPP. Taken together, our results suggest that the induction of ferroptosis contributed to statin-induced cell senescence in adipose tissue and may contributed to obesity disease. | ||||
Vascular dementia [ICD-11: 6D81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [210] | ||||
| Responsed Drug | Gastrodin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male Sprague-Dawley rats (weight 260 ± 20 g; Guizhou Medical University Experimental Animal Center; Certificate No. SCXK2018-0001; Grant No. 2200483) were reared in a specific pathogen-free environment with 12 h light/dark cycle and 55% ± 10% humidity at a temperature of 20~25 , were provided with sufficient feed and sterile drinking water and fasted for 6 h before and after surgery. All animal experiments were performed in accordance with the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Gastrodin (GAS) inhibited ferroptosis in hippocampal neurons by activating the Nrf2/Keap1-GPx4 signaling pathway, suggesting its possible application as a functional food for improving vascular dementia by inhibiting ferroptosis. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [211] | ||||
| Responsed Drug | Oxidopamine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
The AB strain of wild-type zebrafish (Danio rerio) was applied in this study. Zebrafish larvae at 4 dpf (days post-fertilization) were co-incubated with 250 uM 6-OHDA or 1.5 ug/mL nomifensine (Nomi, a dopamine transporter inhibitor) in 6-well plates at a density of 30 zebrafish embryos per group for 2 days and the medium was refreshed every day. The swimming total distance of each fish was recorded for 10 min and was analyzed by an automated video tracking system.
Click to Show/Hide
|
||||
| Response Description | 6-hydroxydopamine (6-OHDA) treatment-induced ferroptosis in SH-SY5Y cells mainly by disturbing the protein expression of GPX4 and ACSL4. Collectively, the activation of the p62-Keap1-Nrf2 pathway prevents 6-OHDA-induced ferroptosis in SH-SY5Y cells, targeting this pathway in combination with a pharmacological inhibitor of ferroptosis can be a potential approach for parkinson's disease therapy. | ||||
Alzheimer disease [ICD-11: 8A20]
| In total 4 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [212] | ||||
| Responsed Drug | Forsythoside A | Investigative | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| Neuro-2a cells | Neuroblastoma | Mus musculus | CVCL_0470 | ||
| BV-2 cells | Normal | Mus musculus | CVCL_0182 | ||
| In Vivo Model |
All animal experiments were approved by the Animal Ethics Committee of Jilin University (permit No. SY201905013) and were conducted in compliance with the ARRIVE guidelines. Eight-month-old B6C3-Tg (APPswePSEN1dE9)/Nju double transgenic male mice (APP/PS1) (genotype: (Appswe) T, (Psen1) T) and age-matched wild-type (WT) (genotype: (Appswe) W, (Psen1) W) male mice were purchased from Nanjing Biomedical Research Institute of Nanjing University. All mice were individually housed at 24 with food and drinking water availablead libitum. After 1 week of adaption in the new environment, WT mice received oral administration of normal saline (10 mL/kg) and were designated as the control group (n = 12). APP/PS1 mice were randomly divided into two groups: the model group (n = 12) received oral administration of normal saline (10 mL/kg) and the agent-treated group (n = 12) received oral treatment with 30 mg/kg FA (L-012-171216, 98.83% purity, Chengdu Herbpurify Co., Ltd., Chengdu, China) beginning on day 8. After 30-day treatment, behavioral experiments were serially performed. The entire treatment protocol lasted for 42 days. Blood samples were collected from the caudal vein. After euthanasia via CO2 inhalation, organs including the brain, liver, spleen, and kidney were collected for further analysis.
Click to Show/Hide
|
||||
| Response Description | Forsythoside A treatment exerted anti-ferroptosis and anti-neuroinflammatory effects in erastin-stimulated HT22 cells, and the Nrf2/GPX4 axis played a key role in these effects. Collectively, these results demonstrate the protective effects of FA and highlight its therapeutic potential as a drug component for AD ( Alzheimer's disease) treatment. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [213] | ||||
| Responsed Drug | ginkgolide-B | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
Male 6-month-old senescence-resistant R1 (SAMR1) and SAMP8 mice (weight, 28-35 g) were purchased from Beijing SPF Biotechnology. First, mice were placed in the center of an empty testing arena (40 x 40 x 40 cm) and allowed to move freely for adaptation. Next, in the training stage, two similar objects were presented in the testing arena and mice were allowed to explore for 10 min, for 3 consecutive days. On day 4, one of the two familiar objects was replaced by a new object. The time of exploring a novel object or familiar object in 10 min was recorded.
Click to Show/Hide
|
||||
| Response Description | Ginkgolide B attenuated Alzheimer's disease (AD)-related cognitive impairment through the regulation of oxidative stress, neuroinflammation and ferroptosis, and that GB-induced protection in AD is dependent on the inhibition of ferroptosis. Furthermore, the involvement of Nrf2/GPX4 pathway-regulated ferroptosis in the GB-related protective effects on the AD mouse model. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [214] | ||||
| Responsed Drug | Salidroside | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CD8T cells (Mouse CD8+ T cells) | ||||
| In Vivo Model |
SAMP8 mice were employed as an AD model and were treated with salidroside for 12 weeks. Behavioral tests, immunohistochemistry, HE and Nissl staining, immunofluorescence, transmission electron microscopy, quantitative proteomics, bioinformatic analysis, flow cytometry, iron staining,western blotting, andmolecular dockingwere performed.
Click to Show/Hide
|
||||
| Response Description | Salidroside alleviates cognitive impairment and inhibits neuronal ferroptosis in Alzheimer's disease. The underlying mechanisms may involve the Nrf2/GPX4 axis activation and reduction in CD8T cells infiltration. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [215] | ||||
| Responsed Drug | Tetrahydroxy stilbene glycoside | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Pathways in cancer | hsa05200 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
APPswe/PSEN1dE9 (APP/PS1) double transgene mice, which were generated by the introduction of human APPswe and PS1-dE9 mutations onto the C57BL/6 background and also wild type (WT) littermates, aged 5 months, were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China). The mice received food and water ad libitum under standard husbandry conditions (22-25, 55-65% relative humidity, and 12h/12h lightdark cycle) and acclimated 1 week for the experiments. Mice were randomly divided into 5 groups, including WT control group, APP/PS1 model group, and APP/PS1 + TSG (60, 120 and 180 mg/kg) different dosage groups. Mice were orally treated with TSG every other day for 2 months. WT and model groups were treated with equivalent vehicle.
Click to Show/Hide
|
||||
| Response Description | Tetrahydroxy stilbene glycoside (TSG) promoted the activation of GSH/GPX4/ROS and Keap1/Nrf2/ARE signaling pathways. Notably, markers related to ferroptosis including increased lipid peroxidation, enhanced neuroinflammation such as NLRP3, and also the expression of DMT1, ACSL4 and NCOA4, were reduced by TSG administration. In addition, TSG enhanced antioxidative stress via the upregulation of SOD, and the expression of FTH1, CD98 and xCT. Hence, TSG should be taken into consideration during treatment of Alzheimer's disease in the future. | ||||
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [216] | ||||
| Responsed Drug | Lapatinib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from the Animal Unit of Central South University. After anesthetization by intraperitoneal injection of 10% chloral hydrate (v/w), the mice were fixed on a stereotactic instrument and stereotactically injected with KA (250 ng/ul) into the hippocampus. KA (1 ul) was injected slowly for 5 min and positioned in the hippocampus (AP-2.0 mm, ML-1.3 mm, V-1.2 mm). After injection, the needle was left in place for additional 10 min to avoid drug reflux. The mice were randomly divided into six experimental groups: 1) sham operation group that received 1 ul PBS injection (5 animals); 2) mice were pretreated p. o. for 21 days on a twice-daily schedule with 100 mg/kg lapatinib alone before PBS administration (5 animals); 3) KA-treated group was injected KA (5 animals); 4) and 5) lapatinib groups were received with 50 mg/kg (5 animals) and 100 mg/kg (5 animals) lapatinib for 21 days before KA treatment, respectively; 6) this group was given i. p. for 14 days with ferroptosis inhibitor (3 mg/kg Fer-1) before KA administration.
Click to Show/Hide
|
||||
| Response Description | Lapatinib exerted neuroprotection via restoring glutathione peroxidase 4 (GPX4). Treatment with GPX4 inhibitor ras-selective lethal small molecule 3 (RSL3) abrogated its anti-ferroptotic potential. It is concluded that lapatinib has neuroprotective potential against epileptic seizures via suppressing GPX4-mediated ferroptosis. | ||||
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 5 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [217] | ||||
| Responsed Drug | 20-Hydroxyeicosatetraenoic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mOHSCs (Mouse organotypic hippocampal slice cultures) | ||||
| In Vivo Model |
Adult male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME USA). Mice at 10-12 weeks of age were anesthetized by 1-3% isoflurane inhalation and ventilated with oxygen-enriched air (20% O2:80% air). The right striatum of mice was injected with 0.5 ul of 0.075 U collagenase VII-S (MilliporeSigma, St. Louis, MO, USA) at 0.1 ul per minute. Injections were administered at 0.5 mm anterior and 2.2 mm lateral of the bregma, and 3.0 mm in depth, as previously described. Sham-operated mice received the same treatment, including needle insertion, but were not injected with collagenase. Mice that died before the end of the surgery or shortly thereafter were excluded.
Click to Show/Hide
|
||||
| Response Description | 20-hydroxyeicosatetraenoic acid induces ferroptosis in OHSCs, and inhibition of 20-HETE synthesis improves Intracerebral hemorrhage (ICH) outcome and attenuates markers of ferroptosis, such as mobile iron, lipid peroxidation, and decreased GPX4. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [218] | ||||
| Responsed Drug | Baicalin | Terminated | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In Vivo Model |
A total of 60 male C57BL/6 mice (10weeks old, 25-28g) were purchased from Guangzhou University of Chinese Medicine Experimental Animal Center (Guangzhou, China). The mice were maintained with enough food and water at 24, 60% relative humidity and 12/12h light/dark cycle. The mice were randomly divided into three groups: sham operation group (Sham), ICH model group (Mod) and baicalin group (Bai) (n = 20/group). Baicalin was suspended in 0.5% carboxymethylcellulose sodium solution. Given the extremely low solubility of baicalin, the concentration of baicalin solution was 0.5 mg/ml. To achieve 20 mg/kg/day dosage, the baicalin solution was administered to the mice in the Bai group by oral route twice at an interval of 1 h within 2 h after ICH injury onset. The remaining two groups received an equal volumes of saline through oral gavage. Since the second day after ICH, mice in the Bai group received 20 mg/kg of baicalin solution while those in the remaining two groups received equal volumes of saline once a day for three consecutive days.
Click to Show/Hide
|
||||
| Response Description | Baicalin significantly increased the mRNA expression of GPX4 and SLC7A11 in the perihematoma brain tissues of intracerebral hemorrhage (ICH) model mice. Baicalin can inhibit the development of ferroptosis in ICH. Baicalin is a potential therapeutic drug for ICH treatment. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [219] | ||||
| Responsed Drug | Dauricine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Adult male C57BL/6 mice weighing 20-28 g were maintained in the specific pathogen-free (SPF) facility to be used in this study. Mice were subjected to a 12-h light/dark cycle at a constant ambient temperature (22 ± 1 ). Mice were randomly assigned into the following five groups based on random numbers generated using SPSS. The sham group(n = 20, of which 20 survived) was subjected to mock surgery (craniotomy without collagenase) and treated with 0.1 mL 0.9% saline. The intracerebral hemorrhage(ICH) group (n = 29, of which 23 survived) was subjected to ICH surgery, then treated with 0.9% saline. The low Dauricine(Dau) group (n = 24, of which 20 survived) was subjected to ICH surgery, then immediately treated with 5 mg/kg Dau via tail vein injection. The medium Dau group (n = 25, of which 22 survived)was subjected to ICH surgery, then treated with 10 mg/kg Dau. The high Dau group (n = 24, of which 22 survived)was subjected to ICH surgery, then immediately treated with 15 mg/kg Dau.
Click to Show/Hide
|
||||
| Response Description | Dauricine (Dau) could inhibit ferroptosis of nerve cells and alleviate brain injury after intracerebral hemorrhage by upregulating glutathione peroxidase 4 (GPX4) and glutathione reductase (GSR) co-expression. Therefore, Dau may be an effective drug for inhibiting ferroptosis and treating intracerebral hemorrhage. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [220] | ||||
| Responsed Drug | Fucoidan | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 | |
| Omm1 cells | Uveal melanoma | Homo sapiens | CVCL_6939 | ||
| SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | ||
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| Response Description | Iron-dependent oxidative stress (ferroptosis) is a main hallmark of retinal and brain diseases, including hemorrhage. Fucoidans can abrogate the decrease in the protein levels of the antioxidant enzyme GPX4 that is crucial for ferroptosis. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [221] | ||||
| Responsed Drug | Rotenone | Approved | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPCNs (Rat primary cortical neurons) | ||||
| In Vivo Model |
Six-to-eight week old male ICR mice were purchased from the Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China). Herein, a total of 51 mice were randomly divided into 3 groups: (i) sham group (n = 15), (ii) ICH group (n = 18), and (iii) ICH + Rot group (n = 18). All mice were euthanized at 3 d after operation and brain samples were harvested, as per our previously described reports.
Click to Show/Hide
|
||||
| Response Description | Single rotenone administration markedly inhibited neuronal viability, promoted iron accumulation, increased malondialdehyde (MDA) contents, decreased total superoxide dismutase (SOD) activity, and downregulated ferroptosis-related proteins RPL8, COX-2, xCT, ASCL4, and GPX4 in primary neurons. Together, our data revealed that intracerebral hemorrhage induced significant mitochondrial dysfunction and that mitochondrial inhibitor rotenone can trigger and enhance neuronal ferroptosis. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 5 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [222] | ||||
| Responsed Drug | Naotaifang Extract | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Specific pathogen-free adult male SD rats, (80 ± 5) days old and weighing 220-250 g, were provided by the Hunan Slack Jingda Experimental Animal Co., Ltd (Hunan, China). SD rats were randomly divided into 4 groups with 15 in each group: sham operation group, MCAO group, MCAO + DFP group and MCAO + NTE group. The rats were treated with drugs via oral gavage. According to the average body weight, the MCAO + NTE rats were given NTE at 27 g/kg, and the sham operation and the MCAO rats were given the same volume of saline (2.5 mL) for 7 consecutive days. The MCAO + DFP rats were given DFP at a dose of 125 mg/kg for 3 consecutive days.
Click to Show/Hide
|
||||
| Response Description | Acute cerebral ischemia induces neuronal ferroptosis and the effects of treating MCAO rats with naotaifang extract involved inhibition of ferroptosis through the TFR1/DMT1 and SCL7A11/GPX4 pathways. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [223] | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
The mice (23-25 g, 8-10 weeks old) were subjected to transientmiddle cerebral artery occlusion (tMCAO) to induce cerebral ischemia as previously described protocol . Briefly, mice were anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg) and subcutaneous injection of meloxicam (10mg/kg) during tMCAO operation. Monofilament with a silicon coating on the tip and a diameter of 0.12 mm (A5-122, Beijing Cinontech Co. Ltd., China) was inserted into the ICA from CCA to occlude the middle cerebral artery (MCA) for 1.5 h. The suture was then removed to restore blood flow for another 22.5 h reperfusion. Sham control mice were subjected to similar surgical operations without MCA occlusion. Specifically, the monofilament was inserted only 5 mm above the carotid bifurcation and withdrew immediately in the Sham group.
Click to Show/Hide
|
||||
| Response Description | Baicalein inhibited the ferroptosis by regulating on the expression levels of GPX4, ACSL4 and ACSL3 in OGD/R cells, tMCAO mice and RSL3-stimulated HT22 cells. Our findings demonstrated that baicalein reversed the cerebral ischemia-reperfusion injury via anti-ferroptosis, which was regulated by GPX4/ACSL4/ACSL3 axis. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [224] | ||||
| Responsed Drug | Carvacrol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
A total of 108 gerbils (male; body weight, 70-90 g; age, 12 to 16 weeks) were used in this study. The gerbils were randomly divided into the following five groups: the vehicle-treated group (sham group),which was given an equal volume of physiological saline; the carvacrol (CAR)-treated group (CAR group); the model group, which underwent the ligation of the bilateral carotid artery for 5 min followed by the loosening of the arterial clamp for reperfusion; the model + CAR-treated groups, which included the CAR-treated group and the model + CAR-treated groups that were treated with CAR (25, 50 and 100 mg/kg/day, i.p.) for 2 consecutive weeks and the model + DFO-treated groups that were treated with DFO (150 mg/kg/day, i.p.) for 2 consecutive weeks as the positive drug group.
Click to Show/Hide
|
||||
| Response Description | Carvacrol provides protection for hippocampal neurons against cerebral ischemia reperfusion in gerbils by inhibiting ferroptosis through increasing the expression of GPx4. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [225] | ||||
| Responsed Drug | Propofol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Male C57BL/6 mice weighing 20-25 g each were obtained from the Animal Experimental Center of Yisi (Changchun, China). Mice were group-housed in a 12 h light/dark cycle (light between 08:00 and 20:00 h) in a temperature-controlled environment room (23-25 ). Mice had ad libitum access to food and water. All surgical procedures were carried out on animals anesthetized with sodium pentobarbital (30 mg/kg) via intraperitoneal injection. MCAO was achieved by inserting a silicone rubber-coated nylon monofilament into the internal carotid artery through the external carotid artery and temporary ligation of the right common carotid artery with a suture. After 45 min of ischemia, blood flow was restored by removing the filament and the suture, and the mice were allowed to recover for 24 h.
Click to Show/Hide
|
||||
| Response Description | Our data support a protective role of propofol against ferroptosis as a cause of cell death in mice with cerebral ischemia-reperfusion injury. Propofol protected against cerebral ischemia-reperfusion injury-induced ferroptosis partly by regulating the Nrf2/Gpx4 signaling pathway. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [226] | ||||
| Responsed Drug | Chrysin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [227] | |||
| Responsed Drug | Kaempferol | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
mPCNs (Mouse primary cortical neurons) | |||
| Response Description | Kaempferol provides protection from OGD/R-induced ferroptosis, at least in part, by activating Nrf2/SLC7A11/GPX4 signaling pathway. Therefore, pharmacological inhibition of ferroptosis may be an attractive therapeutic target for the treatment of ischemic stroke. | |||
Nervous system disease [ICD-11: 8E7Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [228] | |||
| Responsed Drug | moracin N | Investigative | ||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Moracin N was a good ferroptosis inhibitor in Neurodegenerative diseases. The neuroprotective mechanisms of moracin N included inhibition of glutathione depletion, glutathione peroxidase 4 (GPx4) inactivation, reactive oxygen species (ROS) overproduction and iron accumulation, as well as improvement of intracellular antioxidant enzyme activities. | |||
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [229] | ||||
| Responsed Drug | 4-(cyclohexylamino)-3-[(phenylmethyl)amino]-N-[2-(1-piperazinyl)ethyl]-benzenesulfonamide | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
Male Sprague-Dawley rats (450 g-550 g) were purchased from Envigo (Frederick, Md). Animals were kept under standard conditions with a 12/12-h day/night cycle and received food and waterad libitum. Following induction with inhaling low flow CO2 for 30 s, animals were anesthetized by intraperitoneal injection of pentobarbital (45 mg/kg). Additional doses (10 mg/kg) were administered as needed based on tail pinch/withdrawal reflex to maintain anesthesia.
Click to Show/Hide
|
||||
| Response Description | Treatment with ferroptosis inhibitor, UAMC-3203 or/and DFO, reduced severity of myocardial dysfunction, and we further found that GPX4 and 4-HNE were significantly changed after CPR. Therefore, UAMC-3203 and DFO alleviated myocardial dysfunction via inhibiting ferroptosis, which could be a novel possible target for post-resuscitation myocardial dysfunction (PRMD) treatment. | ||||
Cardiomyopathy [ICD-11: BC43]
| In total 7 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [230] | ||||
| Responsed Drug | Berberine | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response Description | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [231] | ||||
| Responsed Drug | Canagliflozin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57BL/6J mice aged 6-8 weeks with weights of 18-20 g were obtained from the Slack Laboratory Animal Co., Ltd. (Shanghai, China). Mice were allowed to acclimatize in the laboratory environment for 1 week before the beginning of the experiment. DCM model establishment: The mice were given a single intraperitoneal injection of 150 mg/kg 1% streptozotocin (STZ, V900890, Sigma, USA, dissolved in 0.1 mol/L sodium citrate buffer, pH = 4.4 - 4.6). Mouse blood from the tail vein was collected in each group of the model mice and tested by glucose meter (Accu-Chek Performa test strips, Roche, Accu-Chek Performa Combo, Roche, USA) on day 3, 5 and 7 after injection.
Click to Show/Hide
|
||||
| Response Description | Canagliflozin (Cana) promotes upregulation of SLC7A11 and downregulation of TfR1 and FTN-H, which protect the cardiomyocytes from ferroptosis. These finding suggests that Cana inhibit ferroptosis by balancing cardiac iron homeostasis and promoting the system Xc/GSH/GPX4 axis in diabetic cardiomyopathy. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [232] | ||||
| Responsed Drug | Curcumin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Two-month-old male New Zealand rabbits purchased from the Medical Experimental Animal Center of Bengbu Medical College were used as experimental subjects. Streptozotocin was dissolved in sterile saline and intraperitoneally injected into the rabbits at a dose of 80 mg/kg. The rabbits were allowed to eat freely after receiving the injection. The fasting blood glucose levels of the rabbits were monitored regularly. The diabetic rabbit model was considered successfully established when the fasting blood glucose level was measured as 11 mmol/L twice or 14 mmol/L once. Following successful modelling, grouping was performed as follows: blank control group (Con-Group), diabetic rabbit group (DM-Group), diabetic rabbit + every other day curcumin administration group (Qod-Group), and diabetic rabbit + daily administration group (Qd-Group).
Click to Show/Hide
|
||||
| Response Description | Curcumin can promote the nuclear translocation of Nrf2, increase the expression of oxidative scavenging factors, such as HO-1, reduce excessive Gpx4 loss, and inhibit glucose-induced ferroptosis in cardiomyocytes. This highlights a potentially new therapeutic route for investigation for the treatment diabetic cardiomyopathy. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [233] | ||||
| Responsed Drug | Doxorubicin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
Male C57BL/6J mice were housed in a temperature- and humidity-controlled room, fed a commercial diet (CRF-1; Oriental Yeast Co. Ltd.), and given free access to water. GPx4 Tg mice and GPx4 hetKO mice were produced as previously described. In these gene-manipulated mice, GPx4 was systemically overexpressed or absent, respectively. These strains were backcrossed with C57BL/6J mice in our laboratory. The DIC model was reproduced as previously reported, with some modification. Briefly, DOX (6 mg/kg, body weight) was administered to mice via tail vein at days 0, 2, and 4.
Click to Show/Hide
|
||||
| Response Description | Doxorubicin (DOX) downregulated glutathione peroxidase 4 (GPx4) and induced excessive lipid peroxidation through DOX-Fe2+ complex in mitochondria, leading to mitochondria-dependent ferroptosis. The findings suggest that mitochondria-dependent ferroptosis plays a key role in progression of doxorubicin-induced cardiomyopathy (DIC) and that ferroptosis is the major form of regulated cell death in DOX cardiotoxicity. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [234] | ||||
| Responsed Drug | Liquiritin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
The set of animal experiments was designed to evaluate the effectiveness of liquiritin on doxorubicin-induced cardiotoxicity as were as ferroptosis were explored. Mice were randomly divided into 5 groups: (1) the control group; (2) doxorubicin group; (3) the doxorubicin plus liquiritin group (20 mg/kg); (4) the doxorubicin plus liquiritin group (40 mg/kg); (5) the doxorubicin plus liquiritin group (80 mg/kg) (Han et al.2022; Mou et al.2021). The control group and doxorubicin group were given equal volume of 0.5% sodium carboxymethylcellulose; the doxorubicin plus liquiritin groups were given different doses of liquiritin (0.5%) sodium carboxymethylcellulose co-suspension) by intragastric administration 7 days in advance once a daily. On day 8, groups (2), (3), (4), and (5) were given a single intraperitoneal injection of 15 mg/kg of DOX to establish a model of doxorubicin-induced cardiotoxicity; and group (1) was given an equal volume of saline intraperitoneally.
Click to Show/Hide
|
||||
| Response Description | Liquiritin can protect the doxorubicin-induce mice's cardiotoxicity, and its beneficial effect is related to the reduction of ferroptosis through a mechanism involving the regulation of the SLC7A11/GPX4 pathway. | ||||
| Experiment 6 Reporting the Ferroptosis-centered Disease Response of This Regulator | [235] | ||||
| Responsed Drug | Resveratrol | Phase 3 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Adult male C57BL/6J mice weighing 20 ± 2 g were purchased from Chongqing Tengxin Biotechnology. Mice were housed at 22 with a 12 h light/dark cycle with free access to food and water. The cardiotoxicity mice model was induced by intraperitoneal injection of 5-FU (30 mg/kg) for 7 days. The cardiotoxicity mice were randomly divided into five groups: model group (normal saline), Res low, medium, high dose group (1, 2, 4 mg/kg) and Fer-1 positive control group (2.5 mg/kg). These mice were given Res or Fer-1 once a day for 3 weeks, with the body weight being recorded. Then, the mice were euthanized, blood samples and heart tissue were collected.
Click to Show/Hide
|
||||
| Response Description | Resveratrol (Res) attenuated 5-FU-induced bodyweight reduction, restored the cardiac dysfunction and reduced the activity of oxidative stress. Furthermore, inhibition of GPX4-mediated ferroptosis was the protective mechanisms of Res against 5-FU-induced cardiotoxicity. | ||||
| Experiment 7 Reporting the Ferroptosis-centered Disease Response of This Regulator | [236] | ||||
| Responsed Drug | Ethoxyquin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
DOX (D1515, Sigma-Aldrich, St Louis, MO, USA; 6 mg/kg, dissolved in distilled water) was administrated to C57BL/6J Male mice (8-10 weeks old, 21-24 g) via the tail vein on days 0, 2, and 4.Ethoxyquin(E0237, Tokyo Chemical Industry, Tokyo, Japan; 100 umol/kg, once a day) was orally administrated every day from days 0 to 14. Ethoxyquinwas dissolved in polyethylene glycol (PEG; 28214-05, Nacalai Tesque, Kyoto, Japan,ethoxyquin10 uL in 990 uL PEG), and the solution was then diluted in the same amount of normal saline (873311, Otsuka Pharmaceutical, Tokyo, Japan).
Click to Show/Hide
|
||||
| Response Description | The inhibitory action of ethoxyquin against GPx4-deficient ferroptosis and its therapeutic efficacy against DOX-induced cell death in cultured cardiomyocytes and cardiotoxicity in a murine model of doxorubicin-induced cardiomyopathy (DIC). | ||||
Left ventricular failure [ICD-11: BD11]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [237] | ||||
| Responsed Drug | Levosimendan | Approved | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response Description | Levosimendan reversed mitochondrial malfunction in heart failure with preserved ejection fraction (HFpEF) mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan reverses cardiac malfunction and cardiomyocyte ferroptosis during heart failure with preserved ejection fraction via connexin 43 signaling activation. | ||||
Cardiovascular diseases [ICD-11: BE2Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [238] | |||
| Responsed Drug | Astragaloside IV | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | Astragaloside IV partially upregulated the levels of SLC7A11 and GPX4 expression which were reduced by LPC. The LPC-suppressed proliferation and LPC-induced apoptosis and senescence of endothelial cells were greatly attenuated by AS-IV treatment. In conclusion, AS-IV could serve as a novel drug for treating ferroptosis-related cardiovascular diseases. | |||
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [239] | ||||
| Responsed Drug | Epigallocatechin Gallate | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
After adaptive feeding, mice were randomly assigned to five groups (n = 10 per group). The details of the groups are as follows: 1) the normal diet (ND) group in which mice were fed ND (18% calories from fat); 2) the HFD group in which mice were fed HFD (60% calories from fat); 3) the HFD-EGCG/L group in which mice received 20 mg/kgbw EGCG by oral gavage daily during HFD feeding; 4) the HFD-EGCG/H group in which mice received 100 mg/kgbw EGCG by oral gavage daily during HFD feeding; and 5) the HFD-Fer-1 group in which mice received intraperitoneal injection of Fer-1 at 1 mg/kg. bw every 3 days during HFD feeding. Mice in the EGCG treatment groups were supplemented with EGCG (20 and 100 mg/kgbw) for 12 weeks. Meanwhile, mice in the ND group and the HFD group were orally gavaged with deionized water daily.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation and Fer-1 treatment apparently increased the protein expression of GPX4 and markedly decreased the protein expression of COX-2 and ACSL4 in the livers of HFD-fed mice. Epigallocatechin gallate may exert protective effects on hepatic lipotoxicity by inhibiting mitochondrial reactive oxygen species-mediated hepatic ferroptosis. Findings from our study provide new insight into prevention and treatment strategies for non-alcoholic fatty liver disease pathological processes. | ||||
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [240] | |||
| Responsed Drug | Chrysophanol | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HSC-T6 cells | Normal | Rattus norvegicus | CVCL_0315 |
| Response Description | Chrysophanol significantly induced HBx-transfected HSC-T6 death by inducing ferroptosis, as demonstrated by lipid ROS accumulation and upregulation of expression of ER markers, such as Bip, CHOP, and p-IRE1, and ferroptotic markers, such as GPX4 and SLC7A11. Therefore, chrysophanol may exert ferroptotic effects on activated HSCs to prevent liver fibrosis. | |||
Drug-induced or toxic liver disease [ICD-11: DB95]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [241] | ||||
| Responsed Drug | Nickel Chloride | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| In Vivo Model |
Totally 128 7-week-old ICR male mice (22-25 g) were provided by Dashuo Biological Technology (Chengdu, China). The animals were divided into four groups (32 mice per group) randomly. The mice in the three experimental groups were gavage administered with Ni (NiCl2·6H2O) at doses of 7.5, 15, and 30 mg/kg body weight respectively, while those in the control group were given distilled water. The Ni dose adopted here was determined according to the value of median lethal dose (LD50, 306.11 mg/kg) attained in the research on acute oral toxicity of male mice. We selected 1/40, 1/20 and 1/10 LD50 (306.11 mg/kg) of NiCl2 in this study.
Click to Show/Hide
|
||||
| Response Description | Nickel chloride caused hepatic ferroptosis accompanied by increased iron content in the liver and up-regulation of cyclooxygenase 2 (COX-2) protein and mRNA expression levels, down-regulation of glutathione eroxidase 4 (GPX4), ferritin heavy chain 1 (FTH1) and nuclear receptor coactivator 4 (NCOA4) protein and mRNA expression levels. Altogether, Mitochondria damage and ferroptosis involved in Ni-induced hepatotoxicity in mice. | ||||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 7 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [242] | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [243] | ||||
| Responsed Drug | Curcumin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| rPTs (Rat pancreas tissues) | |||||
| rHTs (Rat hippocampal tissues) | |||||
| In Vivo Model |
Forty female albino Wistar rats weighing 180-220 g were used in the study. Eight rats in each group were randomly assigned to five different groups: Group I (Sham); Group II (IR); Group III (IR + DMSO); Group IV (IR + Curcumin 100 mg/kg); and Group V (IR + 2 ug/kg LoxBlock-1) were determined. The animals were maintained at a temperature of 21 ± 2 and regulated humidity conditions (50 ± 5%) with a twelve-hour light/dark cycle. Throughout the experiment, the animals were fed standard commercial rat pellets and given tap water. All surgical and anesthesia procedures were performed understerile conditions. In addition, in a case of abnormal symptoms, the animals would be removed from the group and sacrificed under deep anesthesia.
