Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10041)
| Target Name | Nuclear factor erythroid 2-related factor 2 (NFE2L2) | ||||
|---|---|---|---|---|---|
| Synonyms |
Nrf-2; HEBP1; NRF2; NFE2L2; Nuclear factor, erythroid derived 2, like 2; NF-E2-related factor 2; NFE2-related factor 2
Click to Show/Hide
|
||||
| Gene Name | NFE2L2 | ||||
| Sequence |
MMDLELPPPGLPSQQDMDLIDILWRQDIDLGVSREVFDFSQRRKEYELEKQKKLEKERQE
QLQKEQEKAFFAQLQLDEETGEFLPIQPAQHIQSETSGSANYSQVAHIPKSDALYFDDCM QLLAQTFPFVDDNEVSSATFQSLVPDIPGHIESPVFIATNQAQSPETSVAQVAPVDLDGM QQDIEQVWEELLSIPELQCLNIENDKLVETTMVPSPEAKLTEVDNYHFYSSIPSMEKEVG NCSPHFLNAFEDSFSSILSTEDPNQLTVNSLNSDATVNTDFGDEFYSAFIAEPSISNSMP SPATLSHSLSELLNGPIDVSDLSLCKAFNQNHPESTAEFNDSDSGISLNTSPSVASPEHS VESSSYGDTLLGLSDSEVEELDSAPGSVKQNGPKTPVHSSGDMVQPLSPSQGQSTHVHDA QCENTPEKELPVSPGHRKTPFTKDKHSSRLEAHLTRDELRAKALHIPFPVEKIINLPVVD FNEMMSKEQFNEAQLALIRDIRRRGKNKVAAQNCRKRKLENIVELEQDLDHLKDEKEKLL KEKGENDKSLHLLKKQLSTLYLEVFSMLRDEDGKPYSPSEYSLQQTRDGNVFLVPKSKKP DVKKN Click to Show/Hide
|
||||
| Family | bZIP family | ||||
| Function |
Transcription factor that plays a key role in the response to oxidative stress: binds to antioxidant response (ARE) elements present in the promoter region of many cytoprotective genes, such as phase 2 detoxifying enzymes, and promotes their expression, thereby neutralizing reactive electrophiles. In normal conditions, ubiquitinated and degraded in the cytoplasm by the BCR(KEAP1) complex. In response to oxidative stress, electrophile metabolites inhibit activity of the BCR(KEAP1) complex, promoting nuclear accumulation of NFE2L2/NRF2, heterodimerization with one of the small Maf proteins and binding to ARE elements of cytoprotective target genes. The NFE2L2/NRF2 pathway is also activated in response to selective autophagy: autophagy promotes interaction between KEAP1 and SQSTM1/p62 and subsequent inactivation of the BCR(KEAP1) complex, leading to NFE2L2/NRF2 nuclear accumulation and expression of cytoprotective genes. May also be involved in the transcriptional activation of genes of the beta-globin cluster by mediating enhancer activity of hypersensitive site 2 of the beta-globin locus control region. Also plays an important role in the regulation of the innate immune response and antiviral cytosolic DNA sensing. It is a critical regulator of the innate immune response and survival during sepsis by maintaining redox homeostasis and restraint of the dysregulation of pro-inflammatory signaling pathways like MyD88-dependent and -independent and TNF-alpha signaling. Suppresses macrophage inflammatory response by blocking pro-inflammatory cytokine transcription and the induction of IL6. Binds to the proximity of pro-inflammatory genes in macrophages and inhibits RNA Pol II recruitment. The inhibition is independent of the NRF2-binding motif and reactive oxygen species level. Represses antiviral cytosolic DNA sensing by suppressing the expression of the adapter protein STING1 and decreasing responsiveness to STING1 agonists while increasing susceptibility to infection with DNA viruses. Once activated, limits the release of pro-inflammatory cytokines in response to human coronavirus SARS-CoV-2 infection and to virus-derived ligands through a mechanism that involves inhibition of IRF3 dimerization. Also inhibits both SARS-CoV-2 replication, as well as the replication of several other pathogenic viruses including Herpes Simplex Virus-1 and-2, Vaccinia virus, and Zika virus through a type I interferon (IFN)-independent mechanism.
Click to Show/Hide
|
||||
| Gene ID | 4780 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
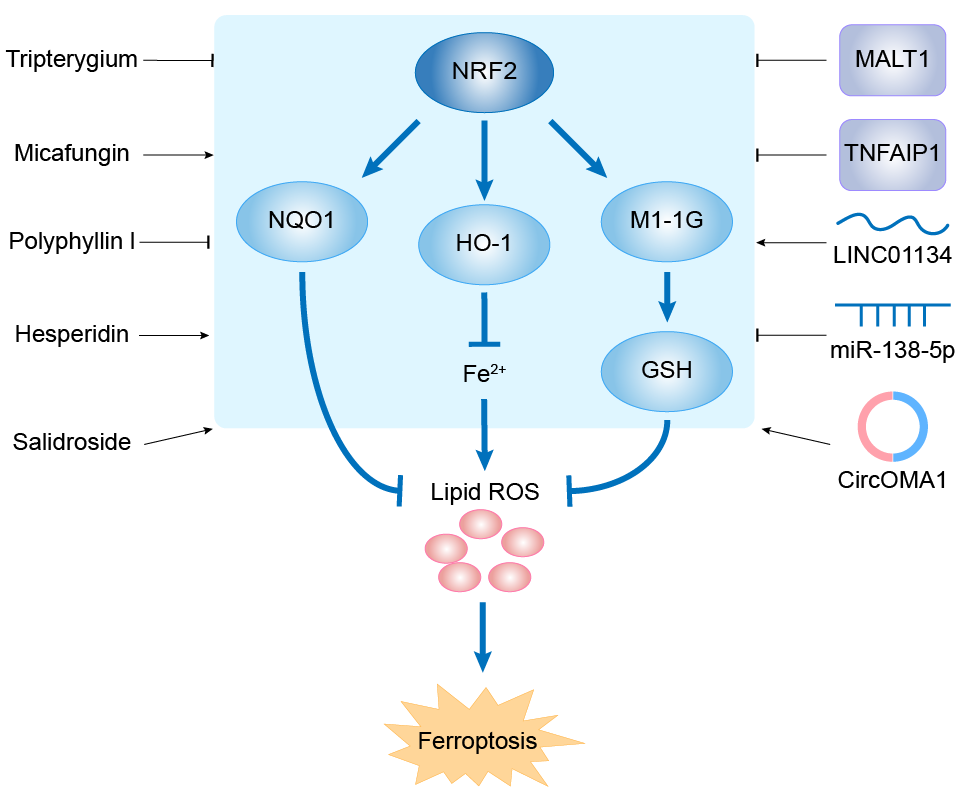
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
NFE2L2 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Vascular endothelial growth factor receptor 2 (KDR)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Apatinib | Investigative | |||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female BALB/c nude mice (age, 4 weeks old) were purchased from Changzhou Cavens Experimental Animal Co., Ltd. (Changzhou, China).The gliomas from the nude mice were fixed in 10% paraformaldehyde at 4 for 12 h and then dehydrated in different concentrations of ethanol. The tumor tissues were permeabilized using xylene and embedded in paraffin. They were then sliced (0.5 um), rehydrated, and stained with HE at 4 for 10 min and sealed. For IHC assessment of Ki-67 in gliomas, the DAKO Envision system (Dako; Agilent Technologies, Inc.) was used.
Click to Show/Hide
|
||||
| Response Description | Apatinib could restrain proliferation of glioma cells through induction of ferroptosis via inhibiting the activation of VEGFR2/Nrf2/Keap1 pathway. Overexpression of Nrf2 could counteract the induction of ferroptosis by apatinib. | ||||
Ubiquitin carboxyl-terminal hydrolase 11 (USP11)
Lung cancer [ICD-11: 2C25]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | RSL3 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| H2122 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1531 | ||
| In Vivo Model |
After two weeks in house, the mice were subcutaneously injected with A549 cells (100 uL containing 5 x 106 cells/injection) and monitored for tumor cell xenografts to reach approximately 100 mm3. The mice were then divided into two groups (n = 5), the RSL3 treatment (100 mg/kg; dissolved in 5% dimethyl sulfoxide/corn oil; administrated intratumorally twice a day for one week) and control (5% dimethyl sulfoxide/corn oil only) groups.
Click to Show/Hide
|
||||
| Response Description | RSL3 was able to directly bind to USP11, a recently identified de-ubiquitinase of NRF2, and inactivate USP11 protein to induce NRF2 protein ubiquitination and degradation in KLK lung adenocarcinoma cells. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [47] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| Response Description | USP11 is highly expressed in patients with non-small cell lung cancer (NSCLC) and positively correlated with NRF2 expression. Together, USP11 stabilizes NRF2 and is thus an important player in cell proliferation and ferroptosis. | ||||
Tyrosine-protein kinase BTK (BTK)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Ibrutinib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| LS513 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1386 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| SW1116 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0544 | ||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| HT-29 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0320 | ||
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| In Vivo Model |
Sixty mice were randomly divided into six groups, (1) the CRC model group (model), (2) mice with RSL3 treatment, (3) mice with Erastin treatment, (4) mice with Ibrutinib treatment, (5) mice with RSL3 and Ibrutinib treatment, and (6) Erastin and Ibrutinib group. Murine subcutaneous tumor model and xenograft tumor mouse model were established and please refer to supplemental method for details. For CRC model group, the mice were treated with PBS for two weeks. For RSL3 group, the mice were intraperitoneal injected with RSL3 (5 mg/kg daily) for two weeks. For Erastin group, the mice were intraperitoneal injected with Erastin (30 mg/kg, twice every other day) for two weeks. For Ibrutinib treatment group, mice were administered in drinking water at a concentration of 0.16 mg/ml for two weeks. Mice were also treated in combination with RSL and Ibrutinib or Erastin and Ibrutinib.
Click to Show/Hide
|
||||
| Response Description | Ibrutinib inhibited BTK, which prevented Nrf2 translocating to cell nucleus and the activation of the Nrf2 dependent antioxidant genes during oxidative stress conditions and eventually enhanced the sensitivity of Colorectal cancer (CRC) cells to ferroptosis. | ||||
Signal transducer and activator of transcription 3 (STAT3)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Peoniflorin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| In Vivo Model |
U251 cells (6 x 106) were inoculated into the flanks of 4-to 5-week-old athymic nude mice (Shanghai Laboratory Animal Company, Shanghai, China) subcutaneously to generate a subcutaneous xenograft tumor model. After 2 weeks, the tumor model was successfully constructed, the mice were treated single and combined with 100 mg/kg RSL3 (2 times/week) and 1.0 g/kg/days PF. Tumor volumes were measured every 4 days to draw the growth curve. Mice were sacrificed 4 weeks after cell injection. Tumor xenografts were collected, photographed, and weighed and the tumor apoptosis was analyzed by Tunel staining.
Click to Show/Hide
|
||||
| Response Description | Paeoniflorin (PF) can function as an antitumor agent for glioma treatment by targeting NEDD4L-dependent STAT3 ubiquitination as well as by regulating the Nrf2/GPX4 signaling axis, which might trigger ferroptosis. | ||||
Sestrin-2 (SESN2)
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Empagliflozin | Approved | |||
| Pathway Response | Autophagy | hsa04140 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hLCs (Liver cells) | ||||
| In Vivo Model |
After a one-week acclimatization period, rats were randomly divided into four experimental groups of six rats each. Group I (the control group) received saline intraperitoneally in the same manner as BLM injections, as well as 1% carboxymethyl cellulose (CMC) orally in the same manner as EMPA. Group II (the BLM-treated group) received BLM (15 mg/kg) intraperitoneally three times per week for four successive weeks in order to induce pulmonary fibrosis. Group III (the EMPA-treated group) received EMPA dissolved in 1% CMC orally via oral gavage at a dose of 10 mg/kg/day throughout the experimental period. Group IV (the combined EMPA and BLM-treated group) received EMPA (10 mg/kg) orally via oral gavage seven days before BLM administration and continued for four weeks after BLM injection.
Click to Show/Hide
|
||||
| Response Description | Empagliflozin has a promising protective effect against BLM-induced liver fibrosis in rats by enhancing autophagy and mitigating ferroptosis, inflammation, and ER stress via modulating the Sesn2/AMPK/Nrf2/HO-1 signaling pathway. | ||||
Serine/threonine-protein kinase TBK1 (TBK1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Tiliroside | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
All animal studies were approved by the Committee on Ethics of Animal Experiments of Binzhou Medical University (approval no: BZMU-IACUC-2021-331, date: 09/10/2021). To generate the ectopic HCC mouse models, HepG2-luciferase cells (HepG2 cells transfected with luciferase gene) were suspended in serum-free media and matrigel (BD Biosciences) at a ratio of 1:1 v/v. A total of 2.5 x 106 HepG2-luciferase cells/100 ul were injected into the left axilla of mice. After reaching a tumor size of 100-150 mm3, all mice were randomly divided into four groups: control (vehicle, intraperitoneal [i.p.]), tiliroside (20 mg/kg,i.p.), sorafenib (30 mg/kg,i.p.), or combination treatment (tiliroside and sorafenib,i.p.). All treatments were administered every 3 d, and the length and width of tumor were measured every 4 d. The formula tumor volume = (length x width2)/2 was used to calculate the tumor volume. Body weight was recorded every 7 d, and the morphology of the tumor was photographed using animal in vivo imaging technology (IVIS Spectrum; PerkinElmer) before the day of sacrifice. The mice were sacrificed 40 d after administration, and the tumors were dissected and weighed. The major organs and xenograft tumors were fixed with 4% paraformaldehyde.
Click to Show/Hide
|
||||
| Response Description | Tiliroside directly binds to TBK1 and inhibits its activity, which inhibits the phosphorylation of Ser349 on p62. Consequently, this decreases the affinity of p62 for Keap1, promotes ubiquitination and degradation of Nrf2 and ferroptosis, and eventually increases the sensitivity of hepatocellular carcinoma cells to sorafenib. | ||||
Sequestosome-1 (SQSTM1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hepa 1-6 cells | Hepatocellular carcinoma | Mus musculus | CVCL_0327 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 1 x 106 Hepa16 cells in control shRNA or NRF2 knockdown cells in 200 ul phosphate buffered saline were injected subcutaneously to the right of the dorsal midline in C57BL/6 mice. Once the tumors reached 80-100 mm3 at day seven, mice were randomly allocated into groups and treated with erastin (30 mg/kg intraperitoneal injection [i.p.], twice every other day) and sorafenib (10 mg/kg i.p., once every other day) for two weeks.
