Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0133)
| Name |
Andrographis
|
||||
|---|---|---|---|---|---|
| Synonyms |
Andrographolide; 5508-58-7; CHEBI:65408; HMPL004; UNII-410105JHGR; DTXSID3045980; 410105JHGR; EINECS 226-852-5; (S,E)-4-Hydroxy-3-(2-((1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethylidene)dihydrofuran-2(3H)-one; NSC 383468; NSC-383468; DTXCID1025980; 3alpha,14,15,18-tetrahydroxy-5b,9bH,10a-labda-8(20),12-dien-16-oic acid gamma-Lactone; 3-(2-(Decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenenaphthyl)ethylidene)dihydro-4-hydroxyfuran-2(3H)-one; (1R-(1-alpha(E(S)),4abeta,5alpha,6alpha,8aalpha))-3-(2-(decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl)ethylidene)dihydro-4-hydroxy-2(3H)-furanone; (3E,4S)-3-[2-[(1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]ethylidene]-4-hydroxyoxolan-2-one; (3E,4S)-3-{2-[(1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-decahydronaphthalen-1-yl]ethylidene}-4-hydroxyoxolan-2-one; ANDROGRAPHOLIDE (USP-RS); ANDROGRAPHOLIDE [USP-RS]; (3E,4S)-3-(2-((1R,4AS,5R,6R,8AS)-DECAHYDRO-6-HYDROXY-5-(HYDROXYMETHYL)-5,8A-DIMETHYL-2-METHYLENE-1-NAPHTHALENYL)ETHYLIDENE)DIHYDRO-4-HYDROXY-2(3H)-FURANONE; (3E,4S)-4-hydroxy-3-{2-[(1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidenedecahydronaphthalen-1-yl]ethylidene}dihydrofuran-2(3H)-one; 2(3H)-FURANONE, 3-(2-((1R,4AS,5R,6R,8AS)-DECAHYDRO-6-HYDROXY-5-(HYDROXYMETHYL)-5,8A-DIMETHYL-2-METHYLENE-1-NAPHTHALENYL)ETHYLIDENE)DIHYDRO-4-HYDROXY-, (3E,4S)-; 2(3H)-Furanone, 3-(2-(decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl)ethylidene)dihydro-4-hydroxy-, (1R-(1-alpha(E(S*)),4a-beta,5-alpha,6-alpha,8a-alpha))-; 2(3H)-Furanone, 3-[2-[(1R,4aS,5R,6R,8aS)-decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl]ethylidene]dihydro-4-hydroxy-, (3E,4S)-; HMPL-004; NSC383468; NCGC00095597-01; (3E,4S)-3-(2-((1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl)ethylidene)-4-hydroxyoxolan-2-one; (3E,4S)-4-hydroxy-3-(2-((1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidenedecahydronaphthalen-1-yl)ethylidene)dihydrofuran-2(3H)-one; Andrographolide, 98%; ANDROGRAPHOLIDE [MI]; ANDROGRAPHOLIDE [INCI]; BIDD:ER0530; CHEMBL186141; GTPL9675; MEGxp0_000978; ANDROGRAPHOLIDE [WHO-DD]; SCHEMBL12056309; ACon1_002113; BOJKULTULYSRAS-OTESTREVSA-N; Andrographolide, analytical standard; HY-N0191; Tox21_111508; BDBM50084419; MFCD07778082; AKOS015920075; CCG-208428; CS-3334; DB05767; NCGC00179817-01; NCGC00179817-02; AS-13637; CAS-5508-58-7; C20214; A830479; ANDROGRAPHOLIDE (CONSTITUENT OF ANDROGRAPHIS); Q-100624; Q4759444; BRD-K89282837-001-01-0; ANDROGRAPHOLIDE (CONSTITUENT OF ANDROGRAPHIS) [DSC]; Andrographolide, United States Pharmacopeia (USP) Reference Standard; (1R-(1-alpha(E(S)),4a-beta,5alpha,6alpha,8a-alpha))-3-(2-(decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl)ethylidene)dihydro-4-hydroxy-2(3H)-furanone; (3E)-3-[2-[(1R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-decalin-1-yl]ethylidene]-4-hydroxy-tetrahydrofuran-2-one;Andrographolide; (3E,4S)-3-[2-[(1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-decalin-1-yl]ethylidene]-4-hydroxy-tetrahydrofuran-2-one; (S,E)-4-Hydroxy-3-(2-((1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylenedecahydronaphthalen-1-yl)ethylidene)dihydrofuran-2; 2(3H)-Furanone,3-[2-[(1R,4aS,5R,6R,8aS)-decahydro-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylene-1-naphthalenyl]ethylidene]dihydro-4-hydroxy-,(3E,4S)-; 3.ALPHA.,14,15,18-TETRAHYDROXY-5.BETA.,9.BETA.H,10.ALPHA.-LABDA-8(20),12-DIEN-16-OIC ACID .GAMMA.-LACTONE; 3alpha,14,15,18-TETRAHYDROXY-5beta,9betaH,10alpha-LABDA-8(20),12-DIEN-16-OIC ACID gamma-LACTONE
