Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10029)
| Target Name | Heme oxygenase 1 (HMOX1) | ||||
|---|---|---|---|---|---|
| Gene Name | HMOX1 | ||||
| Sequence |
MERPQPDSMPQDLSEALKEATKEVHTQAENAEFMRNFQKGQVTRDGFKLVMASLYHIYVA
LEEEIERNKESPVFAPVYFPEELHRKAALEQDLAFWYGPRWQEVIPYTPAMQRYVKRLHE VGRTEPELLVAHAYTRYLGDLSGGQVLKKIAQKALDLPSSGEGLAFFTFPNIASATKFKQ LYRSRMNSLEMTPAVRQRVIEEAKTAFLLNIQLFEELQELLTHDTKDQSPSRAPGLRQRA SNKVQDSAPVETPRGKPPLNTRSQAPLLRWVLTLSFLVATVAVGLYAM Click to Show/Hide
|
||||
| Family | Heme oxygenase family | ||||
| Function |
Catalyzes the oxidative cleavage of heme at the alpha-methene bridge carbon, released as carbon monoxide (CO), to generate biliverdin IXalpha, while releasing the central heme iron chelate as ferrous iron . Affords protection against programmed cell death and this cytoprotective effect relies on its ability to catabolize free heme and prevent it from sensitizing cells to undergo apoptosis.
Click to Show/Hide
|
||||
| Gene ID | 3162 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
HMOX1 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Supraventricular tachycardia [ICD-11: BC81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Icariin | Phase 3 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response Description | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Ulinastatin | Phase 3 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Ulinastatin (UT1) plays a role in mitigation of Acetaminophen (APAP)-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
Mitogen-activated protein kinase 14 (MAPK14)
Multiple myeloma [ICD-11: 2A83]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Andrographis | Approved | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 |
| U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 | |
| AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| Response Description | Andrographolide (Andro) may block the Nrf2/HO-1 signaling pathway by activating P38 ( MAPK14), thereby inducing ferroptosis. Moreover, inhibition of P38 expression rescued Andro-induced cell death, changes in the level of Nrf2 and HO-1 expression, Fe2+ and lipid peroxidation. Taken together, our findings suggest that Andro induces ferroptosis in Multiple myeloma (MM) cells via the P38/Nrf2/HO-1 pathway, providing a potential preventative and therapeutic approach for MM. | |||
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Cetuximab | Approved | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
The DLD-1 cell suspension (4 x 106 cells/200 ul) was injected subcutaneously into the right dorsal flank of 5-week-old male BALB/c nude mice (Charles River, China). The mice were randomly divided into four groups (5 mice/group): 1) the control group, 2) the RSL3 group, 3) the cetuximab group, and 4) the RSL3 + cetuximab group. Both RSL3 (5 mg/kg) and cetuximab (13 mg/kg) were administered by intraperitoneal injection in a volume of 100 ul once per day. The tumour volume was calculated as 0.5 x length x width2. After 17 days of treatment, the mice were sacrificed, and the tumours were removed. Then, tumour tissue obtained from the different treated groups was subjected to western blotting and immunohistochemical experiments.
Click to Show/Hide
|
||||
| Response Description | Our work reveals that cetuximab enhances the cytotoxic effect of RSL3 on KRAS mutant Colorectal cancer (CRC) cells and that cetuximab enhances RSL3-induced ferroptosis by inhibiting the Nrf2/HO-1 axis through the activation of p38 MAPK. | ||||
Kelch-like ECH-associated protein 1 (KEAP1)
Degenerative arthritis [ICD-11: FA05]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
C57BL/6J (WT) mice (8 weeks old, male) were purchased from Nanjing Medical university, and AMPK-KO mice were purchased from Shanghai Model Organisms. They were used to create an OA model by destabilization of the medial meniscus surgery (DMM) (n = 6 per group). Briefly, after the mice were anaesthetized, a medial articular incision was made to expose the leftjoint cavity, and then the tibial collateral ligament was transected. Finally, the articular incision was closed. In the control group, only the joint cavity was opened. One week after surgery, 1 mg/kg baicalein (MCE, HY-N0196) per knee, 1 mg/kg ML385 (MCE, HY-100523) per knee, 1 mg/kg AICAR (MCE, HY-13417) per knee or 1 mg/kg of the ferroptosis inhibitor ferrostatin-1 (Fer-1, MCE, HY-100579) was injected into the joint cavity of the mice once a week. Meanwhile, saline was injected into the control group. Mice were sacrificed after surgery 10 weeks.
Click to Show/Hide
|
||||
| Response Description | Baicalein alleviated osteoarthritis (OA) development by improving the activity of AMPKa/Nrf2/HO-1 signaling to inhibit chondrocyte ferroptosis, revealing baicalein to be a potential therapeutic strategy for OA. AMPKa preserved Nrf2 abundance in chondrocytes and promoted Nrf2 into nucleus by promoting Keap1 degradation | ||||
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Astaxanthin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | |
| In Vivo Model |
6-week-Babl/c female mice were randomized to the following three groups of seven mice each: vehicle group, LPS group, Astaxanthin plus LPS group. Astaxanthin plus LPS group mice were pretreated with astaxanthin (20 mg/kg) byi.v injectionfor daily for 7 consecutive days. Astaxanthin was dissolved in 2%DMSO (vol/vol), 40% PEG-400 (vol/vol), 2% Tween 80 (vol/vol), and 56% PBS (vol/vol). On the last day, the mice were intraperitoneally injected with 5 mg/kg LPS or normal saline 2 h after the injection of astaxanthin. After 6 h of LPS stimulation, mice were euthanized to collect the BALF, and lung tissue samples. BALF was collected three times through a tracheal cannula with autoclaved normal saline, instilled up to a total volume of 1.8 ml.
Click to Show/Hide
|
||||
| Response Description | Astaxanthin protected LPS-induced cell inflammation and acute lung injury (ALI) in mice by inhibiting ferroptosis, and its effect was achieved through Keap1-Nrf2/HO-1 pathway. Therefore, our study indicates that ferroptosis will become a new target for the treatment of ALI, and astaxanthin is a potential drug for the treatment of ALI. | ||||
Hypoxia-inducible factor 1-alpha (HIF1A)
Testicular injury [ICD-11: NB9Z]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Diethylhexylphthalate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| HIF-1 signaling pathway | hsa04066 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
TM3 cells | Normal | Mus musculus | CVCL_4326 | |
| TM4 cells | Normal | Mus musculus | CVCL_4327 | ||
| In Vivo Model |
Specific pathogen-free (SPF) pregnant C57BL/6 mice were purchased from the Experimental Animal Center of Chongqing Medical University. Under SPF conditions, all mice had free access to food and water. All mice were fed under a 12-hour light/dark cycle at 25 ± 2 , and the relative humidity was 50 ± 5%. Male neonatal mice were selected at postnatal day (PND) 21. Since we aimed to mimic the prepubertal testicular injury induced byDEHPexposure, all male mice were randomly divided into four groups and treated by oral gavage from PND22 to PND35 with corn oil (Aladdin, C116023, China), or DEHP at a dose of 100 mg/kg/day (D100), 250 mg/kg/day (D250), or 500 mg/kg/day (D500).
Click to Show/Hide
|
||||
| Response Description | Di-(2-ethylhexyl) phthalate (DEHP) exposure led to testicular injury including excessive ROS accumulation. Prepubertal DEHP exposure induces ferroptosis in mouse testes. In particular, MEHP exposure leads to ferroptosis in Leydig and Sertoli cells via the HIF-1/HO-1 signaling pathway. | ||||
Fibronectin type III domain-containing protein 5 (FNDC5)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Iridin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hCMs (Human cardiomyocytes) | |||
| Response Description | Myocardial infarction is characterized by cardiomyocyte death and mitochondrial dysfunction induced by ischemia. FNDC5 overexpression and/or irisin administration elevated cell viability, decreased ferroptosis, and reversed mitochondrial impairments induced by hypoxia. Mechanistically, FNDC5/irisin reduced ferroptosis and reversed mitochondrial impairments by Nrf2/HO-1 axis in hypoxic cardiomyocytes. | |||
5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Ascorbic Acid | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
PaTu 8988t cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1847 | |
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| mEFs (Mouse embryonic fibroblasts) | |||||
| Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | ||
| In Vivo Model |
All animal experiments were approved by the Ethics Committee of Jiangsu University. To investigate the role of the combination of erastin and vitamin C in inducing ferroptosis, Panc02 cells (1 x 105 cells/site) were transfected and subcutaneously injected into 4-week-old C57BL/6 mice to generate xenografts. When the tumors reached a volume of 50-100 mm3, the mice were randomly divided into four groups (five mice per group) and treated with DMSO (control), imidazole ketone erastin (IKE, MedChemExpress), vitamin C, or a combination of erastin and vitamin C. Mice were treated with 80 ul (400M) erastin by intratumoral injection and/or 4 g/kg vitamin C by intraperitoneal injection every 2 days.
Click to Show/Hide
|
||||
| Response Description | The combination of erastin and vitamin C mainly increases the levels of ferrous iron through the AMPK/NRF2/HMOX1 signaling pathway. Cotreatment with erastin and vitamin C also exhibited a synergistic effect in a pancreatic cancer xenograft model in mice. | ||||
Ubiquitin carboxyl-terminal hydrolase 7 (USP7)
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rSCs (Rat spinal cords) | ||||
| In Vivo Model |
Forty-eight adult female Sprague-Dawley rats weighing 200-220 g were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Hunan, China) and divided into the following eight groups: sham (n = 6), SCI (n = 6), SCI + lentivirus (LV)-negative control 1 (NC1) (n = 6), SCI + LV-HMOX-1 (n = 6), SCI + LV-NC2 (n = 6), SCI + LV-USP7 (n = 6), SCI + LV-USP7 + sh-NC (n = 6), and SCI + LV-USP7 + sh-HMOX-1 (n = 6) groups. One week after animal adaptation and 30 min before operation, a subcutaneous injection of buprenorphine (0.05 mg/kg) was administered to the rats, along with inhalation of 5% isoflurane and an intraperitoneal injection of ketamine (75 mg/kg) and thiazide (10 mg/kg).
Click to Show/Hide
|
||||
| Response Description | In spinal cord injury (SCI) rats, USP7 directly bound to HMOX-1 and its overexpression promoted HMOX-1 expression via deubiquitination. To sum up, USP7 overexpression facilitated the expression of HMOX-1 through deubiquitination, thereby reducing ferroptosis and alleviating SCI. | ||||
Transcription factor AP-2-alpha (TFAP2A)
Gallbladder cancer [ICD-11: 2C13]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
H69 cells | Normal | Homo sapiens | CVCL_8121 |
| GBC-SD cells | Gallbladder carcinoma | Homo sapiens | CVCL_6903 | |
| Response Description | In vitro, gallbladder carcinoma (GBC) exhibited upregulated expression of TFAP2A, whose inhibition reduced GBC cell proliferation, migration, and invasion. Fe2+ and MDA levels were elevated. TFAP2A silencing attenuated the expression of key genes associated with oxidative stress such as heme oxygenase 1 (HO-1), nuclear factor erythroid 2 like 2 (Nrf2), ferritin heavy chain 1 (FTH1) and NAD(P)H quinone dehydrogenase 1 (NQO1). | |||
Pannexin-1 (PANX1)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
C57BL/6 mice (male, 10-15 weeks old) were food-deprived for 12 h before the procedures and were anesthetized with intraperitoneal injection of 1% sodium pentobarbital solution (40 mg/kg). Using a midline abdominal incision, bilateral renal IRI was induced by clamping renal pedicles for 30 min. After removal of the clamp, the kidneys were inspected to confirm reperfusion.
Click to Show/Hide
|
||||
| Response Description | Panx1 deletion induced the expression of a cytoprotective chaperone, heme oxygenase-1 (HO-1), and inhibited ferroptinophagy via the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway. In summary, Panx1 deletion protects against renal ischemia/reperfusion injury (IRI) by attenuating MAPK/ERK activation in a ferroptotic pathway. | ||||
Nuclear receptor subfamily 1 group D member 2 (NR1D2)
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MRTEpiC (Mouse renal tubular epithelial cells) | ||||
| mRTECs (Mouse renal tubular epithelial cells) | |||||
| M4100-57 (Mouse renal tubular epithelial cells) | |||||
| In Vivo Model |
Gene knockout (Rev-erb-a-/-,Rev-erb-b-/-and icDKO) mice and wild-type littermates were treated with folic acid (i.p., 100 mg/kg, once daily for seven consecutive days) at ZT6 or ZT18 to induce acute kidney injury.
Click to Show/Hide
|
||||
| Response Description | Rev-erb-b (NR1D2) promoted ferroptosis by repressing the transcription of Slc7a11 and HO1 (two ferroptosis-inhibitory genes) via direct binding to a RORE cis-element. Targeted inhibition of Rev-erb-b limits ferroptosis to ameliorate folic acid-induced acute kidney injury in mice. | ||||
Nuclear receptor subfamily 1 group D member 1 (NR1D1)
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MRTEpiC (Mouse renal tubular epithelial cells) | ||||
| mRTECs (Mouse renal tubular epithelial cells) | |||||
| M4100-57 (Mouse renal tubular epithelial cells) | |||||
| In Vivo Model |
Gene knockout (Rev-erb-a-/-,Rev-erb-b-/-and icDKO) mice and wild-type littermates were treated with folic acid (i.p., 100 mg/kg, once daily for seven consecutive days) at ZT6 or ZT18 to induce acute kidney injury.