Click to Show/Hide
|
||||
| Response Description | Curcumin attenuates liver, pancreas and cardiac ferroptosis, oxidative stress and injury in ischemia/reperfusion-damaged rats by facilitating ACSL/GPx4 signaling. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [244] | ||||
| Responsed Drug | Gossypol acetic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
A total of 55 adult male Sprague-Dawley rat (350-450 g) were anesthetized with urethane (1.5 g/kg, i.p.), then the hearts were perfused in a Langendorff system. After 30 min of stabilization, hearts were subjected to 30 min of global no-flow ischemia by stopping the perfusion. Reperfusion was followed with Krebs Henseleit (KH) buffer and GAA together for 2 h. A thermoregulated chamber kept the heart at 37 throughout the experiment. Control hearts were not subjected to I/R. The heart slices were sectioned at a thickness of 2 mm and stained with triphenyltetrazolium chloride (25 mg/100 mL) for 10 min and then fixed with 4% formaldehyde solution for 48 h to enhance color contrast.
Click to Show/Hide
|
||||
| Response Description | Gossypol acetic acid significantly attenuated myocardial infarct size, reduced lipid peroxidation, decreased the mRNA levels of the ferroptosis markers Ptgs2 and Acsl4, decreased the protein levels of ACSL4 and NRF2, and increased the protein levels of GPX4 in I/R-induced ex vivo rat hearts. Thus, GAA may play a cytoprotectant role in ferroptosis-induced cardiomyocyte death and myocardial ischemia/reperfusion-induced ferroptotic cell death. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [245] | ||||
| Responsed Drug | Histochrome | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mCMs (Mouse cardiomyocytes) | ||||
| In Vivo Model |
Male Fischer 344 rats (8 weeks old and 160 to 180 g; KOATECH, Pyeongtaek-si, Korea) were anesthetized by inhalation with 2% isoflurane and intubated using an 18-gauge intravenous catheter. The rats were mechanically ventilated with medical-grade oxygen. Surgery was performed on a 37 heating pad to prevent the body from getting cold. A left thoracotomy was performed after the chest was shaved to prevent contamination during surgery.
Click to Show/Hide
|
||||
| Response Description | Histochrome treatment significantly increased GPx4 and free GSH levels, but decreased Cox-2 level. HC treatment significantly decreased intracellular and mitochondrial ROS levels by upregulating the expression of Nrf2 and antioxidant genes. The substantial cardioprotective effects of HC against myocardia I/R injury by reducing ferroptosis-associated myocardial injury. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [246] | ||||
| Responsed Drug | Hydroxysafflor Yellow A | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| Response Description | Hydroxysafflor yellow A (HSYA) and anhydrosafflor yellow B (AHSYB) limited ferroptosis and parthanatos to alleviate oxidative stress in PC12 cells. Oxygen glucose deprivation and reperfusion injury reduced GSH/GSSG level in PC12 cells, but the reduction of GSH/GSSG ratio was regained by HSYA or AHSYB. HSYA and AHSYB activated GPX4 and system Xc- to alleviate ferroptosis. | ||||
| Experiment 6 Reporting the Ferroptosis-centered Disease Response of This Regulator | [242] | ||||
| Responsed Drug | Luteolin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
| Experiment 7 Reporting the Ferroptosis-centered Disease Response of This Regulator | [246] | ||||
| Responsed Drug | Anhydrosafflor yellow B | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| Response Description | Hydroxysafflor yellow A (HSYA) and anhydrosafflor yellow B (AHSYB) limited ferroptosis and parthanatos to alleviate oxidative stress in PC12 cells. Oxygen glucose deprivation and reperfusion injury reduced GSH/GSSG level in PC12 cells, but the reduction of GSH/GSSG ratio was regained by HSYA or AHSYB. HSYA and AHSYB activated GPX4 and system Xc- to alleviate ferroptosis. | ||||
Acute pancreatitis [ICD-11: DC31]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [247] | ||||
| Responsed Drug | Ulinastatin | Phase 3 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
266-6 cells | Normal | Mus musculus | CVCL_3481 | |
| In Vivo Model |
For cerulein-induced acute pancreatitis, male mice (age, 8-10 wk) received 7 hourly intraperitoneal injections of 50 g/kg cerulein in sterile saline. Olanzapine was repeatedly administered orally by gavage at a dose of 5 mg/kg to mice at 3 and 12 hours after the first cerulein injection, while controls were treated by oral administration with vehicle (smooth peanut butter).The parameters of acute pancreatitis were assessed 12 hours after the last cerulein treatment. For the induction of chronic pancreatitis, male mice (age, 8-10 wk) were fed a LieberDeCarli ethanol (5% vol/vol) liquid diet for 4 weeks (F1258; Bio-Serv, Flemington,NJ).In parallel, olanzapine was administered orally by gavage at a dose of 5 mg/kg to mice (3 times per week, over 4 weeks), while controls were treated by oral administration with vehicle. The parameters of chronic pancreatitis were assessed in mice 4 weeks after feeding them the LieberDeCarli ethanol liquid diet.
Click to Show/Hide
|
||||
| Response Description | Trypsin-mediated sensitization of pancreatic acinar cells to ferroptosis may be targeted for the prevention and treatment of pancreatitis in mice. Conversely, olanzapine administration protected against pancreatic ferroptotic damage and experimental pancreatitis in Gpx4-deficient mice. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [248] | ||||
| Responsed Drug | Wedelolactone | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell pyroptosis | |||||
In Vitro Model |
AR42J cells | Digestive system neoplasms | Rattus norvegicus | CVCL_0143 | |
| In Vivo Model |
The 8-weeks old male Sprague-Dawley rats (bodyweight 250-300 g) were purchased from Liaoning changsheng biotechnology co. LTD (Benxi, China). The rats in the taurocholate-induced acute pancreatitis group (Taur, n = 6) were fasted overnight, after anesthesia the hepatic portal of the bile duct was clamped and 3.5% sodium taurocholate (Aladdin, Shanghai, China) in a volume of 1 ml/kg were retrogradely injected into the biliopancreatic duct at a constant speed (0.1 ml/min). The rats in the Sham group (n = 6) were received the laparotomy and the same volume of saline solution. The rats in the disulfiram treatment group (Taur + Disul, n = 6) were administrated with 100 mg/kg pyroptosis antagonist disulfiram (Aladdin) by intraperitoneal (i.p.) injection before the surgery. The rats in the ferrostatin-1 treatment group (Taur + Fer-1, n = 6) were i.p. administered 2.5 mol/kg ferroptosis antagonist ferrostatin-1 (Aladdin) 1 h before the surgery.
Click to Show/Hide
|
||||
| Response Description | Wedelolactone promoted the transcriptional activity and the selenium sensitivity of GPX4. Moreover, the protective effects of Wed in caerulein-stimulated pancreatic acinar cells were markedly abrogated by the down-regulation of GPX4. Wed mitigated Acute pancreatitis (AP) and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [247] | ||||
| Responsed Drug | Olanzapine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
266-6 cells | Normal | Mus musculus | CVCL_3481 | |
| In Vivo Model |
For cerulein-induced acute pancreatitis, male mice (age, 8-10 wk) received 7 hourly intraperitoneal injections of 50 g/kg cerulein in sterile saline. Olanzapine was repeatedly administered orally by gavage at a dose of 5 mg/kg to mice at 3 and 12 hours after the first cerulein injection, while controls were treated by oral administration with vehicle (smooth peanut butter).The parameters of acute pancreatitis were assessed 12 hours after the last cerulein treatment. For the induction of chronic pancreatitis, male mice (age, 8-10 wk) were fed a LieberDeCarli ethanol (5% vol/vol) liquid diet for 4 weeks (F1258; Bio-Serv, Flemington,NJ).In parallel, olanzapine was administered orally by gavage at a dose of 5 mg/kg to mice (3 times per week, over 4 weeks), while controls were treated by oral administration with vehicle. The parameters of chronic pancreatitis were assessed in mice 4 weeks after feeding them the LieberDeCarli ethanol liquid diet.
Click to Show/Hide
|
||||
| Response Description | Trypsin-mediated sensitization of pancreatic acinar cells to ferroptosis may be targeted for the prevention and treatment of pancreatitis in mice. Conversely, olanzapine administration protected against pancreatic ferroptotic damage and experimental pancreatitis in Gpx4-deficient mice. | ||||
Ulcerative colitis [ICD-11: DD71]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [249] | ||||
| Responsed Drug | Curculigoside | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
IEC-6 cells | Normal | Rattus norvegicus | CVCL_0343 | |
| In Vivo Model |
Male C57BL/6J mice (8 weeks) were housed in a controlled condition at 25 , 45-55% humidity and 12 h light/dark cycle. All mice were randomly divided into five groups: Vehicle group, mice received dextran sulfate sodium (DSS group), DSS mice received ferrostatin-1 (DSS + Fer-1 group), DSS mice received low dose of CUR (DSS + CUR-L group), and DSS mice received high dose of CUR (DSS + CUR-H group). In the experiments, 3% DSS (D122347, Aladdin, Shanghai, China) in drinking water for 7 days was prepared to induce UC models. Mice in DSS + Fer-1 group were intraperitoneally injected with 5 mg/kg Fer-1 (S7243, Selleck, Shanghai, China) every two days from the day before DSS induction. In addition, mice in DSS + CUR-L or DSS + CUR-H group received intragastric administration with CUR (HY-N0705, Med Chem Express, Shanghai, China) at 50 mg/kg or 100 mg/kg once a day for 7 days during DSS administration.
Click to Show/Hide
|
||||
| Response Description | Curculigoside (CUR) could increase the selenium sensitivity and promote GPX4 transcription level in IEC-6 cells. Knockdown of GPX4 significantly blocked the protective effects of CUR on cell death, GSH and MDA contents as well as LDH activity in ferroptotic IEC-6 cells. Taken together, CUR protects against ferroptosis in ulcerative colitis (UC) by the induction of GPX4, which presents a potential agent for UC treatment. | ||||
Vitiligo [ICD-11: ED63]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [250] | |||
| Responsed Drug | Baicalein | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hMCs (Human mesangial cells) | |||
| Response Description | Baicalein up-regulated GPX4 and reduced TFR1 level in melanocytes treated with RSL3+FAC. Baicalein protected melanocytes against ferroptosis through up-regulating GPX4. Ferroptosis might be pervasive in the occurrence and development of vitiligo, and could be proposed as the potential therapeutic target. | |||
Knee osteoarthritis [ICD-11: FA01]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [251] | ||||
| Responsed Drug | Biochanin A | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
Male mice were purchased from Guangzhou University of Chinese Medicine's Experimental Animal Center C57BL/6 mice (7-week-old, 20 g) (Guangzhou, China). After one week of adaptively feeding with chow meals and sterilized water, the animals were separated into five groups of ten mice randomly assigned to the negative control (NC); model, positive control (PC); model group; high dosage of BCA treatment (BCA-H) group; and low dosage of BCA treatment (BCA-L) group. The iron overload mice model was designed based on earlier research. Except for the NC group, mice were administered ID intraperitoneally (500 mg/kg) once a week for eight weeks. In the right knee joints, OA was induced with the initial injection of iron dextran two weeks after the injection by destabilizing the medial meniscus (DMM) using a microscope. After the operation, the positive control group was administered with NAC intragastrically (100 mg/kg) for eight weeks. BCA-H and BCA-L groups were administered 20 mg/kg and 40 mg/kg of BCA separately for eight weeks according to previous studies.
Click to Show/Hide
|
||||
| Response Description | Biochanin A (BCA) could directly reduce intracellular iron concentration by inhibiting TfR1 and promoting FPN but also target the Nrf2/system xc-/GPX4 signaling pathway to scavenge free radicals and prevent lipid peroxidation. The results of this research indicate that BCA regulates iron homeostasis during the progression of osteoarthritis, which can open a new field of treatment for knee osteoarthritis. | ||||
Intervertebral disc degeneration [ICD-11: FA80]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [252] | ||||
| Responsed Drug | L-Homocysteine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell necrosis | |||||
In Vitro Model |
hNPCs (Human nucleus pulposus cells) | ||||
| In Vivo Model |
Thirty male C57BL/6 mice (8 weeks old, average weight 25 g) were divided into three groups. Blank group: Ten mice were fed with normal diet and water. HHcy group: Ten mice were fed with a high Met diet (normal diet with 2% l-methionine). HHcy + Folic Acidrescue group: Ten mice were fed withhigh Met diet (normal diet with 2% l-methionine), and injected with Folic Acid (1.0 umol/kg/d) twice a week two months later. At the beginning of treatment, mice were anesthetized with anintraperitoneal injectionof 0.3% (w/v)pentobarbital sodium(20 uL/g) before surgery and disc degeneration was induced by stabbing the C5-6 and C6-7 discs with a 32G needle.
Click to Show/Hide
|
||||
| Response Description | Homocysteine (Hcy) is an amino acid involved in gene methylation. Hcy upregulates oxidative stress and ferroptosis in the nucleus pulposus via enhancing GPX4 methylation, and is a new contributing factor in intervertebral disc degeneration (IVDD). | ||||
Endometriosis [ICD-11: GA10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [253] | |||
| Responsed Drug | Baicalein | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 |
| hMPs (Human macrophages) | ||||
| Response Description | Baicalein, a potential anti-ferroptosis compound, increased GPX4 expression, significantly inhibited ferroptosis, and restored phagocytosis of THP-1 cells in vitro. Collectively, our study reveals that ferroptosis triggered by high iron in cyst fluid promotes the development of endometriosis by impairing macrophage phagocytosis and producing more angiogenic cytokines (e.g., IL8 and VEGFA). | |||
Male infertility [ICD-11: GB04]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [254] | ||||
| Responsed Drug | Busulfan | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mTTs (Mouse testicular tissues) | ||||
| In Vivo Model |
Eight-week-old healthy ICR male mice, weighted 20-24 g, were provided by Experimental Animal Center of Nantong University (Nantong, China). For the first animal study, eight-week-old ICR male mice were randomly assigned to four groups: control, busulfan, busulfan plus Fer-1 and busulfan plus DFO groups (n = 6 per group). Mice were anesthetized and then given testicular injection of busulfan on both sides at the dose of 4 mg/kg body weight. The solution containing busulfan was directly injected from the scrotum into testicular transverse diameter. Fer-1 and DFO were administered by intraperitoneal injectionat concentrations of 1 mg/kg and 30 mg/kg respectively three times a week after busulfan injection. Four weeks later, the epididymal spermatozoa and testes from all mice were collected for assessment.
Click to Show/Hide
|
||||
| Response Description | Busulfan treatment induced spermatogenic cells ferroptosis by down-regulating nuclear factor-E2-related factor 2 (Nrf2) and glutathione peroxidase 4 (GPX4) expressions, and decreasing iron efflux through reduction of ferroportin 1 (FPN1) expression. Targeting ferroptosis serves as a potential strategy for prevention of busulfan-induced damage and male infertility. | ||||
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [255] | ||||
| Responsed Drug | Polydatin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks of age, weight 20-25 g) were purchased from Experimental Animal Center of the Fourth Military Medical University (Xi'an, China) and bred in an experimental animal room of SPF grade. They were randomly divided into four groups: control (equivalent saline containing 1% DMSO) group (n = 5), cisplatin (20 mg/kg dissolved in saline) only group (n = 7), cisplatin + polydatin (40 mg/kg dissolved in 1% DMSO) group (n = 7), and cisplatin+ Fer-1 (5 mg/kg dissolved in 1% DMSO) group (n = 7) were administered intraperitoneally. Mice were injected with cisplatin once; PD or Fer-1 was given 1 h before and 24 h after cisplatin. Animals were ethically sacrificed by dislocating their spines at 48 h after cisplatin injection, and whole blood and kidneys were collected for further analysis.
Click to Show/Hide
|
||||
| Response Description | In vitro and in vivo experiments indicated the prominent nephroprotective effects of polydatin against ferroptosis in cisplatin-induced acute kidney injury models, occurred at least partly through inhibiting excessive intracellular free iron accumulation and ROS production, rescuing GSH consumption, and enhancing GPx4 activity, thereby decreasing lipid peroxidation and ferroptosis sensitivity and ultimately attenuating the pathological progression of AKI. | ||||
Chronic kidney disease [ICD-11: GB61]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [256] | ||||
| Responsed Drug | Aspirin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
These mice were on eight weeks old male DBA/2J background (n = 36, HFK Bioscience, Beijing, China). They were randomized one of the six groups: control normal mice group (NC); diabetic mice group (DM); diabetic mice group (Fer-1), who intraperitoneal injected Fer-1 (Selleck, Houston, TX, USA); diabetic mice group (vehicle-P), who intraperitoneal injected 1% dimethyl sulfoxide (DMSO); diabetic mice group (As), who intragastric administrated Aspirin (Solarbio, Beijing, China); diabetic mice group (vehicle-G), who intragastric administrated 0.5% sodium carboxymethyl cellulose (Na-CMC; Solarbio, Beijing, China). Diabetes models were induced with 5 consecutive days of a single intraperitoneal injection of streptozotocin 40 mg/kg (dissolved in 0.1 M citrate buffer, pH 4.5; SigmaAldrich, St Louis, MO, USA). Control mice only was injected the same volume of citrate buffer. In the Fer-1 or vehicle-P groups, the diabetic mice were treated respectively with Fer-1 (2.5 umol/kg, dissolved in 1% DMSO) or 1% DMSO during the duration of treatment for 12-week every day. And in the AS and vehicle-G groups, the diabetic mice were treated respectively with aspirin (50 mg/kg, dissolved in 0.5% Na-CMC) or 0.5% Na-CMC for 12-week every day.
Click to Show/Hide
|
||||
| Response Description | Aspirin can upregulate SLC7A11 and GPX4 expression by suppressing COX2. Our results demonstrated that ferroptosis in renal tubular cells contributes to Diabetic kidney disease (DKD) development and that diabetes-related ferroptosis was inhibited through the downregulation of COX2 by aspirin, thus retarding the progression of DKD. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [257] | ||||
| Responsed Drug | Platycodin D | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| Response Description | Ferroptosis in HK-2 cells was induced by HG and was suppressed by Platycodin D (PD). GPX4 expression was downregulated in HG-induced cells and was upregulated by PD. Thus, PD suppressed ferroptosis of HK-2 cells by upregulating GPX4 expression, suggesting that PD might be an effective drug for Diabetic nephropathy therapy. | ||||
Lung injury [ICD-11: NB32]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [258] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hT2AECs (Type II alveolar epithelial cells) | ||||
| In Vivo Model |
The animals were randomly assigned to six groups (7 mice in each) as follows: (I) Normal saline (NS) group, (II) Ast-IV 100 mg/kg (Ast) group, (III) PM2.5 group, (IV) Ast-IV 50 mg/kg + PM2.5 (Ast-L) group, (V) Ast 100 mg/kg + PM2.5 (Ast-H) group, and (VI) Ast-IV 100 mg/kg + erastin 20 mg/kg + PM2.5 (Era) group. Based on our previous results, this study adopted anintraperitoneal injection(i.p.) of Ast-IV (dissolved in normal saline containing 0.1% DMSO for preventive treatment. After all the mice were adaptively fed for 5 days, in the NS and PM2.5 groups, mice received the normal saline containing 0.1% DMSO viai.p.once a day for the next three consecutive days. Similar to the NS group, in the Ast, Ast-H, and Era groups, mice received Ast-IV (100 mg/kg) viai.p. Ast-L group received Ast-IV (50 mg/kg) viai.p. To evaluate the effect of Ast-IV on ferroptosis in PM2.5-induced lung injury, we used the ferroptosis agonist erastin to activate ferroptosisin vivo. In the Era group, mice received erastin (20 mg/kg, 10% DMSO + 40% PEG300 + 5%Tween80 + 45% normal saline) 30 min before each preventive treatment of Ast-IV.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (Ast-IV) reduced the lung wet-dry ratio and the levels of interleukin 6 (IL-6), tumor necrosis factor- (TNF-) and interleukin 1 (IL-1) in serum. Ast-IV could also improve the oxidative stress level in BALF, restore the GSH level in the lung tissue, and reduce the iron content in the lung tissue. Western blot outcomes revealed that Ast-IV regulated the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to protect PM2.5-mediated lung injury. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [259] | ||||
| Responsed Drug | Lipopolysaccharide | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
The male C57BL/6 mice were divided randomly into 4 groups (n = 4 per group, 8-10 weeks old, weight = 23-25 g): the control group receiving 0.9% NaCl (containing 0.1% DMSO), the LPS group receiving LPS plus 0.9% NaCl (containing 0.1% DMSO), the Fer-1 group receiving Fer-1 only, and the LPS + Fer-1 group receiving both Fer-1 and LPS. The LPS-induced ALI model was induced by instilling intratracheally 50 ul of LPS solution (0.2 g/L), then Fer-1 (0.8 mg/kg) was administered after LPS challenge via tail vein injection. The Fer-1 was dissolved in DMSO first, and diluted with 0.9% NaCl. The final concentration of Fer-1 and DMSO was 0.2 mg/ml and 0.1% respectively.
Click to Show/Hide
|
||||
| Response Description | The cell viability of BEAS-2B was down-regulated by lipopolysaccharide (LPS) treatment, together with the ferroptosis markers SLC7A11 and GPX4, while the levels of MDA, 4-HNE and total iron were increased by LPS treatment in a dose-dependent manner, which could be rescued by ferrostatin-1. Fer-1 exerted therapeutic action against LPS-induced acute lung injury, and down-regulated the ferroptosis level in lung tissues. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [259] | ||||
| Responsed Drug | Ferrostatin-1 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
The male C57BL/6 mice were divided randomly into 4 groups (n = 4 per group, 8-10 weeks old, weight = 23-25 g): the control group receiving 0.9% NaCl (containing 0.1% DMSO), the LPS group receiving LPS plus 0.9% NaCl (containing 0.1% DMSO), the Fer-1 group receiving Fer-1 only, and the LPS + Fer-1 group receiving both Fer-1 and LPS. The LPS-induced ALI model was induced by instilling intratracheally 50 ul of LPS solution (0.2 g/L), then Fer-1 (0.8 mg/kg) was administered after LPS challenge via tail vein injection. The Fer-1 was dissolved in DMSO first, and diluted with 0.9% NaCl. The final concentration of Fer-1 and DMSO was 0.2 mg/ml and 0.1% respectively.
Click to Show/Hide
|
||||
| Response Description | The cell viability of BEAS-2B was down-regulated by lipopolysaccharide (LPS) treatment, together with the ferroptosis markers SLC7A11 and GPX4, while the levels of MDA, 4-HNE and total iron were increased by LPS treatment in a dose-dependent manner, which could be rescued by ferrostatin-1. Fer-1 exerted therapeutic action against LPS-induced acute lung injury, and down-regulated the ferroptosis level in lung tissues. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 6 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [260] | ||||
| Responsed Drug | Bicyclol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
The mice were treated with intraperitoneal administration (i.p.) of oil (control group) or a mixture of CCl4 (50%) and oil (50%) at a dosage of 2 ml/kg body weight. In the bicyclol-treated group, mice accepted administration of 200 mg/kg (using 0.5% carboxymethyl cellulose as solvent) by gavage three times a day 1 h before CCl4 exposure, while other groups accepted vehicles of the equal volume. Fer-1 was prepared in DMSO (5 mg/kg), andi.p. injected into mice once 1 h before CCl4 exposure. The dosage of bicyclol was consistent with our previous work. The mice were then sacrificed to collect liver and serum samples after 24 or 48 h.
Click to Show/Hide
|
||||
| Response Description | Bicyclol exerted its hepatoprotection by preventing the aforesaid ferroptotic process. Furthermore, bicyclol alleviated erastin-induced cellular inviability, destruction, and lipid peroxidation in vitro. Knockdown of GPX4 diminished these protective activities against perturbations associated with ferroptosis in L-O2 hepatocytes. Additionally, Nrf2 silencing drastically reduced GPX4 levels, and further impeded the medicinal effects of bicyclol. In summary, positively regulating Nrf2-GPX4 axis by bicyclol can prevent ferroptosis in CCl4-induced acute liver injury in mice. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [261] | ||||
| Responsed Drug | Disulfiram | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| 769-P cells | Renal cell carcinom | Homo sapiens | CVCL_1050 | ||
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| MDA231-LM2-4175 cells | Breast adenocarcinoma | Homo sapiens | CVCL_5998 | ||
| In Vivo Model |
C57BL/6J male mice aged 8 weeks were purchased from Charles River Laboratories International, Inc., and housed in a specific pathogen-free animal facility. DMSO or DSF (21 mg/kg) was injected intraperitoneally into mice for 0.5 h, followed by ConA injection via the tail vein at 15 mg/kg. Mice were sacrificed at 24 h post ConA injection. Liver and blood samples were collected at this time point for H&E staining, IHC staining, and measurement of AST/ALT (Dian Diagnostics Co., Ltd).
Click to Show/Hide
|
||||
| Response Description | Disulfiram (DSF) is conjugated to multiple cysteine residues in GPX4 and disrupts GPX4 interaction with HSC70, an adaptor protein for chaperone mediated autophagy, thus preventing GPX4 degradation induced by erastin. In addition, DSF ameliorates concanavalin A induced acute liver injury by suppressing ferroptosis in a mouse model. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [262] | ||||
| Responsed Drug | Epigallocatechin Gallate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [263] | ||||
| Responsed Drug | Kaempferol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male BALB/c mice (8-week-old, 20-22 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). The experimental animals were fed adaptively for one week in the Experimental Animal Center of Guangdong Pharmaceutical University (Guangzhou, China). Feeding conditions were set at 26 , humidity 65% and a lightdark cycle for 12 hours. All animal experiments were performed following the Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Research Ethical Committee of Guangdong Pharmaceutical University (gdpulacspf2020007).
Click to Show/Hide
|
||||
| Response Description | Kaempferol (KA) activated the Nrf2 pathway and upregulated Gpx4 in mouse livers and L02 cells to inhibit ferroptosis induced by APAP. Finally, molecular docking indicated the potential interaction of KA with Keap1. Taken together, KA ameliorated oxidative stress and ferroptosis-mediated acetaminophen-induced liver injury by activating Nrf2 signaling. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [264] | ||||
| Responsed Drug | Schisandrin B | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
rPHs (Rat primary hepatocytes) | ||||
| In Vivo Model |
A total of 40 male SD rats (180~200 g, 6~8 weeks) were purchased from the CMU experimental animal center. The rats were randomly divided into four groups: control (CON) group (normal diet rats were injected with equal volume of normal saline through caudal vein once a week, n = 10), SchB group (SchB diet rats, 50 mg/kg/day, were injected with equal volume of normal saline through caudal vein once a week, n = 10), THP group (normal diet rats were injected with 3 mg/kg/day THP through caudal vein once a week, n = 10), and SchB+THP group (SchB diet rats, 50 mg/kg/day, were injected with 3 mg/kg/day THP through caudal vein once a week, n = 10). CON and THP rats were fed an AIN-76A feed (12.4% fat, 68.8% carbohydrate, and 18.8% protein). SchB and SchB+THP rats were fed an SchB feed (approximately 0.5 SchB was added into AIN-76A feed). After conversion, 0.5 SchB in feed = 50 mg/kg in rats.
Click to Show/Hide
|
||||
| Response Description | Schisandrin B (SchB) increased the levels of SOD, GSH, GSH-px, CAT, and T-AOC, decreased the level of MDA, and inhibited the abnormal oxidative stress in the liver. And SchB as a natural molecule depends on reducing the level of oxidative stress, thereby inhibiting lipid peroxidation, ferroptosis, and apoptosis. The expression of NRF2, GPX4, SOD2, and Bcl-2/Bax decreased, while the expression of NOX2/4 and cleaved caspase-3 increased in pirarubicin-treated hepatocytes. However, the above changes were significantly reversed after SchB or Fer-1 treatment. SchB has obvious protective effect on pirarubicin-induced hepatotoxicity. | ||||
| Experiment 6 Reporting the Ferroptosis-centered Disease Response of This Regulator | [265] | ||||
| Responsed Drug | Apigenin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| Response Description | DEHP caused oxidative stress and increased the Fe2+ content, finally resulting in ferroptosis in AML12 cells. Apigenin restrained the toxicity of DEHP and antagonized DEHP-induced ferroptosis in AML12 cells. The protective effects of APG on DEHP-induced liver injury were achieved by activating GPX4 and suppressing intracellular iron accumulation. | ||||
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [266] | ||||
| Responsed Drug | Edaravone | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rSCTs (Rat spinal cord tissues) | ||||
| In Vivo Model |
The rats were initially anesthetized with 5% isoflurane (RWD life science, Shenzhen, China) and then maintained with 22.5% isoflurane. A 1-cm midline incision was made over the thoracic vertebrae, and laminectomy on T10 and the caudal half of T9 vertebrae was performed. Spinal cord contusion injury was conducted by NYU Impactor Model III (W.M. Keck Center for Collaborative Neuroscience Rutgers, The State University of New Jersey, United States) using a 10-g node dropping freely from a height of 2.5 cm and muscles and skin sutured in layers. Sham controls underwent laminectomy without the contusion. To prevent infection at the incision, cefuroxime sodium was applied for 3 days after injury. The bladders were emptied manually twice daily in the first week after injury.
Click to Show/Hide
|
||||
| Response Description | Edaravone not only rescues the ferroptosis negative regulators, xCT and GPX4, but also downregulates those pro-ferroptosis factors, ACSL4 and 5-LOX. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. And in the acute phase of spinal cord injury (SCI), edaravone reduced neuronal cell death and neuroinflammation. | ||||
Skin injury [ICD-11: ND56]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [267] | ||||
| Responsed Drug | Nicotinamide mononucleotide | Preclinical | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HaCaT cells | Normal | Homo sapiens | CVCL_0038 | |
| In Vivo Model |
The skin injury model was created using ultraviolet B (UVB) 250 mJ/cm2 irradiation onto BALB/c mice after their back shaved. A total of 26 mice were used in this study; 8 mice without treatment served as controls, while 18 mice were irradiated under the UVB lamp and administered with PBS (200 ul per injection area), Lip-1 (Selleck, S7699) (10 mg/kg every other day per injection area), or NMN (Chalet Healthy PTY Ltd, Jiangsu Chengxin Pharmaceutical Co., Ltd) (400 mg/kg/day via drinking water at pH 7.2).