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
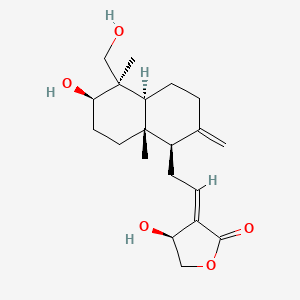 |
||||
| Formula |
C20H30O5
|
||||
| IUPAC Name |
(3E,4S)-3-[2-[(1R,4aS,5R,6R,8aS)-6-hydroxy-5-(hydroxymethyl)-5,8a-dimethyl-2-methylidene-3,4,4a,6,7,8-hexahydro-1H-naphthalen-1-yl]ethylidene]-4-hydroxyoxolan-2-one
|
||||
| Canonical SMILES |
CC12CCC(C(C1CCC(=C)C2CC=C3C(COC3=O)O)(C)CO)O
|
||||
| InChI |
InChI=1S/C20H30O5/c1-12-4-7-16-19(2,9-8-17(23)20(16,3)11-21)14(12)6-5-13-15(22)10-25-18(13)24/h5,14-17,21-23H,1,4,6-11H2,2-3H3/b13-5+/t14-,15-,16+,17-,19+,20+/m1/s1
|
||||
| InChIKey |
BOJKULTULYSRAS-OTESTREVSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Marker/Suppressor | |||
| Responsed Disease | Multiple myeloma | ICD-11: 2A83 | ||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 |
| U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 | |
| AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| Response regulation | Andrographolide (Andro) may block the Nrf2/HO-1 signaling pathway by activating P38 ( MAPK14), thereby inducing ferroptosis. Moreover, inhibition of P38 expression rescued Andro-induced cell death, changes in the level of Nrf2 and HO-1 expression, Fe2+ and lipid peroxidation. Taken together, our findings suggest that Andro induces ferroptosis in Multiple myeloma (MM) cells via the P38/Nrf2/HO-1 pathway, providing a potential preventative and therapeutic approach for MM. | |||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Marker/Suppressor | |||
| Responsed Disease | Multiple myeloma | ICD-11: 2A83 | ||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 |
| U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 | |
| AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| Response regulation | Andrographolide (Andro) may block the Nrf2/HO-1 signaling pathway by activating P38 (MAPK14), thereby inducing ferroptosis. Moreover, inhibition of P38 expression rescued Andro-induced cell death, changes in the level of Nrf2 and HO-1 expression, Fe2+ and lipid peroxidation. Taken together, our findings suggest that Andro induces ferroptosis in Multiple myeloma (MM) cells via the P38/Nrf2/HO-1 pathway, providing a potential preventative and therapeutic approach for MM. | |||
Heme oxygenase 1 (HMOX1)
| In total 4 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Multiple myeloma | ICD-11: 2A83 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 | |
| U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 | ||
| AML12 cells | Normal | Mus musculus | CVCL_0140 | ||
| Response regulation | Andrographolide (Andro) may block the Nrf2/HO-1 signaling pathway by activating P38 ( MAPK14), thereby inducing ferroptosis. Moreover, inhibition of P38 expression rescued Andro-induced cell death, changes in the level of Nrf2 and HO-1 expression, Fe2+ and lipid peroxidation. Taken together, our findings suggest that Andro induces ferroptosis in Multiple myeloma (MM) cells via the P38/Nrf2/HO-1 pathway, providing a potential preventative and therapeutic approach for MM. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Multiple myeloma | ICD-11: 2A83 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 | |
| U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 | ||
| AML12 cells | Normal | Mus musculus | CVCL_0140 | ||