Click to Show/Hide
|
||||
| Response Description | Rev-erb-a (NR1D1) promoted ferroptosis by repressing the transcription of Slc7a11 and HO1 (two ferroptosis-inhibitory genes) via direct binding to a RORE cis-element. Targeted inhibition of Rev-erb-a limits ferroptosis to ameliorate folic acid-induced acute kidney injury in mice. | ||||
mmu-miR-7212-5p (miRNA)
Sepsis [ICD-11: 1G40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
TCMK-1 cells | Normal | Mus musculus | CVCL_2772 | |
| In Vivo Model |
C57BL/6 mice (male, 6-8 weeks old) were purchased from Guangdong Yaokang Biotechnology Co., Ltd. (China). According to a common sepsis protocol, we used the cecal ligation and puncture (CLP) method to construct a model of sepsis. For RNA sequencing, 5 mice were randomly divided into 2 groups: the CLP group (n = 3) and the sham group (n = 2). We anesthetized mice in the CLP group with 24% isoflurane. Under aseptic conditions, a 2-cm midline laparotomy was created below the diaphragm to expose the cecum. Two-thirds of the cecum was ligated with a 5-0 silk suture and punctured twice using a 22-gauge needle. The cecum was gently squeezed to extrude a small amount of feces through the perforation site. Animals were resuscitated with 1 mL of subcutaneous saline after CLP. The procedures of the sham group (controls) were the same as the CLP group, except for the ligation and perforation. The mice were sacrificed via neck fracture 6 hours after CLP, and the kidneys were taken for subsequent RNA sequencing.
Click to Show/Hide
|
||||
| Response Description | The mmu-miR-7212-5p-Hmox1 axis in ferroptosis and m6A RNA methylation regulators may have potential clinical significance for the future treatment of Sepsis-associated acute kidney injury (SA-AKI). | ||||
Mitogen-activated protein kinase 8 (MAPK8)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | SP600125 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
BV-2 cells | Normal | Mus musculus | CVCL_0182 |
| Response Description | Following addition of the JNK (MAPK8) inhibitor SP600125, the expression of HO-1 decreased, expression of FTH1 was increased and iron accumulation was decreased. Therefore, it was hypothesized that NPs induced ferroptosis in BV2 cells via the JNK/HO-1/FTH1 pathway. | |||
Fibroblast growth factor 21 (FGF21)
Hereditary haemochromatosis [ICD-11: 5C64]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mPHs (Mouse primary hepatocytes) | ||||
| In Vivo Model |
In the adenovirus-mediated FGF21 over-expression mouse model, 12-week-old C57BL/6J male mice were divided into four groups: (1) EGFP vector overexpression + PBS injection group (n = 10), (2) EGFP vector overexpression + iron dextran injection group (n = 10), (3) FGF21-EGFP overexpression + PBS injection group (n = 10) and (4) FGF21-EGFP overexpression + iron dextran injection group (n = 10). The mice were administered PBS and iron dextran by intraperitoneal injection for 7 days.
Click to Show/Hide
|
||||
| Response Description | FGF21 could protect hepatocytes from developing iron overload-induced ferroptosis by stimulating HO-1 ubiquitination and subsequent degradation. The FGF21HO-1 pathway could be targeted for treating iron overload-induced ferroptosis-related diseases, particularly hereditary haemochromatosis (HH). | ||||
Unspecific Regulator
Sepsis [ICD-11: 1G40]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | ||||
| Responsed Drug | Ferulic acid | Patented | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
Briefly, female BALB/c mice (6-8 weeks, weighing 20-25 g, purchased from Hunan SJA Laboratory Animal Co., Ltd., Changsha, Hunan, China) were raised in specific pathogen-free conditions under controlled temperature (23-25 ) and humidity (40-80%) as well as a 12 h dark/light cycle for 1 week of acclimation. They were fed a standard chow diet and waterad libitum. Mice were anaesthetised with 2% isoflurane inhalation and underwent moderate caecal ligation and puncture in accordance with a previously reported protocol (Rittirsch etal.2009). Meanwhile, mice in the control groups were subjected to a sham operation. Buprenorphin (0.05 mg/kg) was injected for postoperative analgesia, and all mice were placed in cages immediately after the surgical procedures with free access to water and food (Rittirsch etal. 2009).
Click to Show/Hide
|
||||
| Response Description | Collectively, our data highlighted the alleviatory role of ferulic acid in sepsis-induced ALI by activating the Nrf2/HO-1 pathway and inhibiting ferroptosis, offering a new basis for sepsis treatment. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | ||||
| Responsed Drug | Dexmedetomidine | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mVTs (Mouse ventricular tissues) | ||||
| In Vivo Model |
A total of 32 male C57BL/6 mice (25 g, 8 weeks old) were obtained from the Guangdong Medical Lab Animal Center and housed in the Laboratory Animal Service Center (Jinan University, Guangdong, China). Mice were anesthetized with isoflurane (RWD Life Science) inhalation at the concentration of 2.5% for anesthetic induction and then at 1% for anesthetic maintenance until the end of the CLP. During the experiment, the body temperature was kept at 36-38 with a heating pad. Anesthetized mice were subjected to midline laparotomy. The cecum was carefully separated to avoid blood vessels damage and the cecum was identified and punctured twice with a 22-gauge needle. Then, the abdominal cavity was closed with two epithelium layers, followed by a normal saline injection subcutaneously for resuscitation before mice were returned to the cage.
Click to Show/Hide
|
||||
| Response Description | The attenuation of sepsisinduced HO1 overexpression and iron concentration, and the reduction of ferroptosis via enhancing GPX4, may be the major mechanisms via which Dexmedetomidine alleviates sepsis induced myocardial cellular injury. | ||||
Glioblastoma [ICD-11: 2A00]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Responsed Drug | Dihydroartemisinin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
All BALB/C nude mice were purchased from Huafukang Biotechnology (Beijing, China). These mice were 5 weeks old and weighed 14-16 g. We established subcutaneous tumour-forming mouse model by injecting 5 x 106 U87 cells into the lateral abdomen of BALB/C nude mice. Animals were then treated with DHA solvent (50 mg/kg) by intragastric administration once a day for 26 days.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin could promote ferroptosis in glioma cells. Low expression of GPX4 and high expression of HMOX1 were identified in DHA treated glioma cells. MAZ was further identified as the direct target of long noncoding RNA (lncRNA) TUG1 through luciferase assay. Downregulated expression of TUG1 and upregulated expression of MAZ were identified in DHA treated glioma cells. TUG1 overexpression or inhibition of FTH1 expression could enhance the antiglioma effect of DHA in vitro and in vivo, providing a promising strategy to enhance the antitumor effect of DHA in glioma. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Responsed Drug | Siramesine | Terminated | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| Response Description | Lapatinib and siramesine was the most effective tyrosine kinase inhibitor and lysosome disruptor drug combination in inducing synergistic cell death in A549 and U87 cells. This cell death was through ferroptosis mediated by ROS and reduced expression of HO-1 in glioma cells. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Responsed Drug | Lapatinib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| Response Description | Lapatinib and siramesine was the most effective tyrosine kinase inhibitor and lysosome disruptor drug combination in inducing synergistic cell death in A549 and U87 cells. This cell death was through ferroptosis mediated by ROS and reduced expression of HO-1 in glioma cells. | ||||
Central nervous system cancer [ICD-11: 2A02]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | |||
| Responsed Drug | Polygonatum cyrtonemaHua Polysaccharides | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
BV-2 cells | Normal | Mus musculus | CVCL_0182 |
| Response Description | Subsequent studies have revealed that Polygonatum cyrtonemaHua Polysaccharides (PCP) alleviates ferroptosis in microglia due to protein levels of ERASTIN/RSL3 inhibitor SLC7A11/GPX4 by activating the NRF2/HO-1 signaling pathway. PCP has the development potential as a new drug candidate for treating CNS diseases. | |||
Acute myeloid leukaemia [ICD-11: 2A60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | |||
| Responsed Drug | Honokiol | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 |
| U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | |
| SKM-1 cells | Acute myeloid leukemia | Homo sapiens | CVCL_0098 | |
| Response Description | Honokiol decreased the viability of the targeted Acute myeloid leukemia cells, induced their cell cycle arrest at G0/G1 phase, and inhibited their colony-formation capacity. Honokiol also triggers a noncanonical ferroptosis pathway in THP-1 and U-937 cells by upregulating the level of intracellular lipid peroxide and HMOX1 significantly. And HMOX1 was a critical target in honokiol-induced ferroptosis. | |||
Osteosarcoma [ICD-11: 2B51]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | |||
| Responsed Drug | 3,5-bis((E)-2-fluorobenzylidene)piperidin-4-one hydrochloride | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 |
| SAOS-2 cells | Osteosarcoma | Homo sapiens | CVCL_0548 | |
| Response Description | 3,5-bis((E)-2-fluorobenzylidene)piperidin-4-one hydrochloride (EF24) upregulated HMOX1 to suppress GPX4 expression to induce ferroptosis by increasing MDA level, ROS level and intracellular ferric ion level. Thus, EF24 might serve as a potential agent for the treatment of HMOX1-positive osteosarcoma patients. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | |||
| Responsed Drug | Zoledronic acid | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| MNNG/HOS Cl #5 cells | Osteosarcoma | Homo sapiens | CVCL_0439 | |
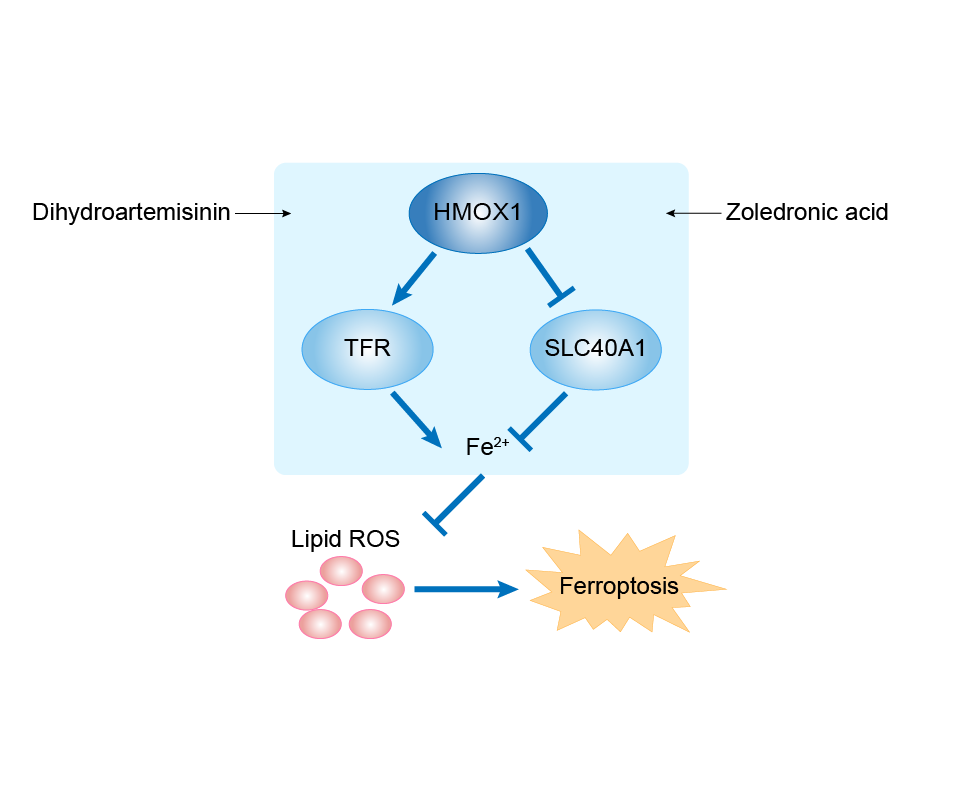
| Response Description | Zoledronic acid treatment decreased cell viability and promoted the increase in lipid peroxide content and PTGS2 expression. Our results indicate that zoledronic acid induces ferroptosis by decreasing ubiquinone content and promoting HMOX1 expression in osteosarcoma cells. | |||
Nasopharyngeal cancer [ICD-11: 2B6B]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [25] | ||||
| Responsed Drug | Cephalosporin | Approved | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| XWLC-05 cells | Lung adenocarcinoma | Homo sapiens | CVCL_IQ71 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| CNE2 cells | Normal | Homo sapiens | CVCL_6889 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| K-562 cells | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | ||
| ECV-304 cells | Bladder carcinoma | Homo sapiens | CVCL_2029 | ||
| In Vivo Model |
6-8 week old male balb/cnude micewere purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Nasopharyngeal carcinoma CNE2 cells were collected during logarithmic growth and rinsed with PBS twice and resuspended to a density of 1 x 107 cells/ml using fresh and cooled DMEM/F12 medium (free of FBS and antibiotics). Each mouse was inoculated subcutaneously with a 0.1 ml cell suspension on the right-side flank. Tumor-bearing mice were used for in vivo anticancer studies 8 days after inoculation when the average tumor volume reached ~200 mm3.