Click to Show/Hide
|
||||
| Response Description | Upon exposure to ferroptosis-inducing compounds (e.g., erastin, sorafenib, and buthionine sulfoximine), p62 (SQSTM1) expression prevented NRF2 degradation and enhanced subsequent NRF2 nuclear accumulation through inactivation of Kelch-like ECH-associated protein 1. The status of NRF2 is a key factor that determines the therapeutic response to ferroptosis-targeted therapies in hepatocellular carcinoma cells. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Rats were randomly divided into the Sham, middle cerebral artery occlusion-reperfusion (MCAO/R), and MCAO/R + AST IV (28 mg/kg) groups. The MCAO/R + AST IV group was intragastrically injected with 10 mL/kg AST IV at 50, 26, and 2 h before modelling (Xiao et al., 2021). The Sham and MCAO/R groups received equal amounts of normal saline. As described previously, the modified Longa method (Longa et al., 1989) was used to establish the MCAO/R model. After anaesthesia with 2%sodium pentobarbital, the left common carotid artery(CCA), the external carotid artery(ECA), and the internal carotid artery(ICA) were isolated. The distal end of the ECA was ligated, a small incision was made at the stump of the ECA, and a suture (Batch number: 2636A2, Beijing Seinong Technology Co., Ltd., Beijing, China; head-end diameter: 0.36 ± 0.02 mm) was inserted into the ICA from the ECA through the bifurcation of the CCA. To achieve cerebral ischaemia, the head-end was used to block blood flow in the middle cerebral artery until the intracranial segment of the ICA was inserted. The suture was removed after 2 h, and follow-up experiments were performed 24 h after reperfusion. In the Sham group, the CCA, ECA, and ICA were exposed and separated, but no sutures were inserted. Penicillin powder was used to fight infection after operation.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AST IV) increased the P62 (SQSTM1) and Nrf2 levels and decreased the Keap1 levels. P62 silencing reduced the effects of AST IV on the P62/Keap1/Nrf2 pathway and ferroptosis. Our findings suggest that AST IV mitigates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via activation of the P62/Keap1/Nrf2 pathway. | ||||
Endometrial hyperplasia [ICD-11: GA16]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Guizhi Fuling Capsule | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
mUTs (Mouse uterine tissues) | ||||
| In Vivo Model |
Female C57BL/6 mice (8-week-old) were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). Fifteen mice were randomly divided into three groups: Olive oil group, Estradiol group and Estradiol + IKE group. The Estradiol group was subcutaneously injected estradiol (50 ug/kg/day), Estradiol + IKE group was subcutaneously injected estradiol and intraperitoneally injected IKE (50 mg/kg) for 21 days, while the Olive oil group received the same volume of olive oil. In the experiment of exploring the improvement of GFC to EH, twenty mice were randomly divided into four groups: Olive oil group, Estradiol group, 75 mg/kg GFC group and 150 mg/kg GFC group. Except for Olive oil group, mice were subcutaneously daily injected with estradiol (50 ug/kg/day) for 21 days, while the Olive oil group received the same volume of olive oil. 75 mg/kg GFC group and 150 mg/kg GFC group were treated with GFC intragastrical administration.
Click to Show/Hide
|
||||
| Response Description | Guizhi Fuling Capsule (GFC) may attenuate estrogen-induced endometrial hyperplasia in mice through triggering ferroptosis via inhibiting p62 (SQSTM1)-Keap1-NRF2 pathway. GFC might act as a promising traditional Chinese medicine to treat endometrial hyperplasia. | ||||
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Entacapone | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks; 20-25 g) were purchased from LINGCHANG BIOTECH (China). Mice were divided into four groups: (i) sham, (ii) I/R, (iii) I/R+entacapone, and (iv) I/R + Fer-1. Entacapone (15 mg/kg bodyweight) was dissolved in sodium carboxymethyl cellulose (0.5%) and administered (i.g.) to mice. Mice in the sham group were administered (i.g.) an equal volume of solvent. Fer-1 was dissolved in 5% dimethyl sulfoxide + 30% polyethylene glycol-400 + 60% saline and injected (i.p.). Mice were treated three times per day for 3 days in advance. Before I/R, mice were fasted for 12 h and anesthetized (1% pentobarbital sodium, i.p.). The abdomen was exposed and bilateral renal pedicles were clamped to induce renal I/R. After 25 min, the arterial clamps were removed. A body temperature of 37 was maintained throughout the procedure. The sham group underwent the same procedure except for clamping of the renal pedicle. Mice were killed 24 h after reperfusion, and kidney and blood samples were collected for experimentation.
Click to Show/Hide
|
||||
| Response Description | Entacapone upregulates p62 (SQSTM1) expression and affects the p62-KEAP1-NRF2 pathway, thereby upregulating nuclear translocation of NRF2. This action results in increased expression of the downstream SLC7A11, and significant suppression of oxidative stress and ferroptosis. Entacapone may serve as a novel strategy to improve treatment of, and recovery from, ischemia/reperfusion-induced acute kidney injury (I/R-AKI). | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Baicalin | Terminated | |||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
C57BL/6 mice at 6-8 weeks were intraperitoneally injected with D-GalN/LPS (1772-03-8/L2880, Sigma-Aldrich, USA) at a dose of 700 mg/kg and 10 ug/kg, respectively. The constructed D-GaIN/LPS-induced ALI model mice were named the model group, and the normal mice injected with phosphate-buffered saline (PBS) were named the blank group. After 1 h of LPS/D-GalN treatment, Exo and Ba-Exo (150 ug/mice) were injected into the tail vein of the mice in the Exo and Ba-Exo groups, respectively. Mice were sacrificed via anesthesia overdose 12 h after the intervention. Half of the liver tissue was fixed in paraformaldehyde, while the other half was frozen at 80 . Peripheral blood serum was stored at -80 .
Click to Show/Hide
|
||||
| Response Description | Baicalin-pretreated MSCs (Ba-Exo) exerts a protective effect on liver function and activates the Keap1-NRF2 pathway via P62 (SQSTM1), thereby inhibiting ROS production and lipid peroxide-induced ferroptosis. Therefore, baicalin pretreatment is an effective and promising approach in optimizing the therapeutic efficacy of Exo in acute liver injury (ALI). | ||||
RAC-alpha serine/threonine-protein kinase (AKT1)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Fraxetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Wistar rats (200-250 g) were obtained from Slac Laboratory Animal Center (Shanghai, China) and kept in cages. The rats were anesthetized with 1% pentobarbital and then lied on its back. Thereafter, the left precordial area of the rats were shaved and disinfected, followed by trachea intubation for artificial ventilation. After the left thoracotomy, the heart was fully exposed and the left coronary artery (LAD) was ligated with a 6-0 prolene suture at 2-3 mm from its origin between the pulmonary artery conus and the left atrial appendage. After 30 min, the suture was gently removed to allow reperfusion for 2 h.
Click to Show/Hide
|
||||
| Response Description | Fraxetin activated phosphorylation of AKT and Nrf2 nuclear accumulation in Myocardial infarction in vivoandin vitromodels. Moreover, Fra reduced the activity of serum LDH, the accumulation of iron and the MDA level, and increased GSH and glutathione peroxidase 4 (GPX4) in rats with MI. | ||||
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Roxadustat | Phase 3 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mRTs (Mouse renal tissues) | ||||
| In Vivo Model |
C57BL/6 male mice, 6 to 8 weeks old, were purchased from Liaoning Changsheng Biotechnology Co. (Liaoning, China). The animal experiment was conducted in three parts. In the first part, mice were randomly divided into 4 groups (n = 12/group): (1) control group that received an intraperitoneal injection of saline, (2) FG-4592 group that received intraperitoneal injection of FG-4592 once (10 mg/kg, dissolved in DMSO at 50 mg/ml and then further diluted in sterile phosphate-buffered saline to 1 mg/ml), (3) FA group that received intraperitoneal injection of a single dose of FA (250 mg/kg, dissolved in 0.3 M sodium bicarbonate), and (4) FA + FG-4592 group that received FG-4592 two days prior to FA single-dose injection. Kidney specimens and blood samples were collected on the second day (n = 6/group) and the fourteenth day (n = 6/group) after FA injection for further examination. In the second part, mice were treated with a ferroptosis inhibitor (Fer-1). In the third part, mice were treated with a PI3K inhibitor (wortmannin).
Click to Show/Hide
|
||||
| Response Description | Roxadustat (FG-4592) pretreatment is achieved mainly by decreasing ferroptosis at the early stage of FA-induced kidney injury via Akt/GSK-3-mediated Nrf2 activation, which retards the fibrosis progression. | ||||
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | lipoxin A4 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mPSCNs (Mouse primary spinal cord neurons) | ||||
| In Vivo Model |
Pregnant C57BL/6 mouse was purchased from Laboratory Animal Center of Xinxiang Medical University. pregnant mouse was anesthetized with CO2 and sacrificed by cervical dislocation at embryonic day 15. All embryos were separated from pregnant mouse under aseptic conditions. Under dissection microscope, each embryo was quickly killed by cervical dislocation, and the spinal cord was isolated. The membrane of the spinal cord and dorsal root ganglion was removed from the spinal cord applying microforceps. Subsequently, the spinal cord was quickly cut into small pieces (1 mm3) using ultrafine microscissors.
Click to Show/Hide
|
||||
| Response Description | Lipoxin A4 (LXA4) enhanced the protein expression of p-AKT, nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and haem-oxygenase-1 (HO-1) in primary spinal cord neurons. LXA4 exerted a neuroprotective effect in Erastin-induced ferroptosis of primary spinal cord neurons by activating the Akt/Nrf2/HO-1 signaling pathway. Thus, LXA4 may be a potential therapeutic agent for spinal cord injury (SCI). | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [56] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
Mice weighing between 20 and 23 g were selected and 5 x 106 Hep-G2 cells were subcutaneously injected into their backs. The mice were subsequently divided into the following four groups: control (n = 5), FNDC5 overexpressing (n = 5), FNDC5 overexpressing followed by treatment with the PI3K inhibitor LY294002 (MCE, China), and FNDC5 knockdown (n = 5). Seven days after cell injection, sorafenib (30 mg/kg) was administered to all mice via intraperitoneal injection every alternate day for 4 weeks. The mice in the third group were intraperitoneally injected with LY294002 (25 mg/kg) diluted with DMSO twice a week for 4 weeks.
Click to Show/Hide
|
||||
| Response Description | FNDC5 activated the PI3K/Akt pathway, which in turn promoted the nuclear translocation of Nrf2 and increased the intracellular antioxidant response in Hepatocellular Carcinoma Cells, thereby conferring resistance to ferroptosis. Our study provides novel insights for improving the efficacy of sorafenib. | ||||
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA)
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Thioctic acid | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | a-Lipoic acid (a-LA) suppressed cell viability decline and mitigated ferroptosis in an MPP-induced PC12 cell model of parkinson's disease (PD) via activating the PI3K/Akt/Nrf2 pathway. These results discovered a novel a-LA-based therapy for PD patients, and activating the PI3K/Akt/Nrf2 pathway might be developed as a promising therapeutic approach for PD. | |||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [56] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
Mice weighing between 20 and 23 g were selected and 5 x 106 Hep-G2 cells were subcutaneously injected into their backs. The mice were subsequently divided into the following four groups: control (n = 5), FNDC5 overexpressing (n = 5), FNDC5 overexpressing followed by treatment with the PI3K inhibitor LY294002 (MCE, China), and FNDC5 knockdown (n = 5). Seven days after cell injection, sorafenib (30 mg/kg) was administered to all mice via intraperitoneal injection every alternate day for 4 weeks. The mice in the third group were intraperitoneally injected with LY294002 (25 mg/kg) diluted with DMSO twice a week for 4 weeks.
Click to Show/Hide
|
||||
| Response Description | FNDC5 activated the PI3K/Akt pathway, which in turn promoted the nuclear translocation of Nrf2 and increased the intracellular antioxidant response in Hepatocellular Carcinoma Cells, thereby conferring resistance to ferroptosis. Our study provides novel insights for improving the efficacy of sorafenib. | ||||
Peroxisome proliferator-activated receptor gamma (PPARG)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Pioglitazone | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPNCs (Rat primary nerve cells) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
The rats underwent surgery using an ultraclean table and were fixed in a stereotaxic frame. The scalp was opened to expose the anterior brain region. A dental drill was used to drill a 1-mm-diameter hole in the skull surface. Blood (100 ul) was collected from the rat tail vein and injected into the rat striatum with a microsyringe (stereotaxic coordinates; 2 mm lateral to the midline, 0.2 mm posterior to bregma, and 5.5 mm deep below the skull). First, 60 ul of autogenous blood were injected at a rate of 2 ul/min, and the next 40 ul of blood were injected at 5 ul/min. Finally, the needle was left for 10 min before being removed.
Click to Show/Hide
|
||||
| Response Description | Pioglitazone (PDZ), a PPAR agonist, promotes Gpx4 expression through the interaction between PPAR and the Nrf2 pathway, inhibits ferroptosis of neurons after intracerebral hemorrhage (ICH), and promotes the recovery of neural function. | ||||
NAD-dependent protein deacylase sirtuin-6 (SIRT6)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Isoorientin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
The A549/DDP tumor cells were subcutaneously injected (2 x 106 cells/mL) into BALB/c-nu mice under aseptic conditions. 6 days later, the average diameter of the tumor reaches 0.5 cm, and the mice were randomly divided into the 1 mg/kg DDP group and the 1 mg/kg DDP + 25mg/kg IO group. Six mice from each group were intraperitoneally administered medications every 2 days for a total of 10 doses each.
Click to Show/Hide
|
||||
| Response Description | Isoorientin (IO) can promote ferroptosis and reverse drug resistance in lung cancer through the SIRT6/Nrf2/GPX4 signaling pathway, thus offering a theoretical basis for its potential clinical application. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Sepsis [ICD-11: 1G40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Iridin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
All animals were purchased from the Animal Experimental Center of Wuhan University (ABLS-III Laboratory). C57BL/6 male mice weighing 20-25 g were used for this study. HK-2 cells were seeded into 96-well plates (5 x 105 cells/well) and cultured for 24 h until 80% confluence. Subsequently, we have added LPS (10 ug/ml) into the cultured cells for 22 h to establish the cell model of LPS-induced AKI.
Click to Show/Hide
|
||||
| Response Description | Sepsis-associated acute kidney injury induced ferroptosis by increasing iron and lipid peroxidation. Irisin effectively suppressed ferroptosis and alleviated SA-AKI and improved the mitochondria functionviainduction of the SIRT1/Nrf2 signal axis. | ||||
Depressive disorder [ICD-11: 6A70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Edaravone | Approved | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Male C57BL/6J mice (aged 7-8 weeks) and retired male CD-1 mice (aged 16-20 weeks) were obtained from the Experimental Animal Centre of Chongqing Medical University (Chongqing, China). The experimental animals were housed in cages under a 12 h light/12 h dark cycle (lights on at 8:00 a.m.), 60 ± 5% humidity, and a temperature of 23 ± 1 with access to water and food freely. All experimental procedures were conducted in accordance with the Ethics Committee of Chongqing Medical University. EDA was purchased from Sigma-Aldrich (St. Louis, USA) and was dissolved in Vehicle (NaCl, 0.9%) at a dosage of 10 mg/kg. EX527 (a Sirt1 inhibitor) and ML385 (a Nrf2 inhibitor) were obtained from MedChemExpress (New Jersey, USA).