Click to Show/Hide
|
||||
| Response Description | Nicotinamide mononucleotide recruits GSH to enhance GPX4-mediated ferroptosis defense in UV irradiation-induced skin injury and inhibits oxidative skin damage. NMN or ferroptosis inhibitor might become promising therapeutic approaches for treating oxidative stress-induced Skin injury. | ||||
Health [ICD-11: N.A.]
| In total 4 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [270] | ||||
| Responsed Drug | Alpha-Tocopherol | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mHSPCs (Mouse hematopoietic stem and progenitor cells) | ||||
| In Vivo Model |
C57BL/6 WT mice were purchased from Beijing HFK BioScience Company (Beijing, China). Gpx4flox/flox mice were crossed with Vav-Cre mice and Mx-Cre mice to generate the Gpx4flox/flox Vav-Cre mice and Gpx4flox/flox Mx-Cre mice, respectively. For Gpx4 deletion, Gpx4flox/flox Mx-Cre mice were intraperitoneally injected with 20 mg/kg pIpC (Sigma) every other day for two weeks. CD45.1/45.2 mice and CD45.1 mice on a C57BL/6 background were used as competitor and recipient mice, respectively, in the competitive transplantation assay. Mice were fed natural ingredient diets containing >= 120 IU/kg vitamin E. A fixed formulation diet with or without 75 IU/kg vitamin E (Beijing HFK BioScience Company, Beijing, China) was fed to the mice involved in the vitamin E depletion experiments. For 5-FU treatment, mice were intraperitoneally injected with 150 mg/kg 5-FU (Sigma).
Click to Show/Hide
|
||||
| Response Description | a-Tocopherol, the main component of vitamin E, was shown to rescue the Gpx4-deficient hematopoietic stem and progenitor cells (HSPCs) from ferroptosis in vitro. When Gpx4 knockout mice were fed a vitamin E-depleted diet, a reduced number of HSPCs and impaired function of HSCs were found. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [271] | ||||
| Responsed Drug | Fluvastatin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | ||||
| Response Description | Fluvastatin exerts potent protective effects against ox-LDL-induced endothelial cell dysfunction through regulation of GPx4 and xCT. These data indicated a novel function of fluvastatin in the protection of endothelial cells from ox-LDL-induced ferroptosis, the mechanism of which involves the regulation of GPx4 and xCT. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [268] | ||||
| Responsed Drug | D-2-hydroxyglutarate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| KYSE-170 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1358 | ||
| Response Description | Ectopic expression of mutant IDH1 or treatment of cells with cell-permeable D-2-hydroxyglutarate (D-2-HG) promotes the accumulation of lipid reactive oxygen species (ROS) and subsequently ferroptosis. Mechanistically, mutant IDH1 reduces the protein level of the glutathione peroxidase 4 (GPX4). | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [272] | ||||
| Responsed Drug | Selenium | Approved | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mTCs (Mouse T cells) | ||||
| In Vivo Model |
All mice used in this study were 6-12 weeks old on a C57/BL6/J background. WT or T-KO mice were fed with water supplemented with methionine (1 mgl-1, Sigma) or Se-Met (1 mgl-1, Sigma) and maintained on the diets for 4 weeks before experiments. Alternatively, WT mice were fed with selenium-adequate (0.15 mg/kg) and selenium-high (1 mg/kg) diets that were purchased from Envigo and mice were maintained on the diets for 4 weeks before experiments.
Click to Show/Hide
|
||||
| Response Description | The deletion of GPX4 in T cells selectively abrogated TFH cells and germinal center responses in immunized mice. Selenium supplementation enhanced GPX4 expression in T cells, increased TFH cell numbers and promoted antibody responses in immunized mice and young adults after influenza vaccination. | ||||
Nanotoxicity [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [273] | |||
| Responsed Drug | Thioctic acid | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
BALB/3T3 cells | Normal | Mus musculus | CVCL_0184 |
| Response Description | CoNPs could induce the ferroptosis-like cell death through the enhancement of intracellular reactive oxygen species (ROS) level, cytoplasmic Fe2+ level, lipid peroxidation, and consumption of reduced glutathione (GSH) as well as inhibition of glutathione peroxidase 4 (GPX4) activity. Importantly, a-lipoic acid (ALA), a natural antioxidant with the capability to scavenge free radicals and chelate toxic metals, was found to efficiently alleviate nanotoxicity. | |||
Isocitrate dehydrogenase [NADP] cytoplasmic (IDH1)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [268] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| KYSE-170 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1358 | |
| Response Description | Ectopic expression of mutant IDH1 or treatment of cells with cell-permeable D-2-hydroxyglutarate (D-2-HG) promotes the accumulation of lipid reactive oxygen species (ROS) and subsequently ferroptosis. Mechanistically, mutant IDH1 reduces the protein level of the glutathione peroxidase 4 (GPX4). | |||
Vitamin D3 receptor (VDR)
Paricalcitol
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
A total of 72 male C57BL/6 mice were purchased from Slyke jingda Biotechnology Company. They were randomly divided into five groups: Control group (n = 8), Cisplatin (20 mg/kg dissolved in saline) only group (n = 16), Cisplatin + paricalcitol (0.2 ug/kg dissolved in sterile water for injection and 20% propylene glycol) group (n = 16), Cisplatin + DMSO group (n = 16), Cisplatin + Fer-1 (5 mg/kg dissolved in DMSO) group (n = 16), were administered intraperitoneally. Cisplatin was injected once to mice, while Fer-1 was injected once an hour before cisplatin, and paricalcitol was injected once daily for five consecutive days before cisplatin. Each eight mice were sacrificed at 48 h and 72 h, respectively after cisplatin injection, and eight mice in the control group were sacrificed together with mice at 72 h.
Click to Show/Hide
|
||||
| Response Description | Pretreatment of paricalcitol could also alleviated Erastin (an inducer of ferroptosis) induced cell death in HK-2 cell. Ferroptosis plays an important role in cisplatin induced acute kidney injury. VDR activation can protect against cisplatin induced renal injury by inhibiting ferroptosis partly via trans-regulation of GPX4. | ||||
Transient receptor potential cation channel subfamily V member 1 (TRPV1)
Capsiate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mSIOs (Mouse small intestinal organoids) | ||||
| In Vivo Model |
Six- to eight-week-old specific pathogen-free male C57BL/6 mice were purchased from the animal center of Nanfang Hospital of Southern Medical University (Guangzhou, China). The mice were anesthetized with isoflurane. A noninvasive microvascular artery clip was placed on the superior mesenteric artery (SMA) for 60 min, and the clip was removed for reperfusion for 2 hours. During the study period, body temperature was maintained at 37 with a heating pad, and liquid resuscitation was performed by subcutaneous injection with 0.5 ml of physiological saline immediately after reperfusion.
Click to Show/Hide
|
||||
| Response Description | The gut microbiota metabolite capsiate enhances Gpx4 expression and inhibits ferroptosis by activating TRPV1 in intestinal ischemia/reperfusion (I/R) injury, providing a potential avenue for the management of intestinal ischemia/reperfusion (I/R) injury. | ||||
Thioredoxin (TXN)
Cyperquat
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | ||
| In Vivo Model |
Male C57BL/6 mice wild-type (WT), 8 weeks of age, were from Chongqing Medical University, China. Mice were divided into four groups (n = 10-13 per group), control group, MPTP group, h-Trx-1 Tg group, and h-Trx-1 Tg + MPTP group. Control and h-Trx-1 Tg groups were administered saline only. For the Trx-1 knockdown experiment, mice were divided into six groups (n = 10-13 per group), control + saline group, control + MPTP group, AAV9-vehicle + saline group, AAV9-vehicle + MPTP group, AAV9-shRNA-mTrx-1 + saline group, and AAV9-shRNA-mTrx-1 + MPTP.
Click to Show/Hide
|
||||
| Response Description | 1-methyl-4-phenylpyridinium (Cyperquat) decreased cell viability, GPX4, and Trx-1 (TXN). The decreased GPX4 and GSH, and increased ROS were inhibited by Fer-1 and Trx-1 overexpression. Trx-1 reversed the decreases of GPX4 and tyrosine hydroxylase (TH) induced by MPTP in the substantia nigra pars compacta (SNpc). Trx-1 inhibits ferroptosis in parkinson's disease through regulating GPX4 and GSH. | ||||
Sterol regulatory element-binding protein 1 (SREBF1)
Apatinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | |
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| In Vivo Model |
Female nude mice (BALB/c, nu/nu, 18-22 g, 4-5 weeks old) were obtained from Guangdong Medical Laboratory Animal center, China, and maintained under specific pathogen-free conditions on a 12h/12h light/dark cycle. Each mouse was injected subcutaneously with eight million luciferase-expressing cells resuspended in 50 ul of PBS and 50 ul of Matrigel (BD Biosciences). When a palpable mass had developed, the mice were randomly divided into five groups: apatinib (50 mg/kg/day oral dose for 14 days); RSL3 (100 mg/kg injection of RSL3 twice per week for 2 weeks at the same site); both; apatinib (50 mg/kg/day oral dose for 14 days) plus vitamin E (100 mg/kg/day oral dose for 14 days); and vehicle (DMSO, 100 ul oral dose for 14 days).
Click to Show/Hide
|
||||
| Response Description | Apatinib exerted antitumor effects against gastric cancer cells in vitro and in vivo through the induction of lipid peroxidation mediated by GPX4, then lead to ferroptosis. Furethermore, we found apatinib inhibited transcription of GPX4 via a SREBP1a-mediated pathway. These results indicated that GPX4 may be a potential target for anti-GC efficacy evaluation and treatment of apatinib. | ||||
Sphingosine kinase 1 (SPHK1)
Dihydromyricetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Rats were anesthetized by pentobarbital sodium at a dosage of 40 mg/kg by intraperitoneal injection. Rats were first anchored on to an operating table in the supine position. The fur around the incision was shaved and then disinfected. Subsequently, the neck of each rat was incised in the middle to expose the right common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA). The proximal end of the CCA and ECA were ligated and severed using a 0.285 mm nylon suture. The suture was inserted from the ECA stump through the ICA to reach the MCA. The MCA was then occluded for 2 h to create ischemic conditions. Next, the nylon suture was slowly pulled out to restore blood flow and simulate reperfusion condition.
Click to Show/Hide
|
||||
| Response Description | Dihydromyricetin (DHM) repressed ferroptosis by inhibiting the SPHK1/mTOR signaling pathway, thereby alleviating cerebral ischemia reperfusion injury. Moreover, the expression levels of glutathione peroxidase 4 (GPX4) was enhanced while the levels of acyl-CoA synthetase long-chain family member 4 (ACSL4) and phosphatidylethanolamine binding protein 1 (PEBP1) were reduced in OGD/R-treated HT22 cells in the presence of DHM. | ||||
Sphingomyelin phosphodiesterase (SMPD1)
Erastin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Fibrosarcoma [ICD-11: 2B53] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 |
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| Response Description | Erastin (Era) treatment results in the activation of ASM and generation of ceramide, which are required for the Era-induced reactive oxygen species (ROS) generation and LPO in fibrosarcoma. ASM ( SMPD1)-mediated activation of autophagy plays a critical role in ferroptosis inducers (FINs)-induced glutat hione peroxidase 4 (GPX4) degradation and ferroptosis activation. | |||
Signal transducer and activator of transcription 3 (STAT3)
Peoniflorin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| In Vivo Model |
U251 cells (6 x 106) were inoculated into the flanks of 4-to 5-week-old athymic nude mice (Shanghai Laboratory Animal Company, Shanghai, China) subcutaneously to generate a subcutaneous xenograft tumor model. After 2 weeks, the tumor model was successfully constructed, the mice were treated single and combined with 100 mg/kg RSL3 (2 times/week) and 1.0 g/kg/days PF. Tumor volumes were measured every 4 days to draw the growth curve. Mice were sacrificed 4 weeks after cell injection. Tumor xenografts were collected, photographed, and weighed and the tumor apoptosis was analyzed by Tunel staining.
Click to Show/Hide
|
||||
| Response Description | Paeoniflorin (PF) can function as an antitumor agent for glioma treatment by targeting NEDD4L-dependent STAT3 ubiquitination as well as by regulating the Nrf2/GPX4 signaling axis, which might trigger ferroptosis. | ||||
Serine/threonine-protein kinase mTOR (MTOR)
Fatostatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Cell adhesion molecules | hsa04514 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
After anesthetizing the nude mice with isoflurane inhalation, we injected 1 x 106 U87 cells that were engineered for the expression of luciferase into the right striatum (3.5 mm from the midline of the brain and 2 mm in front of the coronal suture, injection depth of 3 mm from the brain surface) of the nude mice to establish an intracranial xenograft model. For the detection of pharmacokinetics in mice, RhoB-loaded p28-PLGA NPs were injected into the mice (n = 3) through the tail vein. We collected blood samples at predetermined time points, quantified the RhoB concentrations, and plotted them with time. To characterize NPs for GBM treatment, we randomly divided the tumor-bearing mice into four groups (n = 8) treated with PBS, free fatostatin (25 mg/kg), NPs-FAT (fatostatin equivalent dose at 25 mg/kg), and p28-NPs-FAT (fatostatin equivalent dose at 25 mg/kg). After 7 days of tumor inoculation, the treatment was conducted 3 days per week for 4 weeks. In addition, we performed IVIS imaging of intracranial tumors at 1, 3, and 5 weeks after tumor inoculation to observe tumor progression. IVIS was also used to carry out imaging of IR780-loaded NPs. The mice were monitored regularly and euthanized when they exhibited severe neurological symptoms and/or obvious weight loss (>20% of their body weight). We sacrificed a separate cohort of mice five weeks after tumor inoculation for pathological staining (n = 3).
Click to Show/Hide
|
||||
| Response Description | Fatostatin induces ferroptosis by inhibiting the AKT/ mTORC1/GPX4 signaling pathway in glioblastoma. In addition, fatostatin inhibits cell proliferation and the EMT process through the AKT/mTORC1 signaling pathway. | ||||
Curcumin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 |
| Response Description | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/Akt/ mTOR signaling pathway. | |||
Dihydromyricetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Rats were anesthetized by pentobarbital sodium at a dosage of 40 mg/kg by intraperitoneal injection. Rats were first anchored on to an operating table in the supine position. The fur around the incision was shaved and then disinfected. Subsequently, the neck of each rat was incised in the middle to expose the right common carotid artery (CCA), external carotid artery (ECA) and internal carotid artery (ICA). The proximal end of the CCA and ECA were ligated and severed using a 0.285 mm nylon suture. The suture was inserted from the ECA stump through the ICA to reach the MCA. The MCA was then occluded for 2 h to create ischemic conditions. Next, the nylon suture was slowly pulled out to restore blood flow and simulate reperfusion condition.
Click to Show/Hide
|
||||
| Response Description | Dihydromyricetin (DHM) repressed ferroptosis by inhibiting the SPHK1/ mTOR signaling pathway, thereby alleviating cerebral ischemia reperfusion injury. Moreover, the expression levels of glutathione peroxidase 4 (GPX4) was enhanced while the levels of acyl-CoA synthetase long-chain family member 4 (ACSL4) and phosphatidylethanolamine binding protein 1 (PEBP1) were reduced in OGD/R-treated HT22 cells in the presence of DHM. | ||||
RAF proto-oncogene serine/threonine-protein kinase (RAF1)
Tetraarsenic tetrasulfide
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [10] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| In Vitro Model | NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| Response Description | On H23 cells treated with realgar, the expression of GPX4, SCL7A11 decreased while ACSL4 expression increased; this effect could also be amplified by Sorafenib. In conclusion, the present study indicated that realgar may induce ferroptosis by regulating the Raf, and hence plays a role in antiKRAS mutant lung cancer. | |||
RAC-alpha serine/threonine-protein kinase (AKT1)
Fatostatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Cell adhesion molecules | hsa04514 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
After anesthetizing the nude mice with isoflurane inhalation, we injected 1 x 106 U87 cells that were engineered for the expression of luciferase into the right striatum (3.5 mm from the midline of the brain and 2 mm in front of the coronal suture, injection depth of 3 mm from the brain surface) of the nude mice to establish an intracranial xenograft model. For the detection of pharmacokinetics in mice, RhoB-loaded p28-PLGA NPs were injected into the mice (n = 3) through the tail vein. We collected blood samples at predetermined time points, quantified the RhoB concentrations, and plotted them with time. To characterize NPs for GBM treatment, we randomly divided the tumor-bearing mice into four groups (n = 8) treated with PBS, free fatostatin (25 mg/kg), NPs-FAT (fatostatin equivalent dose at 25 mg/kg), and p28-NPs-FAT (fatostatin equivalent dose at 25 mg/kg). After 7 days of tumor inoculation, the treatment was conducted 3 days per week for 4 weeks. In addition, we performed IVIS imaging of intracranial tumors at 1, 3, and 5 weeks after tumor inoculation to observe tumor progression. IVIS was also used to carry out imaging of IR780-loaded NPs. The mice were monitored regularly and euthanized when they exhibited severe neurological symptoms and/or obvious weight loss (>20% of their body weight). We sacrificed a separate cohort of mice five weeks after tumor inoculation for pathological staining (n = 3).
Click to Show/Hide
|
||||
| Response Description | Fatostatin induces ferroptosis by inhibiting the AKT/mTORC1/GPX4 signaling pathway in glioblastoma. In addition, fatostatin inhibits cell proliferation and the EMT process through the AKT/mTORC1 signaling pathway. | ||||
Curcumin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 |
| Response Description | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/ Akt/mTOR signaling pathway. | |||
Lapatinib
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | Lapatinib (LAP) inhibited the cell viability and exacerbated cell injury induced by doxorubicin, as well as increased cell apoptosis. LAP aggravated Dox-induced cardiotoxicity by promoting oxidative stress and ferroptosis in cardiomyocytes via PI3K/AKT-mediated mitochondrial dysfunction. Moreover, GPX4 expression was decreased and ASCL4 level was higher following DOX treatment or the combination therapy of LAP and DOX. | |||
PVT1 (IncRNA)
Ketamine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
BALB/c nude mice (age 6 weeks) were brought from the Laboratory Animal Center of Chinese Academy of Sciences (China). HepG2 cell suspension (100 uL, 5 x 105 per site) was hypodermically inoculated into the fat pad of mice. Tumor volume was calculated as follows: tumor volume (mm3) = 0.5 x width (mm)2 x length (mm). When tumor size reached 100 mm3, mice were treated with ketamine (20 mg/kg) or saline intraperitoneally. The mice were succumbed to death when tumor size reached 1000 mm3. Tumors were isolated and weighted. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Click to Show/Hide
|
||||
| Response Description | LncPVT1 directly interacted with miR-214-3p to impede its role as a sponge of GPX4. Depletion of lncPVT1 accelerated the ferroptosis of liver cancer cells, whereas miR-214-3p inhibition and GPX4 overexpression reversed this effect. In this work, we determined that ketamine suppressed viability of liver cancer cells and induced ferroptosis and identified the possible regulatory mechanism of lncPVT1/miR-214-3p/GPX4 axis. | ||||
Protein lifeguard 4 (TMBIM4)
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| In Vivo Model |
To generate murine subcutaneous tumours, 1 x 107 control shRNA or S1R-knockdown Huh7 cells in 200 uL of PBS were injected subcutaneously to the right of the dorsal midline. At day seven, the mice were randomly divided into groups and treated with sorafenib (10 mg/kg/intraperitoneal injection (i.p.), once every other day) for 2 weeks. On day 28, tumours were removed.
Click to Show/Hide
|
||||
| Response Description | S1R (TMBIM4) protects hepatocellular carcinoma cells against sorafenib and subsequent ferroptosis. Inhibition of S1R by RNAi and antagonists markedly increased the anticancer activity of sorafenib by modulating the expression of GPX4, iron metabolism and ROS. | ||||
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA)
Curcumin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 |
| Response Description | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/Akt/mTOR signaling pathway. | |||
Lapatinib
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | Lapatinib (LAP) inhibited the cell viability and exacerbated cell injury induced by doxorubicin, as well as increased cell apoptosis. LAP aggravated Dox-induced cardiotoxicity by promoting oxidative stress and ferroptosis in cardiomyocytes via PI3K/AKT-mediated mitochondrial dysfunction. Moreover, GPX4 expression was decreased and ASCL4 level was higher following DOX treatment or the combination therapy of LAP and DOX. | |||
Phosphatidylethanolamine-binding protein 1 (PEBP1)
Xiaoyaosan
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [14] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Depressive disorder [ICD-11: 6A70] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
The specific-pathogen free (SPF) male C57BL/6 mice (8-week-old, SCXK (Beijing) 2016-0006) were purchased from Beijing Vital River Laboratory Animal Technology Limited Company. A total of 48 mice were randomly assigned to 4 groups (n = 12): a control group (no stress + physiological saline), a CUMS group (CUMS + physiological saline), a Xiaoyaosan group (CUMS + Xiaoyaosan treatment) and a fluoxetine group (CUMS + fluoxetine treatment).
Click to Show/Hide
|
||||
| Response Description | The activation of ferroptosis might exist in the hippocampi of CUMS-induced mice. The PEBP1-GPX4-mediated ferroptosis could be involved in the antidepressant mechanism of Xiaoyaosan. It also implied that ferroptosis could become a new target for research into the depression mechanism and antidepressant drugs. | ||||
Peroxisome proliferator-activated receptor gamma (PPARG)
Pioglitazone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [15] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rPNCs (Rat primary nerve cells) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
The rats underwent surgery using an ultraclean table and were fixed in a stereotaxic frame. The scalp was opened to expose the anterior brain region. A dental drill was used to drill a 1-mm-diameter hole in the skull surface. Blood (100 ul) was collected from the rat tail vein and injected into the rat striatum with a microsyringe (stereotaxic coordinates; 2 mm lateral to the midline, 0.2 mm posterior to bregma, and 5.5 mm deep below the skull). First, 60 ul of autogenous blood were injected at a rate of 2 ul/min, and the next 40 ul of blood were injected at 5 ul/min. Finally, the needle was left for 10 min before being removed.
Click to Show/Hide
|
||||
| Response Description | Pioglitazone (PDZ), a PPAR agonist, promotes Gpx4 expression through the interaction between PPAR and the Nrf2 pathway, inhibits ferroptosis of neurons after intracerebral hemorrhage (ICH), and promotes the recovery of neural function. | ||||
Nuclear receptor subfamily 1 group D member 1 (NR1D1)
Aristololactam
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Aristolochic acid nephropathy [ICD-11: GB55] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mRTECs (Mouse renal tubular epithelial cells) | ||||
| M4100-57 (Mouse renal tubular epithelial cells) | |||||
| In Vivo Model |
Wild-type C57BL/6 mice (eight-week-old, male) were obtained from SPF Biotechnology (Beijing, China). Three sets of animal experiments were performed. In the first set of experiments, male wild-type mice (eight-week-old) were randomly assigned to three groups (n = 6 per group): control group, 2.5 mg/kg AAI group, and 5 mg/kg AAI group. The AAI groups of mice were intraperitoneally injected with AAI (2.5 or 5 mg/kg) once daily for 5 days. The control group of mice were treated with vehicle (corn oil). In the second set of experiments, male Rev-erbfl/fl and Rev-erbkKO mice (eight-week-old) were treated with AAI (5 mg/kg) or vehicle once daily for 5 days by intraperitoneal injection. In the third set of experiments, male wild-type mice (eight-week-old) were randomly divided into the following four groups (n = 6 per group): AAI + SR8278, AAI + DFO, AAI, and vehicle.
Click to Show/Hide
|
||||
| Response Description | Renal REV-ERB protein was significantly increased in aristolochic acid I-treated mice. Furthermore, knockdown of Rev-erb by siRNA or SR8278 (a REV-ERB antagonist) treatment attenuated ALI-induced ferroptosis in mRTECs. SR8278 treatment enhanced the cell survival and GPX4 expression in ALI-treated mRTECs. Taken together, small molecule antagonism of REV-ERB alleviates aristolochic acid I-induced renal injury probably through inhibiting ferroptosis in mice. | ||||
SR8278
[Preclinical]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Aristolochic acid nephropathy [ICD-11: GB55] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mRTECs (Mouse renal tubular epithelial cells) | ||||
| M4100-57 (Mouse renal tubular epithelial cells) | |||||
| In Vivo Model |
Wild-type C57BL/6 mice (eight-week-old, male) were obtained from SPF Biotechnology (Beijing, China). Three sets of animal experiments were performed. In the first set of experiments, male wild-type mice (eight-week-old) were randomly assigned to three groups (n = 6 per group): control group, 2.5 mg/kg AAI group, and 5 mg/kg AAI group. The AAI groups of mice were intraperitoneally injected with AAI (2.5 or 5 mg/kg) once daily for 5 days. The control group of mice were treated with vehicle (corn oil). In the second set of experiments, male Rev-erbfl/fl and Rev-erbkKO mice (eight-week-old) were treated with AAI (5 mg/kg) or vehicle once daily for 5 days by intraperitoneal injection. In the third set of experiments, male wild-type mice (eight-week-old) were randomly divided into the following four groups (n = 6 per group): AAI + SR8278, AAI + DFO, AAI, and vehicle.
Click to Show/Hide
|
||||
| Response Description | Renal REV-ERB protein was significantly increased in aristolochic acid I-treated mice. Furthermore, knockdown of Rev-erb by siRNA or SR8278 (a REV-ERB antagonist) treatment attenuated ALI-induced ferroptosis in mRTECs. SR8278 treatment enhanced the cell survival and GPX4 expression in ALI-treated mRTECs. Taken together, small molecule antagonism of REV-ERB alleviates aristolochic acid I-induced renal injury probably through inhibiting ferroptosis in mice. | ||||
Nonsense-mediated mRNA decay factor SMG9 (SMG9)
RSL3
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [17] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 cells in 100 ul PBS were injected subcutaneously to the right of the dorsal midline in 6- to 8-week-oldathymic nude mice(n = 5 mice/group). After the tumor reached 60-80 mm3 on day 7, the mice were randomly grouped and then given intratumoral treatment with RSL3 (50 mg/kg, once every other day) at day 7 for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | SMG9, a component of the NMD machinery, is a selective driver for ferroptosis in pancreatic cancer cells. SMG9 is a direct binding protein of GPX4 to promote the degradation of GPX4 in response to RSL3 (a GPX4 inhibitor), but not erastin (a SLC7A11 inhibitor). | ||||
NAD-dependent protein deacylase sirtuin-6 (SIRT6)
Melatonin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [18] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cataract [ICD-11: 9B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | B-3 cells | Normal | Homo sapiens | CVCL_6367 | |
| In Vivo Model |
Six-week-old albino Sprague Dawley (SD) male rats were provided by the Experimental Animal Centre of the Second Affiliated Hospitalof Harbin Medical University. Fifteen minutes before exposure, the rats were anaesthetized by intraperitoneal injection of a mixture of 90 mg/kg ketamine and 15 mg/kg xylazine. Then, tropicamide phenylephrine was dropped in both eyes; at the same time, the rats that received drug treatment were injected subconjunctivally (5 ul/eye) with 500 mM Fer-1, 200 mM MT or the same dose of DMSO used to dissolve the drug using a 28-gauge needle and a Hamilton microinjector. After another 5 min, a single eye of every experimental group rat was exposed to UVB (312 nm) 5 W/m2 for 30 min. Every time, UVB exposure was synchronized with the drug injection, and the frequency was every other day until it was stopped 9 weeks later.
Click to Show/Hide
|
||||
| Response Description | Melatonin inhibited ferroptosis through the SIRT6/p-Nrf2/GPX4 and SIRT6/COA4/FTH1 pathways to neutralize lipid peroxidation toxicity, which protected cells against ferroptotic stress in vitro and delayed cataract formation caused by UVB exposure in rats. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Quercetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from Gempharmatech Co., Ltd (Changzhou, China). All mice were housed in cages with standard laboratory conditions: a consistent temperature of 24 , a 12 h light/dark cycle, and free access to water and food. The mice were randomized into four groups: 1) the KA group (n = 6), injected intraperitoneally with 20 mg/kg KA, as described in a previous study; while 2) the control group (n = 6), injected intraperitoneally with an equal volume of PBS; 3) the KA + QCT group (n = 6): this group was givenintragastric administrationof 50 mg/kg of QCT once daily for 21 days before KA injection based on the literature; and 4) the KA+ferrostatin1 (Fer-1) group (n = 6), injected intraperitoneally with a well-known ferroptosis inhibitor (3 mg/kg Fer-1) for 21 days before KA administration, as described in a previous study.
Click to Show/Hide
|
||||
| Response Description | The association between the Nrf2-mediated ferroptosis pathway and seizures in a clinical setting. Quercetin effectively protects against seizure-induced neuron death in vivo and in vitro and alleviates cognitive function impairment via the SIRT1/Nrf2/SLC7A11/GPX4 pathway. | ||||
Icariin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Supraventricular tachycardia [ICD-11: BC81] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response Description | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Ferric ammonium citrate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [21] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Atherosclerosis [ICD-11: BD40] | ||||
| Pathway Response | Autophagy | hsa04140 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| In Vivo Model |
A total of 20 male Apoe-/-mice (6-8 weeks of age, 18-22 g) were purchased from Charles River (Beijing, China). Mice were randomly assigned to a control group (normal diet: 4% fat, cholesterol free, and sodium cholate) and an AS group (high-fat diet: 20% fat, 1.25% cholesterol, and 0.5% sodium cholate).
Click to Show/Hide
|
||||
| Response Description | Ferric ammonium citrate(FAC) can induce a decrease in foam cell activity rather than macrophage activity, increase lipid ROS levels, decrease GPX4 expression and inhibit SIRT1 expression. Activation of SIRT1 can inhibit the ferroptosis and IL-1 and IL-18 levels of foam cells in excess iron by autophagy, providing a novel therapeutic target for atherosclerosis(AS). | ||||
Cadmium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [22] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Kidney injury [ICD-11: NB92] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/ Sirt1 axis. | |||
mmu_circRNA_0000309 (circRNA)
Germacrone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | MPC-5 cells | Normal | Mus musculus | CVCL_AS87 | |
| In Vivo Model |
C57BL/6J mice were purchased from Three Gorges University (Yichang, China), and C57BL/KsJ and male db/db mice were from Changzhou Cavins Laboratory Animal Co. Ltd. (Changzhou, China). All experiments were approved by the Animal Ethics Committee of Zhejiang Provincial People's Hospital, and performed according to specific institutional and national guidelines. The mice were divided into three groups: control C57BL/6J mice, db/db mice, and germacrone-treated db/db mice (db/db + Ger) (n = 10/each group). The db/db + Ger mice received germacrone treatment at a dosage of 10 mg/kg/day, while C57BL/6J mice and db/db mice had been given the same volumes of 0.9% saline simultaneously.
Click to Show/Hide
|
||||
| Response Description | mmu_circRNA_0000309 silence mediates drug resistance to germacrone in Diabetic nephropathy mice. mmu_circRNA_0000309 sponges miR-188-3p, and subsequently upregulates GPX4 expression, inactivating ferroptosis-dependent mitochondrial function and podocyte apoptosis. | ||||
Mitogen-activated protein kinase 8 (MAPK8)
Seratrodast
[Discontinued in Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [24] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Drugs were dissolved in vehicle (0.1% DMSO + 20% PEG 300 + 0.5% CMC-Na + ddH2O). Mice in Control and PTZ groups were administered for five days with an equivalent volume of vehicle. PTZ-induced seizure model was done for the subsequent 1 h after the last administration of drugs. We performed a preliminary doseresponse trial, the dose of 60 mg/kg was established as being sufficient to trigger seizures with lower mortality and chosen as the optimal dose. One mouse in PTZ group was dead due to a severe seizure. At the end of the experiment, the mice were anesthetized or euthanized. For histopathological studies, the mice were anesthetized and intracardially perfused with 0.9% saline, followed by 0.4% paraformaldehyde for fixation of the brain. For immunoblot analysis, the hippocampus was rapidly isolated.