Click to Show/Hide
|
||||
| Response Description | Cephalosporin antibiotics showed highly specific and selective anticancer activity on nasopharyngeal carcinoma CNE2 cells both in vitro and vivo with minimal toxicity. Pathway analyses indicate apoptotic and the ErbB-MAPK-p53 signaling pathways are significantly enriched. HMOX1 represents the top one ranked upregulated gene by COS and overlaps with 16 of 42 enriched apoptotic signaling pathways. Inhibition of HMOX1 significantly reduced the anticancer efficacy of cefotaxime in CNE2 cells. | ||||
Oral squamous cell carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | |||
| Responsed Drug | Carnosic acid | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Response Description | The current findings highlight that carnosic acid may re-sensitize cisplatin-resistant cells to cisplatin by inducing ferroptosis, which involves the inactivation of Nrf2/HO-1/xCT pathway. Hence, this research may support a promising therapeutic approach to overcome chemoresistance in Oral squamous cell carcinoma. | |||
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [27] | ||||
| Responsed Drug | 5-aminolevulinic acid | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
KYSE30 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1351 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
KYSE30 cells were subcutaneously inoculated with 5 x 106 cells per site into both flanks on day 0. At 1 week after transplantation, tumor-bearing mice were randomly assigned to one of the following three groups: (1) saline as a control, (2) 10 mg/kg/day of 5-ALA, or (3) 30 mg/kg/day of 5-ALA. The treatment groups were orally administered 5-ALA once daily for 4 weeks, and the control group was orally administered saline during the same period.
Click to Show/Hide
|
||||
| Response Description | Modulation of GPX4 and HMOX1 by 5-aminolevulinic acid (5-ALA) induced ferroptosis in esophageal squamous cell carcinoma (ESCC). Furthermore, 5-ALA led to an increase in lipid peroxidation and exerted an antitumor effect in various cancer cell lines, which was inhibited by ferrostatin-1. Thus, 5-ALA could be a promising new therapeutic agent for ESCC. | ||||
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [28] | |||
| Responsed Drug | Andrographis | Approved | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
MKN74 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_2791 |
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | |
| Response Description | Andrographis exerted antitumor effects in gastric cancer cell lines (MKN74 and NUGC4) by inhibiting proliferation, reducing colony formation and enhancing apoptotic activity. Moreover, andrographis treatment altered the expression of ferroptosis-associated genes, including HMOX1, GCLC, and GCLM. | |||
Colorectal cancer [ICD-11: 2B91]
| In total 4 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [29] | ||||
| Responsed Drug | Propofol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response Description | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [30] | ||||
| Responsed Drug | Talaroconvolutin A | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| In Vivo Model |
5 x 106 HCT116 cells were inoculated subcutaneously in the underarm of Balb/c nude female mice (5-week old). The inoculated mice were randomly divided into two groups (6 mice each group). When the tumor reached 300 mm3, the drug group was given TalaA intraperitoneally at a dose of 6.0 mg/kg, and the control group was given the same dose of cosolvent-corn oil. The drug (or cosolvent) was injected every 2 days. Body weight and tumor volume were measured every 2 days.
Click to Show/Hide
|
||||
| Response Description | Talaroconvolutin A (TalaA) downregulated the expression of the channel protein solute carrier family 7 member 11 (SLC7A11) but upregulated arachidonate lipoxygenase 3 (ALOXE3), promoting ferroptosis. TalaA causes upregulation of HMOX1 which lead to the degradation of heme and the release of free iron, accumulating in mitochondria and giving rise to lipid peroxidation. TalaA could be a new potential powerful drug candidate for colorectal cancer therapy. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [31] | ||||
| Responsed Drug | tagitinin C | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| Response Description | Tagitinin C induces ferroptosis in colorectal cancer cells and has synergistic effect together with erastin. Mechanistically, tagitinin C induces ferroptosis through ER stress-mediated activation of PERK-Nrf2-HO-1 signaling pathway. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [32] | ||||
| Responsed Drug | Andrographis | Approved | |||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Seven-week-old male athymic nude mice (Envigo, Houston, TX) were housed under controlled conditions of light and fed ad libitum. Approximately 5 x 106 parental and 5FUR HCT116 cells were suspended in the matrigel matrix (BD Biosciences, Franklin Lakes, NJ) and subcutaneously injected into mice using a 27-gauge needle (n = 10 per group). Mice were randomly assigned to different treatment groups and 5FU (30 mg/kg body weight) or andrographis (125 mg/kg body weight) or their combination were given intraperitoneally on alternative days for up to 15 days.
Click to Show/Hide
|
||||
| Response Description | Combined treatment with andrographis was significantly more effective than 5FU and andrographis alone and that these effects were in part orchestrated through dysregulated expression of key genes (including HMOX1, GCLC, GCLM and TCF7L2) within the ferroptosis and Wnt-signaling pathways. Andrographis might offer a safe and inexpensive adjunctive therapeutic option in the management of colorectal cancer patients. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [33] | ||||
| Responsed Drug | Haloperidol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| Response Description | The study demonstrated that haloperidol strengthened sorafenib-induced ferroptosis in Hepatocellular carcinoma cell lines for the first time. During ferroptosis, haloperidol substantially increased the cellular levels of Fe2+, GSH and lipid peroxidation. Additionally, haloperidol induced the expression of HO-1. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [34] | ||||
| Responsed Drug | Eupalinolide B | Investigative | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell migration | |||||
| Cell proliferation | |||||
In Vitro Model |
SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | |
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| In Vivo Model |
Four-week-old nude mice (BALB/c) were used, and they were housed for 1 week in a specific pathogen-free (SPF) environment before experimentation. Each mouse received subcutaneous injections of 1 x 106 SMMC-7721 or HCCLM3 cells in 200 uL phosphate-buffered saline (PBS) into both flanks. One group was intraperitoneally injected every two days with EB at 25 or 50 mg/mouse body weight, for a total of 3 weeks. As a control, DMSO was used in another group. Tumor size was measured to calculate tumor volume, and mouse body weights were monitored every two days.
Click to Show/Hide
|
||||
| Response Description | Eupalinolide B (EB) exerts anti-proliferative activity in hepatic carcinoma by blocking cell cycle arrest at S phase and inducing ferroptosis mediated by endoplasmic reticulum (ER) stress, as well as HO-1 activation. And EB has the ability to inhibit cell proliferation and migration in hepatic carcinoma. | ||||
Lung cancer [ICD-11: 2C25]
| In total 4 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [35] | ||||
| Responsed Drug | Ginkgetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SPC-A1 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | ||
| In Vivo Model |
Briefly, when tumours on transplanted nude mice reached around 100 mm3, the mice were randomized divided into eight groups: control, ginkgetin, DDP, ginkgetin + DDP, UAMC 3203, ginkgetin + UAMC 3203, DDP + UAMC 3203, ginkgetin + DDP + UAMC 3203. Both DDP (3 mg/kg) and ginkgetin (30 mg/kg) were administered by intraperitoneal injection, with 2 - 3 times per week and once per day, respectively. UAMC 3203 (10 mg/kg) was administered 5 days/week by intraperitoneally injection. Tumour size and body weight were measured 3 times per week. After dosing 31 days, the nude mice were sacrificed, and tumours were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | The induction of ferroptosis mediated by ginkgetin was further confirmed by the decreased expression of SLC7A11 and GPX4, and a decreased GSH/GSSG ratio. Simultaneously, ginkgetin disrupted redox hemostasis in DDP-treated cells, as demonstrated by the enhanced ROS formation and inactivation of the Nrf2/HO-1 axis. Ginkgetin also enhanced DDP-induced mitochondrial membrane potential (MMP) loss and apoptosis in cultured non-small cell lung cancer (NSCLC) cells. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [36] | ||||
| Responsed Drug | Gefitinib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRASmutant non-small-cell lung cancer (NSCLC) cells. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [36] | ||||
| Responsed Drug | Betulin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRASmutant non-small-cell lung cancer (NSCLC) cells. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [37] | ||||
| Responsed Drug | S-3'-hydroxy-7', 2', 4'-trimethoxyisoxane | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
When tumor volumes in xenograft nude mice reached an average of roughly 100 mm3, the mice were randomly divided into 3 groups of 6 mice each: control, ShtIX, and ShtIX + Fer-1. The treated group received ShtIX or ShtIX combined with Fer-1 injections into the tail vein of the mice every three days for 7 times, whereas the control group received saline. Every four days, the volume and weight of the tumors were measured. As soon as the test was completed, the nude mice were slaughtered, and the tumor tissues were retrieved. The in vivo experiments were approved by the Animal Care and Use Committee of Hainan Medical College and following the animal rules.
Click to Show/Hide
|
||||
| Response Description | S-3'-hydroxy-7', 2', 4'-trimethoxyisoxane (ShtIX) caused ferroptosis in Non-small cell lung cancer (NSCLC) cells, and inhibiting the Nrf2/HO-1 pathway can considerably exacerbate the effect of ShtIX-induced ferroptosis. | ||||
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [38] | ||||
| Responsed Drug | Shuganning injection | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
SK-BR-3 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| MDA-MB-468 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| WPMY-1 cells | Normal | Homo sapiens | CVCL_3814 | ||
| U-87MG cells | Glioblastoma | Homo sapiens | CVCL_0022 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| In Vivo Model |
MDA-MB-231 cells resuspended in PBS (2 x 106/100 m1) were injected subcutaneously into bothhind limbsof 4-6 weeks old femalenude mice. A week later, SGNI was administrated byintraperitoneal injectionat a dose of 112.5 mg/kg/3 d.
Click to Show/Hide
|
||||
| Response Description | Shuganning injection (SGNI) induced a ferroptotic cell death of Triple-negative breast cancer (TNBC) cells. Mechanistically, SGNI induced ferroptosis was dependent on HO-1, which promotes intracellular labile iron pool accumulation, and was alleviated by HO-1 knockdown and inhibition by tin protoporphyrin IX. | ||||
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [39] | ||||
| Responsed Drug | Norcantharidin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Athymic nu/nu female mice aged 6-8 weeks (n = 9; mean weight, 20.21 ± 1.54 g) were purchased from the specific pathogen SPF (Beijing) Lab Animals Technology Co. Ltd. Mice were housed in a temperature- and humidity-controlled environment (20-24 , 45-55% humidity), with free access to food and water and in groups of three. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC ID: 17-3256) at Nantong University and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Nuclear factor erythroid 2-related factor 2 (NRF2), heme oxygenase 1 (HO-1), glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (xCT) expression levels were significantly decreased following norcantharidin (NCTD) treatment. Collectively, NCTD may represent a potent anticancer agent in ovarian cancer cells, and NCTD-induced ferroptotic cell death may be achieved by inhibiting the NRF2/ HO-1/GPX4/xCT axis. | ||||
Corpus uteri cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [40] | |||
| Responsed Drug | Juglone | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| Cell migration | ||||
In Vitro Model |
Ishikawa cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 |
| Response Description | Juglone as a natural quinone from C. cathayensisgreen peel could exert its anti-cancer activity by inducing iron dependent autophagy and inhibiting the migration of endometrial cancer (EC) cells. Subsequently, Fe2+ accumulation, lipid peroxidation, GSH depletion, the upregulation of HMOX1, and heme degradation to Fe2+ were reported. Juglone was involved in inducing autophagy and inhibiting cell migration and endoplasmic reticulum stress. | |||
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [41] | |||
| Responsed Drug | Isothiocyanate-containing hybrid AR antagonist 13 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
VCaP cells | Prostate carcinoma | Homo sapiens | CVCL_2235 |
| LNCaP cells | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| LNCaP C4-2 cells | Prostate carcinoma | Homo sapiens | CVCL_4782 | |
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | |
| RWPE-1 cells | Normal | Homo sapiens | CVCL_3791 | |
| MDA-kb2 cells | Breast adenocarcinoma | Homo sapiens | CVCL_6421 | |
| Response Description | ITC-ARi 13 and buthionine sulfoximine (BSO) cooperatively downregulate AR and induce ferroptosis likely through increasing the accessibility of 13/12b to cellular targets, escalating free intracellular ferrous iron and attenuating GSH-centered cellular defense and adaptation. Further studies on the combination of ITC-ARi and GSH synthesis inhibitor could result in a new modality against castration-resistant prostate cancer (CRPC). Collectively, the combination of ITC-ARi 13 and BSO reveals a pro-ferroptotic role of Nrf2 through upregulating HO-1 under GSH-deficient conditions. | |||
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [42] | |||
| Responsed Drug | Curcumin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 |
| Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | |
| FTC 238 cells | Thyroid gland follicular carcinoma | Homo sapiens | CVCL_2447 | |
| Response Description | Knockdown of HO-1 inhibits ferroptosis by upregulating the GPX4 expression in follicular thyroid cancer cells. We conclude that curcumin inhibits the tumorigenesis of follicular thyroid cancer via HO-1-induced activation of the ferroptosis signalling pathway. | |||
Testicular dysfunction [ICD-11: 5A81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [43] | ||||
| Responsed Drug | Cadmium | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
TM3 cells | Normal | Mus musculus | CVCL_4326 | |
| In Vivo Model |
Eight-week-old adult male C57BL/6J mice were raised for 7 days before the study in a temperature and humidity controlled animal facility (22-25 , 50% relative humidity) with a12-h light/dark cycles. A total of 10 mice were randomly assigned into two groups. As described in our previous study, the control group was treated with 0.9% NaCl (0 mg CdCl2), and the treatment group was intraperitoneally injected with CdCl2 at a dose of 1.0 mg per kg of body weight for 1 week.