Click to Show/Hide
|
||||
| Response Description | The inflammation and oxidative stress (OS) have been considered crucial components of the pathogenesis of depression. Edaravone possesses potent antidepressant and anxiolytic properties through Sirt1/Nrf2/HO-1/Gpx4 axis and Gpx4-mediated ferroptosis may play a key role in this effect. | ||||
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Quercetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from Gempharmatech Co., Ltd (Changzhou, China). All mice were housed in cages with standard laboratory conditions: a consistent temperature of 24 , a 12 h light/dark cycle, and free access to water and food. The mice were randomized into four groups: 1) the KA group (n = 6), injected intraperitoneally with 20 mg/kg KA, as described in a previous study; while 2) the control group (n = 6), injected intraperitoneally with an equal volume of PBS; 3) the KA + QCT group (n = 6): this group was givenintragastric administrationof 50 mg/kg of QCT once daily for 21 days before KA injection based on the literature; and 4) the KA+ferrostatin1 (Fer-1) group (n = 6), injected intraperitoneally with a well-known ferroptosis inhibitor (3 mg/kg Fer-1) for 21 days before KA administration, as described in a previous study.
Click to Show/Hide
|
||||
| Response Description | The association between the Nrf2-mediated ferroptosis pathway and seizures in a clinical setting. Quercetin effectively protects against seizure-induced neuron death in vivo and in vitro and alleviates cognitive function impairment via the SIRT1/Nrf2/SLC7A11/GPX4 pathway. | ||||
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Astragaloside IV | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Astragaloside IV (AS-IV) inhibited miR-138-5p expression, subsequently increasing Sirt1/Nrf2 activity and cellular antioxidant capacity to alleviate ferroptosis, resulting decreased cell death, which potentially inhibits the diabetic retinopathy pathological process. | |||
Supraventricular tachycardia [ICD-11: BC81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Icariin | Phase 3 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response Description | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Ulinastatin | Phase 3 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Ulinastatin plays a role in mitigation of Acetaminophen (APAP)-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Micafungin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
The surgical procedure for establishing the myocardial I/R injury rat model was carried out as we did before. Briefly, a left thoracotomy was performed in the fourth intercostal space and the heart was exposed via opening thepericardium. The left coronary artery was surrounded with a 4-0 silk suture and a snare was formed by passing both ends of the suture via a short polyethylene tubing. Blockage of the coronary artery was conducted via clamping the snare against the heart surface. Reperfusion was performed by release of the snare. The sham group conducted the same procedure but without ischemia (the snare was not tightened). To establish the I/R injury model, the rat hearts were subjected to 1 h-ischemia plus 3 h-reperfusion. At the end, the blood and hearts were collected for assay of the creatine kinase(CK) activity and infarct size to determine the success of I/R injury model. To explore the role of MALT1 in myocardial I/R injury the underlying mechanisms, three sets of experiment were performed.
Click to Show/Hide
|
||||
| Response Description | The inhibition of MALT1 can reduce ischemia/reperfusion-induced myocardial ferroptosis through enhancing the Nrf2/SLC7A11 pathway; and MALT1 may be used as a potential target to seek novel or existing drugs (such as micafungin) for treating myocardial infarction. | ||||
Mitogen-activated protein kinase 14 (MAPK14)
Multiple myeloma [ICD-11: 2A83]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [25] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Andrographis | Approved | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 |
| U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 | |
| AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| Response Description | Andrographolide (Andro) may block the Nrf2/HO-1 signaling pathway by activating P38 ( MAPK14), thereby inducing ferroptosis. Moreover, inhibition of P38 expression rescued Andro-induced cell death, changes in the level of Nrf2 and HO-1 expression, Fe2+ and lipid peroxidation. Taken together, our findings suggest that Andro induces ferroptosis in Multiple myeloma (MM) cells via the P38/Nrf2/HO-1 pathway, providing a potential preventative and therapeutic approach for MM. | |||
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Cetuximab | Approved | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
The DLD-1 cell suspension (4 x 106 cells/200 ul) was injected subcutaneously into the right dorsal flank of 5-week-old male BALB/c nude mice (Charles River, China). The mice were randomly divided into four groups (5 mice/group): 1) the control group, 2) the RSL3 group, 3) the cetuximab group, and 4) the RSL3 + cetuximab group. Both RSL3 (5 mg/kg) and cetuximab (13 mg/kg) were administered by intraperitoneal injection in a volume of 100 ul once per day. The tumour volume was calculated as 0.5 x length x width2. After 17 days of treatment, the mice were sacrificed, and the tumours were removed. Then, tumour tissue obtained from the different treated groups was subjected to western blotting and immunohistochemical experiments.
Click to Show/Hide
|
||||
| Response Description | Our work reveals that cetuximab enhances the cytotoxic effect of RSL3 on KRAS mutant Colorectal cancer (CRC) cells and that cetuximab enhances RSL3-induced ferroptosis by inhibiting the Nrf2/HO-1 axis through the activation of p38 MAPK. | ||||
LINC01134 (IncRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [27] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Oxaliplatin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response Description | LINC01134 was positively correlated with GPX4 or Nrf2, demonstrating the clinical significance of LINC01134, Nrf2 and GPX4 in OXA resistance of hepatocellular carcinoma. Silenced LINC01134 enhances Oxaliplatin sensitivity by facilitating ferroptosis through GPX4 in hepatocarcinoma. | |||
Krueppel-like factor 15 (KLF15)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [28] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Elabela | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
rAFs (Rat adventitial fibroblasts) | |||
| Response Description | KLF15 siRNA impeded the beneficial roles of elabela (ELA) in DOX-pretreated rat aortic AFs by suppressing the Nrf2/SLC7A11/GPX4 signaling. In conclusion, ELA prevents DOX-triggered promotion of cytotoxicity, and exerts anti-oxidative and anti-ferroptotic effects in rat aortic AFs via activation of the KLF15/GPX4 signaling, indicating a promising therapeutic value of ELA in antagonizing DOX-mediated cardiovascular abnormality and disorders. | |||
Kelch-like ECH-associated protein 1 (KEAP1)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Apatinib | Investigative | |||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female BALB/c nude mice (age, 4 weeks old) were purchased from Changzhou Cavens Experimental Animal Co., Ltd. (Changzhou, China).The gliomas from the nude mice were fixed in 10% paraformaldehyde at 4 for 12 h and then dehydrated in different concentrations of ethanol. The tumor tissues were permeabilized using xylene and embedded in paraffin. They were then sliced (0.5 um), rehydrated, and stained with HE at 4 for 10 min and sealed. For IHC assessment of Ki-67 in gliomas, the DAKO Envision system (Dako; Agilent Technologies, Inc.) was used.
Click to Show/Hide
|
||||
| Response Description | Apatinib could restrain proliferation of glioma cells through induction of ferroptosis via inhibiting the activation of VEGFR2/Nrf2/ Keap1 pathway. Overexpression of Nrf2 could counteract the induction of ferroptosis by apatinib. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [29] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Withaferin A | Investigative | |||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell adhesion molecules | hsa04514 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| SNU-449 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| Response Description | Withaferin A may attenuate the metastatic potential and sorafenib resistance by regulating Keap1/Nrf2-associated EMT and ferroptosis. Thus, Withaferin A may serve as a promising agent for Hepatocellular carcinoma therapy, especially for advanced hepatocellular carcinoma. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Tiliroside | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
All animal studies were approved by the Committee on Ethics of Animal Experiments of Binzhou Medical University (approval no: BZMU-IACUC-2021-331, date: 09/10/2021). To generate the ectopic HCC mouse models, HepG2-luciferase cells (HepG2 cells transfected with luciferase gene) were suspended in serum-free media and matrigel (BD Biosciences) at a ratio of 1:1 v/v. A total of 2.5 x 106 HepG2-luciferase cells/100 ul were injected into the left axilla of mice. After reaching a tumor size of 100-150 mm3, all mice were randomly divided into four groups: control (vehicle, intraperitoneal [i.p.]), tiliroside (20 mg/kg,i.p.), sorafenib (30 mg/kg,i.p.), or combination treatment (tiliroside and sorafenib,i.p.). All treatments were administered every 3 d, and the length and width of tumor were measured every 4 d. The formula tumor volume = (length x width2)/2 was used to calculate the tumor volume. Body weight was recorded every 7 d, and the morphology of the tumor was photographed using animal in vivo imaging technology (IVIS Spectrum; PerkinElmer) before the day of sacrifice. The mice were sacrificed 40 d after administration, and the tumors were dissected and weighed. The major organs and xenograft tumors were fixed with 4% paraformaldehyde.
Click to Show/Hide
|
||||
| Response Description | Tiliroside directly binds to TBK1 and inhibits its activity, which inhibits the phosphorylation of Ser349 on p62. Consequently, this decreases the affinity of p62 for Keap1, promotes ubiquitination and degradation of Nrf2 and ferroptosis, and eventually increases the sensitivity of hepatocellular carcinoma cells to sorafenib. | ||||
Melanoma [ICD-11: 2C30]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [30] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Nobiletin | Investigative | ||
| Pathway Response | Pathways in cancer | hsa05200 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
SK-MEL-28 cells | Cutaneous melanoma | Homo sapiens | CVCL_0526 |
| Response Description | Nobiletin could induce ferroptosis by regulating the GSK3B-mediated Keap1/Nrf2/HO-1 signalling pathway in human melanoma cells. Hence, nobiletin stands as a promising drug candidate for melanoma treatment with development prospects. | |||
Vascular dementia [ICD-11: 6D81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [31] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Gastrodin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male Sprague-Dawley rats (weight 260 ± 20 g; Guizhou Medical University Experimental Animal Center; Certificate No. SCXK2018-0001; Grant No. 2200483) were reared in a specific pathogen-free environment with 12 h light/dark cycle and 55% ± 10% humidity at a temperature of 20~25 , were provided with sufficient feed and sterile drinking water and fasted for 6 h before and after surgery. All animal experiments were performed in accordance with the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Gastrodin (GAS) inhibited ferroptosis in hippocampal neurons by activating the Nrf2/ Keap1-GPx4 signaling pathway, suggesting its possible application as a functional food for improving vascular dementia by inhibiting ferroptosis. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [32] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | L. lactis MG1363-pMG36e-GLP-1 | Investigative | |||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Colon tissues (Mouse colon tissues) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
Fifty male C57BL/6 mice provided by Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China) resided in an animal house (temperature 26 ± 1 , humidity 50 ± 10%), in which the light was on for 12 h and off for 12 h. Mice were acclimatised for 1 week and allowed water and animal food with no limitations. Then, all mice were stochastically divided into 5 groups using random number tables available online (https://www.random-online.com/, accessed on 26 December 2021), including: (1) C group, a control group treated with normal saline for 7 consecutive days (n = 10); (2) M group, a model group with intraperitoneal injection of 20 mg/kg/day MPTP (Sigma-Aldrich, Taufkirchen, Germany, M0896) for 7 consecutive days (n = 10); (3) L group, treated with MPTP and 0.4 mg/kg/day liraglutide for 7 consecutive days (n = 10); (4) R group, treated with MPTP and 109 colony-forming unit (CFU) L. lactis MG1363 for 7 consecutive days via gavage (n = 10); (5) RG group, treated with MPTP and 109 CFUL. lactis MG1363-pMG36e-GLP-1 for 7 consecutive days via gavage (n = 10). All animals survived treatment and all animal experiments were administered from 9:00 to 12:00 in the morning to reduce systematic errors.
Click to Show/Hide
|
||||
| Response Description | L. lactis MG1363-pMG36e-GLP-1 exerts neurotrophic effects via activating the Keap1/Nrf2/GPX4 signalling pathway to down-regulate ACSL4 and up-regulate FSP1 to suppress ferroptosis. These results indicated that the neurotrophic effects of the next-generation probiotics L. lactis MG1363-pMG36e-GLP-1 against MPTP-induced Parkinsonism are mediated by modulating oxidative stress, inhibiting ferroptosis, and redressing dysbiosis. | ||||
Subarachnoid Hemorrhage [ICD-11: 8B01]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
SAH model was constructed by applying endovascular perforation in the rats, according to the protocol introduced in a previous study (Wei et al., 2020), except for slight modifications. Briefly, after performing intraperitoneal anesthesia with 40 mg/kg sodium pentobarbital, the right common carotid, external and internal carotid arteries of the rats were exposed and isolated. The right external carotid artery was ligated, and a 4-0 single-strand nylon thread was used to insert the right internal carotid artery through the stump of the external carotid artery and the bifurcation of the common carotid artery. When resistance is felt when the suture enters the intracranial segment, proceed approximately 3 mm to penetrate internal carotid artery at the bifurcation of middle cerebral artery. The suture was held in this position for 10 s and was then withdrawn. The rats in the Sham group went through an identical procedure, without the suture at the point of resistance. Throughout the experiment, the body temperature of the rats was sustained at around 37 by using a thermal blanket. After the wounds were sutured, the rats were placed in a separate cage and neurological function was closely observed.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AS-IV) triggered Nrf2/HO-1 signaling pathway and alleviated ferroptosis due to the induction of subarachnoid hemorrhage (SAH). The Nrf2 inhibitor ML385 blocked the beneficial effects of neuroprotection. Ferroptosis is profoundly implicated in facilitating EBI in SAH, and that AS-IV thwarts the process of ferroptosis in SAH by activating Nrf2/HO-1 pathway. The liberation of Nrf2 from Keap1, its cytoplasmic repressor will provoke Nrf2 accumulation in the nucleus. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Rats were randomly divided into the Sham, middle cerebral artery occlusion-reperfusion (MCAO/R), and MCAO/R + AST IV (28 mg/kg) groups. The MCAO/R + AST IV group was intragastrically injected with 10 mL/kg AST IV at 50, 26, and 2 h before modelling (Xiao et al., 2021). The Sham and MCAO/R groups received equal amounts of normal saline. As described previously, the modified Longa method (Longa et al., 1989) was used to establish the MCAO/R model. After anaesthesia with 2%sodium pentobarbital, the left common carotid artery(CCA), the external carotid artery(ECA), and the internal carotid artery(ICA) were isolated. The distal end of the ECA was ligated, a small incision was made at the stump of the ECA, and a suture (Batch number: 2636A2, Beijing Seinong Technology Co., Ltd., Beijing, China; head-end diameter: 0.36 ± 0.02 mm) was inserted into the ICA from the ECA through the bifurcation of the CCA. To achieve cerebral ischaemia, the head-end was used to block blood flow in the middle cerebral artery until the intracranial segment of the ICA was inserted. The suture was removed after 2 h, and follow-up experiments were performed 24 h after reperfusion. In the Sham group, the CCA, ECA, and ICA were exposed and separated, but no sutures were inserted. Penicillin powder was used to fight infection after operation.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AST IV) increased the P62 (SQSTM1) and Nrf2 levels and decreased the Keap1 levels. P62 silencing reduced the effects of AST IV on the P62/ Keap1/Nrf2 pathway and ferroptosis. Our findings suggest that AST IV mitigates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via activation of the P62/ Keap1/Nrf2 pathway. | ||||
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [34] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Dehydroabietic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Pathways in cancer | hsa05200 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
The male C57BL/6J mice (6-8 weeks, Beijing Vital River Laboratory Animal Technology Co., Ltd., China) were exposed to 12 h of light and darkness at temperature (22 ± 2 ), humidity (55%) with free access to water and food. All the mice were acclimated for 1 week before the experiment, then the mice were fed normal chow diet (NCD) and high-fat diet (HFD, D12492) for 12 weeks. The HFD group was divided into 3 groups (HFD, low dose of DA (DA-L, 10 mg/kg/d), high dose of DA (DA-H, 20 mg/kg/d),n = 8)). DA was administered by gavage for 9 weeks, and 0.5% CMC-Na was administered by NCD and HFD.
