Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0046)
| Name |
Dimethyl fumarate
|
||||
|---|---|---|---|---|---|
| Synonyms |
Dimethyl fumarate; 624-49-7; Tecfidera; Methyl fumarate; Fumaderm; (E)-Dimethyl fumarate; Fumaric acid, dimethyl ester; Fumaric acid dimethyl ester; BG-12; Dimethyl (E)-but-2-enedioate; Boletic acid dimethyl ester; BG 12 compound; Panaclar; Dimethylfumarate; Dimethyl trans-ethylenedicarboxylate; trans-Butenedioic acid dimethyl ester; Allomaleic acid dimethyl ester; dimethyl (2E)-but-2-enedioate; BG00012; trans-1,2-Ethylenedicarboxylic acid dimethyl ester; 23055-10-9; 2-Butenedioic acid (E)-, dimethyl ester; BG 00012; BG-00012; Dimethyl 2-butenedioate; FAG-201; AZL O 211089; AZL-O-211089; NSC-25942; Dimethylester kyseliny fumarove; NSC-167432; Dimethyl fumar; CHEBI:76004; (E)-but-2-enedioic acid dimethyl ester; FAG 201; BG 12; FP187; Dimethyl fumarate [USAN]; 2-Butenedioic acid (2E)-, dimethyl ester; LAS41008; EINECS 210-849-0; UNII-FO2303MNI2; FP-187; LAS-41008; NSC 25942; TL 353; NSC 167432; AZL-0211089; BRN 0774590; FO2303MNI2; 1,2-bis(methoxycarbonyl)-trans-ethylene; AI3-07872; HSDB 7725; BG 12 [Fumarate]; BG-12 [Fumarate]; Ethylene, 1,2-bis(methoxycarbonyl)-, trans-; Fumaric acid-dimethyl ester; 2-BUTENEDIOIC ACID, DIMETHYL ESTER, (E)-; Dimethyl (2e)-2-butenedioate; 2-butenedioic acid, (2E)-, dimethyl ester; 2-Butenedioic acid, dimethyl ester; dimethyl (~{E})-but-2-enedioate; 4-02-00-02205 (Beilstein Handbook Reference); But-2-enedioic acid, dimethyl ester; BG 12 (Fumarate); BG-12 (Fumarate); AZL 0 211089; Dimethyl fumarate (USAN); 2-Butenedioic acid (2E)-, 1,4-dimethyl ester; WLN: 1OV1U1VO1 -T; Fumaric acid-dimethyl ester 1000 microg/mL in Acetonitrile; BIS-METHYL ESTER; MFCD00064438; Fumarate, Dimethyl; Ethylene,2-bis(methoxycarbonyl)-, trans-; FAG201; Dimethylester kyseliny fumarove [Czech]; 1,4-dimethyl but-2-enedioate; dimethyl-fumarate; FUMARIC ACID DIMETHYL ESTER (1,1,1,8,8,8-D6); Tecfidera (TN); Dimethyl fumarate, 97%; (E/Z)-Dimethyl fumarate; dimethyl trans-butenedioate; Dimethyl (E)-Butenedioate; Dimethyl (E)-butenedionate; SCHEMBL41835; SCHEMBL41836; DIMETHYL FUMARATE [MI]; Dimethyl fumarate (JAN/USAN); GTPL7045; DIMETHYL FUMARATE [JAN]; CHEMBL2107333; DIMETHYL FUMARATE [HSDB]; DTXSID4060787; DIMETHYL FUMARATE [VANDF]; 2-Butenedioic acid, dimethylester; But-2-enedioic aciddimethyl ester; HMS3264D14; Pharmakon1600-01506154; DIMETHYL FUMARATE [WHO-DD]; NSC25942; BDBM50504654; Fumaric acid, dimethyl ester (8CI); NSC167432; NSC760139; s2586; STK039379; Dimethyl ester(E)-2-Butenedioic acid; (E)-CH3OC(O)CH=CHC(O)OCH3; 1,4-dimethyl (2E)-but-2-enedioate; AKOS000121333; CCG-213618; CS-0909; DB08908; Dimethyl ester(2E)-2-Butenedioic acid; DIMETHYL FUMARATE [ORANGE BOOK]; NSC-760139; (E)-2-Butenedioic Acid, Dimethyl Ester; HY-17363; 2(E)-Butenedioic acid 1,4-dimethyl ester; 2-Butenedioic acid, dimethyl ester, (2E)-; CS-0369103; F0069; SW219154-1; EN300-16090; D03846; EN300-305306; H11241; AB00172980_03; AB00172980_04; Dimethyl fumarate, Vetec(TM) reagent grade, 97%; Q418123; SR-01000944222; SR-01000944222-1; trans-1, 2-Ethylenedicarboxylic acid dimethyl ester; BRD-K31111078-001-01-8; Z49500377; F0001-1675; Dimethyl fumarate, certified reference material, TraceCERT(R); 12287-98-8; EOU
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
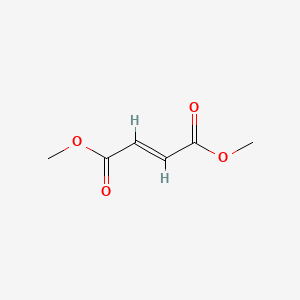 |
||||
| Formula |
C6H8O4
|
||||
| IUPAC Name |
dimethyl (E)-but-2-enedioate
|
||||
| Canonical SMILES |
COC(=O)C=CC(=O)OC
|
||||
| InChI |
InChI=1S/C6H8O4/c1-9-5(7)3-4-6(8)10-2/h3-4H,1-2H3/b4-3+
|
||||
| InChIKey |
LDCRTTXIJACKKU-ONEGZZNKSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Diffuse large B-cell lymphoma | ICD-11: 2A81 | |||
| Responsed Regulator | Signal transducer and activator of transcription 3 (STAT3) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | A-431 cells | Skin squamous cell carcinoma | Homo sapiens | CVCL_0037 | |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| HaCaT cells | Normal | Homo sapiens | CVCL_0038 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| SK-MEL-28 cells | Cutaneous melanoma | Homo sapiens | CVCL_0526 | ||
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
For the VFN-D1 patient-derived and the HBL-1 xenograft mouse models, adult female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were implanted subcutaneously with 1 x 107 cells in PBS (300 ul). Once mice developed palpable tumors, the animals were randomized into control, DMF (daily i.p. treatment with 500 ul of 3 mg/ml DMF in PBS), ABT-199 (daily p.o. treatment with 100 mg/kg in ethanol/PhosalG/PEG400) or DMF+ABT-199 treated groups.