Click to Show/Hide
|
||||
| Response Description | Cadmium induced ferroptosis by iron homeostasis dysregulation, mediated by excessive activation of HMOX-1. The disruption of autophagy flow contributed to Cd-induced testicular dysfunction and attenuated testosterone synthesis. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [44] | |||
| Responsed Drug | Gastrodin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
C6 cells | Malignant glioma | Rattus norvegicus | CVCL_0194 |
| Response Description | Gastrodin induced GPX4, Nrf2 and HO-1 expression to protect C6 cells from H2O2-induced ferroptosis. Gastrodin pretreatment effectively reduced H2O2-induced oxidative damage, indicating gastrodin is a potential antioxidant that reduced cytotoxic ROS. The role of gastrodin in ferroptosis presents a new perspective for understanding parkinson's disease. | |||
Alzheimer disease [ICD-11: 8A20]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [45] | ||||
| Responsed Drug | Salidroside | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
B6.1291-Nfe2l2tm1Ywk/J (Nrf2-/-mice, 017009)and wild-type C57BL/6 were originally from the Jackson Laboratory. Grouping and administration were started when weighing approximately 28-33 g. All mice were housed in a laboratory environment with free access to adequate food and water under a 12 h/12 h light/dark cycle at 22 ± 1 and 55 ± 5% humidity. All procedures conformed to the protocols of the Animal Welfare Commission and Ethical Committee of Southern Medical University. The Nrf2-/- mice were identified by genotyping as shown in Fig.1B. WT and Nrf2-/- mice were randomly assigned to 32 groups (3 groups for WT mice and 3 groups for Nrf2-/- mice) as follows: a sham group, sham + Ab1-42 group, and Salidroside + Ab1-42 group.
Click to Show/Hide
|
||||
| Response Description | Salidroside plays a neuroprotective role by inhibiting neuronal ferroptosis in A1-42-induced Alzheimer's disease mice and Glu-injured HT22 cells, and its mechanism is related to activation of the Nrf2/HO1 signaling pathway. | ||||
Subarachnoid Hemorrhage [ICD-11: 8B01]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [46] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
SAH model was constructed by applying endovascular perforation in the rats, according to the protocol introduced in a previous study (Wei et al., 2020), except for slight modifications. Briefly, after performing intraperitoneal anesthesia with 40 mg/kg sodium pentobarbital, the right common carotid, external and internal carotid arteries of the rats were exposed and isolated. The right external carotid artery was ligated, and a 4-0 single-strand nylon thread was used to insert the right internal carotid artery through the stump of the external carotid artery and the bifurcation of the common carotid artery. When resistance is felt when the suture enters the intracranial segment, proceed approximately 3 mm to penetrate internal carotid artery at the bifurcation of middle cerebral artery. The suture was held in this position for 10 s and was then withdrawn. The rats in the Sham group went through an identical procedure, without the suture at the point of resistance. Throughout the experiment, the body temperature of the rats was sustained at around 37 by using a thermal blanket. After the wounds were sutured, the rats were placed in a separate cage and neurological function was closely observed.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AS-IV) triggered Nrf2/HO-1 signaling pathway and alleviated ferroptosis due to the induction of subarachnoid hemorrhage (SAH). The Nrf2 inhibitor ML385 blocked the beneficial effects of neuroprotection. These results consistently suggest that ferroptosis is profoundly implicated in facilitating EBI in SAH, and that AS-IV thwarts the process of ferroptosis in SAH by activating Nrf2/HO-1 pathway. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [47] | ||||
| Responsed Drug | Caryophyllene | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPAs (Rat primary astrocytes) | ||||
| In Vivo Model |
Rats were anesthetized withisoflurane(2-3% oxygen) and placed in asupine position. And theright common carotid artery (CCA),external carotid artery (ECA), andinternal carotid artery (ICA) were exposed in sequence and separated carefully. Then we ligated the CCA and the ECA in turn, and at the same time, we clamped the internal carotid artery with an arterial clamp. Finally, we inserted a silicone nylon monofilament from the CCA into themiddle cerebral arteryand temporarily fixed it. After 1.5 h ofischemia, the monofilament was taken out and the blood vessels were ligated at theincision. The neck wound was sutured with surgical sutures. Subsequent experiments were performed after 12 h ofreperfusion. In thesham operationrats, except for the absence of the monofilament, the sham operation rats underwent the same surgical procedures as the MCAO/R model rats.
Click to Show/Hide
|
||||
| Response Description | Our results indicated the critical role of ferroptosis in cerebral ischemia reperfusion injury. For the first time, we showed that the significant neuroprotective effects of b-Caryophyllene (BCP) in attenuating ischemic stroke injury are correlated with ferroptosis regulation, and its mechanism is associated with activation of the NRF2/HO-1 axis. | ||||
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [48] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
1% sodium pentobarbital (40 mg/kg) was administered to the rats intraperitoneally to anesthetize them before placing them in a brain stereotaxic device. An incision was created in the midline of the neck to expose the common internal and external carotid arteries. After ligating and cutting the external carotid artery on the left side, a 3-mm stump was exposed. We then perforated the carotid artery at the bifurcation of the middle and anterior cerebral arteries utilizing an 18-20-mm-long surgical filament (0.26 mm diameter; Beijing Cinontech Co. Ltd., China) was threaded through the external carotid artery stump into the internal carotid artery and left in situ for 120 min. After that, the filament was withdrawn to facilitate reperfusion. Rats in the sham surgery group received the identical procedure as the other rats but without filament insertion.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AS-IV) administration decreased the infarct volume, brain edema, neurological deficits, and inflammatory cytokines TNF-, interleukin-1 (IL-1), IL-6, and NF-B, increased the levels of SLC7A11 and glutathione peroxidase 4 (GPX4), decreased lipid reactive oxygen species (ROS) levels, and prevented neuronal ferroptosis. Meanwhile, AS-IV triggered the Nrf2/HO-1 signaling pathway and alleviated ferroptosis due to the induction of stroke. | ||||
Thromboembolic stroke [ICD-11: 8B20]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [49] | ||||
| Responsed Drug | ADA-409-052 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BV-2 cells | Normal | Mus musculus | CVCL_0182 | |
| Neuro-2a cells | Neuroblastoma | Mus musculus | CVCL_0470 | ||
| RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | ||
| In Vivo Model |
Brain penetration study was carried out by TCG Lifesciences Ltd (Kolkata, India) in male BALB/c mice (6-8 weeks old; 18-20 g, in-house breeding). Mice were housed individually under 12/12 h lightdark cycle with free access to food and water. In two experiments 10 mg/kg or 30 mg/kg of ADA-409-052 (HPLC Purity: 99.7%) dissolved in Tween-80 (0.5%, Merck, Germany) -methylcellulose (Sigma) solution was administered as a single bolus by oral gavage (p.o.). ADA-409-052- or vehicle-administered mice were sacrificed 0.75, 4, and 24 h later (n = 3/group).
Click to Show/Hide
|
||||
| Response Description | ADA-409-052 inhibits tert-Butyl hydroperoxide (TBHP)-induced lipid peroxidation (LP) and protects against ferroptotic cell death triggered by glutathione (GSH) depletion or glutathione peroxidase 4 (GPx4) inhibition in neuronal cell lines. Moreover, ADA-409-052 efficiently reduces infarct volume, edema and expression of pro-inflammatory genes in a mouse model of thromboembolic stroke. In addition, ADA-409-052 reduced the expression of stroke-induced Hmox1. | ||||
Nervous system disease [ICD-11: 8E7Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [50] | |||
| Responsed Drug | Gastrodin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Gastrodin (GAS) is a component of Gastrodia elata Blume, with strong antioxidant activity in neurodegenerative diseases. GAS increased the nuclear translocation of Nrf2, up-regulated the downstream HO-1 protein expression in HT-22 cells following treatment with glutamate. | |||
Age-related macular degeneration [ICD-11: 9B75]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [51] | ||||
| Responsed Drug | Sodium iodate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 | |
| In Vivo Model |
In this study, sixteen-week-old male C57BL/6 mice were used and housed in a standard laboratory environment. Forin vivostudies, left eyes were pretreated with a single intravitreal injection of DMSO (1 uL) or 1X PBS (1 uL), and right eyes were pretreated with Fer-1 (30 uM, a single intravitreal injection, 1 uL), DFO (100 uM, a single intravitreal injection, 1 uL), or ZnPP (50 mg/kg, intraperitoneal injection once a week). 15 min later, injection of NaIO3(35 mg/kg, a single injectionviatail vein), or 1X PBS (a single injectionviatail vein, 75 uL) has been performed. After 2 weeks, mice were used for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Sodium iodate-induced oxidative stress model has been identified to be heme oxygenase-1 (HO-1)-regulated ferroptosis, which is controlled by the Nrf2-SLC7A11-HO-1 hierarchy. These findings highlight that targeting HO-1-mediated RPE ferroptosis could serve as an effectively retinal-protective strategy for retinal degenerative diseases prevention, including age-related macular degeneration (AMD). | ||||
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [52] | ||||
| Responsed Drug | Curcumin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Two-month-old male New Zealand rabbits purchased from the Medical Experimental Animal Center of Bengbu Medical College were used as experimental subjects. Streptozotocin was dissolved in sterile saline and intraperitoneally injected into the rabbits at a dose of 80 mg/kg. The rabbits were allowed to eat freely after receiving the injection. The fasting blood glucose levels of the rabbits were monitored regularly. The diabetic rabbit model was considered successfully established when the fasting blood glucose level was measured as 11 mmol/L twice or 14 mmol/L once. Following successful modelling, grouping was performed as follows: blank control group (Con-Group), diabetic rabbit group (DM-Group), diabetic rabbit + every other day curcumin administration group (Qod-Group), and diabetic rabbit + daily administration group (Qd-Group).
Click to Show/Hide
|
||||
| Response Description | Curcumin can promote the nuclear translocation of Nrf2, increase the expression of oxidative scavenging factors, such as HO-1, reduce excessive Gpx4 loss, and inhibit glucose-induced ferroptosis in cardiomyocytes. This highlights a potentially new therapeutic route for investigation for the treatment diabetic cardiomyopathy. | ||||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [53] | ||||
| Responsed Drug | Iridin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
In vivo, the LIRI model was established as described earlier. All mice were anesthetized with pentobarbital administered intraperitoneally (50 mg/kg, Sigma-Aldrich, MO, USA). After endotracheal intubation, the mice were ventilated using a rodent ventilator (MiniVent, Harvard Apparatus, USA), with the title volume set to 7 ml/kg, the respiratory rate set to 120 times/min, and the inspiratory/expiratory ratio set to 1: 2. A noninvasive clamp was used to interrupt the left pulmonary hilum, causing lung ischemia. The clamp was released after 60 minutes of ischemia, and the left lung was reperfused for 120 minutes. Animals were euthanized via cervical dislocation at the end of the experiment. Following that, lung specimens and bronchoalveolar lavage fluid were harvested for analysis. All procedures except lung ischemia were performed on mice in the sham group.
Click to Show/Hide
|
||||
| Response Description | As a result, irisin postconditioning may protect against lung I/R damage by suppressing ferroptosis via the Nrf2/HO-1 signaling axis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Responsed Drug | Apigenin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | ||||
| In Vivo Model |
Mice were exposed to 12/12 h of light/darkness and given free access to food and water. All C57BL/6J mice were fasted for 12 h and anesthetized by intraperitoneal injection of 1% sodium pentobarbital before modeling. Later, the superior mesenteric artery (SMA) was subjected to 45 min of global no-flow ischemia, followed by 90 min of reperfusion to induce IIRI. The successful model could be revealed by the microcirculation detector. In order to investigate the effects of APG, mice were randomly divided into different groups: Sham group, IIRI group, APG groups (2 mg/kg, 4 mg/kg and 8 mg/kg), ZnPPIX group (Protoporphyrin Zinc(), Dalian Meilun Biotech Co., Ltd., China, 10mg/kg), Selegiline group (10 mg/kg, Shandong Topscience Biotech Co., Ltd., China) and the ZnPPIX + Selegiline group (10 mg/kg ZnPPIX and 10 mg/kg Selegiline). As mentioned above, drug was intraperitoneal injected after a 10-min-ischemia.
Click to Show/Hide
|
||||
| Response Description | MST analysis suggested that Apigenin could specifically bind to heme oxygenase 1 (HO-1) and monoamine oxidase b (MAO-B). Simultaneously, APG could attenuate ROS generation and Fe2+ accumulation, maintain mitochondria function thus inhibit ferroptosis and alleviate intestinal ischemia-reperfusion injury. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [55] | ||||
| Responsed Drug | Dimethyl fumarate | Approved | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
The mice were randomly divided into four groups of six: sham + vehicle, sham + DMF, IR + vehicle, and IR + DMF. The mice were supplemented with DMF at a concentration of 100 mg/kg or DMSO by daily oral gavage for a week before surgery, as previously reported. As stated in a prior study, the partial warm liver IRI model was developed. Briefly, the sham group only had free hepatic portal blood vessels after laparotomy, and the blood flow was not obstructed. As for the hepatic IR group, the blood supply to the left and mid-hepatic lobes was blocked, resulting in 70% mouse liver IRI for 90 min. The mice were put on a heated blanket after surgery in order to maintain body temperature and monitor vital signs. Blood supply was restored for 6 h. Died mice were eliminated for testing prior to sample collection. The mice were euthanized after the sample were obtained. The same experimenter carried out all surgeries.
Click to Show/Hide
|
||||
| Response Description | NRF2 knockdown notably decreased the expression of SLC7A11 and HO-1 and blocked the anti-ferroptosis effects of dimethyl fumarate (DMF). DMF inhibits ferroptosis by activating the NRF2/SLC7A11/HO-1 axis and exerts a protective effect against hepatic ischemia-reperfusion injury. | ||||
Acute pancreatitis [ICD-11: DC31]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [56] | ||||
| Responsed Drug | Ginsenoside Rg3 | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AR42J cells | Digestive system neoplasms | Rattus norvegicus | CVCL_0143 | |
| In Vivo Model |
Male C57BL/6 mice (8 weeks old; SPF; weighing 24-26 g, n = 16 in total) were purchased from SPF (Beijing) Biotechnology Co., Ltd. All mice were housed in an environmentally controlled room at a temperature ranging from 20 to 24 on a 12 h light/dark cycle and used in the experiments following an overnight fast with water, availablead libitum. All procedures followed the Principles of Laboratory Animal Care (NIH publication number 85Y23, revised in 1996), and the experimental protocol was approved by the Animal Care Committee, Nanjing Medical University (NMU-2021JK-085).