Click to Show/Hide
|
||||
| Response Description | Dehydroabietic acid (DA) inhibited ferroptosis and increased the expression of key genes such as ferroptosis suppressor protein 1 (FSP1) in vitro and vivo. In all, DA may bind with Keap1, activate Nrf2-ARE, induce its target gene expression, inhibit ROS accumulation and lipid peroxidation, and reduce HFD-induced nonalcoholic fatty liver disease (NAFLD). | ||||
Degenerative arthritis [ICD-11: FA05]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [35] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
C57BL/6J (WT) mice (8 weeks old, male) were purchased from Nanjing Medical university, and AMPK-KO mice were purchased from Shanghai Model Organisms. They were used to create an OA model by destabilization of the medial meniscus surgery (DMM) (n = 6 per group). Briefly, after the mice were anaesthetized, a medial articular incision was made to expose the leftjoint cavity, and then the tibial collateral ligament was transected. Finally, the articular incision was closed. In the control group, only the joint cavity was opened. One week after surgery, 1 mg/kg baicalein (MCE, HY-N0196) per knee, 1 mg/kg ML385 (MCE, HY-100523) per knee, 1 mg/kg AICAR (MCE, HY-13417) per knee or 1 mg/kg of the ferroptosis inhibitor ferrostatin-1 (Fer-1, MCE, HY-100579) was injected into the joint cavity of the mice once a week. Meanwhile, saline was injected into the control group. Mice were sacrificed after surgery 10 weeks.
Click to Show/Hide
|
||||
| Response Description | Baicalein alleviated osteoarthritis (OA) development by improving the activity of AMPKa/Nrf2/HO-1 signaling to inhibit chondrocyte ferroptosis, revealing baicalein to be a potential therapeutic strategy for OA. AMPKa preserved Nrf2 abundance in chondrocytes and promoted Nrf2 into nucleus by promoting Keap1 degradation | ||||
Endometrial hyperplasia [ICD-11: GA16]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Guizhi Fuling Capsule | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
mUTs (Mouse uterine tissues) | ||||
| In Vivo Model |
Female C57BL/6 mice (8-week-old) were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). Fifteen mice were randomly divided into three groups: Olive oil group, Estradiol group and Estradiol + IKE group. The Estradiol group was subcutaneously injected estradiol (50 ug/kg/day), Estradiol + IKE group was subcutaneously injected estradiol and intraperitoneally injected IKE (50 mg/kg) for 21 days, while the Olive oil group received the same volume of olive oil. In the experiment of exploring the improvement of GFC to EH, twenty mice were randomly divided into four groups: Olive oil group, Estradiol group, 75 mg/kg GFC group and 150 mg/kg GFC group. Except for Olive oil group, mice were subcutaneously daily injected with estradiol (50 ug/kg/day) for 21 days, while the Olive oil group received the same volume of olive oil. 75 mg/kg GFC group and 150 mg/kg GFC group were treated with GFC intragastrical administration.
Click to Show/Hide
|
||||
| Response Description | Guizhi Fuling Capsule (GFC) may attenuate estrogen-induced endometrial hyperplasia in mice through triggering ferroptosis via inhibiting p62 (SQSTM1)- Keap1-NRF2 pathway. GFC might act as a promising traditional Chinese medicine to treat endometrial hyperplasia. | ||||
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Entacapone | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks; 20-25 g) were purchased from LINGCHANG BIOTECH (China). Mice were divided into four groups: (i) sham, (ii) I/R, (iii) I/R+entacapone, and (iv) I/R + Fer-1. Entacapone (15 mg/kg bodyweight) was dissolved in sodium carboxymethyl cellulose (0.5%) and administered (i.g.) to mice. Mice in the sham group were administered (i.g.) an equal volume of solvent. Fer-1 was dissolved in 5% dimethyl sulfoxide + 30% polyethylene glycol-400 + 60% saline and injected (i.p.). Mice were treated three times per day for 3 days in advance. Before I/R, mice were fasted for 12 h and anesthetized (1% pentobarbital sodium, i.p.). The abdomen was exposed and bilateral renal pedicles were clamped to induce renal I/R. After 25 min, the arterial clamps were removed. A body temperature of 37 was maintained throughout the procedure. The sham group underwent the same procedure except for clamping of the renal pedicle. Mice were killed 24 h after reperfusion, and kidney and blood samples were collected for experimentation.
Click to Show/Hide
|
||||
| Response Description | Entacapone upregulates p62 (SQSTM1) expression and affects the p62- KEAP1-NRF2 pathway, thereby upregulating nuclear translocation of NRF2. This action results in increased expression of the downstream SLC7A11, and significant suppression of oxidative stress and ferroptosis. Entacapone may serve as a novel strategy to improve treatment of, and recovery from, ischemia/reperfusion-induced acute kidney injury (I/R-AKI). | ||||
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [36] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Formononetin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mPRTECs (Mouse primary renal tubular epithelial cells) | ||||
| In Vivo Model |
For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium(30 mg/kg). Then, UUO surgery orsham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10% DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10% DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO. For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg). Then, UUO surgery or sham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10 % DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10 % DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO.
Click to Show/Hide
|
||||
| Response Description | Formononetin (FN) alleviates chronic kidney disease (CKD) by impeding ferroptosis-associated fibrosis by suppressing the Smad3/ATF3/SLC7A11 signaling and could serve as a candidate therapeutic drug for CKD. In addition, FN also promoted the separation of the Nrf2/ Keap1 complex and enhanced Nrf2 nuclear accumulation. | ||||
Lung injury [ICD-11: NB32]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [37] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Astaxanthin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | |
| In Vivo Model |
6-week-Babl/c female mice were randomized to the following three groups of seven mice each: vehicle group, LPS group, Astaxanthin plus LPS group. Astaxanthin plus LPS group mice were pretreated with astaxanthin (20 mg/kg) byi.v injectionfor daily for 7 consecutive days. Astaxanthin was dissolved in 2%DMSO (vol/vol), 40% PEG-400 (vol/vol), 2% Tween 80 (vol/vol), and 56% PBS (vol/vol). On the last day, the mice were intraperitoneally injected with 5 mg/kg LPS or normal saline 2 h after the injection of astaxanthin. After 6 h of LPS stimulation, mice were euthanized to collect the BALF, and lung tissue samples. BALF was collected three times through a tracheal cannula with autoclaved normal saline, instilled up to a total volume of 1.8 ml.
Click to Show/Hide
|
||||
| Response Description | Astaxanthin protected LPS-induced cell inflammation and acute lung injury (ALI) in mice by inhibiting ferroptosis, and its effect was achieved through Keap1-Nrf2/HO-1 pathway. Therefore, our study indicates that ferroptosis will become a new target for the treatment of ALI, and astaxanthin is a potential drug for the treatment of ALI. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [38] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Panaxydol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Pathways in cancer | hsa05200 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
Specific pathogen-free (SPF) male C57BL/6 mice (6-8 weeks old, 20-24 g body weight) were purchased from the Experimental Animal Center, Anhui Medical University (Hefei, China). All mice were randomly divided into five groups (8 mice every group): control group, LPS group, PX+LPS group (administered 20 mg/kg PX), Fe+LPS group (administered 15 mg/kg Fe-citrate (III)), and PX+Fe+LPS group (administered 20 mg/kg PX and 15 mg/kg Fe-citrate (III)). Fe-citrate (III) was dissolved in stroke-physiological saline solution (SPSS). PX was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich), and further diluted in SPSS. Intravenous injection of Fe or/and intraperitoneal injection of PX were performed from day 0 to day 2. At 1 h after the final Fe and PX treatment, the mice were anesthetized with 30 mg/kg of pentobarbital sodium (Beijing Chemical Co., China) and then LPS (10 ug/mouse; InvivoGen, San Diego, CA, USA) or SPSS was injected into the trachea.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis mediated inflammation in LPS-treated BEAS-2B cells, and panaxydol (PX) might ameliorate LPS-induced inflammation via inhibiting ferroptosis. PX attenuates ferroptosis against LPS-induced acute lung injury via Keap1-Nrf2/HO-1 pathway, and is a promising novel therapeutic candidate for acute lung injury. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [39] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Clausenamide | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Pathways in cancer | hsa05200 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HepaRG cells | Hepatocellular carcinoma | Homo sapiens | CVCL_9720 | |
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | ||
| In Vivo Model |
Male C57BL/6 mice aged 8-10 weeks were purchased from Guangdong Experimental Animal Center (Guangzhou, China). The animals were maintained on a 12 h light-dark cycle in a regulated temperature and humidity environment for 1 week before drug administration. (+)-CLA (50 mg/kg/day, i.g.) or fer-1 (2.5 umol/kg/day, i.p.)were administered for 7 consecutive days. To induce liver injury, mice were injected with erastin (100 mg/kg/day, i.p., twice a day) on both the 6th and 7th day, or a single dose of APAP (600 mg/kg/day, i.p.) on the 7th day after overnight food deprivation. The serum and livers were obtained for analysis.
Click to Show/Hide
|
||||
| Response Description | (+)-clausenamide ((+)-CLA) specifically reacted with the Cys-151 residue of Keap1, which blocked Nrf2 ubiquitylation and resulted in an increased Nrf2 stability. Thus, (+)-CLA protects against acetaminophen-induced hepatotoxicity via inhibiting ferroptosis and activating the Keap1/Nrf2 pathway in a Cys-151-dependent manner. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Baicalin | Terminated | |||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
C57BL/6 mice at 6-8 weeks were intraperitoneally injected with D-GalN/LPS (1772-03-8/L2880, Sigma-Aldrich, USA) at a dose of 700 mg/kg and 10 ug/kg, respectively. The constructed D-GaIN/LPS-induced ALI model mice were named the model group, and the normal mice injected with phosphate-buffered saline (PBS) were named the blank group. After 1 h of LPS/D-GalN treatment, Exo and Ba-Exo (150 ug/mice) were injected into the tail vein of the mice in the Exo and Ba-Exo groups, respectively. Mice were sacrificed via anesthesia overdose 12 h after the intervention. Half of the liver tissue was fixed in paraformaldehyde, while the other half was frozen at 80 . Peripheral blood serum was stored at -80 .
Click to Show/Hide
|
||||
| Response Description | Baicalin-pretreated MSCs (Ba-Exo) exerts a protective effect on liver function and activates the Keap1-NRF2 pathway via P62 (SQSTM1), thereby inhibiting ROS production and lipid peroxide-induced ferroptosis. Therefore, baicalin pretreatment is an effective and promising approach in optimizing the therapeutic efficacy of Exo in acute liver injury (ALI). | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [40] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Xiaojianzhong | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hGCs (Gastric cells) | ||||
| In Vivo Model |
C57BL/6 mice (Male, 6-8 weeks old, 20 g± 2 g) were purchased from Chengdu Yaokang Biotechnology Co., Ltd. (Chengdu, China). All animals were housed in the animal room of Shaanxi University ofTraditional Chinese Medicine, at a temperature of 22 ± 2 and a humidity of 40% ± 5%, alternating between light and dark. In the study, the mice were randomly divided into six groups (n = 10 in each group): the blank group, model group,XJZ high dose group, XJZ medium dose group, XJZ low dose group, and positive control (omeprazole) group. The mice in the model group were given Aspirin (300 mg/kg) via gavage for 14 days; the mice in the XJZ high dose group, XJZ medium dose group, and XJZ low dose group were given aspirin (300 mg/kg) by gavage in the morning and three different concentrations (12 g/kg, 6 g/kg, or 3 g/kg) of XJZ decoction by gavage in the afternoon; the mice in the positive control group were given aspirin (300 mg/kg) by gavage in the morning andomeprazole(20 mg/kg) by gavage in the afternoon. After the model was successfully constructed, the mice were anesthetized with isoflurane and gastric tissues were extracted for analysis.
Click to Show/Hide
|
||||
| Response Description | Xiaojianzhong (XJZ) significantly counteracted aspirin-induced gastric mucosal injury and inhibited oxidative stress and ferroptosis in mice. Upon examining SQSTM1/p62(p62)/ Kelch-like ECH-associated protein 1 (Keap1)/Nuclear Factor erythroid 2-Related Factor 2 (Nrf2), a well-known signaling pathway involved in the regulation of oxidative stress and ferroptosis, we found that its activation was significantly inhibited by aspirin treatment and that this signaling pathway was activated after XJZ intervention. | ||||
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| FHC cells | Normal | Homo sapiens | CVCL_3688 | ||
| In Vivo Model |
To clarify the role of LINC00239 in vivo, we used 4-week-old male BALB/c nude mice provided by the Experimental Animal Center of the Air Force Military Medical University. HCT116 or SW620 cells (1 x 107 cells) were injected subcutaneously into the right flanks of these mice to establish a CRC xenograft model. One week after the injection of cells, the volume of xenografts was continuously monitored (once a week). Four weeks later, the xenografts were removed, and the weights were measured.
Click to Show/Hide
|
||||
| Response Description | LINC00239 plays a novel and indispensable role in ferroptosis by nucleotides 1-315 of LINC00239 to interact with the Kelch domain (Nrf2-binding site) of Keap1, inhibiting Nrf2 ubiquitination and increasing Nrf2 protein stability. And LINC00239 expression has a positive correlation with Nrf2 and GPX4 expression in colorectal cancer tissues. LINC00239 inhibition in combination with ferroptosis induction might be a promising therapeutic strategy for CRC patients. | ||||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [70] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Calcitriol | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Pathways in cancer | hsa05200 | |||
| NF-kappa B signaling pathway | hsa04064 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
ZFL cells | Normal | Danio rerio | CVCL_3276 |
| Response Description | This study confirmed the protective effect of calcitriol on RSL3-induced ferroptosis in zebrafish liver cells, and reported for the first time that calcitriol inhibits ferroptosis in fish cells by regulating the Keap1/Nrf2/GPx4 axis and NF-kB/hepcidin axis. | |||
hsa-miR-138-5p (miRNA)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Astragaloside IV | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Astragaloside IV (AS-IV) inhibited miR-138-5p expression, subsequently increasing Sirt1/Nrf2 activity and cellular antioxidant capacity to alleviate ferroptosis, resulting decreased cell death, which potentially inhibits the diabetic retinopathy pathological process. | |||
High mobility group protein B1 (HMGB1)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [41] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | D-Glucose | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| NF-kappa B signaling pathway | hsa04064 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
SV40 MES 13 cells | Normal | Mus musculus | CVCL_5368 |
| Response Description | Erastin and high glucose both induced ferroptosis in mesangial cells. Suppression of HMGB1 restored cellular proliferation, prevented ROS and LDH generation, decreased ACSL4, PTGS2, and NOX1, and increased GPX4 levels in mesangial cells. Furthermore, nuclear factor E2-related factor 2 (Nrf2) was decreased in diabetic nephropathy (DN) patients and high glucose-mediated translocation of HMGB1 in mesangial cells. | |||
Glycogen synthase kinase-3 beta (GSK3B)
Melanoma [ICD-11: 2C30]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [30] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Nobiletin | Investigative | ||
| Pathway Response | Pathways in cancer | hsa05200 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
SK-MEL-28 cells | Cutaneous melanoma | Homo sapiens | CVCL_0526 |
| Response Description | Nobiletin could induce ferroptosis by regulating the GSK3B-mediated Keap1/Nrf2/HO-1 signalling pathway in human melanoma cells. Hence, nobiletin stands as a promising drug candidate for melanoma treatment with development prospects. | |||
Fibronectin type III domain-containing protein 5 (FNDC5)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [42] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Iridin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hCMs (Human cardiomyocytes) | |||
| Response Description | Myocardial infarction is characterized by cardiomyocyte death and mitochondrial dysfunction induced by ischemia. FNDC5 overexpression and/or irisin administration elevated cell viability, decreased ferroptosis, and reversed mitochondrial impairments induced by hypoxia. Mechanistically, FNDC5/irisin reduced ferroptosis and reversed mitochondrial impairments by Nrf2/HO-1 axis in hypoxic cardiomyocytes. | |||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [56] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
Mice weighing between 20 and 23 g were selected and 5 x 106 Hep-G2 cells were subcutaneously injected into their backs. The mice were subsequently divided into the following four groups: control (n = 5), FNDC5 overexpressing (n = 5), FNDC5 overexpressing followed by treatment with the PI3K inhibitor LY294002 (MCE, China), and FNDC5 knockdown (n = 5). Seven days after cell injection, sorafenib (30 mg/kg) was administered to all mice via intraperitoneal injection every alternate day for 4 weeks. The mice in the third group were intraperitoneally injected with LY294002 (25 mg/kg) diluted with DMSO twice a week for 4 weeks.