Click to Show/Hide
|
||||
| Response Description | Seratrodast could reduce lipid ROS production, regulate the system xc-/glutathione (GSH)/glutathione peroxidase 4 (GPX4) axis, and inhibit JNK (MAPK8) phosphorylation and p53 expression. JNK can directly or indirectly modulate the expression and activation of p53, which could regulate ferroptosis through inhibition of SLC7A11 transcription. Seratrodast increased the latency of seizures and reduced seizure duration in pentylenetetrazole-induced seizures. | ||||
L-F001
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [25] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | L-F001 could restore GPX4 and glutamate-cysteine ligase modifier subunit (GCLM) levels, and significantly deceased Cyclooxygenase (COX-2) levels to rescue the lipid peroxidation imbalance. And L-F001 could reduce RSL3-induced c-Jun N-terminal kinase (JNK) activation, which might be a potential drug target for for the therapy of ferroptosis-related diseases, such as cerebral ischemia. | |||
Mitogen-activated protein kinase 14 (MAPK14)
Artesunate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [26] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response Description | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 ( MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
Lactate
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [27] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H446 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1562 | |
| NCI-H1688 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1487 | |
| Response Description | Lactate derived from metabolic reprogramming increases the expression of glutathione peroxidase 4 (GPX4) to promote ferroptosis resistance in Non-Small Cell Lung Cancer (NSCLC). Mechanistically, Lactate increases mitochondrial ROS generation and drives activation of the p38 (MAPK14)-SGK1 pathway, which attenuates the interaction of NEDD4L with GPX4 and subsequent ubiquitination and degradation of GPX4. | |||
Mitogen-activated protein kinase 1 (MAPK1)
Salidroside
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [28] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mLT (Mouse lung tissue) | ||||
| In Vivo Model |
In our study, the 32 mice were randomly divided for four groups (n = 8 per group): (1) room-air-expose (sham), (2) hyperoxia-expose with Sal (Sal + Hyperoxia), (3) hyperoxia-exposed (Hyperoxia), (4) hyperoxia-exposed with Y-320 (an inhibitor of IL-17) (Y-320 + Hyperoxia). The mice exposed to normoxia groups were placed in room air with 21% oxygen, and the mice exposed to hyperoxia were placed in over 90% oxygen for 24 h. The continue exposure to over 90% oxygen was achieved in a self-made airtight box which attached to a medical oxygen cylinder, and the O2 level inside was continuously monitored with O2 analyzer, mice had free access to food and water. In the first three days before exposure to the hyperoxia, mice in the Sal + Hyperoxia group or Y-320 + Hyperoxia group were treated with Sal (100 mg/Kg) or Y-320 (2 mg/Kg) once orally every day, while the rest of groups were given equal isotonic saline. Based on the above experiments, eight 8-week-old KM mice were randomly divided into two groups: Sal + Hyperoxia group and Sal + Hyperoxia + IL-17A group. Sal + Hyperoxia + IL-17A group, mice were i.v. injected with 50 ug/kg of recombinant mouse IL-17A (210-17, Pepro Tech, USA). Animal were sacrificed following reperfusion, and lungs were stored at -80 until further experimental analysis.
Click to Show/Hide
|
||||
| Response Description | When we applied recombinant IL-17A in Sal+hyperoxia group mice, the protein levels of IL-17RA, Act1, TRAF6, p38 MAPK and p-p38 MAPK increased significantly, and the expression level of GPX4 significantly decreased. Therefore, we demonstrated that IL-17A/IL-17RA mediates ferroptosis of AECII, least in part, via Act1/TRAF6/p38 MAPK pathway, which is responsible for the protective effects of salidroside on hyperoxia-induced acute lung injury (HALI). | ||||
LINC01134 (IncRNA)
Oxaliplatin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [29] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response Description | LINC01134 was positively correlated with GPX4 or Nrf2, demonstrating the clinical significance of LINC01134, Nrf2 and GPX4 in OXA resistance of hepatocellular carcinoma (HCC). Silenced LINC01134 enhances Oxaliplatin sensitivity by facilitating ferroptosis through GPX4 in hepatocarcinoma. | |||
Legumain (LGMN)
RR-11a
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [30] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
The genetic background of embryonic stem cells and the Flp mice used in this experiment was C57BL/6. Mice were randomly separated into experimental groups and control groups. (1) Bilateral IRI: mice (male, 8-10 weeks old) on the lgmnKO background or littermate control mice were anesthetized by an intraperitoneal (i.p.) injection of chloral hydrate and placed on a warm pad to retain their body temperature. A bilateral flank incision was made, both sides of the renal vessels were occluded with clamps for 40 min followed by removing the clamps to induce blood reperfusion. The same procedure was performed in the control group without vessel clamping. (2) Nephrotoxic folic acid-induced AKI: mice (female, 12-14 weeks old) received a single i.p. injection of folic acid at 250 mg/kg in 0.3 mol/L sodium bicarbonate or the vehicle. For therapeutic experiments, RR-11a was freshly dissolved in saline. Mice were administered an i.p. injection of 20 mg/kg RR-11a or the vehicle before ischemia.
Click to Show/Hide
|
||||
| Response Description | Legumain promotes chaperone-mediated autophagy of GPX4 therefore facilitates tubular ferroptosis in acute kidney injury (AKI). Legumain inhibitor RR-11a attenuates ferroptosis and tubular injury induced by ischemia-reperfusion injury (IRI). | ||||
L-seryl-tRNA(Sec) kinase (PSTK)
Punicalin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [31] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| SNU-387 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0250 | ||
| SNU-182 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0090 | ||
| SNU-398 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0077 | ||
| WRL 68 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0581 | ||
| HUVECs (Human umbilical vein endothelial cells) | |||||
| JHH-2 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_2786 | ||
| JHH-7 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_2805 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| Li-7 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_3840 | ||
| In Vivo Model |
Female Nod-SCID mice of 6-8 weeks old were purchased from HFK BIOSCIENCE (Beijing). Hep3B-vehicle/Hep3B-PSTK-KO cells were harvested and injected subcutaneously (1 x 107 cells in 200 uL PBS) into Nod-SCID mice (upper flank). Treatments were started when tumor volumes reached around 50 mm3. Included mice were randomly divided into four groups and injected intraperitoneally with Abemaciclib (50 mg/kg, every other day) or vehicle. Mice were sacrificed when the tumor volume exceeded 2000 mm3. PSTK-KO or vehicle Hep3B cells were implanted and treated with Sorafenib (50 mg/kg, every other day) or Erastin (50 mg/kg, every other day) for 42 days. Tumor volumes were monitored and quantified by the modified ellipsoidal formula, tumor volume = (length x width2)/2. To check the efficacities and appraisal the side effects of PSTK inhibitors, Hep3B cells were harvested and in injected subcutaneously (5 x 106 cells in 200 uL PBS) into Nod-SCID mice (upper flank). Treatments were started when tumor volumes reached around 50 mm3. Included mice were randomly divided into six groups and intragastrically treated with Punicalin (100 mg/kg, every day), Geraniin (100 mg/kg, every day), Sorafenib (50 mg/kg, every day) with or without PSTK inhibitors (Punicalin/Geraniin) for 30 days. Tumor volumes and mice weights were measured every three days.
Click to Show/Hide
|
||||
| Response Description | The depletion of PSTK resulted in the inactivation of glutathione peroxidative 4 (GPX4) and the disruption of glutathione (GSH) metabolism owing to the inhibition of selenocysteine and cysteine synthesis, thus enhancing the induction of ferroptosis upon targeted chemotherapeutic treatment. Punicalin, an agent used to treat hepatitis B virus (HBV), was identified as a possible PSTK inhibitor that exhibited synergistic efficacy when applied together with Sorafenib to treat Hepatocellular carcinoma in vitro and in vivo. | ||||
Krueppel-like factor 15 (KLF15)
Elabela
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [32] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | rAFs (Rat adventitial fibroblasts) | |||
| Response Description | KLF15 siRNA impeded the beneficial roles of elabela (ELA) in DOX-pretreated rat aortic AFs by suppressing the Nrf2/SLC7A11/GPX4 signaling. In conclusion, ELA prevents DOX-triggered promotion of cytotoxicity, and exerts anti-oxidative and anti-ferroptotic effects in rat aortic AFs via activation of the KLF15/GPX4 signaling, indicating a promising therapeutic value of ELA in antagonizing DOX-mediated cardiovascular abnormality and disorders. | |||
hsa-miR-744-5p (miRNA)
Propofol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
BALB/c nude mice (5 weeks) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (license no: SYXK (Beijing) 20170033). For tumor formation, 8 x 106 A549/Cis cells were subcutaneously injected into the right axilla of each mouse. On the 7th d, Cis (4.0 mg/kg) was intraperitoneally injected into each mouse every 4 days. Then, mice were allocated into 3 groups: Control group (no additional injection); SO group (intraperitoneal injection of soybean oil); and Propofol group [intraperitoneal injection of soybean oil-dissolved propofol (35 mg/kg)]. The volume of the tumor was measured by a caliper every 7 days. Tumor volume was measured according to the formula: V (mm3) = 1/2 ab2 (a: the longest axis of tumor; b: the shortest axis of tumor). Then 35 d after transplantation, mice were euthanatized to measure tumor weight using an electronic balance. A part of transplanted tumors was immediately conserved at liquid nitrogen and -80 . The rest was used for paraffin-embedding and immunohistochemical staining.
Click to Show/Hide
|
||||
| Response Description | In summary, propofol inhibited GPX4-mediated ferroptosis and reduces CR of non-small cell lung cancer (NSCLC) cells to Cis through the miR-744-5p/miR-615-3p axis. | ||||
hsa-miR-615-3p (miRNA)
Propofol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
BALB/c nude mice (5 weeks) were provided by Beijing Vital River Laboratory Animal Technology Co., Ltd. (license no: SYXK (Beijing) 20170033). For tumor formation, 8 x 106 A549/Cis cells were subcutaneously injected into the right axilla of each mouse. On the 7th d, Cis (4.0 mg/kg) was intraperitoneally injected into each mouse every 4 days. Then, mice were allocated into 3 groups: Control group (no additional injection); SO group (intraperitoneal injection of soybean oil); and Propofol group [intraperitoneal injection of soybean oil-dissolved propofol (35 mg/kg)]. The volume of the tumor was measured by a caliper every 7 days. Tumor volume was measured according to the formula: V (mm3) = 1/2 ab2 (a: the longest axis of tumor; b: the shortest axis of tumor). Then 35 d after transplantation, mice were euthanatized to measure tumor weight using an electronic balance. A part of transplanted tumors was immediately conserved at liquid nitrogen and -80 . The rest was used for paraffin-embedding and immunohistochemical staining.
Click to Show/Hide
|
||||
| Response Description | In summary, propofol inhibited GPX4-mediated ferroptosis and reduces CR of non-small cell lung cancer (NSCLC) cells to Cis through the miR-744-5p/miR-615-3p axis. | ||||
hsa-miR-34a-5p (miRNA)
Cadmium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [22] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Kidney injury [ICD-11: NB92] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/Sirt1 axis. | |||
hsa-miR-324-3p (miRNA)
Metformin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [34] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| In Vivo Model |
Six-week-old athymic nude mice were obtained from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Mice were divided into five groups: sham group, metformin group, metformin + NC group, metformin + miR-324-3p overexpression group, and metformin + miR-324-3p knockdown group (n = 6 in each group). Mice were injected with 3 x 106 MDA-MB-231 cells subcutaneously into the right flank. For the miR-324-3p overexpression or knockdown in the mice, two groups of mice were treated with miR-324-3p overexpression or knockdown lentivirus (GenePharma), respectively, by intratumoral injection of 50 ul of lentivirus (4 x 107 IU/ml) after the tumor cell injection. One day after tumor cell inoculation, the sham-treated group was treated with PBS and metformin-treated groups were treated with 200 mg/kg metformin every 2 days through intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Metformin promotes ferroptosis of breast cancer by targeting the miR-324-3p/GPX4 axis. The effect of miR-324-3p was mediated by directly targeting glutathione peroxidase 4 (GPX4). Metformin could act as a potential anti-cancer agent through the induction of ferroptosis. | ||||
Icariside II
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [35] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Hereditary Leiomyomatosis [ICD-11: 2C90] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | ACHN cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_1067 | |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
A total of 30 male BALB/c nude mice (4-6 weeks old; 18-23 g) were randomized into four groups (7-8 mice per group): i) control group; ii) treated with 15 mg/kg ICS II; iii) treated with 25 mg/kg ICS II; and, iv) treated with 35 mg/kg ICS II. ACHN and Caki-1 cells (1 x 107) were suspended in 50 ul MEM media mixed with 50 ul Matrigel (BD Biosciences) and injected subcutaneously into the right flank of mice with 1.5%pentobarbital sodium (60 mg/kg body weight; intraperitoneal injection) under anesthesia. Weight lossof more than 20% was considered a humane endpoint.
Click to Show/Hide
|
||||
| Response Description | Icariside II (ICS II) treatment triggered ferroptosis in renal cell carcinoma (RCC) cells by downregulating GPX4 in a p53-independent manner. Furthermore, ICS II treatment resulted in upregulation of miR-324-3p, which negatively regulated the expression of GPX4. | ||||
hsa-miR-214-3p (miRNA)
Ketamine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
BALB/c nude mice (age 6 weeks) were brought from the Laboratory Animal Center of Chinese Academy of Sciences (China). HepG2 cell suspension (100 uL, 5 x 105 per site) was hypodermically inoculated into the fat pad of mice. Tumor volume was calculated as follows: tumor volume (mm3) = 0.5 x width (mm)2 x length (mm). When tumor size reached 100 mm3, mice were treated with ketamine (20 mg/kg) or saline intraperitoneally. The mice were succumbed to death when tumor size reached 1000 mm3. Tumors were isolated and weighted. All animal experiments were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
Click to Show/Hide
|
||||
| Response Description | LncPVT1 directly interacted with miR-214-3p to impede its role as a sponge of GPX4. Depletion of lncPVT1 accelerated the ferroptosis of liver cancer cells, whereas miR-214-3p inhibition and GPX4 overexpression reversed this effect. In this work, we determined that ketamine suppressed viability of liver cancer cells and induced ferroptosis and identified the possible regulatory mechanism of lncPVT1/miR-214-3p/GPX4 axis. | ||||
hsa-miR-188-3p (miRNA)
Germacrone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | MPC-5 cells | Normal | Mus musculus | CVCL_AS87 | |
| In Vivo Model |
C57BL/6J mice were purchased from Three Gorges University (Yichang, China), and C57BL/KsJ and male db/db mice were from Changzhou Cavins Laboratory Animal Co. Ltd. (Changzhou, China). All experiments were approved by the Animal Ethics Committee of Zhejiang Provincial People's Hospital, and performed according to specific institutional and national guidelines. The mice were divided into three groups: control C57BL/6J mice, db/db mice, and germacrone-treated db/db mice (db/db + Ger) (n = 10/each group). The db/db + Ger mice received germacrone treatment at a dosage of 10 mg/kg/day, while C57BL/6J mice and db/db mice had been given the same volumes of 0.9% saline simultaneously.
Click to Show/Hide
|
||||
| Response Description | mmu_circRNA_0000309 silence mediates drug resistance to germacrone in Diabetic nephropathy mice. mmu_circRNA_0000309 sponges miR-188-3p, and subsequently upregulates GPX4 expression, inactivating ferroptosis-dependent mitochondrial function and podocyte apoptosis. | ||||
Histone acetyltransferase KAT5 (KAT5)
Ketamine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [36] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | |
| Response Description | The treatment of Ketamine induced the levels of MDA, lipid ROS, and Fe2+, while KAT5 or GPX4 overexpression could reverse this effect in breast cancer cells. Ketamine suppresses proliferation and induces ferroptosis and apoptosis of breast cancer cells by targeting KAT5/GPX4 axis. Ketamine may serve as a potential therapeutic strategy for breast cancer. | |||
High mobility group protein B1 (HMGB1)
Glycyrrhizin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [37] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Hypoxic ischemic brain injury [ICD-11: 8B24] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rPCNs (Rat primary cortical neurons) | ||||
| In Vivo Model |
Male and female neonatal SpragueDawley rats on postpartum day 7 (P7) were provided by SPF Biotechnology (Beijing, China). Each animal was anesthetized with isoflurane (4% for induction, 2% for maintenance), the skin was incised, and the left common carotid artery was exposed. This artery was ligated with a 5-0 suture and cut, and the skin was sutured closed. Next, the pups recovered for 1 h with their mother. Subsequently, the pups were placed in a hypoxia chamber (8% O2 + 92% N2 mixture) for 2 h. After 2 h of hypoxia, the animals were placed back with their dam.
Click to Show/Hide
|
||||
| Response Description | Glycyrrhizin (GL) not only inhibited ferroptosis induced by RSL3 and oxygen-glucose deprivation in vitro but also inhibited ferroptosis induced by hypoxic-ischemic brain damage (HIBD) in vivo. GL could suppress the occurrence of neuronal ferroptosis and reduce neuronal loss in HIBD via the HMGB1/GPX4 pathway. | ||||
Isoliquiritigenin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [38] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (aged 6-8 weeks and weighing 22-25g) were obtained from the Experimental Animal Center, Sichuan Provincial Peoples Hospital, and were fed a standard laboratory diet. LPS and ISL were dissolved in normal saline and 0.5% Tween-20/saline, respectively. AKI mice were developed by intraperitoneal (i.p.) LPS injection. A total of 30 mice were randomly divided into six groups (n = 5): control, ISL, Fer, LPS, LPS plus ISL, and LPS plus Fer. An intraperitoneal injection of LPS (10 mg/kg) was made to induce septic AKI. ISL was administered via gavage at 50 mg/kg 30 min before LPS injection. Mice were dosed intraperitoneally with Fer (Ferrostatin-1, SML0583, Sigma-Aldrich, St. Louis, MO) at 5 mg/kg. Mice were sacrificed by cervical dislocation 8 h after LPS injection. Kidney tissue and serum samples were collected concurrently.
Click to Show/Hide
|
||||
| Response Description | Isoliquiritigenin (ISL) attenuates septic acute kidney injury by regulating ferritinophagy-mediated ferroptosis. ISL inhibited Fe2+ and lipid peroxidation accumulation in LPS-stimulated HK2 cells. It also increased the expression of GPX4 and xCT, reduced the expression of HMGB1 and NCOA4 then attenuated mitochondria injury in renal tubular following LPS stimulation. | ||||
HCG18 (IncRNA)
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [39] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
BALB/c nude mice (4-6 weeks old) from Beijing Vital River Laboratory Animal Technology (Beijing, China) were reared in a standard laboratory with free access to food and water. Lentivirus LV-sh-NC and LV-sh-HCG18 were from GenePharma (Shanghai, China). In order to establish subcutaneous xenograft tumor models, Huh7-SR cells were infected with lentivirus LV-sh-NC or LV-sh-HCG18 and then resuspended in PBS at 5 x 105/mL. Totally 100 uL cells were subcutaneously injected into the right dorsal area of each nude mouse. When the tumor volume reached 150 mm3, sorafenib (10 mg/kg) was orally administered to nude mice once a day to the end. Tumor volume (V) was calculated: V = 0.5 x L x W2, where L and W were defined as tumor length (L) and width (W). After 28 days of cell injection, the nude mice were euthanized by intraperitoneal injection of excessive pentobarbital sodium (100 mg/kg).
Click to Show/Hide
|
||||
| Response Description | HCG18 sponged miR-450b-5p to regulate GPX4. Collectively, Silencing HCG18 inhibits GPX4 by binding to miR-450b-5p, promotes GPX4-inhibited ferroptosis, and averts sorafenib resistance in hepatocellular carcinoma (HCC). Silencing HCG18 inhibited cell proliferation, promoted apoptosis, and impaired sorafenib resistance. | ||||
Glutathione hydrolase 1 proenzyme (GGT1)
Oridonin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [40] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Oesophageal cancer [ICD-11: 2B70] | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 |
| Response Description | The levels of intracellular iron, malondialdehyde, and reactive oxygen species after oridonin (Ori) treatment, while interfering with the effects of Ori with ferroptosis inhibitor, demonstrating that Ori's inhibition of TE1( esophageal cancer cell) cell proliferation is associated with ferroptosis. Ori can inhibit the gamma-glutamyl cycle by inhibiting the activity of GGT1 and binding to cysteine, thereby inducing ferroptosis to exert anti-cancer activity. Eventually, the value of intracellular GSH/GSSG was reduced, and the enzymatic activity of the glutathione peroxidase 4 (GPX4) was significantly decreased. | |||
Ferritin, mitochondrial (FTMT)
Carbonyl cyanide-m-chlorophenyl-hydrazine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [41] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Osteoporosis [ICD-11: FB83] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hFOB 1.19 cells | Normal | Homo sapiens | CVCL_3708 | |
| In Vivo Model |
Forty-five SD rats (3 months old, 200 ± 20 g) were obtained from the Department of Experimental Animals in China Medical University (Animal Certificate Number: SCXK (Liaoning) 2008-0005). Fifteen rats grew as control while other thirty rats were established T2DOP model. The model rats were given a high-fat feed and 12 h/day water for 2 months. Then streptozotocin was intraperitoneally injected at 30 mg/kg. Seventy-two hours later, the model was successfully established when insulin sensitivity index decreased and fasting plasma glucose exceeded 7.8 mmol/L. Then all rats continue grew 3 months to cause osteoporosis. Thirty model rats were divided into two groups. One was fifteen T2DOP rats only, and other was fifteen T2DOP rats with deferoxamine (DFO) treatment (60 mg/kg/day, intraperitoneally inject, last for the last 1 month).
Click to Show/Hide
|
||||
| Response Description | Carbonyl cyanide-m-chlorophenyl-hydrazine (CCCP) is a mitophagy agonist. Through adding mitophagy agonist CCCP to osteoblasts, we found the increase of ROS and lipid peroxidation while GPX4 decreased. FtMt inhibited the occurrence of ferroptosis in osteoblasts by reducing oxidative stress caused by excess ferrous ions, and FtMt deficiency induced mitophagy in the pathogenesis of type 2 diabetic osteoporosis (T2DOP). | ||||
Endoplasmic reticulum chaperone BiP (HSPA5)
Dihydroartemisinin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [42] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| In Vivo Model |
Specific pathogen-free athymic nude BALB/c mice (4-6 weeks old) were obtained from Guangdong Experimental Animal Centre (Guangzhou, China). To generate murine subcutaneous tumors, cells (for U251: 2 x 106 cells; for U373: 2 x 106 cells) were suspended in 0.2 ml PBS and injected into the flanks of mice (n = 6/group). Tumor volume was measured once every 3 days using calipers.
Click to Show/Hide
|
||||
| Response Description | HSPA5 upregulation increased the expression and activity of glutathione peroxidase 4 (GPX4), which neutralized Dihydroartemisinin-induced lipid peroxidation and thus protected glioma cells from ferroptosis. Ferroptosis might be a novel anticancer mechanism of DHA in glioma and HSPA5 may serve as a negative regulator of DHA-induced ferroptosis. | ||||
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP2)
Doxorubicin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [43] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | ENPP2 was transcriptionally regulated by FoxO4 to protect cardiomyocytes from doxorubicin-induced cardiotoxicity by inhibiting ferroptosis. In addition, the inhibitory effects of ENPP2 on Dox-induced ferroptosis were significantly reduced by FoxO4 overexpression, as demonstrated by increased Fe2+ and lipid ROS activity levels, decreased SLC7A11, GPX4 and FPN1 expression, and increased NOX4 expression, which were observed following FoxO4 overexpression. | |||
E3 ubiquitin-protein ligase TRIM21 (TRIM21)
Fedratinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [44] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Mice were fasted for 12 h and anesthetized (1% pentobarbital sodium, i.p.) before surgery. Bilateral renal pedicles were clamped for 30 min, then remove the arterial clamps. The sham groups were treated in the same way, except for the clamping of the renal pedicle. Blood samples were collected 24 h after reperfusion, mice were killed, and kidney were collected for follow-up experiments. Fedratinib (5 mg/kg body weight) was injected (i.p.) into mice 24 h once in advance before surgery.
Click to Show/Hide
|
||||
| Response Description | A JAK2 inhibitor Fedratinib downregulated TRIM21 expression and reduced damage both in vivo and in vitro, which is correlated with the upregulation of GPX4. Our study showed that loss of TRIM21 could alleviate ferroptosis induced by I/R, revealed the mechanism of ubiquitination degradation of GPX4 by TRIM21 and suggested TRIM21 is a potential target for the treatment of acute kidney injury (AKI). | ||||
E3 ubiquitin-protein ligase NEDD4-like (NEDD4L)
Peoniflorin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| In Vivo Model |
U251 cells (6 x 106) were inoculated into the flanks of 4-to 5-week-old athymic nude mice (Shanghai Laboratory Animal Company, Shanghai, China) subcutaneously to generate a subcutaneous xenograft tumor model. After 2 weeks, the tumor model was successfully constructed, the mice were treated single and combined with 100 mg/kg RSL3 (2 times/week) and 1.0 g/kg/days PF. Tumor volumes were measured every 4 days to draw the growth curve. Mice were sacrificed 4 weeks after cell injection. Tumor xenografts were collected, photographed, and weighed and the tumor apoptosis was analyzed by Tunel staining.
Click to Show/Hide
|
||||
| Response Description | Paeoniflorin (PF) can function as an antitumor agent for glioma treatment by targeting NEDD4L-dependent STAT3 ubiquitination as well as by regulating the Nrf2/GPX4 signaling axis, which might trigger ferroptosis. | ||||
Lactate
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [27] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H446 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1562 | |
| NCI-H1688 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1487 | |
| Response Description | Lactate derived from metabolic reprogramming increases the expression of glutathione peroxidase 4 (GPX4) to promote ferroptosis resistance in Non-Small Cell Lung Cancer (NSCLC). Mechanistically, Lactate increases mitochondrial ROS generation and drives activation of the p38 (MAPK14)-SGK1 pathway, which attenuates the interaction of NEDD4L with GPX4 and subsequent ubiquitination and degradation of GPX4. | |||
E3 ubiquitin-protein ligase MIB2 (MIB2)
Sevoflurane
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [45] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Cognition disorder [ICD-11: MB21] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | mPRs (Mouse primary neurons) | ||||
| In Vivo Model |
Male C57BL/6 mice were obtained from Beijing HFK Bioscience Co., Ltd., China. The mice were then randomly separated into sham and sevoflurane administrated (SEV) groups, with each group containing 20 animals. In SEV groups, mice were placed in an anesthetizing chamber and exposed to 2.5% sevoflurane (CAS No. 28523-86-6, no. S2464, Selleck, Shanghai, China) with complete oxygen for 2 h, and sham group mice were conducted with the same procedure without sevoflurane exposure.
Click to Show/Hide
|
||||
| Response Description | Postoperative cognitive dysfunction (POCD) is a complication of the central nervous system (CNS) often occurred after surgery or anesthesia in the elder patients. Downregulation of MIB2 could alleviate the sevoflurane-anesthesia-induced cognitive dysfunction and neuron injury through reducing ferroptosis via GPX4. | ||||
Cystathionine beta-synthase (CBS)
Sodium hydrosulfide
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [46] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Depressive disorder [ICD-11: 6A70] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | BV-2 cells | Normal | Mus musculus | CVCL_0182 | |
| In Vivo Model |
Adult male 22-24 g C57BL/6J mice were purchased from the Vital River Laboratory Animal Technology Co., Ltd. Mice were randomly divided into four groups, the control group (CON group, n = 8), diabetes mellitus group (DM group, n = 8), DM + sodium hydrosulfide (DM + 5.6 mg/kg NaHS, n = 8) group, and CON + sodium hydrosulfide (CON + 5.6 mg/kg NaHS, n = 8) group. In this experiment, mice have received daily intraperitoneally injection of NaHS during the last 4 weeks. Then, all mice were tested by the open field test (OFT), elevated plus maze test (EPM test), forced swimming test (FST), and tail suspension test (TST).
Click to Show/Hide
|
||||
| Response Description | Sodium hydrosulfide (NaHS) ameliorated the ferroptosis via increasing the protein expressions of SLC7A11, glutathione peroxidase 4 (GPX4), and cystathionine -synthase (CBS), reducing the pro-inflammatory cytokines, decreasing the levels of Fe2+, MDA, ROS, and lipid ROS. In conclusion, NaHS did alleviate anxiety and depression. | ||||
CircOMA1 (circRNA)
Cabergoline
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [47] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Prolactinoma [ICD-11: 2F37] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
Cellular tumor antigen p53 (TP53)
Quisinostat
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [48] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Oral squamous cell carcinoma [ICD-11: 2B6E] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
| Cell pyroptosis | |||||
| In Vitro Model | CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | |
| Tca8113 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6851 | ||
| In Vivo Model |
Adult male athymic BALB/c nude mice (20-22 g of 5-week-old mice) were housed in a controlled environment at 23 ± 2 and 40%-70% humidity under a 12 h dark/light cycle with free access to irradiated food and sterile water. A suspension of 6 x 106/100 uL TCA-8113 cells was inoculated subcutaneously into the hind flank region of each nude mouse. The average tumor volume in nude mice reached 100 mm3, and mice were randomly divided into three groups. Quisinostat was formulated in normal saline and administered at 3 and 10 mg/kg/day byintraperitoneal injection. Control mice were given equal volume saline intraperitoneally. The tumor volume and the bodyweight of mice were monitored every three days.
Click to Show/Hide
|
||||
| Response Description | Quisinostat could increase the apoptosis rate in the tumor tissues of nude mice. Up-regulation of the expression of p53 and down-regulated expression of GPX4 in cell lines were observed by immunofluorescent staining, and the expression locations of p53 and GPX4 proteins in TSCC cells were observed. Quisinostat may be a potential drug for the treatment of tongue squamous cell carcinoma. | ||||
Apigenin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [49] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
5-weeks-old kainate (KA)-induced BALB/c nude mice, a widely used epilepsy mouse model, were performed with intraperitoneal (i.p.) injection of KA (6 mg/kg). Pre-treatment 21 with antioxidant apigenin (60 mg/Kg, 2 days) or post-treatment with apigenin (60 mg/Kg, 1 day), mice were injected with KA (6 mg/kg) via intraperitoneal (i.p.) injection, and then HCP (0.5 mg/Kg) were injected by intravenous (i.v.) injection. In vivo and Ex vivo fluorescence images of relative ClO levels in mice brains 5, 15, 30, 45, and 60 min post injection of HCP were further performed by using the IVIS Spectrum imaging system (Nanjing University) with an excitation filter of 430 nm and the collection wavelength range is from 500-600 nm.