Click to Show/Hide
|
||||
| Response Description | Taken together, the present study, to the best of our knowledge, is the first to reveal a protective role for Ginsenoside Rg3 in mice with acute pancreatitis by suppressing oxidative stressrelated ferroptosis and the activation of the NRF2/HO1 pathway. | ||||
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [57] | ||||
| Responsed Drug | Dioscin | Preclinical | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Six-week-old male Wistar rats (170-200 g) were obtained from Changsheng Biotechnology Co., Ltd. (Changchun, China), and all of them were fed under SPF-conditions. The rats were acclimatized to natural light/dark cycles at a controlled temperature of 22 + 2 with free access to food and water. The experiment was comprised of four groups: the C group (0.5% carboxymethyl cellulose sodium [CMC-Na], n = 6); the Dio group (dioscin-treated rats, n = 6); the CP group (cisplatin-treated mice, n = 6); and the Dio + CP group (dioscin plus cisplatin-treated rats, n = 6). Rats were gavaged with dioscin (60 mg/kg) for ten days, and cisplatin (10 mg/kg) was intraperitoneally injected once on the seventh day.
Click to Show/Hide
|
||||
| Response Description | Dioscin exerts a reno-protective effect by decreasing renal oxidative injury, apoptosis and ferroptosis through the Nrf2/HO-1 signaling pathway, providing a new insight into acute kidney injury prevention. | ||||
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [58] | ||||
| Responsed Drug | Sevoflurane | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
Male C57BL/6 mice (8 weeks, 23-25 g) were obtained from Beijing Hua Fu Kang Biotechnology Co. LTD (Beijing, China). A total of 96 mice were randomly divided into 6 groups (n = 16 per group): Sham group, LPS group, Fer-1 group, Fe group, Sev group, and Fe + Sev group. Mice were given 5 mg/kg LPS intranasally to construct LPS-induced ALI model. To study the role of ferroptosis in ALI, the mice in the Fer-1 or Fe groups were administered with Fer-1 (0.8 mg/kg; Ferrostatin-1, the inhibitor of ferroptosis) or Fe (8 mg/kg; Fe-citrate (III), ferroptosis inducer) via tail vein injection once a day for 3 consecutive days before treatment with LPS, respectively. To study the effect of Sev on ALI mice, the mice in Sev group were treated with LPS for 2 h, and then Sev, delivered by gaseous admixture (oxygen) at a concentration of 3% via a calibrated vaporizer, was administered via an endotracheal tube for 4 h. In order to study the effect of Sev on ferroptosis, the mice in Fe + Sev group were administered with Fe via tail vein injection once a day for 3 consecutive days, and then treated with LPS and Sev. Sham group was given 0.9% NaCl (containing 0.1% DMSO).
Click to Show/Hide
|
||||
| Response Description | Sevoflurane (Sev) could eliminate the worsening effects of ferroptosis inducer Fe-citrate on LPS-induced acute lung injury (ALI) to a certain extent. Sev inhibited ferroptosis by up-regulating HO-1 expression to reduce LPS-induced ALI, which may provide a possible mechanism for the application of Sev in clinical anesthesia. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [59] | ||||
| Responsed Drug | Astaxanthin | Investigative | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Mice were randomly divided into four groups as follows (n = 5): (1) control, (2) APAP (MCE, Monmouth Junction, NJ, USA), (3) olive oil + APAP (oil + APAP), and (4) ASX (Energy Chemical, Shanghai, China) dissolved in olive oil + APAP (ASX + APAP). Astaxanthin was dissolved in olive oil to obtain a mixture of 20 mg/mL. Mice in groups 3 and 4 were given a dose of olive oil and a mixture of 5 mL/kgBW by gavage every day for 2 weeks. On day 15, mice in groups 2, 3, and 4 were given a peritoneal injection of 500 mg/kg APAP to induce liver injury. The mice were fasted for 12 h before the administration of APAP. Ten hours after APAP administration, blood and liver tissue were collected for further examination and analyses. Blood was centrifuged to obtain supernatants,which were stored at -80. Liver tissues were immediately removed from each animal, and homogenates were processed with formaldehyde and glutaraldehyde for protein and histological analysis.
Click to Show/Hide
|
||||
| Response Description | Astaxanthin reduced inflammation through the NF-B pathway, inhibited oxidative stress and ferroptosis, and increased autophagy through the Nrf2/HO-1 pathway, ameliorating acetaminophen-induced liver injury in vivo and in vitro. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Responsed Drug | Ulinastatin | Phase 3 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Ulinastatin plays a role in mitigation of APAP-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [60] | ||||
| Responsed Drug | Abietic acid | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
APAP-induced liver injury model was induced byintraperitoneal injection 300 mg/kg APAP. The mice of APAP + abietic acid (10, 20, 40 mg/kg) were given abietic acid by intraperitoneal injection 1 h before APAP treatment. The doses of abietic acid used in this study were based on previous studies. Twelve hours later, the mice were sacrificed after anesthesia with 1%pentobarbital (50 mg/kg) injected intraperitoneally and the samples were collected.
Click to Show/Hide
|
||||
| Response Description | APAP could increase malondialdehyde (MDA) and Fe2+ levels, and decrease ATP and glutathione (GSH) levels, as well as glutathione peroxidase 4 (GPX4) and xCT expression. However, these changes induced by APAP were prevented by abietic acid, indicating abietic acid could inhibit APAP-induced ferroptosis. Furthermore, abietic acid inhibited APAP-induced liver injury, NF-B activation and increased the expression of Nrf2 and HO-1. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Icariin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Supraventricular tachycardia [ICD-11: BC81] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response Description | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Ulinastatin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Ulinastatin (UT1) plays a role in mitigation of Acetaminophen (APAP)-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
Mitogen-activated protein kinase 14 (MAPK14)
Andrographis
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Multiple myeloma [ICD-11: 2A83] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 |
| U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 | |
| AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| Response Description | Andrographolide (Andro) may block the Nrf2/HO-1 signaling pathway by activating P38 ( MAPK14), thereby inducing ferroptosis. Moreover, inhibition of P38 expression rescued Andro-induced cell death, changes in the level of Nrf2 and HO-1 expression, Fe2+ and lipid peroxidation. Taken together, our findings suggest that Andro induces ferroptosis in Multiple myeloma (MM) cells via the P38/Nrf2/HO-1 pathway, providing a potential preventative and therapeutic approach for MM. | |||
Cetuximab
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
The DLD-1 cell suspension (4 x 106 cells/200 ul) was injected subcutaneously into the right dorsal flank of 5-week-old male BALB/c nude mice (Charles River, China). The mice were randomly divided into four groups (5 mice/group): 1) the control group, 2) the RSL3 group, 3) the cetuximab group, and 4) the RSL3 + cetuximab group. Both RSL3 (5 mg/kg) and cetuximab (13 mg/kg) were administered by intraperitoneal injection in a volume of 100 ul once per day. The tumour volume was calculated as 0.5 x length x width2. After 17 days of treatment, the mice were sacrificed, and the tumours were removed. Then, tumour tissue obtained from the different treated groups was subjected to western blotting and immunohistochemical experiments.
Click to Show/Hide
|
||||
| Response Description | Our work reveals that cetuximab enhances the cytotoxic effect of RSL3 on KRAS mutant Colorectal cancer (CRC) cells and that cetuximab enhances RSL3-induced ferroptosis by inhibiting the Nrf2/HO-1 axis through the activation of p38 MAPK. | ||||
Kelch-like ECH-associated protein 1 (KEAP1)
Baicalein
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Degenerative arthritis [ICD-11: FA05] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCDs (Chondrocytes) | ||||
| In Vivo Model |
C57BL/6J (WT) mice (8 weeks old, male) were purchased from Nanjing Medical university, and AMPK-KO mice were purchased from Shanghai Model Organisms. They were used to create an OA model by destabilization of the medial meniscus surgery (DMM) (n = 6 per group). Briefly, after the mice were anaesthetized, a medial articular incision was made to expose the leftjoint cavity, and then the tibial collateral ligament was transected. Finally, the articular incision was closed. In the control group, only the joint cavity was opened. One week after surgery, 1 mg/kg baicalein (MCE, HY-N0196) per knee, 1 mg/kg ML385 (MCE, HY-100523) per knee, 1 mg/kg AICAR (MCE, HY-13417) per knee or 1 mg/kg of the ferroptosis inhibitor ferrostatin-1 (Fer-1, MCE, HY-100579) was injected into the joint cavity of the mice once a week. Meanwhile, saline was injected into the control group. Mice were sacrificed after surgery 10 weeks.
Click to Show/Hide
|
||||
| Response Description | Baicalein alleviated osteoarthritis (OA) development by improving the activity of AMPKa/Nrf2/HO-1 signaling to inhibit chondrocyte ferroptosis, revealing baicalein to be a potential therapeutic strategy for OA. AMPKa preserved Nrf2 abundance in chondrocytes and promoted Nrf2 into nucleus by promoting Keap1 degradation | ||||
Astaxanthin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | |
| In Vivo Model |
6-week-Babl/c female mice were randomized to the following three groups of seven mice each: vehicle group, LPS group, Astaxanthin plus LPS group. Astaxanthin plus LPS group mice were pretreated with astaxanthin (20 mg/kg) byi.v injectionfor daily for 7 consecutive days. Astaxanthin was dissolved in 2%DMSO (vol/vol), 40% PEG-400 (vol/vol), 2% Tween 80 (vol/vol), and 56% PBS (vol/vol). On the last day, the mice were intraperitoneally injected with 5 mg/kg LPS or normal saline 2 h after the injection of astaxanthin. After 6 h of LPS stimulation, mice were euthanized to collect the BALF, and lung tissue samples. BALF was collected three times through a tracheal cannula with autoclaved normal saline, instilled up to a total volume of 1.8 ml.
Click to Show/Hide
|
||||
| Response Description | Astaxanthin protected LPS-induced cell inflammation and acute lung injury (ALI) in mice by inhibiting ferroptosis, and its effect was achieved through Keap1-Nrf2/HO-1 pathway. Therefore, our study indicates that ferroptosis will become a new target for the treatment of ALI, and astaxanthin is a potential drug for the treatment of ALI. | ||||
Hypoxia-inducible factor 1-alpha (HIF1A)
Diethylhexylphthalate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Testicular injury [ICD-11: NB9Z] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| HIF-1 signaling pathway | hsa04066 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | TM3 cells | Normal | Mus musculus | CVCL_4326 | |
| TM4 cells | Normal | Mus musculus | CVCL_4327 | ||
| In Vivo Model |
Specific pathogen-free (SPF) pregnant C57BL/6 mice were purchased from the Experimental Animal Center of Chongqing Medical University. Under SPF conditions, all mice had free access to food and water. All mice were fed under a 12-hour light/dark cycle at 25 ± 2 , and the relative humidity was 50 ± 5%. Male neonatal mice were selected at postnatal day (PND) 21. Since we aimed to mimic the prepubertal testicular injury induced byDEHPexposure, all male mice were randomly divided into four groups and treated by oral gavage from PND22 to PND35 with corn oil (Aladdin, C116023, China), or DEHP at a dose of 100 mg/kg/day (D100), 250 mg/kg/day (D250), or 500 mg/kg/day (D500).
Click to Show/Hide
|
||||
| Response Description | Di-(2-ethylhexyl) phthalate (DEHP) exposure led to testicular injury including excessive ROS accumulation. Prepubertal DEHP exposure induces ferroptosis in mouse testes. In particular, MEHP exposure leads to ferroptosis in Leydig and Sertoli cells via the HIF-1/HO-1 signaling pathway. | ||||
Fibronectin type III domain-containing protein 5 (FNDC5)
Iridin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Acute myocardial infarction [ICD-11: BA41] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hCMs (Human cardiomyocytes) | |||
| Response Description | Myocardial infarction is characterized by cardiomyocyte death and mitochondrial dysfunction induced by ischemia. FNDC5 overexpression and/or irisin administration elevated cell viability, decreased ferroptosis, and reversed mitochondrial impairments induced by hypoxia. Mechanistically, FNDC5/irisin reduced ferroptosis and reversed mitochondrial impairments by Nrf2/HO-1 axis in hypoxic cardiomyocytes. | |||
5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1)
Ascorbic Acid
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | PaTu 8988t cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1847 | |
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| mEFs (Mouse embryonic fibroblasts) | |||||
| Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | ||
| In Vivo Model |
All animal experiments were approved by the Ethics Committee of Jiangsu University. To investigate the role of the combination of erastin and vitamin C in inducing ferroptosis, Panc02 cells (1 x 105 cells/site) were transfected and subcutaneously injected into 4-week-old C57BL/6 mice to generate xenografts. When the tumors reached a volume of 50-100 mm3, the mice were randomly divided into four groups (five mice per group) and treated with DMSO (control), imidazole ketone erastin (IKE, MedChemExpress), vitamin C, or a combination of erastin and vitamin C. Mice were treated with 80 ul (400M) erastin by intratumoral injection and/or 4 g/kg vitamin C by intraperitoneal injection every 2 days.