Click to Show/Hide
|
||||
| Response Description | FNDC5 activated the PI3K/Akt pathway, which in turn promoted the nuclear translocation of Nrf2 and increased the intracellular antioxidant response in Hepatocellular Carcinoma Cells, thereby conferring resistance to ferroptosis. Our study provides novel insights for improving the efficacy of sorafenib. | ||||
E3 ubiquitin-protein ligase NEDD4-like (NEDD4L)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Peoniflorin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| In Vivo Model |
U251 cells (6 x 106) were inoculated into the flanks of 4-to 5-week-old athymic nude mice (Shanghai Laboratory Animal Company, Shanghai, China) subcutaneously to generate a subcutaneous xenograft tumor model. After 2 weeks, the tumor model was successfully constructed, the mice were treated single and combined with 100 mg/kg RSL3 (2 times/week) and 1.0 g/kg/days PF. Tumor volumes were measured every 4 days to draw the growth curve. Mice were sacrificed 4 weeks after cell injection. Tumor xenografts were collected, photographed, and weighed and the tumor apoptosis was analyzed by Tunel staining.
Click to Show/Hide
|
||||
| Response Description | Paeoniflorin (PF) can function as an antitumor agent for glioma treatment by targeting NEDD4L-dependent STAT3 ubiquitination as well as by regulating the Nrf2/GPX4 signaling axis, which might trigger ferroptosis. | ||||
CircOMA1 (circRNA)
Prolactinoma [ICD-11: 2F37]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [43] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Cabergoline | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [44] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Ascorbic Acid | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
PaTu 8988t cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1847 | |
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| mEFs (Mouse embryonic fibroblasts) | |||||
| Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | ||
| In Vivo Model |
All animal experiments were approved by the Ethics Committee of Jiangsu University. To investigate the role of the combination of erastin and vitamin C in inducing ferroptosis, Panc02 cells (1 x 105 cells/site) were transfected and subcutaneously injected into 4-week-old C57BL/6 mice to generate xenografts. When the tumors reached a volume of 50-100 mm3, the mice were randomly divided into four groups (five mice per group) and treated with DMSO (control), imidazole ketone erastin (IKE, MedChemExpress), vitamin C, or a combination of erastin and vitamin C. Mice were treated with 80 ul (400M) erastin by intratumoral injection and/or 4 g/kg vitamin C by intraperitoneal injection every 2 days.
Click to Show/Hide
|
||||
| Response Description | The combination of erastin and vitamin C mainly increases the levels of ferrous iron through the AMPK/NRF2/HMOX1 signaling pathway. Cotreatment with erastin and vitamin C also exhibited a synergistic effect in a pancreatic cancer xenograft model in mice. | ||||
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [45] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | zero-valent-iron nanoparticle | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| mTOR signaling pathway | hsa04150 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| A9 cells | Lung carcinoma | Mus musculus | CVCL_S007 | ||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| IMR-90 cells | Normal | Homo sapiens | CVCL_0347 | ||
| In Vivo Model |
5-6-week-old BALB/c nude mice (ZVI@Ag treatment) or NOD/SCID mice (ZVI@CMC treatment) were subcutaneously implanted with 1 x 106 H460 cells. For A549 xenograft model of immunodeficient mouse and spontaneous lung metastasis model, 5-6-week-old NOD/SCID mice were subcutaneously implanted with 5 x 106 A549 cells. For experimental lung metastasis model, H460 cells (1 x 106 cells/200 uL) were resuspended in serum-free medium and injected intravenously (i.v.) into tail-vein of NOD/SCID mice. For subcutaneous model of immunocompetent mouse, LLC cells (5 x 105) were injected into both flank of 6-week-old C57BL/6 mice.
Click to Show/Hide
|
||||
| Response Description | Zero-valent-iron nanoparticle (ZVI-NP) triggered ferroptosis selectively in lung cancer cells by suppressing NRF2-mediated cytoprotection program, which was attributed to the ZVI-NP-induced disruption of PRKAA1 (AMPK)/mTOR signaling and activation of GSK3/-TrCP-dependent degradation system. | ||||
Ubiquitin carboxyl-terminal hydrolase 35 (USP35)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [46] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ubiquitin mediated proteolysis | hsa04120 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| 769-P cells | Renal cell carcinom | Homo sapiens | CVCL_1050 | ||
| OS-RC-2 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_1626 | ||
| In Vivo Model |
Female Balb/c nude mice aged 6 weeks were obtained from Vital River Laboratory (Beijing, China). To generate xenografts of RCC, mice were randomized into two groups of 8 that were subcutaneously inoculated with 1 x 10^7 of OS-RC-2 cells stably transfected with USP35 Tet-on shRNA constructs or empty vector. After 7 days, mice were administered with doxycycline every day (20 mg/kg) to induce shRNA expression through oral gavage without blinding.
Click to Show/Hide
|
||||
| Response Description | USP35 functions to maintain NRF2 levels by catalyzing its deubiquitylation and thus antagonizing degradation. NRF2 reduction imposed by USP35 silencing rendered renal clear cell carcinoma cells increased sensitivity to ferroptosis induction. | ||||
Tyrosine-protein kinase receptor TYRO3 (TYRO3)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [48] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
4T1 cells | Mammary carcinoma | Mus musculus | CVCL_0125 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| B16-F10 cells | Melanoma | Mus musculus | CVCL_0159 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| Py8119 cells | Breast carcinoma | Mus musculus | CVCL_AQ09 | ||
| In Vivo Model |
Six-week-old BALB/c mice were purchased from The Jackson Laboratory. Mouse 4T1 cells (5 x 104 cells) in 50 uL of 50% Matrigel (47743-720, Corning) were injected into the mammary fat pad. Three days after inoculation, 100 ug mouse anti-PD-1 antibody (BE0146, Bio X Cell) or IgG control (BE0089, Bio X Cell) was injected intraperitoneally twice a week for a total of 5 injections. For the TYRO3 inhibitor and antimPD-1 combination treatment, mice were also treated daily with vehicle control (90% polyethylene glycol 400 and 10% DMSO) or LDC1267 (20 mg/kg, S7638, Selleck Chemical) for 10 days by intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | The study proposed a model in which high expression of TYRO3 or TYRO3 activation by its ligands on apoptotic cells triggers the downstream AKT/NRF2 pathway, followed by the transcription of genes that inhibit ferroptosis in Mammary carcinoma. | ||||
Tumor suppressor ARF (CDKN2A)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [49] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| HEK293 cells | Normal | Homo sapiens | CVCL_0045 | ||
| SAOS-2 cells | Osteosarcoma | Homo sapiens | CVCL_0548 | ||
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | ||
| In Vivo Model |
Pooled stable cell line was derived from H1299 tet-on ARF cells by transfecting either empty vector or vector overexpressing NRF2. Cells were selected by G418 (1 mg /ml) for 2 weeks and then re-transfected the same vectors again. After additional treatment with or without doxycycline (1.0 mg/ml) for 60 h, 1.0 x 106 of cells were then mixed with Matrigel (BD Biosciences) at 1:1 ratio (volume) and injected subcutaneously into nude mice (NU/NU, Charles River). The mice were fed with the food containing doxycycline hyclate (Harlan, 625 mg/kg) or control food.
Click to Show/Hide
|
||||
| Response Description | ARF (CDKN2A) expression sensitizes cells to ferroptosis in a p53-independent manner while ARF depletion induces NRF2 activation and promotes cancer cell survival in response to oxidative stress. NRF2 is a major target of p53-independent tumor suppression by ARF and also suggest that the ARF-NRF2 interaction acts as a new checkpoint for oxidative stress responses in lung large cell carcinoma cell lines. | ||||
Transcription factor AP-2-alpha (TFAP2A)
Gallbladder cancer [ICD-11: 2C13]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [50] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
H69 cells | Normal | Homo sapiens | CVCL_8121 |
| GBC-SD cells | Gallbladder carcinoma | Homo sapiens | CVCL_6903 | |
| Response Description | In vitro, gallbladder carcinoma (GBC) exhibited upregulated expression of TFAP2A, whose inhibition reduced GBC cell proliferation, migration, and invasion. Fe2+ and MDA levels were elevated. TFAP2A silencing attenuated the expression of key genes associated with oxidative stress such as heme oxygenase 1 (HO-1), nuclear factor erythroid 2 like 2 (Nrf2), ferritin heavy chain 1 (FTH1) and NAD(P)H quinone dehydrogenase 1 (NQO1). | |||
Torsin-2A (TOR2A)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [51] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 |
| Response Description | Interaction of salusin- ( TOR2A) with ferroptosis formed a positive feedback, thereby contributing to HG-induced HK-2 cell injury and diabetic nephropathy. Mechanistically, salusin inactivated nuclear factor erythroidderived 2like 2 (Nrf2) signaling, thus contributing to HGinduced ferroptosisrelated changes in HK2 cells. | |||
Sulfhydryl oxidase 1 (QSOX1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [52] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MHCC97-H cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_4972 | |
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| In Vivo Model |
Male 6-week-old BALB/c nude mice were purchased from Charles River Company (Shanghai, China). For subcutaneous mouse model, 5 x106 MHCC97H vector control cells or MHCC97H/QSOX1 cells were implanted into right flanks subcutaneously. At the 7th day following implantation, the mice bear with MHCC97H vector control cells or MHCC97H/QSOX1 cells respectively were randomly separated into two groups: one group were treated with vehicle (0.9% NaCl i.p., once every other day) and another group sorafenib (10 mg/kg i.p., once every other day) for two weeks.
Click to Show/Hide
|
||||
| Response Description | QSOX1 disrupts redox homoeostasis and sensitizes hepatocellular carcinoma cells to oxidative stress by inhibiting activation of the master antioxidant transcription factor NRF2. Mechanistically, QSOX1 restrains EGF-induced EGFR activation by promoting ubiquitination-mediated degradation of EGFR and accelerating its intracellular endosomal trafficking, leading to suppression of NRF2 activity. | ||||
Serine/arginine-rich splicing factor 1 (SRSF1)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [53] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
LN-229 cells | Glioblastoma | Homo sapiens | CVCL_0393 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| hACs (Normal human astrocyte cells) | |||||
| In Vivo Model |
BALB/c nude mice (female, four-week-old) were purchased from the Nanjing Medical University Experimental Animal Department. Female mice were randomly divided into test group and control group. 2.5 x 105 LN229/TMZ cells transfected with sh-MAPK8-1 or sh-LINC01564-1 were injected into the brain of mice in test group, taking the mice injected with sh-NC-transfected ones as control. Seven days later, the mice were treated with TMZ (66 mg/kg per day, 5 days/cycle, 4 cycles in total) as a monotherapy. Tumor volume was monitored every three days in the period of TMZ treatment. The mice were killed 28 days after the injection. Tumors were excised from mice for observation and weighing as well as the detection of the level of ROS, iron (Fe2+) and proteins (i.e., NFE2L2, NQO1, FTH1 and HO-1).
Click to Show/Hide
|
||||
| Response Description | LINC01564 promotes the temozolomide (TMZ) resistance of glioma cells by upregulating NFE2L2 expression to inhibit ferroptosis. LINC01564 promotes MAPK8 mRNA stability by recruiting SRSF1, and MAPK8 was positively correlated with NFE2L2 and its targets, proving its mediation of NFE2L2. | ||||
rno-miR-23a-3p (miRNA)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rNCs (Rat neuronal cells) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
Male 8-week-old Sprague-Dawley rats were chosen for this study. These animals were randomized to 3, 4, or 5 groups: Design for 3 groups: Sham group, rats underwent sham operation; ICH group, rats underwent ICH operation; ICH + Ac group, rats underwent ICH operation and treated with acupuncture at target acupoints. Design for 4 groups: Sham group; ICH group; ICH + Ac group; ICH + sAc group, rats underwent ICH operation and treated with acupuncture at non-acupoints located 1 cm posterior and parallel to Baihui-penetrating-Qubin needling as previously described. Another design for 4 groups: Sham group; ICH group; ICH + NC group, rats underwent ICH operation and injected with antagomiR-NC; ICH + antagomiR group, rats underwent ICH operation and injected with antagomiR-23a-3p.
Click to Show/Hide
|
||||
| Response Description | Acupuncture alleviated ferroptosis and decreased miR-23a-3p expression, as evidenced by the increased NFE2L2 nuclear translocation and expressions of heme oxygenase-1 and glutathione peroxidase 4 and the decreased iron and malondialdehyde contents and reactive oxygen species accumulation. Notably, the binding site of miR-23a-3p existed in NFE2L2. Taken together, acupuncture may alleviate the neuronal cell death, inflammation, and ferroptosis after intracerebral hemorrhage (ICH) by down-regulating miR-23a-3p. | ||||
RelA-associated inhibitor (PPP1R13L)
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [55] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MLE-2 (Mouse lung epithelial cells) | ||||
| mLT (Mouse lung tissue) | |||||
| In Vivo Model |
8-week-old Nrf2-knockout (Nrf2-/-) and wild-type (WT) littermate male mice on a C57BL/6J background (provided by the RIKEN Bio-Resource Center through the National Bio-Resource Project of MEXT, Japan) were used for in vivo experiments. Intestinal ischemia was induced by clamping of the superior mesenteric artery after the intraperitoneal injection of 50 mg/kg of sodium pentobarbital. After 90 min, the intestine was reperfused for the times indicated. Sham control mice underwent the same procedure without vascular occlusion.
Click to Show/Hide
|
||||
| Response Description | iASPP (PPP1R13L) mediated its protective effects against acute lung injury through the Nrf2/HIF-1/TF signaling pathway. Ferroptosis contributes to intestinal ischemia/reperfusion-induced acute lung injury (ALI), and iASPP treatment inhibits ferroptosis in part via Nrf2. | ||||
Putative metallothionein MT1DP (MT1DP)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [57] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
A total of 6 x 106 A549 cells were subcutaneously injected into the right flank of the athymic BALB/c nude mice (aged 4 weeks, weight 12-16 g; Vital River, Beijing, China). Once tumors reached about 80 mm3, the mice were randomly divided into four groups. Mice were treated with 50 uM/kg erastin by intraperitoneal injection every 2 days for eight times. Tumor size was measured.