Click to Show/Hide
|
||||
| Response regulation | Dimethyl fumarate (DMF) induces lipid peroxidation and thus ferroptosis, particularly in GCB diffuse large B-cell lymphoma (DLBCL). In ABC DLBCL cells, which are addicted to NF-kB and STAT3 survival signaling, DMF treatment efficiently inhibits the activity of the IKK complex and Janus kinases . | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
The mice were randomly divided into four groups of six: sham + vehicle, sham + DMF, IR + vehicle, and IR + DMF. The mice were supplemented with DMF at a concentration of 100 mg/kg or DMSO by daily oral gavage for a week before surgery, as previously reported. As stated in a prior study, the partial warm liver IRI model was developed. Briefly, the sham group only had free hepatic portal blood vessels after laparotomy, and the blood flow was not obstructed. As for the hepatic IR group, the blood supply to the left and mid-hepatic lobes was blocked, resulting in 70% mouse liver IRI for 90 min. The mice were put on a heated blanket after surgery in order to maintain body temperature and monitor vital signs. Blood supply was restored for 6 h. Died mice were eliminated for testing prior to sample collection. The mice were euthanized after the sample were obtained. The same experimenter carried out all surgeries.
Click to Show/Hide
|
||||
| Response regulation | NRF2 knockdown notably decreased the expression of SLC7A11 and HO-1 and blocked the anti-ferroptosis effects of dimethyl fumarate (DMF). DMF inhibits ferroptosis by activating the NRF2/SLC7A11/HO-1 axis and exerts a protective effect against hepatic ischemia-reperfusion injury. | ||||
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
The mice were randomly divided into four groups of six: sham + vehicle, sham + DMF, IR + vehicle, and IR + DMF. The mice were supplemented with DMF at a concentration of 100 mg/kg or DMSO by daily oral gavage for a week before surgery, as previously reported. As stated in a prior study, the partial warm liver IRI model was developed. Briefly, the sham group only had free hepatic portal blood vessels after laparotomy, and the blood flow was not obstructed. As for the hepatic IR group, the blood supply to the left and mid-hepatic lobes was blocked, resulting in 70% mouse liver IRI for 90 min. The mice were put on a heated blanket after surgery in order to maintain body temperature and monitor vital signs. Blood supply was restored for 6 h. Died mice were eliminated for testing prior to sample collection. The mice were euthanized after the sample were obtained. The same experimenter carried out all surgeries.
Click to Show/Hide
|
||||
| Response regulation | NRF2 knockdown notably decreased the expression of SLC7A11 and HO-1 and blocked the anti-ferroptosis effects of dimethyl fumarate (DMF). DMF inhibits ferroptosis by activating the NRF2/SLC7A11/HO-1 axis and exerts a protective effect against hepatic ischemia-reperfusion injury. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
The mice were randomly divided into four groups of six: sham + vehicle, sham + DMF, IR + vehicle, and IR + DMF. The mice were supplemented with DMF at a concentration of 100 mg/kg or DMSO by daily oral gavage for a week before surgery, as previously reported. As stated in a prior study, the partial warm liver IRI model was developed. Briefly, the sham group only had free hepatic portal blood vessels after laparotomy, and the blood flow was not obstructed. As for the hepatic IR group, the blood supply to the left and mid-hepatic lobes was blocked, resulting in 70% mouse liver IRI for 90 min. The mice were put on a heated blanket after surgery in order to maintain body temperature and monitor vital signs. Blood supply was restored for 6 h. Died mice were eliminated for testing prior to sample collection. The mice were euthanized after the sample were obtained. The same experimenter carried out all surgeries.
Click to Show/Hide
|
||||
| Response regulation | NRF2 knockdown notably decreased the expression of SLC7A11 and HO-1 and blocked the anti-ferroptosis effects of dimethyl fumarate (DMF). DMF inhibits ferroptosis by activating the NRF2/SLC7A11/HO-1 axis and exerts a protective effect against hepatic ischemia-reperfusion injury. | ||||
References