Click to Show/Hide
|
||||
| Response Description | The combination of erastin and vitamin C mainly increases the levels of ferrous iron through the AMPK/NRF2/HMOX1 signaling pathway. Cotreatment with erastin and vitamin C also exhibited a synergistic effect in a pancreatic cancer xenograft model in mice. | ||||
Mitogen-activated protein kinase 8 (MAPK8)
SP600125
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [15] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Health [ICD-11: N.A.] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | BV-2 cells | Normal | Mus musculus | CVCL_0182 |
| Response Description | Following addition of the JNK (MAPK8) inhibitor SP600125, the expression of HO-1 decreased, expression of FTH1 was increased and iron accumulation was decreased. Therefore, it was hypothesized that NPs induced ferroptosis in BV2 cells via the JNK/HO-1/FTH1 pathway. | |||
Unspecific Regulator
Ferulic acid
[Patented]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [17] | ||||
| Responsed Disease | Sepsis [ICD-11: 1G40] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
Briefly, female BALB/c mice (6-8 weeks, weighing 20-25 g, purchased from Hunan SJA Laboratory Animal Co., Ltd., Changsha, Hunan, China) were raised in specific pathogen-free conditions under controlled temperature (23-25 ) and humidity (40-80%) as well as a 12 h dark/light cycle for 1 week of acclimation. They were fed a standard chow diet and waterad libitum. Mice were anaesthetised with 2% isoflurane inhalation and underwent moderate caecal ligation and puncture in accordance with a previously reported protocol (Rittirsch etal.2009). Meanwhile, mice in the control groups were subjected to a sham operation. Buprenorphin (0.05 mg/kg) was injected for postoperative analgesia, and all mice were placed in cages immediately after the surgical procedures with free access to water and food (Rittirsch etal. 2009).
Click to Show/Hide
|
||||
| Response Description | Collectively, our data highlighted the alleviatory role of ferulic acid in sepsis-induced ALI by activating the Nrf2/HO-1 pathway and inhibiting ferroptosis, offering a new basis for sepsis treatment. | ||||
Dexmedetomidine
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [18] | ||||
| Responsed Disease | Sepsis [ICD-11: 1G40] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mVTs (Mouse ventricular tissues) | ||||
| In Vivo Model |
A total of 32 male C57BL/6 mice (25 g, 8 weeks old) were obtained from the Guangdong Medical Lab Animal Center and housed in the Laboratory Animal Service Center (Jinan University, Guangdong, China). Mice were anesthetized with isoflurane (RWD Life Science) inhalation at the concentration of 2.5% for anesthetic induction and then at 1% for anesthetic maintenance until the end of the CLP. During the experiment, the body temperature was kept at 36-38 with a heating pad. Anesthetized mice were subjected to midline laparotomy. The cecum was carefully separated to avoid blood vessels damage and the cecum was identified and punctured twice with a 22-gauge needle. Then, the abdominal cavity was closed with two epithelium layers, followed by a normal saline injection subcutaneously for resuscitation before mice were returned to the cage.
Click to Show/Hide
|
||||
| Response Description | The attenuation of sepsisinduced HO1 overexpression and iron concentration, and the reduction of ferroptosis via enhancing GPX4, may be the major mechanisms via which Dexmedetomidine alleviates sepsis induced myocardial cellular injury. | ||||
Dihydroartemisinin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [19] | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
All BALB/C nude mice were purchased from Huafukang Biotechnology (Beijing, China). These mice were 5 weeks old and weighed 14-16 g. We established subcutaneous tumour-forming mouse model by injecting 5 x 106 U87 cells into the lateral abdomen of BALB/C nude mice. Animals were then treated with DHA solvent (50 mg/kg) by intragastric administration once a day for 26 days.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin could promote ferroptosis in glioma cells. Low expression of GPX4 and high expression of HMOX1 were identified in DHA treated glioma cells. MAZ was further identified as the direct target of long noncoding RNA (lncRNA) TUG1 through luciferase assay. Downregulated expression of TUG1 and upregulated expression of MAZ were identified in DHA treated glioma cells. TUG1 overexpression or inhibition of FTH1 expression could enhance the antiglioma effect of DHA in vitro and in vivo, providing a promising strategy to enhance the antitumor effect of DHA in glioma. | ||||
Siramesine
[Terminated]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [20] | |||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| Response Description | Lapatinib and siramesine was the most effective tyrosine kinase inhibitor and lysosome disruptor drug combination in inducing synergistic cell death in A549 and U87 cells. This cell death was through ferroptosis mediated by ROS and reduced expression of HO-1 in glioma cells. | |||
Lapatinib
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [20] | |||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| Response Description | Lapatinib and siramesine was the most effective tyrosine kinase inhibitor and lysosome disruptor drug combination in inducing synergistic cell death in A549 and U87 cells. This cell death was through ferroptosis mediated by ROS and reduced expression of HO-1 in glioma cells. | |||
Polygonatum cyrtonemaHua Polysaccharides
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [21] | |||
| Responsed Disease | Central nervous system cancer [ICD-11: 2A02] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | BV-2 cells | Normal | Mus musculus | CVCL_0182 |
| Response Description | Subsequent studies have revealed that Polygonatum cyrtonemaHua Polysaccharides (PCP) alleviates ferroptosis in microglia due to protein levels of ERASTIN/RSL3 inhibitor SLC7A11/GPX4 by activating the NRF2/HO-1 signaling pathway. PCP has the development potential as a new drug candidate for treating CNS diseases. | |||
Honokiol
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [22] | |||
| Responsed Disease | Acute myeloid leukaemia [ICD-11: 2A60] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 |
| U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | |
| SKM-1 cells | Acute myeloid leukemia | Homo sapiens | CVCL_0098 | |
| Response Description | Honokiol decreased the viability of the targeted Acute myeloid leukemia cells, induced their cell cycle arrest at G0/G1 phase, and inhibited their colony-formation capacity. Honokiol also triggers a noncanonical ferroptosis pathway in THP-1 and U-937 cells by upregulating the level of intracellular lipid peroxide and HMOX1 significantly. And HMOX1 was a critical target in honokiol-induced ferroptosis. | |||
3,5-bis((E)-2-fluorobenzylidene)piperidin-4-one hydrochloride
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 |
| SAOS-2 cells | Osteosarcoma | Homo sapiens | CVCL_0548 | |
| Response Description | 3,5-bis((E)-2-fluorobenzylidene)piperidin-4-one hydrochloride (EF24) upregulated HMOX1 to suppress GPX4 expression to induce ferroptosis by increasing MDA level, ROS level and intracellular ferric ion level. Thus, EF24 might serve as a potential agent for the treatment of HMOX1-positive osteosarcoma patients. | |||
Zoledronic acid
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [24] | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| MNNG/HOS Cl #5 cells | Osteosarcoma | Homo sapiens | CVCL_0439 | |
| Response Description | Zoledronic acid treatment decreased cell viability and promoted the increase in lipid peroxide content and PTGS2 expression. Our results indicate that zoledronic acid induces ferroptosis by decreasing ubiquinone content and promoting HMOX1 expression in osteosarcoma cells. | |||
Cephalosporin
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [25] | ||||
| Responsed Disease | Nasopharyngeal cancer [ICD-11: 2B6B] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| XWLC-05 cells | Lung adenocarcinoma | Homo sapiens | CVCL_IQ71 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| CNE2 cells | Normal | Homo sapiens | CVCL_6889 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| K-562 cells | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | ||
| ECV-304 cells | Bladder carcinoma | Homo sapiens | CVCL_2029 | ||
| In Vivo Model |
6-8 week old male balb/cnude micewere purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Nasopharyngeal carcinoma CNE2 cells were collected during logarithmic growth and rinsed with PBS twice and resuspended to a density of 1 x 107 cells/ml using fresh and cooled DMEM/F12 medium (free of FBS and antibiotics). Each mouse was inoculated subcutaneously with a 0.1 ml cell suspension on the right-side flank. Tumor-bearing mice were used for in vivo anticancer studies 8 days after inoculation when the average tumor volume reached ~200 mm3.
Click to Show/Hide
|
||||
| Response Description | Cephalosporin antibiotics showed highly specific and selective anticancer activity on nasopharyngeal carcinoma CNE2 cells both in vitro and vivo with minimal toxicity. Pathway analyses indicate apoptotic and the ErbB-MAPK-p53 signaling pathways are significantly enriched. HMOX1 represents the top one ranked upregulated gene by COS and overlaps with 16 of 42 enriched apoptotic signaling pathways. Inhibition of HMOX1 significantly reduced the anticancer efficacy of cefotaxime in CNE2 cells. | ||||
Carnosic acid
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [26] | |||
| Responsed Disease | Oral squamous cell carcinoma [ICD-11: 2B6E] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Response Description | The current findings highlight that carnosic acid may re-sensitize cisplatin-resistant cells to cisplatin by inducing ferroptosis, which involves the inactivation of Nrf2/HO-1/xCT pathway. Hence, this research may support a promising therapeutic approach to overcome chemoresistance in Oral squamous cell carcinoma. | |||
5-aminolevulinic acid
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [27] | ||||
| Responsed Disease | Oesophageal cancer [ICD-11: 2B70] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | KYSE30 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1351 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
KYSE30 cells were subcutaneously inoculated with 5 x 106 cells per site into both flanks on day 0. At 1 week after transplantation, tumor-bearing mice were randomly assigned to one of the following three groups: (1) saline as a control, (2) 10 mg/kg/day of 5-ALA, or (3) 30 mg/kg/day of 5-ALA. The treatment groups were orally administered 5-ALA once daily for 4 weeks, and the control group was orally administered saline during the same period.
Click to Show/Hide
|
||||
| Response Description | Modulation of GPX4 and HMOX1 by 5-aminolevulinic acid (5-ALA) induced ferroptosis in esophageal squamous cell carcinoma (ESCC). Furthermore, 5-ALA led to an increase in lipid peroxidation and exerted an antitumor effect in various cancer cell lines, which was inhibited by ferrostatin-1. Thus, 5-ALA could be a promising new therapeutic agent for ESCC. | ||||
Andrographis
[Approved]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [28] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | MKN74 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_2791 | |
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| Response Description | Andrographis exerted antitumor effects in gastric cancer cell lines (MKN74 and NUGC4) by inhibiting proliferation, reducing colony formation and enhancing apoptotic activity. Moreover, andrographis treatment altered the expression of ferroptosis-associated genes, including HMOX1, GCLC, and GCLM. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [32] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Seven-week-old male athymic nude mice (Envigo, Houston, TX) were housed under controlled conditions of light and fed ad libitum. Approximately 5 x 106 parental and 5FUR HCT116 cells were suspended in the matrigel matrix (BD Biosciences, Franklin Lakes, NJ) and subcutaneously injected into mice using a 27-gauge needle (n = 10 per group). Mice were randomly assigned to different treatment groups and 5FU (30 mg/kg body weight) or andrographis (125 mg/kg body weight) or their combination were given intraperitoneally on alternative days for up to 15 days.
Click to Show/Hide
|
||||
| Response Description | Combined treatment with andrographis was significantly more effective than 5FU and andrographis alone and that these effects were in part orchestrated through dysregulated expression of key genes (including HMOX1, GCLC, GCLM and TCF7L2) within the ferroptosis and Wnt-signaling pathways. Andrographis might offer a safe and inexpensive adjunctive therapeutic option in the management of colorectal cancer patients. | ||||
Propofol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [29] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response Description | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
Talaroconvolutin A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [30] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| In Vivo Model |
5 x 106 HCT116 cells were inoculated subcutaneously in the underarm of Balb/c nude female mice (5-week old). The inoculated mice were randomly divided into two groups (6 mice each group). When the tumor reached 300 mm3, the drug group was given TalaA intraperitoneally at a dose of 6.0 mg/kg, and the control group was given the same dose of cosolvent-corn oil. The drug (or cosolvent) was injected every 2 days. Body weight and tumor volume were measured every 2 days.
Click to Show/Hide
|
||||
| Response Description | Talaroconvolutin A (TalaA) downregulated the expression of the channel protein solute carrier family 7 member 11 (SLC7A11) but upregulated arachidonate lipoxygenase 3 (ALOXE3), promoting ferroptosis. TalaA causes upregulation of HMOX1 which lead to the degradation of heme and the release of free iron, accumulating in mitochondria and giving rise to lipid peroxidation. TalaA could be a new potential powerful drug candidate for colorectal cancer therapy. | ||||
tagitinin C
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [31] | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| Response Description | Tagitinin C induces ferroptosis in colorectal cancer cells and has synergistic effect together with erastin. Mechanistically, tagitinin C induces ferroptosis through ER stress-mediated activation of PERK-Nrf2-HO-1 signaling pathway. | |||
Haloperidol
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [33] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response Description | The study demonstrated that haloperidol strengthened sorafenib-induced ferroptosis in Hepatocellular carcinoma cell lines for the first time. During ferroptosis, haloperidol substantially increased the cellular levels of Fe2+, GSH and lipid peroxidation. Additionally, haloperidol induced the expression of HO-1. | |||
Eupalinolide B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [34] | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell migration | |||||
| Cell proliferation | |||||
| In Vitro Model | SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | |
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| In Vivo Model |
Four-week-old nude mice (BALB/c) were used, and they were housed for 1 week in a specific pathogen-free (SPF) environment before experimentation. Each mouse received subcutaneous injections of 1 x 106 SMMC-7721 or HCCLM3 cells in 200 uL phosphate-buffered saline (PBS) into both flanks. One group was intraperitoneally injected every two days with EB at 25 or 50 mg/mouse body weight, for a total of 3 weeks. As a control, DMSO was used in another group. Tumor size was measured to calculate tumor volume, and mouse body weights were monitored every two days.