Click to Show/Hide
|
||||
| Response Description | Previous findings indicated that metallothionein 1D pseudogene (MT1DP), a long noncoding RNA (lncRNA), functioned to aggravate oxidative stress by repressing antioxidation. RNA pulldown assay and dual-luciferase reporter assay confirmed that MT1DP modulated the expression of NRF2 via stabilizing miR-365a-3p. In conclusion, MT1DP sensitized non-small cell lung cancer (NSCLC) cells to erastin-induced ferroptosis by regulating the miR-365a-3p/NRF2 signaling pathway. | ||||
Proprotein convertase subtilisin/kexin type 9 (PCSK9)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [58] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| HuH-6 cells | Hepatoblastoma | Homo sapiens | CVCL_4381 | ||
| In Vivo Model |
Zebrafish were maintained at 28 and in light cycle conditions (12 h). The Casper mutant fish line was purchased from the Zebrafish International Resource Center (ZIRC). For zebrafish xenotransplantation, 48 hours post-fertilization (hpf) zebrafish embryos were dechorionated and anesthetized in egg water solution containing 0.04 mg/mL tricaine (Sigma-Aldrich, St. Louis, MO, USA) before human cell injection. Approximately 200 to 500 fluorescent cells were injected (Eppendorf Femtojet microinjector) into the ducts of Cuvier of each embryo, and the zebrafish were maintained in 0.3X Danieaus solution for 1 h at 28 . After confirmation of a visible cell mass at the injection site, the zebrafish were transferred to a 24-well plate in 500 uL of a 0.3X Danieaus solution incubator and maintained at 34 . The zebrafish with already formed metastasis at 1 hour post-injection (hpi) were discarded.
Click to Show/Hide
|
||||
| Response Description | The disruption of PCSK9 inhibits the anti-ferroptosis p62-Keap1-Nrf2 pathway, one could speculate that a combination therapy of anti-PCSK9 with sorafenib would alleviate drug resistance and improve prognosis. We provide strong evidence supporting the drug repositioning of anti-PCSK9 approaches to treat hepatocellular carcinoma. | ||||
Progestin and adipoQ receptor family member 3 (PAQR3)
Myeloid leukaemia [ICD-11: 2B33]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [59] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
MOLT-3 cells | T-lymphoblastic leukemia | Homo sapiens | CVCL_0624 |
| MOLT4 cells | Adult T acute lymphoblastic leukemia | Homo sapiens | CVCL_0013 | |
| CEM/C1 cells | T acute lymphoblastic leukemia | Homo sapiens | CVCL_3496 | |
| Jurkat cells | T acute lymphoblastic leukemia | Homo sapiens | CVCL_0065 | |
| Response Description | PAQR3 inhibited proliferation and aggravated ferroptosis in acute lymphoblastic leukemia through modulation Nrf2 stability. This study suggested that PAQR3 may serve as an effective biological marker for ALL treatment. | |||
Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN (PTEN)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [60] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCC1419 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1251 | |
| HCC1395 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1249 | ||
| NCI-H226 cells | Pleural epithelioid mesothelioma | Homo sapiens | CVCL_1544 | ||
| NCI-H446 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1562 | ||
| ZR-75-1 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0588 | ||
| HCC1937 cells | Breast ductal carcinoma | Homo sapiens | CVCL_0290 | ||
| HCC1187 cells | Breast ductal carcinoma | Homo sapiens | CVCL_1247 | ||
| HCC1806 cells | Breast squamous cell carcinoma | Homo sapiens | CVCL_1258 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| NCI-H520 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | ||
| mEFs (Mouse embryonic fibroblasts) | |||||
| In Vivo Model |
A cross between 12 week old, B6.129S4 Ptenfl/fl mice obtained from Jackson Laboratory was set up and 14 days later both male and female embryos were harvested from the pregnant females. Highly vascularized sections of the embryos were removed - head, extremities, and liver. The remainder was minced via a scalpel and resuspended in 0.25% trypsin using a pipet. The cells were then incubated for 10 min in an incubator at 37 with 5% CO2 before being further resuspended into single cell suspension. The cells were then spun down at 1500 RPM for 5 min to remove the trypsin, after which they were resuspended in 10 mL of fresh media and transferred to a 10 cm dish.
Click to Show/Hide
|
||||
| Response Description | Loss of PTEN activates AKT kinase to inhibit GSK3, increasing NF-E2 p45-related factor 2 (NRF2) along with transcription of one of its known target genes encoding xCT in breast cancers. | ||||
Pannexin-2 (PANX2)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [61] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
LNCaP cells | Prostate carcinoma | Homo sapiens | CVCL_0395 |
| PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| DU145 cells | Prostate carcinoma | Homo sapiens | CVCL_0105 | |
| RWPE-1 cells | Normal | Homo sapiens | CVCL_3791 | |
| Response Description | PANX2 is implicated in the pathogenesis of prostate cancer (PCa), which regulates malignant phenotypes and ferroptosis through Nrf2 signaling pathway (Nrf2, HO-1, and FTH1), and maybe a potential therapeutic target for PCa. Blocking expression of PANX2 resulted in suppression of proliferation, migration, and invasion in PCa cells, while increasing ferrous iron and MDA levels. | |||
NUAK family SNF1-like kinase 1 (NUAK1)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [62] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| NCI-H716 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1581 | ||
| In Vivo Model |
HCT116-Or or H716 cells (7*106) were suspended into 200 ul matrigel (BD Bioscience), and injected into the subcutaneous tissues of mouse right lower limbs. Animals were grouped randomly (eight mouse per group) and medication was initiated when the average xenograft size was over 100 mm3: Oxaliplatin was given alone weekly through intraperitoneal injection (5 mg/kg), or in combination with liproxstatin-1 through intraperitoneal injection for twice a week (125 mg/kg) and RSL3 through intra-tumor injection (100 mg/kg, in order to achieve better local concentration and reduce the probable systemic toxicity of RSL3) weekly.
Click to Show/Hide
|
||||
| Response Description | KIF20A was highly expressed in the oxaliplatin-resistant cell lines and was strongly correlated with survival among colorectal cancer patients. Silencing KIF20A enhanced cellular sensitivity to oxaliplatin both in vivo and in vitro, and silencing KIF20A also suppressed NUAK1 activation. Moreover, silencing NUAK1 up-regulated the expression of PP1, down-regulated the phosphorylation of downstream GSK3Ser9, suppressed the nuclear import of Nrf2, inhibited the expression of a ferroptosis key negative regulatory protein (GPX4), and blocked cellular resistance. | ||||
Mucin-1 (MUC1)
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [63] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6J male mice (6-8 weeks) were purchased from Slac Lab Animals (Shanghai, China). The basic principle of the CLP method was to find the caecum through anatomy and puncture at the blind end and extrude the contents into the abdominal cavity. Diffuse peritonitis was formed, and systemic infection appeared in mice. Mice in the control group were only treated with laparotomy.
Click to Show/Hide
|
||||
| Response Description | Inhibition of MUC1 dimerization could increase the expression level of Keap1, reduce the phosphorylation level of GSK3, inhibit the entry of Nrf2 into the nucleus, further inhibit the expression level of GPX4, enhance the lipid peroxidation level of lung tissues, trigger ferroptosis, and aggravate lung injury. And inhibiting MUC1 reversed the alleviating effect of vitamin E on acute lung injury caused by sepsis. | ||||
Mitogen-activated protein kinase 8 (MAPK8)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [53] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
LN-229 cells | Glioblastoma | Homo sapiens | CVCL_0393 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| hACs (Normal human astrocyte cells) | |||||
| In Vivo Model |
BALB/c nude mice (female, four-week-old) were purchased from the Nanjing Medical University Experimental Animal Department. Female mice were randomly divided into test group and control group. 2.5 x 105 LN229/TMZ cells transfected with sh-MAPK8-1 or sh-LINC01564-1 were injected into the brain of mice in test group, taking the mice injected with sh-NC-transfected ones as control. Seven days later, the mice were treated with TMZ (66 mg/kg per day, 5 days/cycle, 4 cycles in total) as a monotherapy. Tumor volume was monitored every three days in the period of TMZ treatment. The mice were killed 28 days after the injection. Tumors were excised from mice for observation and weighing as well as the detection of the level of ROS, iron (Fe2+) and proteins (i.e., NFE2L2, NQO1, FTH1 and HO-1).
Click to Show/Hide
|
||||
| Response Description | LINC01564 promotes the temozolomide (TMZ) resistance of glioma cells by upregulating NFE2L2 expression to inhibit ferroptosis. LINC01564 promotes MAPK8 mRNA stability by recruiting SRSF1, and MAPK8 was positively correlated with NFE2L2 and its targets, proving its mediation of NFE2L2. | ||||
Microsomal glutathione S-transferase 1 (MGST1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [64] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CFPAC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_1119 | |
| Panc 02.03 cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1633 | ||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 CFPAC1 cells in 100 ul PBS were injected subcutaneously to the right of the dorsal midline in 6- to 8-week-old athymic nude female mice. Once the tumors reached around 70-80 mm3 at day 7, mice were randomly allocated into groups and then treated with imidazole ketone erastin (IKE; 40 mg/kg, i.p., once every other day) in the absence or presence of liproxstatin-1 (10 mg/kg, i.p., once every other day) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | MGST1 inhibits ferroptotic cancer cell death partly by binding to ALOX5, resulting in reduced lipid peroxidation. The expression of MGST1 is positively correlated with NFE2L2 expression in pancreatic tumors, which is implicated in the poor prognosis of patients with pancreatic ductal adenocarcinoma (PDAC). | ||||
Maleylacetoacetate isomerase (GSTZ1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [65] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| SNU-449 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
Mice were divided into five groups as follows: WT + DMSO (control), WT + Sora, Gstz1-/-+DMSO, Gstz1-/-+Sora, and Gstz1-/-+Sora + RSL3. Each group included three male and three female mice. At 2 weeks of age, all mice were administered an intraperitoneal injection of diethylnitrosamine (DEN; Sigma, St. Louis, MO, USA) at a dose of 75 mg/kg. At the third week, the mice were intraperitoneally administered carbon tetrachloride (CCl4; Macklin, Shanghai, China) at 2 ml/kg twice a week for 12 weeks. In the WT + Sora and Gstz1-/-+Sora group, the mice at 22 weeks were administered intraperitoneally sorafenib (30 mg/kg) every 2 days for 4 weeks until euthanasia. In the Gstz1-/-+Sora + RSL3 group, in addition to sorafenib administration as described above, the mice were injected intraperitoneally with RSL3 (10 mg/kg) every 2 days for 4 weeks at the same weeks.
Click to Show/Hide
|
||||
| Response Description | GSTZ1 enhanced sorafenib-induced ferroptosis by inhibiting the NRF2/GPX4 axis in hepatocellular carcinoma (HCC) cells. Combination therapy of sorafenib and GPX4 inhibitor RSL3 may be a promising strategy in HCC treatment. | ||||
Lysine-specific demethylase 6B (KDM6B)
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [66] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| R-3327-AT-2 cells | Prostate adenocarcinoma | Rattus norvegicus | CVCL_L303 | ||
| In Vivo Model |
A total of 30 male C57/B6J mice (8-10 weeks, 21-23 g) were acquired from Chinese Academy of Medical Sciences (Beijing, China). The sepsis-induced ALI model was constructed by administering LPS intratracheally (5 mg/kg) for 12 h as previously reported. The control groups were given an isovolumetric sterile saline. Then, 12 h after LPS installation, the animals were sacrificed by cervical dislocation under deep anesthesia with an intraperitoneal injection of 2% sodium pentobarbital (60 mg/kg).
Click to Show/Hide
|
||||
| Response Description | JMJD3 ( KDM6B) deficiency may relieve LPS-induced acute lung injury (ALI) by blocking alveolar epithelial ferroptosis in a Nrf2 dependent manner, which may serve as a novel therapeutic target against ALI. | ||||
Long-chain fatty acid transport protein 5 (SLC27A5)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [67] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| In Vivo Model |
Age-matched male BALB/c nude mice (4-6 weeks old) were used for the orthotopic mouse model. Cohorts of mice were randomized into different treatment groups. 4 x 106 tumor cells were suspended in a 50 ul PBS/Matrigel (356234, BD Biosciences) mixture (1:1 (v/v) ratio) for each group of mice and injected into the left liver lobes by surgical implantation.
Click to Show/Hide
|
||||
| Response Description | The combination of sorafenib and carmustine (BCNU), a selective inhibitor of GSR, remarkably hamper tumor growth by enhancing ferroptotic cell death in vivo. SLC27A5 serves as a suppressor in sorafenib resistance and promotes sorafenib-triggered ferroptosis via restraining the NRF2/GSR pathway in hepatocellular carcinoma, providing a potential therapeutic strategy for overcoming sorafenib resistance. | ||||
Lon protease homolog, mitochondrial (LONP1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [68] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Pathways in cancer | hsa05200 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 |
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | |
| Response Description | Elevation of mitochondrial LONP1 in erastin-induced ferroptosis of pancreatic cancer cell lines. Inhibition of LONP1 activates the Nrf2/Keap1 signal pathway and up-regulates the expression of GPX4, a key peroxidase in regulating ferroptosis. | |||
LINC01564 (IncRNA)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [53] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
LN-229 cells | Glioblastoma | Homo sapiens | CVCL_0393 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| hACs (Normal human astrocyte cells) | |||||
| In Vivo Model |
BALB/c nude mice (female, four-week-old) were purchased from the Nanjing Medical University Experimental Animal Department. Female mice were randomly divided into test group and control group. 2.5 x 105 LN229/TMZ cells transfected with sh-MAPK8-1 or sh-LINC01564-1 were injected into the brain of mice in test group, taking the mice injected with sh-NC-transfected ones as control. Seven days later, the mice were treated with TMZ (66 mg/kg per day, 5 days/cycle, 4 cycles in total) as a monotherapy. Tumor volume was monitored every three days in the period of TMZ treatment. The mice were killed 28 days after the injection. Tumors were excised from mice for observation and weighing as well as the detection of the level of ROS, iron (Fe2+) and proteins (i.e., NFE2L2, NQO1, FTH1 and HO-1).
Click to Show/Hide
|
||||
| Response Description | LINC01564 promotes the temozolomide (TMZ) resistance of glioma cells by upregulating NFE2L2 expression to inhibit ferroptosis. LINC01564 promotes MAPK8 mRNA stability by recruiting SRSF1, and MAPK8 was positively correlated with NFE2L2 and its targets, proving its mediation of NFE2L2. | ||||
LINC00239 (IncRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| FHC cells | Normal | Homo sapiens | CVCL_3688 | ||
| In Vivo Model |
To clarify the role of LINC00239 in vivo, we used 4-week-old male BALB/c nude mice provided by the Experimental Animal Center of the Air Force Military Medical University. HCT116 or SW620 cells (1 x 107 cells) were injected subcutaneously into the right flanks of these mice to establish a CRC xenograft model. One week after the injection of cells, the volume of xenografts was continuously monitored (once a week). Four weeks later, the xenografts were removed, and the weights were measured.