Click to Show/Hide
|
||||
| Response Description | Apigenin can efficiently reduce the expression of intracellular MPO and increase the levels of GPX4 and SIRT1, thereby conferring neuroprotection through regulation of kainic acid (KA)-induced ferroptosis. And the level of Ac-p53 inside the brains treated with apigenin was down-regulated, suggesting that the p53-mediated ferroptosis pathway might be blocked. Overall, apigenin was screened and confirmed as an efficient lead compound for epilepsy prevention and treatment. | ||||
Bromodomain-containing protein 4 (BRD4)
JQ1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [50] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| In Vivo Model |
Female athymic BALB/c nude mice (4-6-week old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Approximately 1 x 107 cells (A549) in 200 uL of serum-free medium and Matrigel solution were injected directly into the right axilla of each mouse. Tumor growth was measured with calipers every 3 days.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis was induced under (+)-JQ1 treatment and BRD4 knockdown, indicating that (+)-JQ1 induces ferroptosis via BRD4 inhibition in breast adenocarcinoma. In addition, expression of the ferroptosis-associated genes GPX4, SLC7A11, and SLC3A2 was downregulated under (+)-JQ1 treatment. Moreover, JQ1 treatment and BRD4 knockdown led to decreased FTH1 expression. | ||||
Androgen receptor (AR)
ALZ003
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [51] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 | |
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| In Vivo Model |
NOD-SCID male mice (8-week-old) were purchased from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan). For glioblastoma and TMZ-resistant glioblastoma transplantation, luciferase-expressed U87MG cells (2 x 105) and U87MG-R cells (2 x 105) were injected into the cortex, respectively, at the depth of 3 mm using stereotactic guidance and microprocessor single syringe (Harvard Apparatus, Holliston, MA, USA). After 10 days of transplantation, TMZ (15 mg/kg) and ALZ003 were orally and intravenously administrated three times per week, respectively.
Click to Show/Hide
|
||||
| Response Description | ALZ003 targeting AR for degradation strongly exhibits the therapeutic effect on glioblastoma, including TMZ-resistant tumor,in vitroandin vivo. Particularly, GPX4 was positively regulated by AR, and overexpression of AR also prevented lipid peroxidation. | ||||
Furosine dihydrochloride
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [52] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Kidney injury [ICD-11: NB92] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPKCs (Mouse primary kidney cells) | ||||
| In Vivo Model |
A total of 60 ICR female mice (20 ± 2g, 5 mice/group) were divided into 12 groups (control and 10 furosine treatment groups). Furosine was dissolved in distilled water and a dose of 0.24 g/kg b.w. was administered by gavage or tail vein injection (0.2 mL volume per mouse) once at the beginning. This dose was chosen based on the median lethal dose (LD50) determined in previous acute toxicity experiments, in which the LD50 of furosine was 1.6 g/kg b.w. Mice were fasted for 4 h prior to dosing; animals were sacrificed at 0 (controls), 0.25, 0.5, 1, 2, 3, 4, 6, 8, 10, 12 h after administration, and kidney tissue was dissected and blood samples were collected.
Click to Show/Hide
|
||||
| Response Description | Furosine might decrease the activity of GPX4 via AR, thereby disrupting the conversion of peroxides into non-toxic reduced forms. Once GPX4 loses its reduction activity, excessivelipid peroxidationin kidney cells can lead to cell death by ferroptosis. To conclude, the study demonstrated for the first time the toxicity of furosine toward kidney injury. | ||||
5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1)
Artesunate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [53] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BV-2 cells | Normal | Mus musculus | CVCL_0182 | |
| In Vivo Model |
Rats were anaesthetised through intraperitoneal injection of pentobarbital (40 mg/kg) and placed onto a stereotaxic instrument (RWD Life Science Co., Ltd.). A 1-cm midline incision was performed in the rat scalp to expose the intersection point. Then, a hole 3.2 mm lateral and 1.4 mm anterior to the right bregma was produced. Next, 1.0 ul collagenase type IV (0.25 IU/ul; C5138; Sigma-Aldrich, USA) was injected into the basal ganglia via a microinjection pump (4.2 mm depth below the endocranium) at a rate of 0.2 ul/min. The needle was maintained for 5 min after injection to prevent backflow. Thereafter, the skin incision was closed using sutures. Rats in the sham group received 1.0 ul saline instead of collagenase type IV.
Click to Show/Hide
|
||||
| Response Description | Artesunate alleviates intracerebral haemorrhage secondary injury by inducing ferroptosis in M1-polarized microglia and suppressing inflammation through AMPK/mTORC1/GPX4 pathway | ||||
3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR)
Simvastatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| In Vivo Model |
MDA-MB-231 cells were injected to subcutaneous of mice to build a tumor model. When the tumor volume reaches about 60 mm3, all mice were randomly divided into five groups (n = 5) for various treatments. Then, mice were treated with PBS, Fe3O4@PCBMA, SIM, Fe3O4-SIM and Fe3O4@PCBMA-SIM through injected intravenously. The injected doses of SIM were 4 mg/kg body weight in each mouse on days 0, 3, 6, and 9.
Click to Show/Hide
|
||||
| Response Description | The study presented the ferroptosis nanomedicine by loading simvastatin (SIM), a ferroptosis drugs, into zwitterionic polymer coated of magnetic nanoparticles (Fe3O4@PCBMA), thereby improving the therapeutic effect of triple negative breast cancer. SIM could inhibit the expression of HMGCR to downregulate the mevalonate (MVA) pathway and glutathione peroxidase 4 (GPX4), thereby inducing cancer cell ferroptosis. | ||||
Heat shock factor protein 1 (HSF1)
Palmitic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [126] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Health [ICD-11: N.A.] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Hsf1 and Hsf1+/+-/- mice were kindly given as a present by Dr. Ivor J. Benjamin (Froedtert & Medical College of Wisconsin, Milwaukee, WI, USA). Sex-matched Hsf1-/- mice and Hsf1 littermates were used at 16-20 weeks old. Each mouse was injected intraperitoneally with 2.5 umol PA (dissolved in 0.5 mL 10% BSA) or an equal volume of BSA twice daily for 7 days.
Click to Show/Hide
|
||||
| Response Description | Palmitic acid (PA) decreased the protein expression levels of both heat shock factor 1 (HSF1) and glutathione peroxidase 4 (GPX4) in a dose- and time-dependent manner, which were restored by different ferroptosis inhibitors. Altogether, HSF1 may function as a key defender against PA-induced ferroptosis in cardiomyocytes by maintaining cellular iron homeostasis and GPX4 expression. | ||||
Unspecific Regulator
Methamphetamine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [149] | |||
| Responsed Disease | HIV Infection [ICD-11: 1C60] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | BV-2 cells | Normal | Mus musculus | CVCL_0182 |
| Response Description | Methamphetamine (METH) and HIV-1 lead to oxidative stress and their combined effect increases the risk of HIV-associated neurocognitive disorder (HAND), which may be related to the synergistic ferroptotic impairment in microglia. We found that METH and HIV-1 Tat reduced the expression of ferroptotic protein GPX4 and the cell viability and enhanced the expression of P53 and the level of ferrous iron, while the above indices were significantly improved with pretreatment of ferrostatin-1. | |||
Acetaminophen
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [150] | ||||
| Responsed Disease | Sepsis [ICD-11: 1G40] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hHCs (Hippocampal cells) | ||||
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| In Vivo Model |
Healthy male C57BL/6J mice, weighing 22-24 g, 6 weeks old, were purchased from Tianyao Biotechnology Company (Tianjin, China) and housed in an environment free of specific pathogenic bacteria: temperature 22-24 , relative humidity 50%-70%, alternating day and night every 12 h, and free access to water. The cecal ligation and puncture (CLP) approach was used to establish septic mouse models. The survival rates for 7 days were determined. The Morris water maze (MWM) was utilized to assess cognitive function. Hematoxylin and eosin (HE) staining identified histopathologic alterations in hippocampal tissue.
Click to Show/Hide
|
||||
| Response Description | In both animal and cell studies, Acetaminophen reduced iron content, ROS, glutamate antiporter (xCT), 4-hydroxy-2-nonenal (4-HNE) levels but increased GPX4 expression. Our findings suggest that APAP reduces sepsis-induced cognitive impairment by reducing ferroptosis, which is mediated by the GPX4 signaling pathway. | ||||
Iridin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [151] | ||||
| Responsed Disease | Sepsis [ICD-11: 1G40] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Eight-week-old wild-type (WT) and Nrf2-knockout (Nrf2-/-) littermate male mice on a C57BL/6J background were purchased from Cyagen (Suzhou, China.) and maintained at the Centre for Animals of Wuhan University (Wuhan, China). Before the experiment, the mice were separated and given light and dark cycles for 12 h, 22 ± 0.5 temperature, 60 ± 10% humidity, and free accessed to food and water for at least 1 week. Mice were randomly distributed into sham, CLP, CLP + Irisin (Ir group) and CLP + Irisin + Era (Ir + Era group) groups.
Click to Show/Hide
|
||||
| Response Description | In conclusion, irisin could ameliorate inflammatory microenvironment in Sepsis-associated encephalopathy by suppressing hippocampus ferroptosis via the Nrf2/GPX4 signaling pathway. | ||||
Dexmedetomidine
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [152] | ||||
| Responsed Disease | Sepsis [ICD-11: 1G40] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mVTs (Mouse ventricular tissues) | ||||
| In Vivo Model |
A total of 32 male C57BL/6 mice (25 g, 8 weeks old) were obtained from the Guangdong Medical Lab Animal Center and housed in the Laboratory Animal Service Center (Jinan University, Guangdong, China). Mice were anesthetized with isoflurane (RWD Life Science) inhalation at the concentration of 2.5% for anesthetic induction and then at 1% for anesthetic maintenance until the end of the CLP. During the experiment, the body temperature was kept at 36-38 with a heating pad. Anesthetized mice were subjected to midline laparotomy. The cecum was carefully separated to avoid blood vessels damage and the cecum was identified and punctured twice with a 22-gauge needle. Then, the abdominal cavity was closed with two epithelium layers, followed by a normal saline injection subcutaneously for resuscitation before mice were returned to the cage.
Click to Show/Hide
|
||||
| Response Description | The attenuation of sepsisinduced HO1 overexpression and iron concentration, and the reduction of ferroptosis via enhancing GPX4, may be the major mechanisms via which Dexmedetomidine alleviates sepsis induced myocardial cellular injury. | ||||
Sodium Selenite
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [153] | |||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| HT-29 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0320 | |
| SVG p12 cells | Normal | Homo sapiens | CVCL_3797 | |
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| Response Description | Sodium selenite (SS) down-regulates ferroptosis regulators; solute carrier family 7 member 11 (SLC7A11), glutathione (GSH), and glutathione peroxidase 4 (GPx4), while it up-regulates iron accumulation and lipid peroxidation (LPO) in Glioblastoma. | |||
RSL3
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [154] | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female B-NDG mice (4-6 weeks old, 16-20 g) were purchased from Biocytogen (Biocytogen Jiangsu Co., Ltd., Jiangsu, China) and housed under specific pathogen-free conditions. 5 x 106 U87 cells were resuspended in 200 uL PBS buffer and then inoculated into the left hind limb of each mouse. Once tumor volumes reached >=50 mm3, the mice were randomly divided into four groups (n = 5): the control, RSL3-only, BAY-only, and RSL3 plus BAY groups. Chemicals were administered through intratumor injection (100 mg/kg for RSL3 and 1 mg/kg for BAY 11-7082) biweekly for two weeks.
Click to Show/Hide
|
||||
| Response Description | NF-kB pathway activation is vital for RSL3-induced ferroptosis in glioblastoma cells both in vitro and in vivo. Furthermore, RNAi-mediated GPX4 silencing cannot trigger ferroptosis in glioblastoma cells unless the NF-kB pathway is activated simultaneously. Finally, NF-kB pathway activation promotes ferroptosis by downregulating the expression of ATF4 and SLC7A11. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [175] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| Response Description | RSL3 triggered ferroptotic cell death by promoting the accumulation of cellular ROS and increasing the cellular LIP level. Mechanismly, we found transferrin expression were elevated in colorectal cancer cells treated with RSL3 accompanied by a decrease in the expression of GPX4, indicating an iron-dependent cell death. | ||||
Dihydroartemisinin
[Investigative]
| In total 3 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [155] | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| Response Description | Dihydroartemisinin (DHA) had a selective killing effect on glioblastoma, which was associated with over-expression of transferrin receptors. The primary mechanism by which DHA caused ferroptosis was down-regulation of GPX4 and the following lipid ROS accumulation. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [183] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
| In Vitro Model | NCI-H292 cells | Lung mucoepidermoid carcinoma | Homo sapiens | CVCL_0455 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| MDA-MB-453 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| In Vivo Model |
GPX4 iKO H292 cells were inoculated by injecting 3 x 106 cells in 0.1 mL PBS subcutaneously in the right flank of six- to eight-week-old female athymic nude Foxn1nu/Foxn1 mice (Envigo, East Millstone, NJ, USA). Following inoculation, the mice were monitored until they have fully recovered and are moving. Mice were randomly allocated into their respective groups (non-blinded). Tumor growth was monitored regularly via external caliper measurements.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin (DAT) can augment GPX4 inhibition-induced ferroptosis in a cohort of cancer cells that are otherwise highly resistant to ferroptosis. Collectively, artemisinin compounds can sensitize cells to ferroptosis by regulating cellular iron homeostasis in Lung mucoepidermoid carcinoma. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Response of This Regulator | [197] | ||||
| Responsed Disease | Cervical cancer [ICD-11: 2C77] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| SiHa cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | ||
| Response Description | Dihydroartemisinin (DHA) treatment initiated ferroptosis, as evidenced by the accumulation of reactive oxygen species (ROS), malondialdehyde (MDA) and liquid peroxidation (LPO) levels and simultaneously depletion of glutathione peroxidase 4 (GPX4) and glutathione (GSH). Moreover, nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy was also induced by DHA leading to subsequent increases of intracellular labile iron pool (LIP), exacerbated the Fenton reaction resulting in excessive ROS production, and enhanced cervical cancer ferroptosis. | ||||
Dihydrotanshinone I
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [157] | |||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HEB (Human glial cells) | |||
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| Response Description | Dihydrotanshinone I (DHI) inhibited the proliferation of human glioma cells. Following treatment of the U251 and U87 cells with DHI, changes in the expression levels of ferroptosis-associated proteins were observed; the expression level of GPX4 decreased and that of ACSL-4 increased. DHI also increased the levels of LDH and MDA in the human glioma cells and reduced the GSH/GSSG ratio. | |||
Decitabine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [158] | ||||
| Responsed Disease | Myelodysplastic syndrome [ICD-11: 2A3Z] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell necroptosis | |||||
| In Vitro Model | SKM-1 cells | Acute myeloid leukemia | Homo sapiens | CVCL_0098 | |
| MUTZ-1 cells | Burkitt lymphoma | Homo sapiens | CVCL_1431 | ||
| In Vivo Model |
C57BL/6 mice were purchased from Vital River (Beijing, China) at 6 to 8 weeks of age. Twenty mice were housed with five individuals per cage and used at a weight of approximately 20.0-22.0 g. They were randomly divided into four groups, five in each group, namely control group, low-dose group, middle-dose group, and high-dose group. The low-, middle-, and high-dose group mice were administered an intraperitoneal injection of 0.2-ml iron dextran at a concentration of 6.25, 12.5, and 25 mg/ml, respectively, every 3 days for 10 weeks to establish iron overload model. At the same time, normal saline was given to the control group.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis may account for the main mechanisms of how decitabine induced death of myelodysplastic syndrome (MDS) cells. Decitabine-induced ROS raise leads to ferroptosis in MDS cells by decreasing GSH level and GPX4 activity. | ||||
Sulforaphane
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [159] | |||
| Responsed Disease | Acute myeloid leukaemia [ICD-11: 2A60] | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell apoptosis | ||||
| In Vitro Model | U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 |
| MV4-11 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0064 | |
| Response Description | Sulforaphane triggers different types of PCD in a concentration-dependent manner on the two tested acute myeloid leukemia cell lines. Deepening the molecular mechanisms on U-937 cells, we discovered that at lower concentrations, SFN induces apoptosis; at higher concentrations, SFN elicits ferroptosis, characterized by the depletion of intracellular GSH, the downregulation of GPX4 protein expression, and lipid peroxidation. | |||
Perillaldehyde
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [160] | |||
| Responsed Disease | Acute myeloid leukaemia [ICD-11: 2A60] | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Jurkat cells | T acute lymphoblastic leukemia | Homo sapiens | CVCL_0065 |
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | |
| SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| Response Description | We investigated and characterized its antileukemic potential in vitro, disclosing its ability to trigger ferroptosis. Specifically, perillaldehyde induced lipid peroxidation, decreased glutathione peroxidase 4 protein expression, and depleted intracellular glutathione on HL-60 promyelocytic leukemia cells. | |||
APR-246
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [161] | ||||
| Responsed Disease | Acute myeloid leukaemia [ICD-11: 2A60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| MOLM-14 cells | Leukemia | Homo sapiens | CVCL_7916 | ||
| SET-2 cells | Acute megakaryoblastic leukemia | Homo sapiens | CVCL_2187 | ||
| MV4-11 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0064 | ||
| OCI-AML-2 cells | Acute myeloid leukemia | Homo sapiens | CVCL_1619 | ||
| OCI-AML3 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_1844 | ||
| K-562 cells | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | ||
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| UT-7/Epo cells | Acute megakaryoblastic leukemia | Homo sapiens | CVCL_5202 | ||
| SKM-1 cells | Acute myeloid leukemia | Homo sapiens | CVCL_0098 | ||
| NB4 cells | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | ||
| Kasumi-1 cells | Acute myeloid leukemia | Homo sapiens | CVCL_0589 | ||
| In Vivo Model |
Xenograft tumors were generated by randomly injecting 1 x 106 MOLM14 shCTRL or shSLC7A11 cells into the tail veins of NOD/SCID IL-2 receptor g-chain-null mice (NSG) aged 6-9 weeks. Fourteen days after injection, doxycycline (200 mg/mL) and sucrose (1% weight:volume) were added to the drinking water of these animals. After 3 days, the mice were randomly treated with a daily intraperitoneal injection of APR-246 (100 mg/kg) or vehicle (phosphate-buffered saline [PBS]) for 4 days.
Click to Show/Hide
|
||||
| Response Description | APR-246 is a promising new therapeutic agent that targets p53 mutated proteins in myelodysplastic syndromes and in acute myeloid leukemia (AML). The association of APR-246 with induction of ferroptosis (either by pharmacological compounds, or genetic inactivation of SLC7A11 or GPX4) had a synergistic effect on the promotion of cell death, both in vivo and ex vivo. | ||||
Baicalin
[Terminated]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [162] | ||||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| 143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | ||
| hBMMSCs (Human bone marrow mesenchymal stem cells) | |||||
| In Vivo Model |
A total of 24 BALB/c-nude mice (4-5 weeks old) were purchased and MG63 cells were injected into the right tibial bone marrow cavity of mice in a volume of 1 x 106/100 ul. When the tumor volume was visible, all animals were randomly divided into four groups (n = 6): the control (10% DMSO + 40% PEG300 + 5% Tween-80 + 45% Saline) group, the baicalin (200 mg/kg/day) group, the Fer-1 (0.8 mg/kg/day) group and Fer-1 + baicalin group. The baicalin and Fer-1 were intraperitoneally administered every day for two consecutive weeks and tumor sizes were measured every two days.
Click to Show/Hide
|
||||
| Response Description | By promoting the Fe accumulation, ROS formation, MDA production and suppressing the ratio of GSH/GSSG, baicalin was found to trigger ferroptosis in Osteosarcoma and ferroptosis inhibitor ferrostatin-1 (Fer-1) successfully reversed these suppressive effects, indicating that ferroptosis participated in the baicalin mediated anti-OS activity. Mechanistically, baicalin physically interacted with Nrf2, a critical regulator of ferroptosis, and influenced its stability via inducing ubiquitin degradation, which suppressed the Nrf2 downstream targets GPX4 and xCT expression, and led to stimulating ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [218] | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In Vivo Model |
A total of 60 male C57BL/6 mice (10weeks old, 25-28g) were purchased from Guangzhou University of Chinese Medicine Experimental Animal Center (Guangzhou, China). The mice were maintained with enough food and water at 24, 60% relative humidity and 12/12h light/dark cycle. The mice were randomly divided into three groups: sham operation group (Sham), ICH model group (Mod) and baicalin group (Bai) (n = 20/group). Baicalin was suspended in 0.5% carboxymethylcellulose sodium solution. Given the extremely low solubility of baicalin, the concentration of baicalin solution was 0.5 mg/ml. To achieve 20 mg/kg/day dosage, the baicalin solution was administered to the mice in the Bai group by oral route twice at an interval of 1 h within 2 h after ICH injury onset. The remaining two groups received an equal volumes of saline through oral gavage. Since the second day after ICH, mice in the Bai group received 20 mg/kg of baicalin solution while those in the remaining two groups received equal volumes of saline once a day for three consecutive days.
Click to Show/Hide
|
||||
| Response Description | Baicalin significantly increased the mRNA expression of GPX4 and SLC7A11 in the perihematoma brain tissues of intracerebral hemorrhage (ICH) model mice. Baicalin can inhibit the development of ferroptosis in ICH. Baicalin is a potential therapeutic drug for ICH treatment. | ||||
1,2-Dioxolane
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [163] | |||
| Responsed Disease | Fibrosarcoma [ICD-11: 2B53] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 |
| BJ1-hTERT cells | Normal | Homo sapiens | CVCL_6573 | |
| BJ-eLR (Human fibroblast cancer cells) | ||||
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| Response Description | FINO2 is an endoperoxide-containing 1,2-dioxolane that can initiate ferroptosis selectively in engineered cancer cells. FINO2 both indirectly inhibits GPX4 enzymatic function and directly oxidizes iron, ultimately causing widespread lipid peroxidation in Fibrosarcoma. | |||
Berberine
[Investigative]
| In total 3 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [164] | ||||
| Responsed Disease | Gastrointestinal cancer [ICD-11: 2B5B] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| TMK-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4384 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
Five-week-old male BALB/c mice were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained in a pathogen-free animal facility under a 12 h light/dark cycle in a temperature (22 )- and humidity-controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following the current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval no. 12924, 5 November 2020). Animals were acclimated to their housing for seven days before the start of the experiment. For the peritoneal dissemination tumor model, CT26 cancer cells (1 x 107 in 0.2 mL per mouse) were injected into the mouse peritoneal cavity. To measure tumor weight, mice were euthanized on Day 12 and the tumors were excised, while the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, with gross removal of non-tumor tissues. The largest tumor was formed on the diaphragm, and paraffin-embedded sections of the excised diaphragmatic tumor were prepared and stained with hematoxylin-eosin. BBR was diluted with distilled water to produce a final concentration of 48 mg/mL. The solutions were ultrasonically treated for 1 h, and fully vortexed for 30 min. BBR solution was administered by free drinking. The intake calculated from the amount of water consumed was 15.2 mg/kg body weight/day.
Click to Show/Hide
|
||||
| Response Description | Berberine induces apoptosis and ferroptosis by inhibiting mitochondrial complex I and promoting autophagy, leading to combined cell death in the GIC and suppressing stemness. BBR induces cell death in gastrointestinal cancer cells accompanied by increased mitochondrial superoxide and ACSL4 levels, decreased SLC7A11, and impaired antioxidant mechanisms, indicated by decreased GPX4 expression and decreased GSH. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [205] | ||||
| Responsed Disease | Diabetes mellitus [ICD-11: 5A10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Response Description | Berberine (BBR) stimulated GPX4 expression to reduce the content of Fe2+ and ROS, thereby repressing the ferroptosis of islet cells in diabetes mellitus, which functioned similarly as ferroptosis inhibitor Fer-1. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Response of This Regulator | [230] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response Description | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
Cucurbitacin B
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [165] | ||||
| Responsed Disease | Nasopharyngeal cancer [ICD-11: 2B6B] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| CNE1 cells | Nasopharyngeal carcinoma | Homo sapiens | CVCL_6888 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| H157 cells | Oral cavity Squamous cell carcinoma | Homo sapiens | CVCL_2458 | ||
| HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | ||
| In Vivo Model |
The animal experiment was performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University. BALB/c nude mice (5 weeks old, female, Guangdong Medical Laboratory Animal Centre, China) were used for animal experiments. Approximately 4 million CNE1 cells were injected subcutaneously into the right flank of each mouse. Palpable solid tumours developed within a month after tumour cell inoculation, and mice were randomly allocated to four different groups (five mice /group) as follows: control (PBS) group, CuB treatment groups [0.5 mg/kg (low-dose) group and 1 mg/kg (high-dose) group] and gemcitabine (GEM, 25 mg/kg) group. Mice received intraperitoneal injections of PBS, CuB, and gemcitabine 3 times weekly.
Click to Show/Hide
|
||||
| Response Description | Cucurbitacin B caused intracellular accumulation of iron ions and depletion of glutathione. Detailed molecular mechanism investigation confirmed that CuB both induced widespread lipid peroxidation and downregulated the expression of GPX4, ultimately initiating a multipronged mechanism of ferroptosis. The study highlighted the therapeutic potential of CuB as a ferroptosis-inducing agent for nasopharyngeal cancer. | ||||
5-aminolevulinic acid
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [166] | ||||
| Responsed Disease | Oesophageal cancer [ICD-11: 2B70] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | KYSE30 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1351 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
KYSE30 cells were subcutaneously inoculated with 5 x 106 cells per site into both flanks on day 0. At 1 week after transplantation, tumor-bearing mice were randomly assigned to one of the following three groups: (1) saline as a control, (2) 10 mg/kg/day of 5-ALA, or (3) 30 mg/kg/day of 5-ALA. The treatment groups were orally administered 5-ALA once daily for 4 weeks, and the control group was orally administered saline during the same period.
Click to Show/Hide
|
||||
| Response Description | Modulation of GPX4 and HMOX1 by 5-aminolevulinic acid (5-ALA) induced ferroptosis in esophageal squamous cell carcinoma (ESCC). Furthermore, 5-ALA led to an increase in lipid peroxidation and exerted an antitumor effect in various cancer cell lines, which was inhibited by ferrostatin-1. Thus, 5-ALA could be a promising new therapeutic agent for ESCC. | ||||
Atranorin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [167] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hGCCs (Gastric cancer cells) | ||||
| In Vivo Model |
NOD-scid mice (NOD.CB17-Prkdcscid/NcrCrl) aged 6-7 weeks and weighing 20-22 g were used in the experiment. The animal study was performed at the Shanghai University of Traditional Chinese Medicine with approval from the Institutional Animal Care and Use Committee in accordance with the institutional guidelines. All mice were randomly divided into two groups, and each group consisted of four mice. In experimental group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul Atranorin@SPION (10 mg/kg) every 2 days. In control group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul SPION (10 mg/kg) alone every 2 days. After 2 months, the mice were sacrificed, and their tumors were excised.
Click to Show/Hide
|
||||
| Response Description | Atranorin@SPIONs significantly reduced the 5-hydroxymethylcytidine modification level of GPX4 and SLC7A11 mRNA 3' untranslated region in gastric cancer cells. This study revealed the molecular biological mechanism by which Atranorin@SPION inhibit the in vitro and in vivo activity of GCSCs, that is, Atranorin@SPION reduced the expression of members of the Xc-/GPX4 axis and reduced their mRNA 5-hydroxymethylcytidine modification, finally induced ferroptosis of GCSCs. | ||||
Thioguanine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [168] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| In Vivo Model |
Female BALB/c nude mice (6-7 weeks, 17-18 g) were purchased from Hunan Slack Scene of Laboratory Animal Co., Ltd. and used to establish the xenograft mouse model with 5 x 106 exponentially growing MGC-803 cells inoculated subcutaneously into the right forelimb for each mouse. Once the volume of tumors reached 100 mm3, the mice were divided into 3 groups: solvent control; 6-TG (10 mg/kg/day); 6-TG (10 mg/kg/day) + Fer-1(50 mg/kg/day).
Click to Show/Hide
|
||||
| Response Description | 6-Thioguanine was identified as a potential ferroptosis inducer in gastric cancer cells for the first time. It could inactivate system xc, block the generation of GSH, down-regulate the expression of GPX4, increase the level of Lipid ROS, and finally trigger the Fe-2+-mediated ferroptosis in MGC-803 and AGS cell lines. | ||||
XN4
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [169] | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 |
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | |
| Response Description | The pro-ferroptotic role of XN4 in gastric cancer (GC) might enable it to become a promising drug for GC treatment in the future despite the need for extensive research. Moreover, GPX4 levels decreased, but NOX4 and ferroptosis-related protein PTGS2 levels increased in GC cells following XN4 treatment, which was nullified by NOX4 knockdown. | |||
(6R,6aR,9S,11bS,14R)-4,4-Dimethyl-8-methylene-7,11,12-trioxododecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-14-yl valinate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [170] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | ||
| In Vivo Model |
Male nude mice (ages 6-8 weeks) used in the studies were purchased from Hunan SJA Laboratory Animal Co. (Changsha, China). Male nude mice were subcutaneously injected with MGC-803cells into the right flank of mouse. Once the tumor volume reached 100-200 mm3, mice were randomly divided into 5 groups (6 mice/group) and administered with saline,a2(5, 10, and 20 mg/kg), or 5-fluorouracil (5-FU, 15mg/kg) once a day for 21 daysviatail vein injection.
Click to Show/Hide
|
||||
| Response Description | (6R,6aR,9S,11bS,14R)-4,4-Dimethyl-8-methylene-7,11,12-trioxododecahydro-1H-6,11b-(epoxymethano)-6a,9-methanocyclohepta[a]naphthalen-14-yl valinate (a2), a new JDA derivative, inhibited the growth of gastric cancer cells. Importantly, compounda2decreased GPX4 expression and overexpressing GPX4 antagonized the anti-proliferative activity ofa2. Furthermore, a2caused ferrous iron accumulation through the autophagy pathway, prevention of which rescueda2induced ferrous iron elevation and cell growth inhibition. | ||||
Actinidia chinensis Planch
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [171] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| In Vitro Model | HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| In Vivo Model |
Wild type AB strain of zebrafish (Danio rerio) was obtained from Southern Medical University. The HGC-27 cells labeled with EGFP were resuspended in PBS in the concentration of 5*107/ml. 10 nl cell suspension containing approximately 300 cells were loaded into capillary needles and injected into the abdominal perivitelline space of zebrafish embryos by a nanoliter injector (Narishige, Tokyo, Japan). After injection, the tumor-bearing embryos were transferred into a 24-well plate and acclimated in embryo water at 35 for 24 h and then incubated at 0, 90, 180 mg/ml ACP decoction for 48 h.
Click to Show/Hide
|
||||
| Response Description | Actinidia chinensis Planch (ACP) increased the accumulation of ROS via inhibited the glutathione peroxidase 4 (GPx4) and xCT (SLC7A11) proteins, while were inhibited by Ferrostatin-1 (Fer-1) significantly. In conclusion, ACP was a promising antineoplastic agent for the treatment of gastric cancer by regulating apoptosis, ferroptosis and mesenchymal phenotype. | ||||
Polyphyllin B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [172] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | NUGC-3 cells | Gastric carcinoma | Homo sapiens | CVCL_1612 | |
| MKN-1 cells | Gastric carcinoma | Homo sapiens | CVCL_1415 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| In Vivo Model |
The nude mice were raised in our laboratory for a week before the experiment. Then, 5 x 106 MKN-1 cells were subcutaneously injected to establish the subcutaneous xenograft tumour model in nude mice. When the maximum diameter of the xenograft tumours grew steadily to 1 cm, they were dissected completely and cut into 1 mm3 tissue fragments. Then, the tissue fragment was inserted into the surface of the serosa on the greater curvature of the stomach. Different doses of PB (2.5 mg/kg or 5.0 mg/kg) were given by intraperitoneal injection once a day for 3 weeks. The control group was given the same volume of vehicle. The positive control group was given 5-Fu at the dose of 10 mg/kg. The body weight and tumour size of nude mice were recorded. Mice were administered fluorescein substrate (150 mg/kg) intraperitoneally for in vivo imaging twice a week on a Xenogen IVIS 200 imaging system (Caliper Life Sciences, USA). The tumour inhibition rate was analysed using LT Living Image 4.3 Software.