Click to Show/Hide
|
||||
| Response Description | Eupalinolide B (EB) exerts anti-proliferative activity in hepatic carcinoma by blocking cell cycle arrest at S phase and inducing ferroptosis mediated by endoplasmic reticulum (ER) stress, as well as HO-1 activation. And EB has the ability to inhibit cell proliferation and migration in hepatic carcinoma. | ||||
Ginkgetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [35] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SPC-A1 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | ||
| In Vivo Model |
Briefly, when tumours on transplanted nude mice reached around 100 mm3, the mice were randomized divided into eight groups: control, ginkgetin, DDP, ginkgetin + DDP, UAMC 3203, ginkgetin + UAMC 3203, DDP + UAMC 3203, ginkgetin + DDP + UAMC 3203. Both DDP (3 mg/kg) and ginkgetin (30 mg/kg) were administered by intraperitoneal injection, with 2 - 3 times per week and once per day, respectively. UAMC 3203 (10 mg/kg) was administered 5 days/week by intraperitoneally injection. Tumour size and body weight were measured 3 times per week. After dosing 31 days, the nude mice were sacrificed, and tumours were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | The induction of ferroptosis mediated by ginkgetin was further confirmed by the decreased expression of SLC7A11 and GPX4, and a decreased GSH/GSSG ratio. Simultaneously, ginkgetin disrupted redox hemostasis in DDP-treated cells, as demonstrated by the enhanced ROS formation and inactivation of the Nrf2/HO-1 axis. Ginkgetin also enhanced DDP-induced mitochondrial membrane potential (MMP) loss and apoptosis in cultured non-small cell lung cancer (NSCLC) cells. | ||||
Gefitinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [36] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRASmutant non-small-cell lung cancer (NSCLC) cells. | ||||
Betulin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [36] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRASmutant non-small-cell lung cancer (NSCLC) cells. | ||||
S-3'-hydroxy-7', 2', 4'-trimethoxyisoxane
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [37] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
When tumor volumes in xenograft nude mice reached an average of roughly 100 mm3, the mice were randomly divided into 3 groups of 6 mice each: control, ShtIX, and ShtIX + Fer-1. The treated group received ShtIX or ShtIX combined with Fer-1 injections into the tail vein of the mice every three days for 7 times, whereas the control group received saline. Every four days, the volume and weight of the tumors were measured. As soon as the test was completed, the nude mice were slaughtered, and the tumor tissues were retrieved. The in vivo experiments were approved by the Animal Care and Use Committee of Hainan Medical College and following the animal rules.
Click to Show/Hide
|
||||
| Response Description | S-3'-hydroxy-7', 2', 4'-trimethoxyisoxane (ShtIX) caused ferroptosis in Non-small cell lung cancer (NSCLC) cells, and inhibiting the Nrf2/HO-1 pathway can considerably exacerbate the effect of ShtIX-induced ferroptosis. | ||||
Shuganning injection
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [38] | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | SK-BR-3 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| MDA-MB-468 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| WPMY-1 cells | Normal | Homo sapiens | CVCL_3814 | ||
| U-87MG cells | Glioblastoma | Homo sapiens | CVCL_0022 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| In Vivo Model |
MDA-MB-231 cells resuspended in PBS (2 x 106/100 m1) were injected subcutaneously into bothhind limbsof 4-6 weeks old femalenude mice. A week later, SGNI was administrated byintraperitoneal injectionat a dose of 112.5 mg/kg/3 d.
Click to Show/Hide
|
||||
| Response Description | Shuganning injection (SGNI) induced a ferroptotic cell death of Triple-negative breast cancer (TNBC) cells. Mechanistically, SGNI induced ferroptosis was dependent on HO-1, which promotes intracellular labile iron pool accumulation, and was alleviated by HO-1 knockdown and inhibition by tin protoporphyrin IX. | ||||
Norcantharidin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [39] | ||||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Athymic nu/nu female mice aged 6-8 weeks (n = 9; mean weight, 20.21 ± 1.54 g) were purchased from the specific pathogen SPF (Beijing) Lab Animals Technology Co. Ltd. Mice were housed in a temperature- and humidity-controlled environment (20-24 , 45-55% humidity), with free access to food and water and in groups of three. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC ID: 17-3256) at Nantong University and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Nuclear factor erythroid 2-related factor 2 (NRF2), heme oxygenase 1 (HO-1), glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (xCT) expression levels were significantly decreased following norcantharidin (NCTD) treatment. Collectively, NCTD may represent a potent anticancer agent in ovarian cancer cells, and NCTD-induced ferroptotic cell death may be achieved by inhibiting the NRF2/ HO-1/GPX4/xCT axis. | ||||
Juglone
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [40] | |||
| Responsed Disease | Corpus uteri cancer [ICD-11: 2C76] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| Cell migration | ||||
| In Vitro Model | Ishikawa cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 |
| Response Description | Juglone as a natural quinone from C. cathayensisgreen peel could exert its anti-cancer activity by inducing iron dependent autophagy and inhibiting the migration of endometrial cancer (EC) cells. Subsequently, Fe2+ accumulation, lipid peroxidation, GSH depletion, the upregulation of HMOX1, and heme degradation to Fe2+ were reported. Juglone was involved in inducing autophagy and inhibiting cell migration and endoplasmic reticulum stress. | |||
Isothiocyanate-containing hybrid AR antagonist 13
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [41] | |||
| Responsed Disease | Prostate cancer [ICD-11: 2C82] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | VCaP cells | Prostate carcinoma | Homo sapiens | CVCL_2235 |
| LNCaP cells | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| LNCaP C4-2 cells | Prostate carcinoma | Homo sapiens | CVCL_4782 | |
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | |
| RWPE-1 cells | Normal | Homo sapiens | CVCL_3791 | |
| MDA-kb2 cells | Breast adenocarcinoma | Homo sapiens | CVCL_6421 | |
| Response Description | ITC-ARi 13 and buthionine sulfoximine (BSO) cooperatively downregulate AR and induce ferroptosis likely through increasing the accessibility of 13/12b to cellular targets, escalating free intracellular ferrous iron and attenuating GSH-centered cellular defense and adaptation. Further studies on the combination of ITC-ARi and GSH synthesis inhibitor could result in a new modality against castration-resistant prostate cancer (CRPC). Collectively, the combination of ITC-ARi 13 and BSO reveals a pro-ferroptotic role of Nrf2 through upregulating HO-1 under GSH-deficient conditions. | |||
Curcumin
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [42] | ||||
| Responsed Disease | Thyroid cancer [ICD-11: 2D10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | |
| Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | ||
| FTC 238 cells | Thyroid gland follicular carcinoma | Homo sapiens | CVCL_2447 | ||
| Response Description | Knockdown of HO-1 inhibits ferroptosis by upregulating the GPX4 expression in follicular thyroid cancer cells. We conclude that curcumin inhibits the tumorigenesis of follicular thyroid cancer via HO-1-induced activation of the ferroptosis signalling pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [52] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Two-month-old male New Zealand rabbits purchased from the Medical Experimental Animal Center of Bengbu Medical College were used as experimental subjects. Streptozotocin was dissolved in sterile saline and intraperitoneally injected into the rabbits at a dose of 80 mg/kg. The rabbits were allowed to eat freely after receiving the injection. The fasting blood glucose levels of the rabbits were monitored regularly. The diabetic rabbit model was considered successfully established when the fasting blood glucose level was measured as 11 mmol/L twice or 14 mmol/L once. Following successful modelling, grouping was performed as follows: blank control group (Con-Group), diabetic rabbit group (DM-Group), diabetic rabbit + every other day curcumin administration group (Qod-Group), and diabetic rabbit + daily administration group (Qd-Group).
Click to Show/Hide
|
||||
| Response Description | Curcumin can promote the nuclear translocation of Nrf2, increase the expression of oxidative scavenging factors, such as HO-1, reduce excessive Gpx4 loss, and inhibit glucose-induced ferroptosis in cardiomyocytes. This highlights a potentially new therapeutic route for investigation for the treatment diabetic cardiomyopathy. | ||||
Cadmium
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [43] | ||||
| Responsed Disease | Testicular dysfunction [ICD-11: 5A81] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | TM3 cells | Normal | Mus musculus | CVCL_4326 | |
| In Vivo Model |
Eight-week-old adult male C57BL/6J mice were raised for 7 days before the study in a temperature and humidity controlled animal facility (22-25 , 50% relative humidity) with a12-h light/dark cycles. A total of 10 mice were randomly assigned into two groups. As described in our previous study, the control group was treated with 0.9% NaCl (0 mg CdCl2), and the treatment group was intraperitoneally injected with CdCl2 at a dose of 1.0 mg per kg of body weight for 1 week.
Click to Show/Hide
|
||||
| Response Description | Cadmium induced ferroptosis by iron homeostasis dysregulation, mediated by excessive activation of HMOX-1. The disruption of autophagy flow contributed to Cd-induced testicular dysfunction and attenuated testosterone synthesis. | ||||
Gastrodin
[Investigative]
| In total 2 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [44] | |||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | C6 cells | Malignant glioma | Rattus norvegicus | CVCL_0194 |
| Response Description | Gastrodin induced GPX4, Nrf2 and HO-1 expression to protect C6 cells from H2O2-induced ferroptosis. Gastrodin pretreatment effectively reduced H2O2-induced oxidative damage, indicating gastrodin is a potential antioxidant that reduced cytotoxic ROS. The role of gastrodin in ferroptosis presents a new perspective for understanding parkinson's disease. | |||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [50] | |||
| Responsed Disease | Nervous system disease [ICD-11: 8E7Z] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Gastrodin (GAS) is a component of Gastrodia elata Blume, with strong antioxidant activity in neurodegenerative diseases. GAS increased the nuclear translocation of Nrf2, up-regulated the downstream HO-1 protein expression in HT-22 cells following treatment with glutamate. | |||
Salidroside
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [45] | ||||
| Responsed Disease | Alzheimer disease [ICD-11: 8A20] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
B6.1291-Nfe2l2tm1Ywk/J (Nrf2-/-mice, 017009)and wild-type C57BL/6 were originally from the Jackson Laboratory. Grouping and administration were started when weighing approximately 28-33 g. All mice were housed in a laboratory environment with free access to adequate food and water under a 12 h/12 h light/dark cycle at 22 ± 1 and 55 ± 5% humidity. All procedures conformed to the protocols of the Animal Welfare Commission and Ethical Committee of Southern Medical University. The Nrf2-/- mice were identified by genotyping as shown in Fig.1B. WT and Nrf2-/- mice were randomly assigned to 32 groups (3 groups for WT mice and 3 groups for Nrf2-/- mice) as follows: a sham group, sham + Ab1-42 group, and Salidroside + Ab1-42 group.
Click to Show/Hide
|
||||
| Response Description | Salidroside plays a neuroprotective role by inhibiting neuronal ferroptosis in A1-42-induced Alzheimer's disease mice and Glu-injured HT22 cells, and its mechanism is related to activation of the Nrf2/HO1 signaling pathway. | ||||
Astragaloside IV
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [46] | ||||
| Responsed Disease | Subarachnoid Hemorrhage [ICD-11: 8B01] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
SAH model was constructed by applying endovascular perforation in the rats, according to the protocol introduced in a previous study (Wei et al., 2020), except for slight modifications. Briefly, after performing intraperitoneal anesthesia with 40 mg/kg sodium pentobarbital, the right common carotid, external and internal carotid arteries of the rats were exposed and isolated. The right external carotid artery was ligated, and a 4-0 single-strand nylon thread was used to insert the right internal carotid artery through the stump of the external carotid artery and the bifurcation of the common carotid artery. When resistance is felt when the suture enters the intracranial segment, proceed approximately 3 mm to penetrate internal carotid artery at the bifurcation of middle cerebral artery. The suture was held in this position for 10 s and was then withdrawn. The rats in the Sham group went through an identical procedure, without the suture at the point of resistance. Throughout the experiment, the body temperature of the rats was sustained at around 37 by using a thermal blanket. After the wounds were sutured, the rats were placed in a separate cage and neurological function was closely observed.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AS-IV) triggered Nrf2/HO-1 signaling pathway and alleviated ferroptosis due to the induction of subarachnoid hemorrhage (SAH). The Nrf2 inhibitor ML385 blocked the beneficial effects of neuroprotection. These results consistently suggest that ferroptosis is profoundly implicated in facilitating EBI in SAH, and that AS-IV thwarts the process of ferroptosis in SAH by activating Nrf2/HO-1 pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [48] | ||||
| Responsed Disease | Cerebral ischaemic stroke [ICD-11: 8B11] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
1% sodium pentobarbital (40 mg/kg) was administered to the rats intraperitoneally to anesthetize them before placing them in a brain stereotaxic device. An incision was created in the midline of the neck to expose the common internal and external carotid arteries. After ligating and cutting the external carotid artery on the left side, a 3-mm stump was exposed. We then perforated the carotid artery at the bifurcation of the middle and anterior cerebral arteries utilizing an 18-20-mm-long surgical filament (0.26 mm diameter; Beijing Cinontech Co. Ltd., China) was threaded through the external carotid artery stump into the internal carotid artery and left in situ for 120 min. After that, the filament was withdrawn to facilitate reperfusion. Rats in the sham surgery group received the identical procedure as the other rats but without filament insertion.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AS-IV) administration decreased the infarct volume, brain edema, neurological deficits, and inflammatory cytokines TNF-, interleukin-1 (IL-1), IL-6, and NF-B, increased the levels of SLC7A11 and glutathione peroxidase 4 (GPX4), decreased lipid reactive oxygen species (ROS) levels, and prevented neuronal ferroptosis. Meanwhile, AS-IV triggered the Nrf2/HO-1 signaling pathway and alleviated ferroptosis due to the induction of stroke. | ||||
Caryophyllene
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [47] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rPAs (Rat primary astrocytes) | ||||
| In Vivo Model |
Rats were anesthetized withisoflurane(2-3% oxygen) and placed in asupine position. And theright common carotid artery (CCA),external carotid artery (ECA), andinternal carotid artery (ICA) were exposed in sequence and separated carefully. Then we ligated the CCA and the ECA in turn, and at the same time, we clamped the internal carotid artery with an arterial clamp. Finally, we inserted a silicone nylon monofilament from the CCA into themiddle cerebral arteryand temporarily fixed it. After 1.5 h ofischemia, the monofilament was taken out and the blood vessels were ligated at theincision. The neck wound was sutured with surgical sutures. Subsequent experiments were performed after 12 h ofreperfusion. In thesham operationrats, except for the absence of the monofilament, the sham operation rats underwent the same surgical procedures as the MCAO/R model rats.