Click to Show/Hide
|
||||
| Response Description | LINC00239 plays a novel and indispensable role in ferroptosis by nucleotides 1-315 of LINC00239 to interact with the Kelch domain (Nrf2-binding site) of Keap1, inhibiting Nrf2 ubiquitination and increasing Nrf2 protein stability. And LINC00239 expression has a positive correlation with Nrf2 and GPX4 expression in CRC tissues. LINC00239 inhibition in combination with ferroptosis induction might be a promising therapeutic strategy for colorectal cancer patients. | ||||
Kinesin-like protein KIF20A (KIF20A)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [62] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| NCI-H716 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1581 | ||
| In Vivo Model |
HCT116-Or or H716 cells (7*106) were suspended into 200 ul matrigel (BD Bioscience), and injected into the subcutaneous tissues of mouse right lower limbs. Animals were grouped randomly (eight mouse per group) and medication was initiated when the average xenograft size was over 100 mm3: Oxaliplatin was given alone weekly through intraperitoneal injection (5 mg/kg), or in combination with liproxstatin-1 through intraperitoneal injection for twice a week (125 mg/kg) and RSL3 through intra-tumor injection (100 mg/kg, in order to achieve better local concentration and reduce the probable systemic toxicity of RSL3) weekly.
Click to Show/Hide
|
||||
| Response Description | KIF20A was highly expressed in the oxaliplatin-resistant cell lines and was strongly correlated with survival among colorectal cancer patients. Silencing KIF20A enhanced cellular sensitivity to oxaliplatin both in vivo and in vitro, and silencing KIF20A also suppressed NUAK1 activation. Moreover, silencing NUAK1 up-regulated the expression of PP1, down-regulated the phosphorylation of downstream GSK3Ser9, suppressed the nuclear import of Nrf2, inhibited the expression of a ferroptosis key negative regulatory protein (GPX4), and blocked cellular resistance. | ||||
hsa-miR-431-3p (miRNA)
Intervertebral disc degeneration [ICD-11: FA80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [71] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hBMCs (Bone marrow cells) | |||
| hBMSCs (Bone marrow stromal cells) | ||||
| Response Description | BMSC-EV-loaded circ_0072464 inhibited NPC ferroptosis to relieve intervertebral disc degeneration (IDD) via upregulation of miR-431-mediated NRF2, therefore providing a potential therapeutic target against IDD. | |||
hsa-miR-365a-3p (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [57] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
A total of 6 x 106 A549 cells were subcutaneously injected into the right flank of the athymic BALB/c nude mice (aged 4 weeks, weight 12-16 g; Vital River, Beijing, China). Once tumors reached about 80 mm3, the mice were randomly divided into four groups. Mice were treated with 50 uM/kg erastin by intraperitoneal injection every 2 days for eight times. Tumor size was measured.
Click to Show/Hide
|
||||
| Response Description | Previous findings indicated that metallothionein 1D pseudogene (MT1DP), a long noncoding RNA (lncRNA), functioned to aggravate oxidative stress by repressing antioxidation. RNA pulldown assay and dual-luciferase reporter assay confirmed that MT1DP modulated the expression of NRF2 via stabilizing miR-365a-3p. In conclusion, MT1DP sensitized non-small cell lung cancer (NSCLC) cells to erastin-induced ferroptosis by regulating the miR-365a-3p/NRF2 signaling pathway. | ||||
hsa-miR-27a-3p (miRNA)
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [72] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
SPF male Sprague Dawley rats aged 8 weeks were purchased from Beijing HFK Bioscience Co., Ltd. and housed in the Experimental Animal Center of North China University of Science and Technology (licence no. SYXK(Ji)2020-007) at 22 ± 2 with 60 ± 5% humidity, 12 h light/dark cycles, and free access to food and water. The rats were raised adaptively for approximately 7 days before experimental manipulation. The animals were handled according to the National Institute of Healths Guide for the Care and Use of Laboratory Animals (1996) guidelines, and all animal experiments were approved by the Animal Care and Use Committee of North China University of Science and Technology. In addition, all efforts were made to minimize the number of animals used and their suffering.
Click to Show/Hide
|
||||
| Response Description | miRNA-27-a inhibited Nrf2 in a targeted manner, which also exacerbated the extent of ferroptosis. Therefore, the present study indicated that miRNA-27-a may aggravate brain tissue ferroptosis during ischaemic stroke, potentially by inhibiting Nrf2. | ||||
hsa-miR-130b-5p (miRNA)
Melanoma [ICD-11: 2C30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [73] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PIG1 cells | Normal | Homo sapiens | CVCL_S410 | |
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | ||
| G-361 cells | Melanoma | Homo sapiens | CVCL_1220 | ||
| HS1-CLS cells | Skin sarcoma | Homo sapiens | CVCL_5978 | ||
| IGR-1 cells | Cutaneous melanoma | Homo sapiens | CVCL_1303 | ||
| MeWo cells | Melanoma | Homo sapiens | CVCL_0445 | ||
| NIS-G cells | Melanoma | Homo sapiens | CVCL_6005 | ||
| WS1-CLS cells | Skin sarcoma | Homo sapiens | CVCL_6211 | ||
| KMM-L1 cells | Normal | Mus musculus | CVCL_XB77 | ||
| In Vivo Model |
NU/NU nude mice were purchased from Hunan Slac Laboratory Animals Co., Ltd. (Changsha, China). Melanoma cells (5 x 106) were injected subcutaneously into the left posterior side of 7-week-old immunodeficient female mice. The tumor growth was monitored by measuring the length (L) and width (W) of the tumor. Tumor volume = 1/2 (length x width2). When the tumor volume reached about 50 mm3, mice were randomly assigned into 8 groups (n = 6) and given intraperitoneal injection of erastin for 20 days. Erastin was dissolved in 5% DMSO + corn oil (C8267, Sigma) in the test tube heated at 37 and gently shaken before use.
Click to Show/Hide
|
||||
| Response Description | MiR-130b-3p is able to inhibit the ferroptosis induced by erastin or RSL3 in melanoma cells by targeting DKK1 and subsequent activation of Nrf2/HO-1 pathway. | ||||
Growth/differentiation factor 15 (GDF15)
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [74] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPNs (Rat primary neurons) | ||||
| In Vivo Model |
Total of 50 C57BL/6J adult mice (males, average weight of 20 g, 8 weeks of age) were acquired from Charles River (Beijing, China). In brief, ketamine (80 mg/kg) was utilized to anesthetize mice before the skin was prepared for disinfection, and then the back skin was incised to expose the lamina at T10. Finally, a moderate contusion (5 g x 5 cm) was created by an impactor (RWD, Shenzhen, China). Spinal cord hemorrhage, hindlimb extension, and delayed paralysis suggest successful modeling. Only laminectomy was performed in the Sham group.
Click to Show/Hide
|
||||
| Response Description | GDF15 effectively alleviated neuronal ferroptosis post spinal cord injury (SCI) via the p62-Keap1-Nrf2 signaling pathway and promoted locomotor recovery of SCI mice, which is suggested as a potential target on SCI pathogenesis and treatment. | ||||
Furin (FURIN)
Ulcerative colitis [ICD-11: DD71]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [75] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
NCM460 cells | Normal | Homo sapiens | CVCL_0460 | |
| In Vivo Model |
Male C57BL/6 mice wild-type (WT), 8 weeks of age, were from Chongqing Medical University, China. Mice were divided into four groups (n = 10-13 per group), control group, MPTP group, h-Trx-1 Tg group, and h-Trx-1 Tg + MPTP group. Control and h-Trx-1 Tg groups were administered saline only. For the Trx-1 knockdown experiment, mice were divided into six groups (n = 10-13 per group), control + saline group, control + MPTP group, AAV9-vehicle + saline group, AAV9-vehicle + MPTP group, AAV9-shRNA-mTrx-1 + saline group, and AAV9-shRNA-mTrx-1 + MPTP.
Click to Show/Hide
|
||||
| Response Description | Furin protects epithelial cells from DSS-induced ferroptosis-like cell injury and alleviates experimental ulcerative colitis by activating the Nrf2-Gpx4 signaling pathway. | ||||
E3 ubiquitin-protein ligase MIB1 (MIB1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [76] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell adhesion molecules | hsa04514 | |||
| Notch signaling pathway | hsa04330 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| Response Description | MIB1 may function as a positive regulator of ferroptosis through targeted degradation of the master antioxidant transcription factor NRF2 and sensitizes lung cancer cells to ferroptosis. | |||
Dickkopf-related protein 1 (DKK1)
Melanoma [ICD-11: 2C30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [73] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PIG1 cells | Normal | Homo sapiens | CVCL_S410 | |
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | ||
| G-361 cells | Melanoma | Homo sapiens | CVCL_1220 | ||
| HS1-CLS cells | Skin sarcoma | Homo sapiens | CVCL_5978 | ||
| IGR-1 cells | Cutaneous melanoma | Homo sapiens | CVCL_1303 | ||
| MeWo cells | Melanoma | Homo sapiens | CVCL_0445 | ||
| NIS-G cells | Melanoma | Homo sapiens | CVCL_6005 | ||
| WS1-CLS cells | Skin sarcoma | Homo sapiens | CVCL_6211 | ||
| KMM-L1 cells | Normal | Mus musculus | CVCL_XB77 | ||
| In Vivo Model |
NU/NU nude mice were purchased from Hunan Slac Laboratory Animals Co., Ltd. (Changsha, China). Melanoma cells (5 x 106) were injected subcutaneously into the left posterior side of 7-week-old immunodeficient female mice. The tumor growth was monitored by measuring the length (L) and width (W) of the tumor. Tumor volume = 1/2 (length x width2). When the tumor volume reached about 50 mm3, mice were randomly assigned into 8 groups (n = 6) and given intraperitoneal injection of erastin for 20 days. Erastin was dissolved in 5% DMSO + corn oil (C8267, Sigma) in the test tube heated at 37 and gently shaken before use.
Click to Show/Hide
|
||||
| Response Description | MiR-130b-3p is able to inhibit the ferroptosis induced by erastin or RSL3 in melanoma cells by targeting DKK1 and subsequent activation of Nrf2/HO-1 pathway. | ||||
Cyclic AMP-dependent transcription factor ATF-2 (ATF2)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [77] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| In Vivo Model |
Athymic nu/nu mice (5 to 6-week-old female) were acquired from the Silaike Experimental Animal Co. Ltd (Shanghai, China). Mice was randomly divided into different groups (n = 6 per group), 1 x 106 MB-231 cells with stable overexpression of ATF2 and control cells were subcutaneously inoculated on the right flanks. Seven days later, mice were injected intraperitoneally with PBS containingdimethyl sulfoxide(DMSO) and JQ1 (50 mg/kg) once every 2-4 days for 8 times, and the tumor volumes and body weight of mice were monitored and recorded every 23 days.
Click to Show/Hide
|
||||
| Response Description | ATF2 inhibited BETi-induced ferroptosis by increasing NRF2 expression. Altogether, ATF2 suppressed ani-tumor effects of BETi in a negative feedback manner by attenuating ferroptosis. BETi combined with ATF2 or NRF2 inhibitor might be a novel strategy for treatment of Breast cancer. | ||||
Circ_0072464 (circRNA)
Intervertebral disc degeneration [ICD-11: FA80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [71] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hBMCs (Bone marrow cells) | |||
| hBMSCs (Bone marrow stromal cells) | ||||
| Response Description | BMSC-EV-loaded circ_0072464 inhibited NPC ferroptosis to relieve intervertebral disc degeneration (IDD) via upregulation of miR-431-mediated NRF2, therefore providing a potential therapeutic target against IDD. | |||
BTB/POZ domain-containing adapter for CUL3-mediated RhoA degradation protein 2 (TNFAIP1)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [78] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Downregulation of TNFAIP1 alleviates OGD/Rinduced neuronal damage by suppressing Nrf2/GPX4 mediated ferroptosis, which might lay the foundation for the investigation of targeted-therapy for cerebral ischemia-reperfusion injury in clinic. | |||
All trans-polyprenyl-diphosphate synthase PDSS2 (PDSS2)
Atherosclerosis [ICD-11: BD40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [79] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hCAECs (Human coronary artery endothelial cells) | ||||
| In Vivo Model |
Eight-week-old male mice PDSS2 wild type [WT (n = 6)], PDSS2/, Nrf2/, Nrf2+/+, on C57BL/6 background (18-22 g) were provided by Nanjing Medical University (Nanjing, China). PDSS2/ mice (n = 12) were crossed with PDSS2/ to obtain PDSS2/, Nrf2/ (n = 6) and PDSS2/, Nrf2+/+ (n = 6) mice. Mice were fed an AIN76A Western diet for 8 weeks to accelerate the development of AS. Mice from each group were euthanized after 8 weeks.
Click to Show/Hide
|
||||
| Response Description | Overexpression of PDSS2 suppressed the ferroptosis of HCAECs by promoting the activation of Nrf2 pathways. Thence PDSS2 may play a cardio-protective role in atherosclerosis (AS). | ||||
A disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13)
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [80] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mKTs (Mouse knee tissues) | ||||
| In Vivo Model |
C57BL/6 mice (8-12 week) mice purchased from Cavens (Changzhou, China) were divided into four groups: control (saline), CDDP only (CP, 20 mg/kg; MCE, United States), CP (20 mg/kg) + ADAMTS13 (0.1 nmol/kg) and CP (20 mg/kg) + ADAMTS13 (0.3 nmol/kg). Each group contained 5 mice. 0.1 and 0.3 nmol/kg rhADAMTS13 were injected into the caudal vein daily for 3 days after surgery.
Click to Show/Hide
|
||||
| Response Description | ADAMTS13 alleviated CP-induced inflammatory response and oxidative stress in acute kidney injury mice, during which the Nrf2 signaling pathway was abnormal. Overall, ADAMTS-13-regulated Nrf2 signaling inhibits ferroptosis to ameliorate CP-induced AKI. | ||||
Unspecific Regulator
Sepsis [ICD-11: 1G40]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [81] | ||||
| Responsed Drug | Ferulic acid | Patented | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
Briefly, female BALB/c mice (6-8 weeks, weighing 20-25 g, purchased from Hunan SJA Laboratory Animal Co., Ltd., Changsha, Hunan, China) were raised in specific pathogen-free conditions under controlled temperature (23-25 ) and humidity (40-80%) as well as a 12 h dark/light cycle for 1 week of acclimation. They were fed a standard chow diet and waterad libitum. Mice were anaesthetised with 2% isoflurane inhalation and underwent moderate caecal ligation and puncture in accordance with a previously reported protocol (Rittirsch etal.2009). Meanwhile, mice in the control groups were subjected to a sham operation. Buprenorphin (0.05 mg/kg) was injected for postoperative analgesia, and all mice were placed in cages immediately after the surgical procedures with free access to water and food (Rittirsch etal. 2009).