| Response regulation | Andrographolide (Andro) may block the Nrf2/HO-1 signaling pathway by activating P38 (MAPK14), thereby inducing ferroptosis. Moreover, inhibition of P38 expression rescued Andro-induced cell death, changes in the level of Nrf2 and HO-1 expression, Fe2+ and lipid peroxidation. Taken together, our findings suggest that Andro induces ferroptosis in Multiple myeloma (MM) cells via the P38/Nrf2/HO-1 pathway, providing a potential preventative and therapeutic approach for MM. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Driver/Suppressor | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | MKN74 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_2791 | |
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| Response regulation | Andrographis exerted antitumor effects in gastric cancer cell lines (MKN74 and NUGC4) by inhibiting proliferation, reducing colony formation and enhancing apoptotic activity. Moreover, andrographis treatment altered the expression of ferroptosis-associated genes, including HMOX1, GCLC, and GCLM. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Driver/Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Seven-week-old male athymic nude mice (Envigo, Houston, TX) were housed under controlled conditions of light and fed ad libitum. Approximately 5 x 106 parental and 5FUR HCT116 cells were suspended in the matrigel matrix (BD Biosciences, Franklin Lakes, NJ) and subcutaneously injected into mice using a 27-gauge needle (n = 10 per group). Mice were randomly assigned to different treatment groups and 5FU (30 mg/kg body weight) or andrographis (125 mg/kg body weight) or their combination were given intraperitoneally on alternative days for up to 15 days.
Click to Show/Hide
|
||||
| Response regulation | Combined treatment with andrographis was significantly more effective than 5FU and andrographis alone and that these effects were in part orchestrated through dysregulated expression of key genes (including HMOX1, GCLC, GCLM and TCF7L2) within the ferroptosis and Wnt-signaling pathways. Andrographis might offer a safe and inexpensive adjunctive therapeutic option in the management of colorectal cancer patients. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response regulation | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
Glutamate--cysteine ligase regulatory subunit (GCLM)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| In Vitro Model | MKN74 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_2791 |
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | |
| Response regulation | Andrographis exerted antitumor effects in gastric cancer cell lines (MKN74 and NUGC4) by inhibiting proliferation, reducing colony formation and enhancing apoptotic activity. Moreover, andrographis treatment altered the expression of ferroptosis-associated genes, including HMOX1, GCLC, and GCLM. | |||
Glutamate--cysteine ligase catalytic subunit (GCLC)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| In Vitro Model | MKN74 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_2791 |
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | |
| Response regulation | Andrographis exerted antitumor effects in gastric cancer cell lines (MKN74 and NUGC4) by inhibiting proliferation, reducing colony formation and enhancing apoptotic activity. Moreover, andrographis treatment altered the expression of ferroptosis-associated genes, including HMOX1, GCLC, and GCLM. | |||
Ferroptosis suppressor protein 1 (AIFM2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response regulation | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
References