Click to Show/Hide
|
||||
| Response Description | Our results indicated the critical role of ferroptosis in cerebral ischemia reperfusion injury. For the first time, we showed that the significant neuroprotective effects of b-Caryophyllene (BCP) in attenuating ischemic stroke injury are correlated with ferroptosis regulation, and its mechanism is associated with activation of the NRF2/HO-1 axis. | ||||
ADA-409-052
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [49] | ||||
| Responsed Disease | Thromboembolic stroke [ICD-11: 8B20] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BV-2 cells | Normal | Mus musculus | CVCL_0182 | |
| Neuro-2a cells | Neuroblastoma | Mus musculus | CVCL_0470 | ||
| RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | ||
| In Vivo Model |
Brain penetration study was carried out by TCG Lifesciences Ltd (Kolkata, India) in male BALB/c mice (6-8 weeks old; 18-20 g, in-house breeding). Mice were housed individually under 12/12 h lightdark cycle with free access to food and water. In two experiments 10 mg/kg or 30 mg/kg of ADA-409-052 (HPLC Purity: 99.7%) dissolved in Tween-80 (0.5%, Merck, Germany) -methylcellulose (Sigma) solution was administered as a single bolus by oral gavage (p.o.). ADA-409-052- or vehicle-administered mice were sacrificed 0.75, 4, and 24 h later (n = 3/group).
Click to Show/Hide
|
||||
| Response Description | ADA-409-052 inhibits tert-Butyl hydroperoxide (TBHP)-induced lipid peroxidation (LP) and protects against ferroptotic cell death triggered by glutathione (GSH) depletion or glutathione peroxidase 4 (GPx4) inhibition in neuronal cell lines. Moreover, ADA-409-052 efficiently reduces infarct volume, edema and expression of pro-inflammatory genes in a mouse model of thromboembolic stroke. In addition, ADA-409-052 reduced the expression of stroke-induced Hmox1. | ||||
Sodium iodate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [51] | ||||
| Responsed Disease | Age-related macular degeneration [ICD-11: 9B75] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 | |
| In Vivo Model |
In this study, sixteen-week-old male C57BL/6 mice were used and housed in a standard laboratory environment. Forin vivostudies, left eyes were pretreated with a single intravitreal injection of DMSO (1 uL) or 1X PBS (1 uL), and right eyes were pretreated with Fer-1 (30 uM, a single intravitreal injection, 1 uL), DFO (100 uM, a single intravitreal injection, 1 uL), or ZnPP (50 mg/kg, intraperitoneal injection once a week). 15 min later, injection of NaIO3(35 mg/kg, a single injectionviatail vein), or 1X PBS (a single injectionviatail vein, 75 uL) has been performed. After 2 weeks, mice were used for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Sodium iodate-induced oxidative stress model has been identified to be heme oxygenase-1 (HO-1)-regulated ferroptosis, which is controlled by the Nrf2-SLC7A11-HO-1 hierarchy. These findings highlight that targeting HO-1-mediated RPE ferroptosis could serve as an effectively retinal-protective strategy for retinal degenerative diseases prevention, including age-related macular degeneration (AMD). | ||||
Iridin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [53] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
In vivo, the LIRI model was established as described earlier. All mice were anesthetized with pentobarbital administered intraperitoneally (50 mg/kg, Sigma-Aldrich, MO, USA). After endotracheal intubation, the mice were ventilated using a rodent ventilator (MiniVent, Harvard Apparatus, USA), with the title volume set to 7 ml/kg, the respiratory rate set to 120 times/min, and the inspiratory/expiratory ratio set to 1: 2. A noninvasive clamp was used to interrupt the left pulmonary hilum, causing lung ischemia. The clamp was released after 60 minutes of ischemia, and the left lung was reperfused for 120 minutes. Animals were euthanized via cervical dislocation at the end of the experiment. Following that, lung specimens and bronchoalveolar lavage fluid were harvested for analysis. All procedures except lung ischemia were performed on mice in the sham group.
Click to Show/Hide
|
||||
| Response Description | As a result, irisin postconditioning may protect against lung I/R damage by suppressing ferroptosis via the Nrf2/HO-1 signaling axis. | ||||
Apigenin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [54] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HUVECs (Human umbilical vein endothelial cells) | ||||
| In Vivo Model |
Mice were exposed to 12/12 h of light/darkness and given free access to food and water. All C57BL/6J mice were fasted for 12 h and anesthetized by intraperitoneal injection of 1% sodium pentobarbital before modeling. Later, the superior mesenteric artery (SMA) was subjected to 45 min of global no-flow ischemia, followed by 90 min of reperfusion to induce IIRI. The successful model could be revealed by the microcirculation detector. In order to investigate the effects of APG, mice were randomly divided into different groups: Sham group, IIRI group, APG groups (2 mg/kg, 4 mg/kg and 8 mg/kg), ZnPPIX group (Protoporphyrin Zinc(), Dalian Meilun Biotech Co., Ltd., China, 10mg/kg), Selegiline group (10 mg/kg, Shandong Topscience Biotech Co., Ltd., China) and the ZnPPIX + Selegiline group (10 mg/kg ZnPPIX and 10 mg/kg Selegiline). As mentioned above, drug was intraperitoneal injected after a 10-min-ischemia.
Click to Show/Hide
|
||||
| Response Description | MST analysis suggested that Apigenin could specifically bind to heme oxygenase 1 (HO-1) and monoamine oxidase b (MAO-B). Simultaneously, APG could attenuate ROS generation and Fe2+ accumulation, maintain mitochondria function thus inhibit ferroptosis and alleviate intestinal ischemia-reperfusion injury. | ||||
Dimethyl fumarate
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [55] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
The mice were randomly divided into four groups of six: sham + vehicle, sham + DMF, IR + vehicle, and IR + DMF. The mice were supplemented with DMF at a concentration of 100 mg/kg or DMSO by daily oral gavage for a week before surgery, as previously reported. As stated in a prior study, the partial warm liver IRI model was developed. Briefly, the sham group only had free hepatic portal blood vessels after laparotomy, and the blood flow was not obstructed. As for the hepatic IR group, the blood supply to the left and mid-hepatic lobes was blocked, resulting in 70% mouse liver IRI for 90 min. The mice were put on a heated blanket after surgery in order to maintain body temperature and monitor vital signs. Blood supply was restored for 6 h. Died mice were eliminated for testing prior to sample collection. The mice were euthanized after the sample were obtained. The same experimenter carried out all surgeries.
Click to Show/Hide
|
||||
| Response Description | NRF2 knockdown notably decreased the expression of SLC7A11 and HO-1 and blocked the anti-ferroptosis effects of dimethyl fumarate (DMF). DMF inhibits ferroptosis by activating the NRF2/SLC7A11/HO-1 axis and exerts a protective effect against hepatic ischemia-reperfusion injury. | ||||
Ginsenoside Rg3
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [56] | ||||
| Responsed Disease | Acute pancreatitis [ICD-11: DC31] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AR42J cells | Digestive system neoplasms | Rattus norvegicus | CVCL_0143 | |
| In Vivo Model |
Male C57BL/6 mice (8 weeks old; SPF; weighing 24-26 g, n = 16 in total) were purchased from SPF (Beijing) Biotechnology Co., Ltd. All mice were housed in an environmentally controlled room at a temperature ranging from 20 to 24 on a 12 h light/dark cycle and used in the experiments following an overnight fast with water, availablead libitum. All procedures followed the Principles of Laboratory Animal Care (NIH publication number 85Y23, revised in 1996), and the experimental protocol was approved by the Animal Care Committee, Nanjing Medical University (NMU-2021JK-085).
Click to Show/Hide
|
||||
| Response Description | Taken together, the present study, to the best of our knowledge, is the first to reveal a protective role for Ginsenoside Rg3 in mice with acute pancreatitis by suppressing oxidative stressrelated ferroptosis and the activation of the NRF2/HO1 pathway. | ||||
Dioscin
[Preclinical]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [57] | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Six-week-old male Wistar rats (170-200 g) were obtained from Changsheng Biotechnology Co., Ltd. (Changchun, China), and all of them were fed under SPF-conditions. The rats were acclimatized to natural light/dark cycles at a controlled temperature of 22 + 2 with free access to food and water. The experiment was comprised of four groups: the C group (0.5% carboxymethyl cellulose sodium [CMC-Na], n = 6); the Dio group (dioscin-treated rats, n = 6); the CP group (cisplatin-treated mice, n = 6); and the Dio + CP group (dioscin plus cisplatin-treated rats, n = 6). Rats were gavaged with dioscin (60 mg/kg) for ten days, and cisplatin (10 mg/kg) was intraperitoneally injected once on the seventh day.
Click to Show/Hide
|
||||
| Response Description | Dioscin exerts a reno-protective effect by decreasing renal oxidative injury, apoptosis and ferroptosis through the Nrf2/HO-1 signaling pathway, providing a new insight into acute kidney injury prevention. | ||||
Sevoflurane
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [58] | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
Male C57BL/6 mice (8 weeks, 23-25 g) were obtained from Beijing Hua Fu Kang Biotechnology Co. LTD (Beijing, China). A total of 96 mice were randomly divided into 6 groups (n = 16 per group): Sham group, LPS group, Fer-1 group, Fe group, Sev group, and Fe + Sev group. Mice were given 5 mg/kg LPS intranasally to construct LPS-induced ALI model. To study the role of ferroptosis in ALI, the mice in the Fer-1 or Fe groups were administered with Fer-1 (0.8 mg/kg; Ferrostatin-1, the inhibitor of ferroptosis) or Fe (8 mg/kg; Fe-citrate (III), ferroptosis inducer) via tail vein injection once a day for 3 consecutive days before treatment with LPS, respectively. To study the effect of Sev on ALI mice, the mice in Sev group were treated with LPS for 2 h, and then Sev, delivered by gaseous admixture (oxygen) at a concentration of 3% via a calibrated vaporizer, was administered via an endotracheal tube for 4 h. In order to study the effect of Sev on ferroptosis, the mice in Fe + Sev group were administered with Fe via tail vein injection once a day for 3 consecutive days, and then treated with LPS and Sev. Sham group was given 0.9% NaCl (containing 0.1% DMSO).
Click to Show/Hide
|
||||
| Response Description | Sevoflurane (Sev) could eliminate the worsening effects of ferroptosis inducer Fe-citrate on LPS-induced acute lung injury (ALI) to a certain extent. Sev inhibited ferroptosis by up-regulating HO-1 expression to reduce LPS-induced ALI, which may provide a possible mechanism for the application of Sev in clinical anesthesia. | ||||
Astaxanthin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [59] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Mice were randomly divided into four groups as follows (n = 5): (1) control, (2) APAP (MCE, Monmouth Junction, NJ, USA), (3) olive oil + APAP (oil + APAP), and (4) ASX (Energy Chemical, Shanghai, China) dissolved in olive oil + APAP (ASX + APAP). Astaxanthin was dissolved in olive oil to obtain a mixture of 20 mg/mL. Mice in groups 3 and 4 were given a dose of olive oil and a mixture of 5 mL/kgBW by gavage every day for 2 weeks. On day 15, mice in groups 2, 3, and 4 were given a peritoneal injection of 500 mg/kg APAP to induce liver injury. The mice were fasted for 12 h before the administration of APAP. Ten hours after APAP administration, blood and liver tissue were collected for further examination and analyses. Blood was centrifuged to obtain supernatants,which were stored at -80. Liver tissues were immediately removed from each animal, and homogenates were processed with formaldehyde and glutaraldehyde for protein and histological analysis.
Click to Show/Hide
|
||||
| Response Description | Astaxanthin reduced inflammation through the NF-B pathway, inhibited oxidative stress and ferroptosis, and increased autophagy through the Nrf2/HO-1 pathway, ameliorating acetaminophen-induced liver injury in vivo and in vitro. | ||||
Ulinastatin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Ulinastatin plays a role in mitigation of APAP-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
Abietic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [60] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
APAP-induced liver injury model was induced byintraperitoneal injection 300 mg/kg APAP. The mice of APAP + abietic acid (10, 20, 40 mg/kg) were given abietic acid by intraperitoneal injection 1 h before APAP treatment. The doses of abietic acid used in this study were based on previous studies. Twelve hours later, the mice were sacrificed after anesthesia with 1%pentobarbital (50 mg/kg) injected intraperitoneally and the samples were collected.
Click to Show/Hide
|
||||
| Response Description | APAP could increase malondialdehyde (MDA) and Fe2+ levels, and decrease ATP and glutathione (GSH) levels, as well as glutathione peroxidase 4 (GPX4) and xCT expression. However, these changes induced by APAP were prevented by abietic acid, indicating abietic acid could inhibit APAP-induced ferroptosis. Furthermore, abietic acid inhibited APAP-induced liver injury, NF-B activation and increased the expression of Nrf2 and HO-1. | ||||
References