Click to Show/Hide
|
||||
| Response Description | We identified a novel GPx4 inhibitor, polyphyllin B (PB), which can induce ferroptosis by down-regulating GPx4 expression in gastric cancer (GC) cells. It has also been shown to inhibit cell proliferation, suppress invasion and migration, induce apoptosis, and block the cell cycle progression in GC cellsin vitro. Then, immunofluorescence and western blotting assay confirmed that PB can regulate the expression of LC3B, TFR1, NOCA4 and FTH1in vitro, which suggested that suggest that PB may increase the level of Fe2+by transporting Fe3+into the cell by TFR1 and promoting NCOA4-dependent iron autophagy. | ||||
Honokiol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [173] | ||||
| Responsed Disease | Colon cancer [ICD-11: 2B90] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SW48 cells | Colon adenocarcinoma | Homo sapiens | CVCL_1724 | |
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| LS174T cells | Colon adenocarcinoma | Homo sapiens | CVCL_1384 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | ||
| RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Ten BALB/c nude mice (male, 4 weeks old, 18.0 ± 2.0 g) were randomly divided into two groups for the in vivo xenograft assay. Mice were injected with 5 x 106 RKO cells with stable overexpression GPX4 (described Lv-GPX4 group), control vector (described Lv-NC group). Cells were subcutaneously injected into the right anterior axilla of mice in both groups. Mice then received HNK (0.5 mg/kg/w) by intraperitoneal injection for 4 weeks. The subcutaneous tumor volumes in the nude mice in the two groups were recorded every two days.
Click to Show/Hide
|
||||
| Response Description | Honokiol reduced the viability of Colon cancer (CC) cell lines by increasing ROS and Fe2+levels. HNK decreased the activity of Glutathione Peroxidase 4 (GPX4) but did not affect system Xc-. Thus, HNK can induce ferroptosis in CC cells by reducing the activity of GPX4. | ||||
Curcumin
[Investigative]
| In total 4 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [174] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response Description | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [204] | ||||
| Responsed Disease | Thyroid cancer [ICD-11: 2D10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | |
| Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | ||
| FTC 238 cells | Thyroid gland follicular carcinoma | Homo sapiens | CVCL_2447 | ||
| Response Description | Knockdown of HO-1 inhibits ferroptosis by upregulating the GPX4 expression in follicular thyroid cancer cells. We conclude that curcumin inhibits the tumorigenesis of follicular thyroid cancer via HO-1-induced activation of the ferroptosis signalling pathway. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Response of This Regulator | [232] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Two-month-old male New Zealand rabbits purchased from the Medical Experimental Animal Center of Bengbu Medical College were used as experimental subjects. Streptozotocin was dissolved in sterile saline and intraperitoneally injected into the rabbits at a dose of 80 mg/kg. The rabbits were allowed to eat freely after receiving the injection. The fasting blood glucose levels of the rabbits were monitored regularly. The diabetic rabbit model was considered successfully established when the fasting blood glucose level was measured as 11 mmol/L twice or 14 mmol/L once. Following successful modelling, grouping was performed as follows: blank control group (Con-Group), diabetic rabbit group (DM-Group), diabetic rabbit + every other day curcumin administration group (Qod-Group), and diabetic rabbit + daily administration group (Qd-Group).
Click to Show/Hide
|
||||
| Response Description | Curcumin can promote the nuclear translocation of Nrf2, increase the expression of oxidative scavenging factors, such as HO-1, reduce excessive Gpx4 loss, and inhibit glucose-induced ferroptosis in cardiomyocytes. This highlights a potentially new therapeutic route for investigation for the treatment diabetic cardiomyopathy. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Drug Response of This Regulator | [243] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| rPTs (Rat pancreas tissues) | |||||
| rHTs (Rat hippocampal tissues) | |||||
| In Vivo Model |
Forty female albino Wistar rats weighing 180-220 g were used in the study. Eight rats in each group were randomly assigned to five different groups: Group I (Sham); Group II (IR); Group III (IR + DMSO); Group IV (IR + Curcumin 100 mg/kg); and Group V (IR + 2 ug/kg LoxBlock-1) were determined. The animals were maintained at a temperature of 21 ± 2 and regulated humidity conditions (50 ± 5%) with a twelve-hour light/dark cycle. Throughout the experiment, the animals were fed standard commercial rat pellets and given tap water. All surgical and anesthesia procedures were performed understerile conditions. In addition, in a case of abnormal symptoms, the animals would be removed from the group and sacrificed under deep anesthesia.
Click to Show/Hide
|
||||
| Response Description | Curcumin attenuates liver, pancreas and cardiac ferroptosis, oxidative stress and injury in ischemia/reperfusion-damaged rats by facilitating ACSL/GPx4 signaling. | ||||
Andrographis
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [174] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response Description | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
Wogonin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [176] | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| AsPC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0152 | ||
| HPDE6-C7 cells | Normal | Homo sapiens | CVCL_0P38 | ||
| In Vivo Model |
Female BALB/c nude mice (5 weeks old) were procured from Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd (Zhejiang, China) and given 5 days to acclimate to their surroundings. PANC-1 cells (1 x 107) in 100 uL PBS at the logarithmic growth phase were administered to mice subcutaneously in the left flank. The mice were treated with indicated treatments after nearly 10 days when the tumour size was approximately 1,000 mm3. In the control group, mice (n = 5) received intraperitoneal injections of the vehicle. In the treatment group, the mice (n = 5) were administered 50 uL of 60 mg/kg body weight of wogonin once a day for 12 days. A slide calliper size was used to measure the tumour size. The equation for calculating tumour volume is as follows: tumour volume = AB2/2, wherein A is the length, and B is the width of the tumour. The mice were sacrificed the next day after the treatment procedure was complete by cervical dislocation. The tumour tissues were harvested and snap-frozen using liquid nitrogen for subsequent analyses.
Click to Show/Hide
|
||||
| Response Description | Wogonin could significantly reduces pancreatic cancer cell proliferation and induce ferroptosisviathe Nrf2/GPX4 axis. Therefore, wogonin could be potentially used for treating patients with pancreatic cancer. | ||||
QD394-Me
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [177] | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| In Vivo Model |
Female Balb/c mice were purchased from Envigo. At the time of implantation, all mice were aged 5-6 weeks. Mice were implanted subcutaneously in the right flank with 1 x 106 CT-26 cells in 100 uL DPBS. Seven days after implantation, mice were randomized into groups (n = 5) with mean tumor volumes ranging from 97 to 117 mm3. The negative control group was dosed daily in the intraperitoneal cavity (IP) with the same vehicle used for QD394. QD394 was dosed at 10 mg/kg IP, and QD394-Me was dosed 3 times weekly intravenously (IV) at 20 mg/kg.
Click to Show/Hide
|
||||
| Response Description | QD394 causes an iron- and ROS-dependent, GPX4 mediated cell death, suggesting ferroptosis as a major mechanism. Importantly, QD394 decreases the expression of LRPPRC and PNPT1. Pharmacokinetics-guided lead optimization resulted in the derivative QD394-Me, which showed improved plasma stability and reduced toxicity in mice compared to QD394. Overall, QD394 and QD394-Me represent novel ROS-inducing drug-like compounds warranting further development for the treatment of pancreatic cancer. | ||||
QD394
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [177] | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| In Vivo Model |
Female Balb/c mice were purchased from Envigo. At the time of implantation, all mice were aged 5-6 weeks. Mice were implanted subcutaneously in the right flank with 1 x 106 CT-26 cells in 100 uL DPBS. Seven days after implantation, mice were randomized into groups (n = 5) with mean tumor volumes ranging from 97 to 117 mm3. The negative control group was dosed daily in the intraperitoneal cavity (IP) with the same vehicle used for QD394. QD394 was dosed at 10 mg/kg IP, and QD394-Me was dosed 3 times weekly intravenously (IV) at 20 mg/kg.
Click to Show/Hide
|
||||
| Response Description | QD394 causes an iron- and ROS-dependent, GPX4 mediated cell death, suggesting ferroptosis as a major mechanism. Importantly, QD394 decreases the expression of LRPPRC and PNPT1. Pharmacokinetics-guided lead optimization resulted in the derivative QD394-Me, which showed improved plasma stability and reduced toxicity in mice compared to QD394. Overall, QD394 and QD394-Me represent novel ROS-inducing drug-like compounds warranting further development for the treatment of pancreatic cancer. | ||||
Solasonine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [178] | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| HepaRG cells | Hepatocellular carcinoma | Homo sapiens | CVCL_9720 | ||
| In Vivo Model |
BALB/c nude mice aged 4-6 weeks, weighing 15~20 g, were purchased from Shanghai SLAC Laboratory Animal Co.,Ltd (Shanghai, China). Following acclimation, the right flank of each experimental mouse was subcutaneously injected with HepG2 cells (2 x 106) suspended in PBS (200 uL) and then randomly assigned to: (i) the control group and received no further treatment or (ii) the intervention group and received solasonine (50 mg/kg body weight) in an equal volume of PBS. Tumor volumes were measured every 5 days. After 30 days, the mice were sacrificed and the tumors were resected, weighed, and processed for histological analysis.
Click to Show/Hide
|
||||
| Response Description | Solasonine increased lipid ROS levels in HepG2 cells by suppression of GPX4 and GSS. However, the use of a ferroptosis inhibitor reversed solasonine-induced ROS production and cell apoptosis. Taken together, solasonine promotes ferroptosis of hepatocellular carcinoma cells via GPX4-induced destruction of the glutathione redox system. | ||||
l-Buthionine sulfoximine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [179] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response Description | Auranofin/buthionine sulfoxime (BSO) and Erastin/BSO cotreatment alters redox homeostasis by increasing levels of Nrf2 and HO-1 and decreasing GPX4 levels. Targeting these two main ferroptotic pathways simultaneously can overcome chemotherapy resistance in hepatocellular carcinoma (HCC). | |||
Atractylodin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [180] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
| In Vitro Model | Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hccm (Human hepatocellular carcinoma cells) | ||||
| Response Description | Atractylodin can inhibit the proliferation, migration, and invasion of Huh7 and Hccm liver cancer cells, and induce cell apoptosis and cell cycle arrest. In addition, atractylodin may induce ferroptosis in hepatocellular carcinoma cells by inhibiting the expression of GPX4 and FTL proteins, and up-regulating the expression of ACSL4 and TFR1 proteins. | |||
Auranofin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [179] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response Description | Auranofin/buthionine sulfoxime (BSO) and Erastin/BSO cotreatment alters redox homeostasis by increasing levels of Nrf2 and HO-1 and decreasing GPX4 levels. Targeting these two main ferroptotic pathways simultaneously can overcome chemotherapy resistance in hepatocellular carcinoma (HCC). | |||
Erastin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [179] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response Description | Auranofin/buthionine sulfoxime (BSO) and Erastin/BSO cotreatment alters redox homeostasis by increasing levels of Nrf2 and HO-1 and decreasing GPX4 levels. Targeting these two main ferroptotic pathways simultaneously can overcome chemotherapy resistance in hepatocellular carcinoma (HCC). | |||
Seco-Lupane Triterpene Derivatives
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [181] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Response Description | A new seco-lupane triterpene derivative, compound21, was found to regulate cell growth through the cell cycle and ferroptosis, which in turn inhibited the proliferation, migration, and invasion of HepG2 cells. And it was found that compound 21 significantly upregulated ACSL4 protein expression and downregulated GPX4 protein expression. It has the potential to become an effective new drug for the treatment of hepatocellular carcinoma. | |||
2-pyridylhydrazone dithiocarbamate s-acetic acid
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [182] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| Cell apoptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Response Description | 2-pyridylhydrazone dithiocarbamate s-acetic acid (PdtaA) induced both apoptosis and cell cycle arrest. Notably, PdtaA also induced ferroptosis via downregulation of GPx4 and xCT in liver cancer cells. Autophagy inhibitor 3-methyladenin or genetic knockdown of NCOA4 was employed to inhibit ferritinophagy, which significantly neutralized the action of PdtaA in both apoptosis and ferroptosis. | |||
Ginkgetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [184] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SPC-A1 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | ||
| In Vivo Model |
Briefly, when tumours on transplanted nude mice reached around 100 mm3, the mice were randomized divided into eight groups: control, ginkgetin, DDP, ginkgetin + DDP, UAMC 3203, ginkgetin + UAMC 3203, DDP + UAMC 3203, ginkgetin + DDP + UAMC 3203. Both DDP (3 mg/kg) and ginkgetin (30 mg/kg) were administered by intraperitoneal injection, with 2 - 3 times per week and once per day, respectively. UAMC 3203 (10 mg/kg) was administered 5 days/week by intraperitoneally injection. Tumour size and body weight were measured 3 times per week. After dosing 31 days, the nude mice were sacrificed, and tumours were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | The induction of ferroptosis mediated by ginkgetin was further confirmed by the decreased expression of SLC7A11 and GPX4, and a decreased GSH/GSSG ratio. Simultaneously, ginkgetin disrupted redox hemostasis in DDP-treated cells, as demonstrated by the enhanced ROS formation and inactivation of the Nrf2/HO-1 axis. Ginkgetin also enhanced DDP-induced mitochondrial membrane potential (MMP) loss and apoptosis in cultured non-small cell lung cancer (NSCLC) cells. | ||||
Gefitinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [185] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRASmutant non-small-cell lung cancer (NSCLC) cells. | ||||
Betulin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [185] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRASmutant non-small-cell lung cancer (NSCLC) cells. | ||||
Capsaicin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [186] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 | |
| Response Description | Capsaicin inhibited the proliferation of A549 and NCI-H23 cells and induced ferroptosis by inactivating SLC7A11/GPX4 signaling. Capsaicin could be used as a potential anticancer agent in the treatment of non-small cell lung cancer (NSCLC). | |||
Orlistat
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [188] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| AMPK signaling pathway | hsa04152 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| LL/2 (LLC1) cells | Lung cancer | Mus musculus | CVCL_4358 | ||
| In Vivo Model |
C57BL/6 mice were anesthetized, and 5 x 105 LLC cells were implanted subcutaneously into the right flank. Five days post-implant, mice were randomized and assigned into two groups and treated with orlistat (10 mg/kg, intraperitoneal injection) or PBS daily for 14 days. The tumor volume was measured twice a week with a caliper, and the tumor volume was calculated according to the formula ((length x width2 )/2).
Click to Show/Hide
|
||||
| Response Description | Orlistat, as a single agent, inhibited the proliferation and viabilities of lung cancer cells and induced ferroptosis-like cell death in vitro. Mechanistically, we found that orlistat reduced the expression of GPX4, a central ferroptosis regulator, and induced lipid peroxidation. | ||||
[4-[Bis(4-chlorophenyl)methyl]piperazin-1-yl]-(5-methyl-4-nitro-1,2-oxazol-3-yl)methanone
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [189] | |||
| Responsed Disease | Melanoma [ICD-11: 2C30] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | LOX-IMVI cells | Melanoma | Homo sapiens | CVCL_1381 |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| HEK293-EBNA1-6E cells | Normal | Homo sapiens | CVCL_HF20 | |
| CJM cells | Melanoma | Homo sapiens | CVCL_U797 | |
| WM88 cells | Melanoma | Homo sapiens | CVCL_6805 | |
| KP-4 cells | Pancreatic carcinoma | Homo sapiens | CVCL_1338 | |
| HCC4006 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1269 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | |
| Caki-2 cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_0235 | |
| Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | |
| MC-38 cells | Colon adenocarcinoma | Homo sapiens | CVCL_B288 | |
| Response Description | ML210 is a prodrug that is converted in cells into a nitrile-oxide electrophile that covalently inhibits GPX4 with remarkable proteome-wide selectivity in melanoma. | |||
DETD-35
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [190] | |||
| Responsed Disease | Melanoma [ICD-11: 2C30] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HaCaT cells | Normal | Homo sapiens | CVCL_0038 |
| CCD-966Sk cells | Normal | Homo sapiens | CVCL_U267 | |
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | |
| A2058 cells | Amelanotic melanoma | Homo sapiens | CVCL_1059 | |
| SK-MEL-2 cells (MEK inhibitor-resistant) cells | Melanoma | Homo sapiens | CVCL_0069 | |
| Response Description | Sesquiterpene lactones DET and DETD-35 significantly reprogram this metabolic adaptation and inhibit GPX4 activity to disturb glutathione metabolism and induce ferroptosis. Targeting ferroptosis and GPX4 could be a novel approach to cope with drug resistance in melanoma cancers. | |||
DET
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [190] | |||
| Responsed Disease | Melanoma [ICD-11: 2C30] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HaCaT cells | Normal | Homo sapiens | CVCL_0038 |
| CCD-966Sk cells | Normal | Homo sapiens | CVCL_U267 | |
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | |
| A2058 cells | Amelanotic melanoma | Homo sapiens | CVCL_1059 | |
| SK-MEL-2 cells (MEK inhibitor-resistant) cells | Melanoma | Homo sapiens | CVCL_0069 | |
| Response Description | Sesquiterpene lactones DET and DETD-35 significantly reprogram this metabolic adaptation and inhibit GPX4 activity to disturb glutathione metabolism and induce ferroptosis. Targeting ferroptosis and GPX4 could be a novel approach to cope with drug resistance in melanoma cancers. | |||
Lycium barbarumpolysaccharide
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [191] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Response Description | Lycium barbarum polysaccharide (LBP) effectively inhibited proliferation of breast cancer cells and promoted ferroptosis by modulation of the xCT/GPX4 pathway. GPX4 inactivity and repression of SLC7A11 (the gene for xCT) result in ROS accumulation, thereby modulating ferroptosis. | |||
Etoposide
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [192] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 |
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Response Description | The combined treatment of etoposide and erastin synergistically induced oxidative stress and lipid peroxidation, while suppressing glutathione peroxidase activity in breast cancer cells. More importantly, the combination treatment synergistically increased iron accumulation, which was associated with altered expression of IREB2/FPN1. Additionally, ferroptosis-regulating proteins ACSF2 and GPX4 were altered more potently by the combination treatment, compared to untreated cells and erastin treatment alone (p<0.05). | |||
Tubastatin A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [193] | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| In Vivo Model |
Female 5-week-old athymic nude mice were obtained from Sun Yat-sen University. All mice were kept under specific-pathogen free conditions in the animal facility of Sun Yat-sen University Cancer Centre. Cancer cells were suspended and counted in 1 x DMEM, and 2 x 106 MDA-MB-231 cells were injected into mice subcutaneously. When the tumours reached 50-100 mm3, the mice were randomly assigned to different treatment groups. Tumours were irradiated with a JL Shepherd Mark I-68A irradiator at a dose of 10 Gy. Tub was dissolved in solvent containing 1% DMSO, 30% polyethylene glycol, 1% Tween 80 and 68% H2O and then subcutaneously administered to mice at a dose of 2.5 mg/kg once a day. Lipro-1 diluted in PBS was intraperitoneally injected daily at a dose of 10 mg/kg. Tub or Lipro-1 was administered three times before irradiation followed by continued daily administration until the endpoint, as indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Tubastatin A (Tub) as a novel GPX4 inhibitor that induced ferroptosis through large-scale drug screening. We showed that IR-mediated GPX4 expression restrained ferroptosis to drive radioresistance in breast cancer. | ||||
Apatinib
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [194] | |||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | |
| Response Description | Apatinib combined with olaparib-induced ferroptosis via a p53-dependent manner in ovarian cancer. Further studies showed that apatinib combined with olaparib-induced ferroptosis by inhibiting the expression of Nrf2 and autophagy, thereby inhibiting the expression of GPX4. The Nrf2 activator RTA408 and the autophagy activator rapamycin rescued the combination drug-induced ferroptosis. | |||
Triptolide
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [195] | ||||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A2780/DDP cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_D619 | |
| In Vivo Model |
All female BALB/cnude mice(4-6 weeks old, 15-20 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). They were raised in specific pathogen-free conditions and allowed to access sterile water and food freely. A2780/DDP cell suspension (100 uL) with a density of 1 x 107 cells/mL was injected subcutaneously into the axilla of the mice. After observing the nude mice for a week, it was confirmed that subcutaneous A2780/DDP cells were inoculated successfully. Sterile saline (100 uL) was injected into the abdominal cavity of the nude mice in the control group for 14 days. The mice in the DDP treatment group were given DDP (4 mg/kg/day) intraperitoneally on the first and eighth days. TG (100 uL, 1 mg/kg) diluted with sterile physiological saline were injected into the abdominal cavity of the nude mice in the TG treatment group for 14 days. In addition, the nude mice in the TG + DDP treatment group were given TG (100 uL, 1 mg/kg) for 14 days and DDP (4 mg/kg/day) intraperitoneally on the first and eighth days.
Click to Show/Hide
|
||||
| Response Description | Tripterygium (TG) can effectively inhibit the proliferation of drug-resistant ovarian tumor cells A2780/DDP and increase the sensitivity to cisplatin chemotherapy both invitro and invivo. In terms of mechanism, TG induces ferroptosis by targeting the NRF2/GPX4 signal axis to weaken the antioxidant capacity of cancer cells. | ||||
Olaparib
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [194] | |||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | |
| Response Description | Apatinib combined with olaparib-induced ferroptosis via a p53-dependent manner in ovarian cancer. Further studies showed that apatinib combined with olaparib-induced ferroptosis by inhibiting the expression of Nrf2 and autophagy, thereby inhibiting the expression of GPX4. The Nrf2 activator RTA408 and the autophagy activator rapamycin rescued the combination drug-induced ferroptosis. | |||
Norcantharidin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [196] | ||||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Athymic nu/nu female mice aged 6-8 weeks (n = 9; mean weight, 20.21 ± 1.54 g) were purchased from the specific pathogen SPF (Beijing) Lab Animals Technology Co. Ltd. Mice were housed in a temperature- and humidity-controlled environment (20-24 , 45-55% humidity), with free access to food and water and in groups of three. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC ID: 17-3256) at Nantong University and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Nuclear factor erythroid 2-related factor 2 (NRF2), heme oxygenase 1 (HO-1), glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (xCT) expression levels were significantly decreased following norcantharidin (NCTD) treatment. Collectively, NCTD may represent a potent anticancer agent in ovarian cancer cells, and NCTD-induced ferroptotic cell death may be achieved by inhibiting the NRF2/HO-1/GPX4/xCT axis. | ||||
Buthionine sulfoximine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [198] | |||
| Responsed Disease | Prostate cancer [ICD-11: 2C82] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | VCaP cells | Prostate carcinoma | Homo sapiens | CVCL_2235 |
| LNCaP cells | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| LNCaP C4-2 cells | Prostate carcinoma | Homo sapiens | CVCL_4782 | |
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | |
| RWPE-1 cells | Normal | Homo sapiens | CVCL_3791 | |
| MDA-kb2 cells | Breast adenocarcinoma | Homo sapiens | CVCL_6421 | |
| Response Description | ITC-ARi 13 and buthionine sulfoximine (BSO) cooperatively downregulate AR and induce ferroptosis likely through increasing the accessibility of 13/12b to cellular targets, escalating free intracellular ferrous iron and attenuating GSH-centered cellular defense and adaptation. Further studies on the combination of ITC-ARi and GSH synthesis inhibitor could result in a new modality against castration-resistant prostate cancer (CRPC). Collectively, the combination of ITC-ARi 13 and BSO reveals a pro-ferroptotic role of Nrf2 through upregulating HO-1 under GSH-deficient conditions. | |||
Lycorine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [199] | |||
| Responsed Disease | Hereditary Leiomyomatosis [ICD-11: 2C90] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | |
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| Response Description | Lycorine could inhibit the proliferation in human renal cell carcinoma (RCC) cells. The anti-tumor effect of lycorine was associated with the induction of ferroptosis. After lycorine treatment, the expression levels of GPX4 in RCC cells decreased, whereas those of ACSL4 increased. | |||
Tetrachlorobenzoquinone
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [200] | |||
| Responsed Disease | Hereditary Leiomyomatosis [ICD-11: 2C90] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Tetrachlorobenzoquinone (TCBQ)-induced ferroptosis occurred as a result of iron accumulation and inhibition of GPX4 expression. Mechanistically, TCBQ promotes the iron import into cells by improving the expression of TF and TFR1, and the complex of TF and TFR1 is internalized by endocytosis in Adrenal gland pheochromocytoma. | |||
Quinazolinyl-arylurea derivatives 7J
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [201] | |||
| Responsed Disease | Bladder cancer [ICD-11: 2C94] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell autophagy | ||||
| Cell proliferation | ||||
| In Vitro Model | BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| HCC827 cells | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | |
| T24 cells | Bladder carcinoma | Homo sapiens | CVCL_0554 | |
| RT4 cells | Bladder carcinoma | Homo sapiens | CVCL_0036 | |
| Response Description | Compound 7j treatment could trigger three different cell death forms including apoptosis, ferroptosis, and autophagy; which form would occur depended on the concentrations and incubation time of 7j. Ferroptosis and autophagy occurred in the case of higher concentrations combining with extended incubation time through effectively regulating the Sxc-/GPx4/ROS and PI3K/Akt/mTOR/ULK1 pathways, respectively. Compound 7j could be a promising lead for molecular-targeted anti- bladder cancer agents' discovery. | |||
FIN56
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [202] | |||
| Responsed Disease | Bladder cancer [ICD-11: 2C94] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | J82 cells | Bladder carcinoma | Homo sapiens | CVCL_0359 |
| 253J cells | Bladder carcinoma | Homo sapiens | CVCL_7935 | |
| T24 cells | Bladder carcinoma | Homo sapiens | CVCL_0554 | |
| RT-112 cells | Bladder carcinoma | Homo sapiens | CVCL_1670 | |
| mEFs (Mouse embryonic fibroblasts) | ||||
| Response Description | Fin56, a type 3 inducer, leads to ferroptosis mainly by promoting GPX4 degradation. Fin56 induces ferroptosis and autophagy in bladder cancer cells and that Fin56-triggered ferroptosis mechanistically depends on the autophagic machinery. | |||
Ascorbic Acid
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [203] | |||
| Responsed Disease | Thyroid cancer [ICD-11: 2D10] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| Cell proliferation | ||||
| In Vitro Model | 8505C cells | Thyroid gland anaplastic carcinoma | Homo sapiens | CVCL_1054 |
| C643 cells | Thyroid gland anaplastic carcinoma | Homo sapiens | CVCL_5969 | |
| Response Description | Vitamin C could significantly inhibit anaplastic thyroid cancer (ATC) cells growth through ferroptosis activation, evidenced by the GPX4 inactivation, ROS accumulation and iron-dependent lipid peroxidation. | |||
Empagliflozin
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [206] | ||||
| Responsed Disease | Diabetes mellitus [ICD-11: 5A10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| HUVECs (Human umbilical vein endothelial cells) | |||||
| MOVAS-1 cells | Normal | Homo sapiens | CVCL_0F08 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
For diabetes induction, C57BL/6 mice were fed with high fat diet (HFD) for 3 weeks (20% kcal protein, 20% kcal carbohydrate, and 60% kcal fat). Intraperitoneal administration of 60 mg/kg body weight streptozotocin (STZ, Sigma-Aldrich, St Louis, MO, USA) diluted in sodium citrate buffer was then performed for the following six days. Mice were fasted overnight prior to each STZ injection and blood glucose level measurement. Blood glucose level was evaluated using Accu-Check Integra (Roche Diagnostics, Shanghai, China). Mice with blood glucose level above 16.6 mM were assumed as diabetic mice, and were used for establishing diabetic HLI model as described previously.
Click to Show/Hide
|
||||
| Response Description | Empagliflozin, a clinical hypoglycemic gliflozin drug, can inhibit ferroptosis and enhance skeletal muscle cell survival and paracrine function under hyperglycemic condition via restoring the expression of GPX4. This study highlights the potential of intramuscular injection of empagliflozin for treating diabetic hindlimb ischemia. | ||||
Salidroside
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [207] | ||||
| Responsed Disease | Diabetes mellitus [ICD-11: 5A10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| HUVECs (Human umbilical vein endothelial cells) | |||||
| MOVAS-1 cells | Normal | Homo sapiens | CVCL_0F08 | ||
| In Vivo Model |
For diabetes induction, C57BL/6 mice were given a high-fat diet for three weeks that contained 20% protein, 20% carbohydrate, and 60% fat. Sodium citratebuffer-diluted 60 mg/kg body weight streptozotocin (STZ; Sigma-Aldrich, St. Louis, MO) were administered intraperitoneally for the next constitutive six days. Prior to each STZ injection and blood glucose testusing Accu-Check Integra (Roche Diagnostics, Shanghai, China), mice were fasted overnight. Mice with blood glucose levels higher than 16.6 mM were considered diabetic and were utilized to establish the diabetic HLI model.
Click to Show/Hide
|
||||
| Response Description | Salidroside/GPX4-mediated ferroptosis inhibition is crucial for promoting angiogenesis and blood perfusion recovery in diabetic hindlimb ischemia mice. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [214] | ||||
| Responsed Disease | Alzheimer disease [ICD-11: 8A20] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CD8T cells (Mouse CD8+ T cells) | ||||
| In Vivo Model |
SAMP8 mice were employed as an AD model and were treated with salidroside for 12 weeks. Behavioral tests, immunohistochemistry, HE and Nissl staining, immunofluorescence, transmission electron microscopy, quantitative proteomics, bioinformatic analysis, flow cytometry, iron staining,western blotting, andmolecular dockingwere performed.
Click to Show/Hide
|
||||
| Response Description | Salidroside alleviates cognitive impairment and inhibits neuronal ferroptosis in Alzheimer's disease. The underlying mechanisms may involve the Nrf2/GPX4 axis activation and reduction in CD8T cells infiltration. | ||||
Metformin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [208] | ||||
| Responsed Disease | Ovarian dysfunction [ICD-11: 5A80] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
n = 6 blank control group was created. The mice in the control group were fed regular food and gavaged with normal saline daily. The mice in the control group were given a high-fat diet and 1 mg/kg of letrozole via gavage for 21 days to establish a PCOS model of insulin resistance and hyperandrogenism. The mice, after successful modeling, were randomly divided into PCOS group and metformin group (n = 6). During the treatment period, the control group continued to be fed with normal feed and given normal saline; the PCOS group was fed with continuous high-fat feed and given letrozole (1 mg/kg/day) by intragastric administration, and the metformin group was given metformin by intragastric administration (200 mg/kg/day). After 30 days of treatment, the experimental mice were euthanized, serum was collected, one mouse ovary was collected for histological examination, and the other was stored in a -80 refrigerator for molecular biology experimental research.
Click to Show/Hide
|
||||
| Response Description | Morphological results showed that after metformin treatment, polycystic lesions in ovaries were reduced, the ovarian function was restored, and the expressions of SIRT3 and GPX4 were elevated. Metformin could regulate ferroptosis to improve polycystic ovary syndrome via the SIRT3/AMPK/mTOR pathway. | ||||
Atorvastatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [209] | ||||
| Responsed Disease | Obesity [ICD-11: 5B81] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell senescence | |||||
| In Vivo Model |
Mice were sacrificed by cervical dislocation. As described previously, epidydimal adipose tissues (EAT) were isolated and minced into ~5-mg pieces in DMEM containing 10% FBS. After 2 h of incubation, 50 mg of small pieces were placed in serum-free DMEM and exposed to 1 umol/L atorvastatin for 18 h, and 0.1% DMSO served as a control. In specific experiments, EAT explants were also treated with GGPP (50 uM; GlpBio), or ferrostatin-1 (Fer-1, 8 uM), and added to the culture medium at the same time as was atorvastatin. Group animal size was n = 6-8 per group. The exact group size is specially described in the Figure legends.
Click to Show/Hide
|
||||
| Response Description | Atorvastatin decreased the level of GPX4 and depleted GGPP production, but not Fer-1. Atorvastatin was able to induce ferroptosis in adipose tissue, which was due to increased ROS and an increase in cellular senescence. Moreover, this effect could be reversed by the supplement of GGPP. Taken together, our results suggest that the induction of ferroptosis contributed to statin-induced cell senescence in adipose tissue and may contributed to obesity disease. | ||||
Gastrodin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [210] | ||||
| Responsed Disease | Vascular dementia [ICD-11: 6D81] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male Sprague-Dawley rats (weight 260 ± 20 g; Guizhou Medical University Experimental Animal Center; Certificate No. SCXK2018-0001; Grant No. 2200483) were reared in a specific pathogen-free environment with 12 h light/dark cycle and 55% ± 10% humidity at a temperature of 20~25 , were provided with sufficient feed and sterile drinking water and fasted for 6 h before and after surgery. All animal experiments were performed in accordance with the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Gastrodin (GAS) inhibited ferroptosis in hippocampal neurons by activating the Nrf2/Keap1-GPx4 signaling pathway, suggesting its possible application as a functional food for improving vascular dementia by inhibiting ferroptosis. | ||||
Oxidopamine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [211] | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
The AB strain of wild-type zebrafish (Danio rerio) was applied in this study. Zebrafish larvae at 4 dpf (days post-fertilization) were co-incubated with 250 uM 6-OHDA or 1.5 ug/mL nomifensine (Nomi, a dopamine transporter inhibitor) in 6-well plates at a density of 30 zebrafish embryos per group for 2 days and the medium was refreshed every day. The swimming total distance of each fish was recorded for 10 min and was analyzed by an automated video tracking system.
Click to Show/Hide
|
||||
| Response Description | 6-hydroxydopamine (6-OHDA) treatment-induced ferroptosis in SH-SY5Y cells mainly by disturbing the protein expression of GPX4 and ACSL4. Collectively, the activation of the p62-Keap1-Nrf2 pathway prevents 6-OHDA-induced ferroptosis in SH-SY5Y cells, targeting this pathway in combination with a pharmacological inhibitor of ferroptosis can be a potential approach for parkinson's disease therapy. | ||||
Forsythoside A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [212] | ||||
| Responsed Disease | Alzheimer disease [ICD-11: 8A20] | ||||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| Neuro-2a cells | Neuroblastoma | Mus musculus | CVCL_0470 | ||
| BV-2 cells | Normal | Mus musculus | CVCL_0182 | ||
| In Vivo Model |
All animal experiments were approved by the Animal Ethics Committee of Jilin University (permit No. SY201905013) and were conducted in compliance with the ARRIVE guidelines. Eight-month-old B6C3-Tg (APPswePSEN1dE9)/Nju double transgenic male mice (APP/PS1) (genotype: (Appswe) T, (Psen1) T) and age-matched wild-type (WT) (genotype: (Appswe) W, (Psen1) W) male mice were purchased from Nanjing Biomedical Research Institute of Nanjing University. All mice were individually housed at 24 with food and drinking water availablead libitum. After 1 week of adaption in the new environment, WT mice received oral administration of normal saline (10 mL/kg) and were designated as the control group (n = 12). APP/PS1 mice were randomly divided into two groups: the model group (n = 12) received oral administration of normal saline (10 mL/kg) and the agent-treated group (n = 12) received oral treatment with 30 mg/kg FA (L-012-171216, 98.83% purity, Chengdu Herbpurify Co., Ltd., Chengdu, China) beginning on day 8. After 30-day treatment, behavioral experiments were serially performed. The entire treatment protocol lasted for 42 days. Blood samples were collected from the caudal vein. After euthanasia via CO2 inhalation, organs including the brain, liver, spleen, and kidney were collected for further analysis.
Click to Show/Hide
|
||||
| Response Description | Forsythoside A treatment exerted anti-ferroptosis and anti-neuroinflammatory effects in erastin-stimulated HT22 cells, and the Nrf2/GPX4 axis played a key role in these effects. Collectively, these results demonstrate the protective effects of FA and highlight its therapeutic potential as a drug component for AD ( Alzheimer's disease) treatment. | ||||
ginkgolide-B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [213] | ||||
| Responsed Disease | Alzheimer disease [ICD-11: 8A20] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
Male 6-month-old senescence-resistant R1 (SAMR1) and SAMP8 mice (weight, 28-35 g) were purchased from Beijing SPF Biotechnology. First, mice were placed in the center of an empty testing arena (40 x 40 x 40 cm) and allowed to move freely for adaptation. Next, in the training stage, two similar objects were presented in the testing arena and mice were allowed to explore for 10 min, for 3 consecutive days. On day 4, one of the two familiar objects was replaced by a new object. The time of exploring a novel object or familiar object in 10 min was recorded.
Click to Show/Hide
|
||||
| Response Description | Ginkgolide B attenuated Alzheimer's disease (AD)-related cognitive impairment through the regulation of oxidative stress, neuroinflammation and ferroptosis, and that GB-induced protection in AD is dependent on the inhibition of ferroptosis. Furthermore, the involvement of Nrf2/GPX4 pathway-regulated ferroptosis in the GB-related protective effects on the AD mouse model. | ||||
Tetrahydroxy stilbene glycoside
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [215] | ||||
| Responsed Disease | Alzheimer disease [ICD-11: 8A20] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Pathways in cancer | hsa05200 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
APPswe/PSEN1dE9 (APP/PS1) double transgene mice, which were generated by the introduction of human APPswe and PS1-dE9 mutations onto the C57BL/6 background and also wild type (WT) littermates, aged 5 months, were purchased from Beijing HFK Bioscience Co., Ltd. (Beijing, China). The mice received food and water ad libitum under standard husbandry conditions (22-25, 55-65% relative humidity, and 12h/12h lightdark cycle) and acclimated 1 week for the experiments. Mice were randomly divided into 5 groups, including WT control group, APP/PS1 model group, and APP/PS1 + TSG (60, 120 and 180 mg/kg) different dosage groups. Mice were orally treated with TSG every other day for 2 months. WT and model groups were treated with equivalent vehicle.
Click to Show/Hide
|
||||
| Response Description | Tetrahydroxy stilbene glycoside (TSG) promoted the activation of GSH/GPX4/ROS and Keap1/Nrf2/ARE signaling pathways. Notably, markers related to ferroptosis including increased lipid peroxidation, enhanced neuroinflammation such as NLRP3, and also the expression of DMT1, ACSL4 and NCOA4, were reduced by TSG administration. In addition, TSG enhanced antioxidative stress via the upregulation of SOD, and the expression of FTH1, CD98 and xCT. Hence, TSG should be taken into consideration during treatment of Alzheimer's disease in the future. | ||||
Lapatinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [216] | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from the Animal Unit of Central South University. After anesthetization by intraperitoneal injection of 10% chloral hydrate (v/w), the mice were fixed on a stereotactic instrument and stereotactically injected with KA (250 ng/ul) into the hippocampus. KA (1 ul) was injected slowly for 5 min and positioned in the hippocampus (AP-2.0 mm, ML-1.3 mm, V-1.2 mm). After injection, the needle was left in place for additional 10 min to avoid drug reflux. The mice were randomly divided into six experimental groups: 1) sham operation group that received 1 ul PBS injection (5 animals); 2) mice were pretreated p. o. for 21 days on a twice-daily schedule with 100 mg/kg lapatinib alone before PBS administration (5 animals); 3) KA-treated group was injected KA (5 animals); 4) and 5) lapatinib groups were received with 50 mg/kg (5 animals) and 100 mg/kg (5 animals) lapatinib for 21 days before KA treatment, respectively; 6) this group was given i. p. for 14 days with ferroptosis inhibitor (3 mg/kg Fer-1) before KA administration.
Click to Show/Hide
|
||||
| Response Description | Lapatinib exerted neuroprotection via restoring glutathione peroxidase 4 (GPX4). Treatment with GPX4 inhibitor ras-selective lethal small molecule 3 (RSL3) abrogated its anti-ferroptotic potential. It is concluded that lapatinib has neuroprotective potential against epileptic seizures via suppressing GPX4-mediated ferroptosis. | ||||
20-Hydroxyeicosatetraenoic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [217] | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mOHSCs (Mouse organotypic hippocampal slice cultures) | ||||
| In Vivo Model |
Adult male C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME USA). Mice at 10-12 weeks of age were anesthetized by 1-3% isoflurane inhalation and ventilated with oxygen-enriched air (20% O2:80% air). The right striatum of mice was injected with 0.5 ul of 0.075 U collagenase VII-S (MilliporeSigma, St. Louis, MO, USA) at 0.1 ul per minute. Injections were administered at 0.5 mm anterior and 2.2 mm lateral of the bregma, and 3.0 mm in depth, as previously described. Sham-operated mice received the same treatment, including needle insertion, but were not injected with collagenase. Mice that died before the end of the surgery or shortly thereafter were excluded.
Click to Show/Hide
|
||||
| Response Description | 20-hydroxyeicosatetraenoic acid induces ferroptosis in OHSCs, and inhibition of 20-HETE synthesis improves Intracerebral hemorrhage (ICH) outcome and attenuates markers of ferroptosis, such as mobile iron, lipid peroxidation, and decreased GPX4. | ||||
Dauricine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [219] | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Adult male C57BL/6 mice weighing 20-28 g were maintained in the specific pathogen-free (SPF) facility to be used in this study. Mice were subjected to a 12-h light/dark cycle at a constant ambient temperature (22 ± 1 ). Mice were randomly assigned into the following five groups based on random numbers generated using SPSS. The sham group(n = 20, of which 20 survived) was subjected to mock surgery (craniotomy without collagenase) and treated with 0.1 mL 0.9% saline. The intracerebral hemorrhage(ICH) group (n = 29, of which 23 survived) was subjected to ICH surgery, then treated with 0.9% saline. The low Dauricine(Dau) group (n = 24, of which 20 survived) was subjected to ICH surgery, then immediately treated with 5 mg/kg Dau via tail vein injection. The medium Dau group (n = 25, of which 22 survived)was subjected to ICH surgery, then treated with 10 mg/kg Dau. The high Dau group (n = 24, of which 22 survived)was subjected to ICH surgery, then immediately treated with 15 mg/kg Dau.
Click to Show/Hide
|
||||
| Response Description | Dauricine (Dau) could inhibit ferroptosis of nerve cells and alleviate brain injury after intracerebral hemorrhage by upregulating glutathione peroxidase 4 (GPX4) and glutathione reductase (GSR) co-expression. Therefore, Dau may be an effective drug for inhibiting ferroptosis and treating intracerebral hemorrhage. | ||||
Fucoidan
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [220] | |||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Omm1 cells | Uveal melanoma | Homo sapiens | CVCL_6939 | |
| SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| Response Description | Iron-dependent oxidative stress (ferroptosis) is a main hallmark of retinal and brain diseases, including hemorrhage. Fucoidans can abrogate the decrease in the protein levels of the antioxidant enzyme GPX4 that is crucial for ferroptosis. | |||
Rotenone
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [221] | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rPCNs (Rat primary cortical neurons) | ||||
| In Vivo Model |
Six-to-eight week old male ICR mice were purchased from the Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China). Herein, a total of 51 mice were randomly divided into 3 groups: (i) sham group (n = 15), (ii) ICH group (n = 18), and (iii) ICH + Rot group (n = 18). All mice were euthanized at 3 d after operation and brain samples were harvested, as per our previously described reports.
Click to Show/Hide
|
||||
| Response Description | Single rotenone administration markedly inhibited neuronal viability, promoted iron accumulation, increased malondialdehyde (MDA) contents, decreased total superoxide dismutase (SOD) activity, and downregulated ferroptosis-related proteins RPL8, COX-2, xCT, ASCL4, and GPX4 in primary neurons. Together, our data revealed that intracerebral hemorrhage induced significant mitochondrial dysfunction and that mitochondrial inhibitor rotenone can trigger and enhance neuronal ferroptosis. | ||||
Naotaifang Extract
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [222] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Specific pathogen-free adult male SD rats, (80 ± 5) days old and weighing 220-250 g, were provided by the Hunan Slack Jingda Experimental Animal Co., Ltd (Hunan, China). SD rats were randomly divided into 4 groups with 15 in each group: sham operation group, MCAO group, MCAO + DFP group and MCAO + NTE group. The rats were treated with drugs via oral gavage. According to the average body weight, the MCAO + NTE rats were given NTE at 27 g/kg, and the sham operation and the MCAO rats were given the same volume of saline (2.5 mL) for 7 consecutive days. The MCAO + DFP rats were given DFP at a dose of 125 mg/kg for 3 consecutive days.
Click to Show/Hide
|
||||
| Response Description | Acute cerebral ischemia induces neuronal ferroptosis and the effects of treating MCAO rats with naotaifang extract involved inhibition of ferroptosis through the TFR1/DMT1 and SCL7A11/GPX4 pathways. | ||||
Baicalein
[Investigative]
| In total 4 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [223] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
The mice (23-25 g, 8-10 weeks old) were subjected to transientmiddle cerebral artery occlusion (tMCAO) to induce cerebral ischemia as previously described protocol . Briefly, mice were anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg) and subcutaneous injection of meloxicam (10mg/kg) during tMCAO operation. Monofilament with a silicon coating on the tip and a diameter of 0.12 mm (A5-122, Beijing Cinontech Co. Ltd., China) was inserted into the ICA from CCA to occlude the middle cerebral artery (MCA) for 1.5 h. The suture was then removed to restore blood flow for another 22.5 h reperfusion. Sham control mice were subjected to similar surgical operations without MCA occlusion. Specifically, the monofilament was inserted only 5 mm above the carotid bifurcation and withdrew immediately in the Sham group.
Click to Show/Hide
|
||||
| Response Description | Baicalein inhibited the ferroptosis by regulating on the expression levels of GPX4, ACSL4 and ACSL3 in OGD/R cells, tMCAO mice and RSL3-stimulated HT22 cells. Our findings demonstrated that baicalein reversed the cerebral ischemia-reperfusion injury via anti-ferroptosis, which was regulated by GPX4/ACSL4/ACSL3 axis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [242] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Response of This Regulator | [250] | ||||
| Responsed Disease | Vitiligo [ICD-11: ED63] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hMCs (Human mesangial cells) | ||||
| Response Description | Baicalein up-regulated GPX4 and reduced TFR1 level in melanocytes treated with RSL3+FAC. Baicalein protected melanocytes against ferroptosis through up-regulating GPX4. Ferroptosis might be pervasive in the occurrence and development of vitiligo, and could be proposed as the potential therapeutic target. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Drug Response of This Regulator | [253] | ||||
| Responsed Disease | Endometriosis [ICD-11: GA10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| hMPs (Human macrophages) | |||||
| Response Description | Baicalein, a potential anti-ferroptosis compound, increased GPX4 expression, significantly inhibited ferroptosis, and restored phagocytosis of THP-1 cells in vitro. Collectively, our study reveals that ferroptosis triggered by high iron in cyst fluid promotes the development of endometriosis by impairing macrophage phagocytosis and producing more angiogenic cytokines (e.g., IL8 and VEGFA). | ||||
Carvacrol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [224] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
A total of 108 gerbils (male; body weight, 70-90 g; age, 12 to 16 weeks) were used in this study. The gerbils were randomly divided into the following five groups: the vehicle-treated group (sham group),which was given an equal volume of physiological saline; the carvacrol (CAR)-treated group (CAR group); the model group, which underwent the ligation of the bilateral carotid artery for 5 min followed by the loosening of the arterial clamp for reperfusion; the model + CAR-treated groups, which included the CAR-treated group and the model + CAR-treated groups that were treated with CAR (25, 50 and 100 mg/kg/day, i.p.) for 2 consecutive weeks and the model + DFO-treated groups that were treated with DFO (150 mg/kg/day, i.p.) for 2 consecutive weeks as the positive drug group.
Click to Show/Hide
|
||||
| Response Description | Carvacrol provides protection for hippocampal neurons against cerebral ischemia reperfusion in gerbils by inhibiting ferroptosis through increasing the expression of GPx4. | ||||
Propofol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [225] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male C57BL/6 mice weighing 20-25 g each were obtained from the Animal Experimental Center of Yisi (Changchun, China). Mice were group-housed in a 12 h light/dark cycle (light between 08:00 and 20:00 h) in a temperature-controlled environment room (23-25 ). Mice had ad libitum access to food and water. All surgical procedures were carried out on animals anesthetized with sodium pentobarbital (30 mg/kg) via intraperitoneal injection. MCAO was achieved by inserting a silicone rubber-coated nylon monofilament into the internal carotid artery through the external carotid artery and temporary ligation of the right common carotid artery with a suture. After 45 min of ischemia, blood flow was restored by removing the filament and the suture, and the mice were allowed to recover for 24 h.
Click to Show/Hide
|
||||
| Response Description | Our data support a protective role of propofol against ferroptosis as a cause of cell death in mice with cerebral ischemia-reperfusion injury. Propofol protected against cerebral ischemia-reperfusion injury-induced ferroptosis partly by regulating the Nrf2/Gpx4 signaling pathway. | ||||
Chrysin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [226] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
Kaempferol
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [227] | ||||
| Responsed Disease | Cerebral ischaemic stroke [ICD-11: 8B11] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPCNs (Mouse primary cortical neurons) | ||||
| Response Description | Kaempferol provides protection from OGD/R-induced ferroptosis, at least in part, by activating Nrf2/SLC7A11/GPX4 signaling pathway. Therefore, pharmacological inhibition of ferroptosis may be an attractive therapeutic target for the treatment of ischemic stroke. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [263] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male BALB/c mice (8-week-old, 20-22 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). The experimental animals were fed adaptively for one week in the Experimental Animal Center of Guangdong Pharmaceutical University (Guangzhou, China). Feeding conditions were set at 26 , humidity 65% and a lightdark cycle for 12 hours. All animal experiments were performed following the Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Research Ethical Committee of Guangdong Pharmaceutical University (gdpulacspf2020007).
Click to Show/Hide
|
||||
| Response Description | Kaempferol (KA) activated the Nrf2 pathway and upregulated Gpx4 in mouse livers and L02 cells to inhibit ferroptosis induced by APAP. Finally, molecular docking indicated the potential interaction of KA with Keap1. Taken together, KA ameliorated oxidative stress and ferroptosis-mediated acetaminophen-induced liver injury by activating Nrf2 signaling. | ||||
moracin N
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [228] | |||
| Responsed Disease | Nervous system disease [ICD-11: 8E7Z] | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Moracin N was a good ferroptosis inhibitor in Neurodegenerative diseases. The neuroprotective mechanisms of moracin N included inhibition of glutathione depletion, glutathione peroxidase 4 (GPx4) inactivation, reactive oxygen species (ROS) overproduction and iron accumulation, as well as improvement of intracellular antioxidant enzyme activities. | |||
4-(cyclohexylamino)-3-[(phenylmethyl)amino]-N-[2-(1-piperazinyl)ethyl]-benzenesulfonamide
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [229] | ||||
| Responsed Disease | Acute myocardial infarction [ICD-11: BA41] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
Male Sprague-Dawley rats (450 g-550 g) were purchased from Envigo (Frederick, Md). Animals were kept under standard conditions with a 12/12-h day/night cycle and received food and waterad libitum. Following induction with inhaling low flow CO2 for 30 s, animals were anesthetized by intraperitoneal injection of pentobarbital (45 mg/kg). Additional doses (10 mg/kg) were administered as needed based on tail pinch/withdrawal reflex to maintain anesthesia.
Click to Show/Hide
|
||||
| Response Description | Treatment with ferroptosis inhibitor, UAMC-3203 or/and DFO, reduced severity of myocardial dysfunction, and we further found that GPX4 and 4-HNE were significantly changed after CPR. Therefore, UAMC-3203 and DFO alleviated myocardial dysfunction via inhibiting ferroptosis, which could be a novel possible target for post-resuscitation myocardial dysfunction (PRMD) treatment. | ||||
Canagliflozin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [231] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57BL/6J mice aged 6-8 weeks with weights of 18-20 g were obtained from the Slack Laboratory Animal Co., Ltd. (Shanghai, China). Mice were allowed to acclimatize in the laboratory environment for 1 week before the beginning of the experiment. DCM model establishment: The mice were given a single intraperitoneal injection of 150 mg/kg 1% streptozotocin (STZ, V900890, Sigma, USA, dissolved in 0.1 mol/L sodium citrate buffer, pH = 4.4 - 4.6). Mouse blood from the tail vein was collected in each group of the model mice and tested by glucose meter (Accu-Chek Performa test strips, Roche, Accu-Chek Performa Combo, Roche, USA) on day 3, 5 and 7 after injection.
Click to Show/Hide
|
||||
| Response Description | Canagliflozin (Cana) promotes upregulation of SLC7A11 and downregulation of TfR1 and FTN-H, which protect the cardiomyocytes from ferroptosis. These finding suggests that Cana inhibit ferroptosis by balancing cardiac iron homeostasis and promoting the system Xc/GSH/GPX4 axis in diabetic cardiomyopathy. | ||||
Doxorubicin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [233] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
Male C57BL/6J mice were housed in a temperature- and humidity-controlled room, fed a commercial diet (CRF-1; Oriental Yeast Co. Ltd.), and given free access to water. GPx4 Tg mice and GPx4 hetKO mice were produced as previously described. In these gene-manipulated mice, GPx4 was systemically overexpressed or absent, respectively. These strains were backcrossed with C57BL/6J mice in our laboratory. The DIC model was reproduced as previously reported, with some modification. Briefly, DOX (6 mg/kg, body weight) was administered to mice via tail vein at days 0, 2, and 4.
Click to Show/Hide
|
||||
| Response Description | Doxorubicin (DOX) downregulated glutathione peroxidase 4 (GPx4) and induced excessive lipid peroxidation through DOX-Fe2+ complex in mitochondria, leading to mitochondria-dependent ferroptosis. The findings suggest that mitochondria-dependent ferroptosis plays a key role in progression of doxorubicin-induced cardiomyopathy (DIC) and that ferroptosis is the major form of regulated cell death in DOX cardiotoxicity. | ||||
Liquiritin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [234] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
The set of animal experiments was designed to evaluate the effectiveness of liquiritin on doxorubicin-induced cardiotoxicity as were as ferroptosis were explored. Mice were randomly divided into 5 groups: (1) the control group; (2) doxorubicin group; (3) the doxorubicin plus liquiritin group (20 mg/kg); (4) the doxorubicin plus liquiritin group (40 mg/kg); (5) the doxorubicin plus liquiritin group (80 mg/kg) (Han et al.2022; Mou et al.2021). The control group and doxorubicin group were given equal volume of 0.5% sodium carboxymethylcellulose; the doxorubicin plus liquiritin groups were given different doses of liquiritin (0.5%) sodium carboxymethylcellulose co-suspension) by intragastric administration 7 days in advance once a daily. On day 8, groups (2), (3), (4), and (5) were given a single intraperitoneal injection of 15 mg/kg of DOX to establish a model of doxorubicin-induced cardiotoxicity; and group (1) was given an equal volume of saline intraperitoneally.
Click to Show/Hide
|
||||
| Response Description | Liquiritin can protect the doxorubicin-induce mice's cardiotoxicity, and its beneficial effect is related to the reduction of ferroptosis through a mechanism involving the regulation of the SLC7A11/GPX4 pathway. | ||||
Resveratrol
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [235] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Adult male C57BL/6J mice weighing 20 ± 2 g were purchased from Chongqing Tengxin Biotechnology. Mice were housed at 22 with a 12 h light/dark cycle with free access to food and water. The cardiotoxicity mice model was induced by intraperitoneal injection of 5-FU (30 mg/kg) for 7 days. The cardiotoxicity mice were randomly divided into five groups: model group (normal saline), Res low, medium, high dose group (1, 2, 4 mg/kg) and Fer-1 positive control group (2.5 mg/kg). These mice were given Res or Fer-1 once a day for 3 weeks, with the body weight being recorded. Then, the mice were euthanized, blood samples and heart tissue were collected.
Click to Show/Hide
|
||||
| Response Description | Resveratrol (Res) attenuated 5-FU-induced bodyweight reduction, restored the cardiac dysfunction and reduced the activity of oxidative stress. Furthermore, inhibition of GPX4-mediated ferroptosis was the protective mechanisms of Res against 5-FU-induced cardiotoxicity. | ||||
Ethoxyquin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [236] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
DOX (D1515, Sigma-Aldrich, St Louis, MO, USA; 6 mg/kg, dissolved in distilled water) was administrated to C57BL/6J Male mice (8-10 weeks old, 21-24 g) via the tail vein on days 0, 2, and 4.Ethoxyquin(E0237, Tokyo Chemical Industry, Tokyo, Japan; 100 umol/kg, once a day) was orally administrated every day from days 0 to 14. Ethoxyquinwas dissolved in polyethylene glycol (PEG; 28214-05, Nacalai Tesque, Kyoto, Japan,ethoxyquin10 uL in 990 uL PEG), and the solution was then diluted in the same amount of normal saline (873311, Otsuka Pharmaceutical, Tokyo, Japan).
Click to Show/Hide
|
||||
| Response Description | The inhibitory action of ethoxyquin against GPx4-deficient ferroptosis and its therapeutic efficacy against DOX-induced cell death in cultured cardiomyocytes and cardiotoxicity in a murine model of doxorubicin-induced cardiomyopathy (DIC). | ||||
Levosimendan
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [237] | ||||
| Responsed Disease | Left ventricular failure [ICD-11: BD11] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response Description | Levosimendan reversed mitochondrial malfunction in heart failure with preserved ejection fraction (HFpEF) mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan reverses cardiac malfunction and cardiomyocyte ferroptosis during heart failure with preserved ejection fraction via connexin 43 signaling activation. | ||||
Astragaloside IV
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [238] | ||||
| Responsed Disease | Cardiovascular diseases [ICD-11: BE2Z] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HUVECs (Human umbilical vein endothelial cells) | ||||
| Response Description | Astragaloside IV partially upregulated the levels of SLC7A11 and GPX4 expression which were reduced by LPC. The LPC-suppressed proliferation and LPC-induced apoptosis and senescence of endothelial cells were greatly attenuated by AS-IV treatment. In conclusion, AS-IV could serve as a novel drug for treating ferroptosis-related cardiovascular diseases. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [258] | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hT2AECs (Type II alveolar epithelial cells) | ||||
| In Vivo Model |
The animals were randomly assigned to six groups (7 mice in each) as follows: (I) Normal saline (NS) group, (II) Ast-IV 100 mg/kg (Ast) group, (III) PM2.5 group, (IV) Ast-IV 50 mg/kg + PM2.5 (Ast-L) group, (V) Ast 100 mg/kg + PM2.5 (Ast-H) group, and (VI) Ast-IV 100 mg/kg + erastin 20 mg/kg + PM2.5 (Era) group. Based on our previous results, this study adopted anintraperitoneal injection(i.p.) of Ast-IV (dissolved in normal saline containing 0.1% DMSO for preventive treatment. After all the mice were adaptively fed for 5 days, in the NS and PM2.5 groups, mice received the normal saline containing 0.1% DMSO viai.p.once a day for the next three consecutive days. Similar to the NS group, in the Ast, Ast-H, and Era groups, mice received Ast-IV (100 mg/kg) viai.p. Ast-L group received Ast-IV (50 mg/kg) viai.p. To evaluate the effect of Ast-IV on ferroptosis in PM2.5-induced lung injury, we used the ferroptosis agonist erastin to activate ferroptosisin vivo. In the Era group, mice received erastin (20 mg/kg, 10% DMSO + 40% PEG300 + 5%Tween80 + 45% normal saline) 30 min before each preventive treatment of Ast-IV.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (Ast-IV) reduced the lung wet-dry ratio and the levels of interleukin 6 (IL-6), tumor necrosis factor- (TNF-) and interleukin 1 (IL-1) in serum. Ast-IV could also improve the oxidative stress level in BALF, restore the GSH level in the lung tissue, and reduce the iron content in the lung tissue. Western blot outcomes revealed that Ast-IV regulated the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to protect PM2.5-mediated lung injury. | ||||
Epigallocatechin Gallate
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [239] | ||||
| Responsed Disease | Nonalcoholic fatty liver disease [ICD-11: DB92] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
After adaptive feeding, mice were randomly assigned to five groups (n = 10 per group). The details of the groups are as follows: 1) the normal diet (ND) group in which mice were fed ND (18% calories from fat); 2) the HFD group in which mice were fed HFD (60% calories from fat); 3) the HFD-EGCG/L group in which mice received 20 mg/kgbw EGCG by oral gavage daily during HFD feeding; 4) the HFD-EGCG/H group in which mice received 100 mg/kgbw EGCG by oral gavage daily during HFD feeding; and 5) the HFD-Fer-1 group in which mice received intraperitoneal injection of Fer-1 at 1 mg/kg. bw every 3 days during HFD feeding. Mice in the EGCG treatment groups were supplemented with EGCG (20 and 100 mg/kgbw) for 12 weeks. Meanwhile, mice in the ND group and the HFD group were orally gavaged with deionized water daily.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation and Fer-1 treatment apparently increased the protein expression of GPX4 and markedly decreased the protein expression of COX-2 and ACSL4 in the livers of HFD-fed mice. Epigallocatechin gallate may exert protective effects on hepatic lipotoxicity by inhibiting mitochondrial reactive oxygen species-mediated hepatic ferroptosis. Findings from our study provide new insight into prevention and treatment strategies for non-alcoholic fatty liver disease pathological processes. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [262] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
Chrysophanol
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [240] | |||
| Responsed Disease | Liver fibrosis [ICD-11: DB93] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HSC-T6 cells | Normal | Rattus norvegicus | CVCL_0315 |
| Response Description | Chrysophanol significantly induced HBx-transfected HSC-T6 death by inducing ferroptosis, as demonstrated by lipid ROS accumulation and upregulation of expression of ER markers, such as Bip, CHOP, and p-IRE1, and ferroptotic markers, such as GPX4 and SLC7A11. Therefore, chrysophanol may exert ferroptotic effects on activated HSCs to prevent liver fibrosis. | |||
Nickel Chloride
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [241] | ||||
| Responsed Disease | Drug-induced or toxic liver disease [ICD-11: DB95] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
Totally 128 7-week-old ICR male mice (22-25 g) were provided by Dashuo Biological Technology (Chengdu, China). The animals were divided into four groups (32 mice per group) randomly. The mice in the three experimental groups were gavage administered with Ni (NiCl2·6H2O) at doses of 7.5, 15, and 30 mg/kg body weight respectively, while those in the control group were given distilled water. The Ni dose adopted here was determined according to the value of median lethal dose (LD50, 306.11 mg/kg) attained in the research on acute oral toxicity of male mice. We selected 1/40, 1/20 and 1/10 LD50 (306.11 mg/kg) of NiCl2 in this study.
Click to Show/Hide
|
||||
| Response Description | Nickel chloride caused hepatic ferroptosis accompanied by increased iron content in the liver and up-regulation of cyclooxygenase 2 (COX-2) protein and mRNA expression levels, down-regulation of glutathione eroxidase 4 (GPX4), ferritin heavy chain 1 (FTH1) and nuclear receptor coactivator 4 (NCOA4) protein and mRNA expression levels. Altogether, Mitochondria damage and ferroptosis involved in Ni-induced hepatotoxicity in mice. | ||||
Gossypol acetic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [244] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
A total of 55 adult male Sprague-Dawley rat (350-450 g) were anesthetized with urethane (1.5 g/kg, i.p.), then the hearts were perfused in a Langendorff system. After 30 min of stabilization, hearts were subjected to 30 min of global no-flow ischemia by stopping the perfusion. Reperfusion was followed with Krebs Henseleit (KH) buffer and GAA together for 2 h. A thermoregulated chamber kept the heart at 37 throughout the experiment. Control hearts were not subjected to I/R. The heart slices were sectioned at a thickness of 2 mm and stained with triphenyltetrazolium chloride (25 mg/100 mL) for 10 min and then fixed with 4% formaldehyde solution for 48 h to enhance color contrast.
Click to Show/Hide
|
||||
| Response Description | Gossypol acetic acid significantly attenuated myocardial infarct size, reduced lipid peroxidation, decreased the mRNA levels of the ferroptosis markers Ptgs2 and Acsl4, decreased the protein levels of ACSL4 and NRF2, and increased the protein levels of GPX4 in I/R-induced ex vivo rat hearts. Thus, GAA may play a cytoprotectant role in ferroptosis-induced cardiomyocyte death and myocardial ischemia/reperfusion-induced ferroptotic cell death. | ||||
Histochrome
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [245] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mCMs (Mouse cardiomyocytes) | ||||
| In Vivo Model |
Male Fischer 344 rats (8 weeks old and 160 to 180 g; KOATECH, Pyeongtaek-si, Korea) were anesthetized by inhalation with 2% isoflurane and intubated using an 18-gauge intravenous catheter. The rats were mechanically ventilated with medical-grade oxygen. Surgery was performed on a 37 heating pad to prevent the body from getting cold. A left thoracotomy was performed after the chest was shaved to prevent contamination during surgery.
Click to Show/Hide
|
||||
| Response Description | Histochrome treatment significantly increased GPx4 and free GSH levels, but decreased Cox-2 level. HC treatment significantly decreased intracellular and mitochondrial ROS levels by upregulating the expression of Nrf2 and antioxidant genes. The substantial cardioprotective effects of HC against myocardia I/R injury by reducing ferroptosis-associated myocardial injury. | ||||
Hydroxysafflor Yellow A
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [246] | |||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Hydroxysafflor yellow A (HSYA) and anhydrosafflor yellow B (AHSYB) limited ferroptosis and parthanatos to alleviate oxidative stress in PC12 cells. Oxygen glucose deprivation and reperfusion injury reduced GSH/GSSG level in PC12 cells, but the reduction of GSH/GSSG ratio was regained by HSYA or AHSYB. HSYA and AHSYB activated GPX4 and system Xc- to alleviate ferroptosis. | |||
Luteolin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [242] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
Anhydrosafflor yellow B
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [246] | |||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Hydroxysafflor yellow A (HSYA) and anhydrosafflor yellow B (AHSYB) limited ferroptosis and parthanatos to alleviate oxidative stress in PC12 cells. Oxygen glucose deprivation and reperfusion injury reduced GSH/GSSG level in PC12 cells, but the reduction of GSH/GSSG ratio was regained by HSYA or AHSYB. HSYA and AHSYB activated GPX4 and system Xc- to alleviate ferroptosis. | |||
Ulinastatin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [247] | ||||
| Responsed Disease | Acute pancreatitis [ICD-11: DC31] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | 266-6 cells | Normal | Mus musculus | CVCL_3481 | |
| In Vivo Model |
For cerulein-induced acute pancreatitis, male mice (age, 8-10 wk) received 7 hourly intraperitoneal injections of 50 g/kg cerulein in sterile saline. Olanzapine was repeatedly administered orally by gavage at a dose of 5 mg/kg to mice at 3 and 12 hours after the first cerulein injection, while controls were treated by oral administration with vehicle (smooth peanut butter).The parameters of acute pancreatitis were assessed 12 hours after the last cerulein treatment. For the induction of chronic pancreatitis, male mice (age, 8-10 wk) were fed a LieberDeCarli ethanol (5% vol/vol) liquid diet for 4 weeks (F1258; Bio-Serv, Flemington,NJ).In parallel, olanzapine was administered orally by gavage at a dose of 5 mg/kg to mice (3 times per week, over 4 weeks), while controls were treated by oral administration with vehicle. The parameters of chronic pancreatitis were assessed in mice 4 weeks after feeding them the LieberDeCarli ethanol liquid diet.
Click to Show/Hide
|
||||
| Response Description | Trypsin-mediated sensitization of pancreatic acinar cells to ferroptosis may be targeted for the prevention and treatment of pancreatitis in mice. Conversely, olanzapine administration protected against pancreatic ferroptotic damage and experimental pancreatitis in Gpx4-deficient mice. | ||||
Wedelolactone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [248] | ||||
| Responsed Disease | Acute pancreatitis [ICD-11: DC31] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell pyroptosis | |||||
| In Vitro Model | AR42J cells | Digestive system neoplasms | Rattus norvegicus | CVCL_0143 | |
| In Vivo Model |
The 8-weeks old male Sprague-Dawley rats (bodyweight 250-300 g) were purchased from Liaoning changsheng biotechnology co. LTD (Benxi, China). The rats in the taurocholate-induced acute pancreatitis group (Taur, n = 6) were fasted overnight, after anesthesia the hepatic portal of the bile duct was clamped and 3.5% sodium taurocholate (Aladdin, Shanghai, China) in a volume of 1 ml/kg were retrogradely injected into the biliopancreatic duct at a constant speed (0.1 ml/min). The rats in the Sham group (n = 6) were received the laparotomy and the same volume of saline solution. The rats in the disulfiram treatment group (Taur + Disul, n = 6) were administrated with 100 mg/kg pyroptosis antagonist disulfiram (Aladdin) by intraperitoneal (i.p.) injection before the surgery. The rats in the ferrostatin-1 treatment group (Taur + Fer-1, n = 6) were i.p. administered 2.5 mol/kg ferroptosis antagonist ferrostatin-1 (Aladdin) 1 h before the surgery.
Click to Show/Hide
|
||||
| Response Description | Wedelolactone promoted the transcriptional activity and the selenium sensitivity of GPX4. Moreover, the protective effects of Wed in caerulein-stimulated pancreatic acinar cells were markedly abrogated by the down-regulation of GPX4. Wed mitigated Acute pancreatitis (AP) and associated lung injury via GPX4 mediated suppression of pyroptosis and ferroptosis. | ||||
Olanzapine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [247] | ||||
| Responsed Disease | Acute pancreatitis [ICD-11: DC31] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | 266-6 cells | Normal | Mus musculus | CVCL_3481 | |
| In Vivo Model |
For cerulein-induced acute pancreatitis, male mice (age, 8-10 wk) received 7 hourly intraperitoneal injections of 50 g/kg cerulein in sterile saline. Olanzapine was repeatedly administered orally by gavage at a dose of 5 mg/kg to mice at 3 and 12 hours after the first cerulein injection, while controls were treated by oral administration with vehicle (smooth peanut butter).The parameters of acute pancreatitis were assessed 12 hours after the last cerulein treatment. For the induction of chronic pancreatitis, male mice (age, 8-10 wk) were fed a LieberDeCarli ethanol (5% vol/vol) liquid diet for 4 weeks (F1258; Bio-Serv, Flemington,NJ).In parallel, olanzapine was administered orally by gavage at a dose of 5 mg/kg to mice (3 times per week, over 4 weeks), while controls were treated by oral administration with vehicle. The parameters of chronic pancreatitis were assessed in mice 4 weeks after feeding them the LieberDeCarli ethanol liquid diet.
Click to Show/Hide
|
||||
| Response Description | Trypsin-mediated sensitization of pancreatic acinar cells to ferroptosis may be targeted for the prevention and treatment of pancreatitis in mice. Conversely, olanzapine administration protected against pancreatic ferroptotic damage and experimental pancreatitis in Gpx4-deficient mice. | ||||
Curculigoside
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [249] | ||||
| Responsed Disease | Ulcerative colitis [ICD-11: DD71] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | IEC-6 cells | Normal | Rattus norvegicus | CVCL_0343 | |
| In Vivo Model |
Male C57BL/6J mice (8 weeks) were housed in a controlled condition at 25 , 45-55% humidity and 12 h light/dark cycle. All mice were randomly divided into five groups: Vehicle group, mice received dextran sulfate sodium (DSS group), DSS mice received ferrostatin-1 (DSS + Fer-1 group), DSS mice received low dose of CUR (DSS + CUR-L group), and DSS mice received high dose of CUR (DSS + CUR-H group). In the experiments, 3% DSS (D122347, Aladdin, Shanghai, China) in drinking water for 7 days was prepared to induce UC models. Mice in DSS + Fer-1 group were intraperitoneally injected with 5 mg/kg Fer-1 (S7243, Selleck, Shanghai, China) every two days from the day before DSS induction. In addition, mice in DSS + CUR-L or DSS + CUR-H group received intragastric administration with CUR (HY-N0705, Med Chem Express, Shanghai, China) at 50 mg/kg or 100 mg/kg once a day for 7 days during DSS administration.
Click to Show/Hide
|
||||
| Response Description | Curculigoside (CUR) could increase the selenium sensitivity and promote GPX4 transcription level in IEC-6 cells. Knockdown of GPX4 significantly blocked the protective effects of CUR on cell death, GSH and MDA contents as well as LDH activity in ferroptotic IEC-6 cells. Taken together, CUR protects against ferroptosis in ulcerative colitis (UC) by the induction of GPX4, which presents a potential agent for UC treatment. | ||||
Biochanin A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [251] | ||||
| Responsed Disease | Knee osteoarthritis [ICD-11: FA01] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCDs (Chondrocytes) | ||||
| In Vivo Model |
Male mice were purchased from Guangzhou University of Chinese Medicine's Experimental Animal Center C57BL/6 mice (7-week-old, 20 g) (Guangzhou, China). After one week of adaptively feeding with chow meals and sterilized water, the animals were separated into five groups of ten mice randomly assigned to the negative control (NC); model, positive control (PC); model group; high dosage of BCA treatment (BCA-H) group; and low dosage of BCA treatment (BCA-L) group. The iron overload mice model was designed based on earlier research. Except for the NC group, mice were administered ID intraperitoneally (500 mg/kg) once a week for eight weeks. In the right knee joints, OA was induced with the initial injection of iron dextran two weeks after the injection by destabilizing the medial meniscus (DMM) using a microscope. After the operation, the positive control group was administered with NAC intragastrically (100 mg/kg) for eight weeks. BCA-H and BCA-L groups were administered 20 mg/kg and 40 mg/kg of BCA separately for eight weeks according to previous studies.
Click to Show/Hide
|
||||
| Response Description | Biochanin A (BCA) could directly reduce intracellular iron concentration by inhibiting TfR1 and promoting FPN but also target the Nrf2/system xc-/GPX4 signaling pathway to scavenge free radicals and prevent lipid peroxidation. The results of this research indicate that BCA regulates iron homeostasis during the progression of osteoarthritis, which can open a new field of treatment for knee osteoarthritis. | ||||
L-Homocysteine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [252] | ||||
| Responsed Disease | Intervertebral disc degeneration [ICD-11: FA80] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Necroptosis | hsa04217 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell necrosis | |||||
| In Vitro Model | hNPCs (Human nucleus pulposus cells) | ||||
| In Vivo Model |
Thirty male C57BL/6 mice (8 weeks old, average weight 25 g) were divided into three groups. Blank group: Ten mice were fed with normal diet and water. HHcy group: Ten mice were fed with a high Met diet (normal diet with 2% l-methionine). HHcy + Folic Acidrescue group: Ten mice were fed withhigh Met diet (normal diet with 2% l-methionine), and injected with Folic Acid (1.0 umol/kg/d) twice a week two months later. At the beginning of treatment, mice were anesthetized with anintraperitoneal injectionof 0.3% (w/v)pentobarbital sodium(20 uL/g) before surgery and disc degeneration was induced by stabbing the C5-6 and C6-7 discs with a 32G needle.
Click to Show/Hide
|
||||
| Response Description | Homocysteine (Hcy) is an amino acid involved in gene methylation. Hcy upregulates oxidative stress and ferroptosis in the nucleus pulposus via enhancing GPX4 methylation, and is a new contributing factor in intervertebral disc degeneration (IVDD). | ||||
Busulfan
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [254] | ||||
| Responsed Disease | Male infertility [ICD-11: GB04] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mTTs (Mouse testicular tissues) | ||||
| In Vivo Model |
Eight-week-old healthy ICR male mice, weighted 20-24 g, were provided by Experimental Animal Center of Nantong University (Nantong, China). For the first animal study, eight-week-old ICR male mice were randomly assigned to four groups: control, busulfan, busulfan plus Fer-1 and busulfan plus DFO groups (n = 6 per group). Mice were anesthetized and then given testicular injection of busulfan on both sides at the dose of 4 mg/kg body weight. The solution containing busulfan was directly injected from the scrotum into testicular transverse diameter. Fer-1 and DFO were administered by intraperitoneal injectionat concentrations of 1 mg/kg and 30 mg/kg respectively three times a week after busulfan injection. Four weeks later, the epididymal spermatozoa and testes from all mice were collected for assessment.
Click to Show/Hide
|
||||
| Response Description | Busulfan treatment induced spermatogenic cells ferroptosis by down-regulating nuclear factor-E2-related factor 2 (Nrf2) and glutathione peroxidase 4 (GPX4) expressions, and decreasing iron efflux through reduction of ferroportin 1 (FPN1) expression. Targeting ferroptosis serves as a potential strategy for prevention of busulfan-induced damage and male infertility. | ||||
Polydatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [255] | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks of age, weight 20-25 g) were purchased from Experimental Animal Center of the Fourth Military Medical University (Xi'an, China) and bred in an experimental animal room of SPF grade. They were randomly divided into four groups: control (equivalent saline containing 1% DMSO) group (n = 5), cisplatin (20 mg/kg dissolved in saline) only group (n = 7), cisplatin + polydatin (40 mg/kg dissolved in 1% DMSO) group (n = 7), and cisplatin+ Fer-1 (5 mg/kg dissolved in 1% DMSO) group (n = 7) were administered intraperitoneally. Mice were injected with cisplatin once; PD or Fer-1 was given 1 h before and 24 h after cisplatin. Animals were ethically sacrificed by dislocating their spines at 48 h after cisplatin injection, and whole blood and kidneys were collected for further analysis.
Click to Show/Hide
|
||||
| Response Description | In vitro and in vivo experiments indicated the prominent nephroprotective effects of polydatin against ferroptosis in cisplatin-induced acute kidney injury models, occurred at least partly through inhibiting excessive intracellular free iron accumulation and ROS production, rescuing GSH consumption, and enhancing GPx4 activity, thereby decreasing lipid peroxidation and ferroptosis sensitivity and ultimately attenuating the pathological progression of AKI. | ||||
Aspirin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [256] | ||||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
These mice were on eight weeks old male DBA/2J background (n = 36, HFK Bioscience, Beijing, China). They were randomized one of the six groups: control normal mice group (NC); diabetic mice group (DM); diabetic mice group (Fer-1), who intraperitoneal injected Fer-1 (Selleck, Houston, TX, USA); diabetic mice group (vehicle-P), who intraperitoneal injected 1% dimethyl sulfoxide (DMSO); diabetic mice group (As), who intragastric administrated Aspirin (Solarbio, Beijing, China); diabetic mice group (vehicle-G), who intragastric administrated 0.5% sodium carboxymethyl cellulose (Na-CMC; Solarbio, Beijing, China). Diabetes models were induced with 5 consecutive days of a single intraperitoneal injection of streptozotocin 40 mg/kg (dissolved in 0.1 M citrate buffer, pH 4.5; SigmaAldrich, St Louis, MO, USA). Control mice only was injected the same volume of citrate buffer. In the Fer-1 or vehicle-P groups, the diabetic mice were treated respectively with Fer-1 (2.5 umol/kg, dissolved in 1% DMSO) or 1% DMSO during the duration of treatment for 12-week every day. And in the AS and vehicle-G groups, the diabetic mice were treated respectively with aspirin (50 mg/kg, dissolved in 0.5% Na-CMC) or 0.5% Na-CMC for 12-week every day.
Click to Show/Hide
|
||||
| Response Description | Aspirin can upregulate SLC7A11 and GPX4 expression by suppressing COX2. Our results demonstrated that ferroptosis in renal tubular cells contributes to Diabetic kidney disease (DKD) development and that diabetes-related ferroptosis was inhibited through the downregulation of COX2 by aspirin, thus retarding the progression of DKD. | ||||
Platycodin D
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [257] | |||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 |
| Response Description | Ferroptosis in HK-2 cells was induced by HG and was suppressed by Platycodin D (PD). GPX4 expression was downregulated in HG-induced cells and was upregulated by PD. Thus, PD suppressed ferroptosis of HK-2 cells by upregulating GPX4 expression, suggesting that PD might be an effective drug for Diabetic nephropathy therapy. | |||
Lipopolysaccharide
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [259] | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
The male C57BL/6 mice were divided randomly into 4 groups (n = 4 per group, 8-10 weeks old, weight = 23-25 g): the control group receiving 0.9% NaCl (containing 0.1% DMSO), the LPS group receiving LPS plus 0.9% NaCl (containing 0.1% DMSO), the Fer-1 group receiving Fer-1 only, and the LPS + Fer-1 group receiving both Fer-1 and LPS. The LPS-induced ALI model was induced by instilling intratracheally 50 ul of LPS solution (0.2 g/L), then Fer-1 (0.8 mg/kg) was administered after LPS challenge via tail vein injection. The Fer-1 was dissolved in DMSO first, and diluted with 0.9% NaCl. The final concentration of Fer-1 and DMSO was 0.2 mg/ml and 0.1% respectively.
Click to Show/Hide
|
||||
| Response Description | The cell viability of BEAS-2B was down-regulated by lipopolysaccharide (LPS) treatment, together with the ferroptosis markers SLC7A11 and GPX4, while the levels of MDA, 4-HNE and total iron were increased by LPS treatment in a dose-dependent manner, which could be rescued by ferrostatin-1. Fer-1 exerted therapeutic action against LPS-induced acute lung injury, and down-regulated the ferroptosis level in lung tissues. | ||||
Ferrostatin-1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [259] | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
The male C57BL/6 mice were divided randomly into 4 groups (n = 4 per group, 8-10 weeks old, weight = 23-25 g): the control group receiving 0.9% NaCl (containing 0.1% DMSO), the LPS group receiving LPS plus 0.9% NaCl (containing 0.1% DMSO), the Fer-1 group receiving Fer-1 only, and the LPS + Fer-1 group receiving both Fer-1 and LPS. The LPS-induced ALI model was induced by instilling intratracheally 50 ul of LPS solution (0.2 g/L), then Fer-1 (0.8 mg/kg) was administered after LPS challenge via tail vein injection. The Fer-1 was dissolved in DMSO first, and diluted with 0.9% NaCl. The final concentration of Fer-1 and DMSO was 0.2 mg/ml and 0.1% respectively.
Click to Show/Hide
|
||||
| Response Description | The cell viability of BEAS-2B was down-regulated by lipopolysaccharide (LPS) treatment, together with the ferroptosis markers SLC7A11 and GPX4, while the levels of MDA, 4-HNE and total iron were increased by LPS treatment in a dose-dependent manner, which could be rescued by ferrostatin-1. Fer-1 exerted therapeutic action against LPS-induced acute lung injury, and down-regulated the ferroptosis level in lung tissues. | ||||
Bicyclol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [260] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
The mice were treated with intraperitoneal administration (i.p.) of oil (control group) or a mixture of CCl4 (50%) and oil (50%) at a dosage of 2 ml/kg body weight. In the bicyclol-treated group, mice accepted administration of 200 mg/kg (using 0.5% carboxymethyl cellulose as solvent) by gavage three times a day 1 h before CCl4 exposure, while other groups accepted vehicles of the equal volume. Fer-1 was prepared in DMSO (5 mg/kg), andi.p. injected into mice once 1 h before CCl4 exposure. The dosage of bicyclol was consistent with our previous work. The mice were then sacrificed to collect liver and serum samples after 24 or 48 h.
Click to Show/Hide
|
||||
| Response Description | Bicyclol exerted its hepatoprotection by preventing the aforesaid ferroptotic process. Furthermore, bicyclol alleviated erastin-induced cellular inviability, destruction, and lipid peroxidation in vitro. Knockdown of GPX4 diminished these protective activities against perturbations associated with ferroptosis in L-O2 hepatocytes. Additionally, Nrf2 silencing drastically reduced GPX4 levels, and further impeded the medicinal effects of bicyclol. In summary, positively regulating Nrf2-GPX4 axis by bicyclol can prevent ferroptosis in CCl4-induced acute liver injury in mice. | ||||
Disulfiram
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [261] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| 769-P cells | Renal cell carcinom | Homo sapiens | CVCL_1050 | ||
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| MDA231-LM2-4175 cells | Breast adenocarcinoma | Homo sapiens | CVCL_5998 | ||
| In Vivo Model |
C57BL/6J male mice aged 8 weeks were purchased from Charles River Laboratories International, Inc., and housed in a specific pathogen-free animal facility. DMSO or DSF (21 mg/kg) was injected intraperitoneally into mice for 0.5 h, followed by ConA injection via the tail vein at 15 mg/kg. Mice were sacrificed at 24 h post ConA injection. Liver and blood samples were collected at this time point for H&E staining, IHC staining, and measurement of AST/ALT (Dian Diagnostics Co., Ltd).
Click to Show/Hide
|
||||
| Response Description | Disulfiram (DSF) is conjugated to multiple cysteine residues in GPX4 and disrupts GPX4 interaction with HSC70, an adaptor protein for chaperone mediated autophagy, thus preventing GPX4 degradation induced by erastin. In addition, DSF ameliorates concanavalin A induced acute liver injury by suppressing ferroptosis in a mouse model. | ||||
Schisandrin B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [264] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | rPHs (Rat primary hepatocytes) | ||||
| In Vivo Model |
A total of 40 male SD rats (180~200 g, 6~8 weeks) were purchased from the CMU experimental animal center. The rats were randomly divided into four groups: control (CON) group (normal diet rats were injected with equal volume of normal saline through caudal vein once a week, n = 10), SchB group (SchB diet rats, 50 mg/kg/day, were injected with equal volume of normal saline through caudal vein once a week, n = 10), THP group (normal diet rats were injected with 3 mg/kg/day THP through caudal vein once a week, n = 10), and SchB+THP group (SchB diet rats, 50 mg/kg/day, were injected with 3 mg/kg/day THP through caudal vein once a week, n = 10). CON and THP rats were fed an AIN-76A feed (12.4% fat, 68.8% carbohydrate, and 18.8% protein). SchB and SchB+THP rats were fed an SchB feed (approximately 0.5 SchB was added into AIN-76A feed). After conversion, 0.5 SchB in feed = 50 mg/kg in rats.
Click to Show/Hide
|
||||
| Response Description | Schisandrin B (SchB) increased the levels of SOD, GSH, GSH-px, CAT, and T-AOC, decreased the level of MDA, and inhibited the abnormal oxidative stress in the liver. And SchB as a natural molecule depends on reducing the level of oxidative stress, thereby inhibiting lipid peroxidation, ferroptosis, and apoptosis. The expression of NRF2, GPX4, SOD2, and Bcl-2/Bax decreased, while the expression of NOX2/4 and cleaved caspase-3 increased in pirarubicin-treated hepatocytes. However, the above changes were significantly reversed after SchB or Fer-1 treatment. SchB has obvious protective effect on pirarubicin-induced hepatotoxicity. | ||||
Apigenin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [265] | |||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 |
| Response Description | DEHP caused oxidative stress and increased the Fe2+ content, finally resulting in ferroptosis in AML12 cells. Apigenin restrained the toxicity of DEHP and antagonized DEHP-induced ferroptosis in AML12 cells. The protective effects of APG on DEHP-induced liver injury were achieved by activating GPX4 and suppressing intracellular iron accumulation. | |||
Edaravone
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [266] | ||||
| Responsed Disease | Spinal cord injury [ICD-11: ND51] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rSCTs (Rat spinal cord tissues) | ||||
| In Vivo Model |
The rats were initially anesthetized with 5% isoflurane (RWD life science, Shenzhen, China) and then maintained with 22.5% isoflurane. A 1-cm midline incision was made over the thoracic vertebrae, and laminectomy on T10 and the caudal half of T9 vertebrae was performed. Spinal cord contusion injury was conducted by NYU Impactor Model III (W.M. Keck Center for Collaborative Neuroscience Rutgers, The State University of New Jersey, United States) using a 10-g node dropping freely from a height of 2.5 cm and muscles and skin sutured in layers. Sham controls underwent laminectomy without the contusion. To prevent infection at the incision, cefuroxime sodium was applied for 3 days after injury. The bladders were emptied manually twice daily in the first week after injury.
Click to Show/Hide
|
||||
| Response Description | Edaravone not only rescues the ferroptosis negative regulators, xCT and GPX4, but also downregulates those pro-ferroptosis factors, ACSL4 and 5-LOX. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. And in the acute phase of spinal cord injury (SCI), edaravone reduced neuronal cell death and neuroinflammation. | ||||
Nicotinamide mononucleotide
[Preclinical]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [267] | ||||
| Responsed Disease | Skin injury [ICD-11: ND56] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HaCaT cells | Normal | Homo sapiens | CVCL_0038 | |
| In Vivo Model |
The skin injury model was created using ultraviolet B (UVB) 250 mJ/cm2 irradiation onto BALB/c mice after their back shaved. A total of 26 mice were used in this study; 8 mice without treatment served as controls, while 18 mice were irradiated under the UVB lamp and administered with PBS (200 ul per injection area), Lip-1 (Selleck, S7699) (10 mg/kg every other day per injection area), or NMN (Chalet Healthy PTY Ltd, Jiangsu Chengxin Pharmaceutical Co., Ltd) (400 mg/kg/day via drinking water at pH 7.2).
Click to Show/Hide
|
||||
| Response Description | Nicotinamide mononucleotide recruits GSH to enhance GPX4-mediated ferroptosis defense in UV irradiation-induced skin injury and inhibits oxidative skin damage. NMN or ferroptosis inhibitor might become promising therapeutic approaches for treating oxidative stress-induced Skin injury. | ||||
Alpha-Tocopherol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [270] | ||||
| Responsed Disease | Health [ICD-11: N.A.] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHSPCs (Mouse hematopoietic stem and progenitor cells) | ||||
| In Vivo Model |
C57BL/6 WT mice were purchased from Beijing HFK BioScience Company (Beijing, China). Gpx4flox/flox mice were crossed with Vav-Cre mice and Mx-Cre mice to generate the Gpx4flox/flox Vav-Cre mice and Gpx4flox/flox Mx-Cre mice, respectively. For Gpx4 deletion, Gpx4flox/flox Mx-Cre mice were intraperitoneally injected with 20 mg/kg pIpC (Sigma) every other day for two weeks. CD45.1/45.2 mice and CD45.1 mice on a C57BL/6 background were used as competitor and recipient mice, respectively, in the competitive transplantation assay. Mice were fed natural ingredient diets containing >= 120 IU/kg vitamin E. A fixed formulation diet with or without 75 IU/kg vitamin E (Beijing HFK BioScience Company, Beijing, China) was fed to the mice involved in the vitamin E depletion experiments. For 5-FU treatment, mice were intraperitoneally injected with 150 mg/kg 5-FU (Sigma).
Click to Show/Hide
|
||||
| Response Description | a-Tocopherol, the main component of vitamin E, was shown to rescue the Gpx4-deficient hematopoietic stem and progenitor cells (HSPCs) from ferroptosis in vitro. When Gpx4 knockout mice were fed a vitamin E-depleted diet, a reduced number of HSPCs and impaired function of HSCs were found. | ||||
Fluvastatin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [271] | |||
| Responsed Disease | Health [ICD-11: N.A.] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | Fluvastatin exerts potent protective effects against ox-LDL-induced endothelial cell dysfunction through regulation of GPx4 and xCT. These data indicated a novel function of fluvastatin in the protection of endothelial cells from ox-LDL-induced ferroptosis, the mechanism of which involves the regulation of GPx4 and xCT. | |||
D-2-hydroxyglutarate
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [268] | |||
| Responsed Disease | Health [ICD-11: N.A.] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| KYSE-170 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1358 | |
| Response Description | Ectopic expression of mutant IDH1 or treatment of cells with cell-permeable D-2-hydroxyglutarate (D-2-HG) promotes the accumulation of lipid reactive oxygen species (ROS) and subsequently ferroptosis. Mechanistically, mutant IDH1 reduces the protein level of the glutathione peroxidase 4 (GPX4). | |||
Selenium
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [272] | ||||
| Responsed Disease | Health [ICD-11: N.A.] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mTCs (Mouse T cells) | ||||
| In Vivo Model |
All mice used in this study were 6-12 weeks old on a C57/BL6/J background. WT or T-KO mice were fed with water supplemented with methionine (1 mgl-1, Sigma) or Se-Met (1 mgl-1, Sigma) and maintained on the diets for 4 weeks before experiments. Alternatively, WT mice were fed with selenium-adequate (0.15 mg/kg) and selenium-high (1 mg/kg) diets that were purchased from Envigo and mice were maintained on the diets for 4 weeks before experiments.
Click to Show/Hide
|
||||
| Response Description | The deletion of GPX4 in T cells selectively abrogated TFH cells and germinal center responses in immunized mice. Selenium supplementation enhanced GPX4 expression in T cells, increased TFH cell numbers and promoted antibody responses in immunized mice and young adults after influenza vaccination. | ||||
Thioctic acid
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [273] | |||
| Responsed Disease | Nanotoxicity [ICD-11: N.A.] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | BALB/3T3 cells | Normal | Mus musculus | CVCL_0184 |
| Response Description | CoNPs could induce the ferroptosis-like cell death through the enhancement of intracellular reactive oxygen species (ROS) level, cytoplasmic Fe2+ level, lipid peroxidation, and consumption of reduced glutathione (GSH) as well as inhibition of glutathione peroxidase 4 (GPX4) activity. Importantly, a-lipoic acid (ALA), a natural antioxidant with the capability to scavenge free radicals and chelate toxic metals, was found to efficiently alleviate nanotoxicity. | |||
References