Click to Show/Hide
|
||||
| Response Description | Collectively, our data highlighted the alleviatory role of ferulic acid in sepsis-induced ALI by activating the Nrf2/HO-1 pathway and inhibiting ferroptosis, offering a new basis for sepsis treatment. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [82] | ||||
| Responsed Drug | Iridin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Eight-week-old wild-type (WT) and Nrf2-knockout (Nrf2-/-) littermate male mice on a C57BL/6J background were purchased from Cyagen (Suzhou, China.) and maintained at the Centre for Animals of Wuhan University (Wuhan, China). Before the experiment, the mice were separated and given light and dark cycles for 12 h, 22 ± 0.5 temperature, 60 ± 10% humidity, and free accessed to food and water for at least 1 week. Mice were randomly distributed into sham, CLP, CLP + Irisin (Ir group) and CLP + Irisin + Era (Ir + Era group) groups.
Click to Show/Hide
|
||||
| Response Description | In conclusion, irisin could ameliorate inflammatory microenvironment in sepsis-associated encephalopathy by suppressing hippocampus ferroptosis via the Nrf2/GPX4 signaling pathway. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [83] | ||||
| Responsed Drug | MCTR1 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice aged 8-12 weeks, weighing about 25 g, were purchased from Shanghai Experimental Animal Center of China. The mice were anesthetized with isoflurane. The abdomen area was disinfected after shaving. A 1 cm midline incision was made on the lower abdomen skin to expose the cecum. A 5-0 silk thread was used to ligate the midpoint between the distal pole and the bottom of the cecum. A 21 g needle was used to penetrate the cecum twice from the mesenteric toward the antimesenteric direction. After the wound was sutured, 0.25% ropivacaine was injected subcutaneously for postoperative analgesia. The mice were immediately resuscitated by injecting prewarmed normal saline 1 ml subcutaneously.
Click to Show/Hide
|
||||
| Response Description | MCTR1 effectively alleviates sepsis-associated acute kidney injury, at least in part, by inhibiting ferroptosis. Mechanistically, MCTR1 activates the Nrf2, which may contribute to the inhibition of ferroptosis. | ||||
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [84] | ||||
| Responsed Drug | Ibuprofen | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
In the intracranial glioma model, U87MG cells (5 x 105) were intracerebrally injected into the left side (bregma: 1 mm; lateral: 2 mm; ventral: 3 mm) of the brains of nude mice. Two weeks after tumor cell transplantation, mouse brains were scanned to detect tumor formation using a 3.0-T scanner (GE Signa HD MRI Systems). Then, mice were divided randomly into two groups (n = 6/group) and treated with vehicle control (PBS), oribuprofen (20 mg/kg), in 100 ul of PBS given i.p. 1x/day, 5 days/week.
Click to Show/Hide
|
||||
| Response Description | Ibuprofen could induce ferroptosis of glioblastoma cells via downregulation of Nrf2 signaling pathway and is a potential drug for glioma treatment. All the data suggested that Nrf2 could regulate the expression of GPX4 and SLC7A11 in glioma cells. | ||||
Central nervous system cancer [ICD-11: 2A02]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [85] | |||
| Responsed Drug | Polygonatum cyrtonemaHua Polysaccharides | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
BV-2 cells | Normal | Mus musculus | CVCL_0182 |
| Response Description | Subsequent studies have revealed that Polygonatum cyrtonemaHua Polysaccharides (PCP) alleviates ferroptosis in microglia due to protein levels of ERASTIN/RSL3 inhibitor SLC7A11/GPX4 by activating the NRF2/HO-1 signaling pathway. PCP has the development potential as a new drug candidate for treating CNS diseases. | |||
Acute myeloid leukaemia [ICD-11: 2A60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [86] | ||||
| Responsed Drug | All-trans retinoic acid derivative | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
NB4 cells | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | |
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | ||
| U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | ||
| In Vivo Model |
Six- to seven-week-old female NCG mice were obtained from the Nanjing model animal research institute (Nanjing, China). Mice were raised in individually ventilated cages of Anhui Medical University (Hefei, China). Then, an injection of NB4 cells (5 x 106/100 ul) suspended in chilled Matrigel and PBS (1:1) was given into the right flank of each mouse. For each experiment, the animals (n = 12) were randomly allocated into the two groups and treated with solvent or 10 mg/kg ATPR (intraperitoneal injection, once every other day) for 7 days.
Click to Show/Hide
|
||||
| Response Description | ATPR, a novel all-trans retinoic acid (ATRA) derivative, has been extensively developed to show superior anticancer effect than ATRA in acute myeloid leukemia (AML). ATPR-induced ferroptosis was regulated by autophagy via iron homeostasis, especially Nrf2. | ||||
Myeloid leukaemia [ICD-11: 2B33]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [87] | |||
| Responsed Drug | Triptolide | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
K-562 cells | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 |
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| Response Description | Triptolide inhibited Nrf2 expression and induced leukemia cell ferroptosis, as evidenced by increased ROS levels and lipid oxidation as well as decreased glutathione peroxidase 4 expression. Therefore, it is plausible that triptolide can promote leukemia cell sensitivity to DOX via downregulation of Nrf2 expression. | |||
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [88] | ||||
| Responsed Drug | Baicalin | Terminated | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| 143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | ||
| hBMMSCs (Human bone marrow mesenchymal stem cells) | |||||
| In Vivo Model |
A total of 24 BALB/c-nude mice (4-5 weeks old) were purchased and MG63 cells were injected into the right tibial bone marrow cavity of mice in a volume of 1 x 106/100 ul. When the tumor volume was visible, all animals were randomly divided into four groups (n = 6): the control (10% DMSO + 40% PEG300 + 5% Tween-80 + 45% Saline) group, the baicalin (200 mg/kg/day) group, the Fer-1 (0.8 mg/kg/day) group and Fer-1 + baicalin group. The baicalin and Fer-1 were intraperitoneally administered every day for two consecutive weeks and tumor sizes were measured every two days.
Click to Show/Hide
|
||||
| Response Description | By promoting the Fe accumulation, ROS formation, MDA production and suppressing the ratio of GSH/GSSG, baicalin was found to trigger ferroptosis in Osteosarcoma and ferroptosis inhibitor ferrostatin-1 (Fer-1) successfully reversed these suppressive effects, indicating that ferroptosis participated in the baicalin mediated anti-OS activity. Mechanistically, baicalin physically interacted with Nrf2, a critical regulator of ferroptosis, and influenced its stability via inducing ubiquitin degradation, which suppressed the Nrf2 downstream targets GPX4 and xCT expression, and led to stimulating ferroptosis. | ||||
Oral squamous cell carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [89] | |||
| Responsed Drug | Carnosic acid | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Response Description | The current findings highlight that carnosic acid may re-sensitize cisplatin-resistant cells to cisplatin by inducing ferroptosis, which involves the inactivation of Nrf2/HO-1/xCT pathway. Hence, this research may support a promising therapeutic approach to overcome chemoresistance in Oral squamous cell carcinoma. | |||
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [90] | ||||
| Responsed Drug | Polyphyllin I | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| In Vivo Model |
A subcutaneous gastric tumor model was established by subcutaneously injecting 1 x 106 AGS cells or 2 x 106 MKN-45 cells near the right axilla of mice. Seven days after tumor cell inoculation, mice received daily i. p. Injection of PPI (3 mg/kg, dissolved in 1% DMSO + 5% PEG300 + 5% Tween 80 + 89% deionized water), as described previously, or the control solution with the same solvent. Mice were weighed at day 15, while tumor volumes were measured every 3 days and calculated using a formula: length x width2/2.
Click to Show/Hide
|
||||
| Response Description | For the first time, our results have demonstrated that Polyphyllin I exerts its antitumor activity on the gastric cancer by, at least partially, inducing cancer cell ferroptosisviaregulating NRF2/FTH1 pathway. | ||||
Colorectal cancer [ICD-11: 2B91]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [91] | ||||
| Responsed Drug | Propofol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response Description | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [92] | ||||
| Responsed Drug | Oxaliplatin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| Response Description | Oxaliplatin promoted ferroptosis and oxidative stress in colorectal cancer cells by inhibiting the Nrf2 signaling pathway. Treatment with oxaliplatin enhanced the effects of erastin on CRC cells by promoting ferroptosis and oxidative stress and inhibiting cell viability. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [93] | ||||
| Responsed Drug | tagitinin C | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| Response Description | Tagitinin C induces ferroptosis in colorectal cancer cells and has synergistic effect together with erastin. Mechanistically, tagitinin C induces ferroptosis through ER stress-mediated activation of PERK-Nrf2-HO-1 signaling pathway. | ||||
Pancreatic cancer [ICD-11: 2C10]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [94] | ||||
| Responsed Drug | Itaconic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 cells in 100 ul PBS were injected subcutaneously into the right of the dorsal midline in 6- to 8-week-old femaleathymic nude mice(n = 6 mice/group). After the tumor reached 60-80 mm3 on day 7, the mice were randomly grouped and then given intraperitoneal injections with itaconic acid (50 mg/kg, once every other day) at day 7 for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Itaconic acid-induced expression and activation of NFE2L2 serves as a defense mechanism to limit ferroptosis by producing antioxidant genes. Consequently, impaired NCOA4 expression prevented, whereas a disrupted NFE2L2 pathway enhanced, sensitivity to itaconic acid-induced ferroptosis in pancreatic cancer cells. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [95] | ||||
| Responsed Drug | Wogonin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| AsPC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0152 | ||
| HPDE6-C7 cells | Normal | Homo sapiens | CVCL_0P38 | ||
| In Vivo Model |
Female BALB/c nude mice (5 weeks old) were procured from Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd (Zhejiang, China) and given 5 days to acclimate to their surroundings. PANC-1 cells (1 x 107) in 100 uL PBS at the logarithmic growth phase were administered to mice subcutaneously in the left flank. The mice were treated with indicated treatments after nearly 10 days when the tumour size was approximately 1,000 mm3. In the control group, mice (n = 5) received intraperitoneal injections of the vehicle. In the treatment group, the mice (n = 5) were administered 50 uL of 60 mg/kg body weight of wogonin once a day for 12 days. A slide calliper size was used to measure the tumour size. The equation for calculating tumour volume is as follows: tumour volume = AB2/2, wherein A is the length, and B is the width of the tumour. The mice were sacrificed the next day after the treatment procedure was complete by cervical dislocation. The tumour tissues were harvested and snap-frozen using liquid nitrogen for subsequent analyses.
Click to Show/Hide
|
||||
| Response Description | Wogonin could significantly reduces pancreatic cancer cell proliferation and induce ferroptosisviathe Nrf2/GPX4 axis. Therefore, wogonin could be potentially used for treating patients with pancreatic cancer. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [96] | |||
| Responsed Drug | Camptothecin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response Description | Sorafenib is a potent inducer of ferroptosis used to manage hepatocellular carcinoma (HCC). Nrf2 inhibition by Camptothecin improves sorafenib's sensitivity and reduces sorafenib's resistance via the augmentation of sorafenib's ferroptosis action. | |||
Lung cancer [ICD-11: 2C25]
| In total 5 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [97] | ||||
| Responsed Drug | Erastin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
A total of 60 BALB/c-nu/nu nude mice (male; age, 4-6 weeks; weight, 16-22 g) were obtained from the Shanghai Laboratory Animal Co., Ltd. N5CP cells (5 x 106) were suspended in 200 ul DMEM and Matrigel mixture at a ratio of 1:1. Subsequently, the mixture was injected subcutaneously into the upper right flank of 20 nude mice. After 10 days, the mice were randomly divided into four groups and were treated with CDDP (5 mg/kg/2 days), erastin (10 mg/kg/2 days), sorafenib (10 mg/kg/2 days) or PBS by intraperitoneal injection. Two days after the third injection, the mice were sacrificed and tumours were carefully removed. For the combination experiment, CDDP (1 mg/kg) and erastin (5 mg/kg) or sorafenib (3 mg/kg) were also injected three times.
Click to Show/Hide
|
||||
| Response Description | The potential mechanism by which sorafenib and erastin induced ferroptosis in cisplatin (CDDP)-resistant non-small cell lung cancer (NSCLC) cells may be associated with inhibition of the expression of the Nrf2 downstream target gene xCT. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [97] | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
A total of 60 BALB/c-nu/nu nude mice (male; age, 4-6 weeks; weight, 16-22 g) were obtained from the Shanghai Laboratory Animal Co., Ltd. N5CP cells (5 x 106) were suspended in 200 ul DMEM and Matrigel mixture at a ratio of 1:1. Subsequently, the mixture was injected subcutaneously into the upper right flank of 20 nude mice. After 10 days, the mice were randomly divided into four groups and were treated with CDDP (5 mg/kg/2 days), erastin (10 mg/kg/2 days), sorafenib (10 mg/kg/2 days) or PBS by intraperitoneal injection. Two days after the third injection, the mice were sacrificed and tumours were carefully removed. For the combination experiment, CDDP (1 mg/kg) and erastin (5 mg/kg) or sorafenib (3 mg/kg) were also injected three times.
Click to Show/Hide
|
||||
| Response Description | The potential mechanism by which sorafenib and erastin induced ferroptosis in cisplatin (CDDP)-resistant non-small cell lung cancer (NSCLC) cells may be associated with inhibition of the expression of the Nrf2 downstream target gene xCT. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [98] | ||||
| Responsed Drug | Ginkgetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SPC-A1 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | ||
| In Vivo Model |
Briefly, when tumours on transplanted nude mice reached around 100 mm3, the mice were randomized divided into eight groups: control, ginkgetin, DDP, ginkgetin + DDP, UAMC 3203, ginkgetin + UAMC 3203, DDP + UAMC 3203, ginkgetin + DDP + UAMC 3203. Both DDP (3 mg/kg) and ginkgetin (30 mg/kg) were administered by intraperitoneal injection, with 2 - 3 times per week and once per day, respectively. UAMC 3203 (10 mg/kg) was administered 5 days/week by intraperitoneally injection. Tumour size and body weight were measured 3 times per week. After dosing 31 days, the nude mice were sacrificed, and tumours were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | The induction of ferroptosis mediated by ginkgetin was further confirmed by the decreased expression of SLC7A11 and GPX4, and a decreased GSH/GSSG ratio. Simultaneously, ginkgetin disrupted redox hemostasis in DDP-treated cells, as demonstrated by the enhanced ROS formation and inactivation of the Nrf2/HO-1 axis. Ginkgetin also enhanced DDP-induced mitochondrial membrane potential (MMP) loss and apoptosis in cultured non-small cell lung cancer (NSCLC) cells. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [99] | ||||
| Responsed Drug | S-3'-hydroxy-7', 2', 4'-trimethoxyisoxane | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
When tumor volumes in xenograft nude mice reached an average of roughly 100 mm3, the mice were randomly divided into 3 groups of 6 mice each: control, ShtIX, and ShtIX + Fer-1. The treated group received ShtIX or ShtIX combined with Fer-1 injections into the tail vein of the mice every three days for 7 times, whereas the control group received saline. Every four days, the volume and weight of the tumors were measured. As soon as the test was completed, the nude mice were slaughtered, and the tumor tissues were retrieved. The in vivo experiments were approved by the Animal Care and Use Committee of Hainan Medical College and following the animal rules.
Click to Show/Hide
|
||||
| Response Description | S-3'-hydroxy-7', 2', 4'-trimethoxyisoxane (ShtIX) caused ferroptosis in Non-small cell lung cancer (NSCLC) cells, and inhibiting the Nrf2/HO-1 pathway can considerably exacerbate the effect of ShtIX-induced ferroptosis. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [100] | ||||
| Responsed Drug | Manoalide | Phase 2 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| H157 cells | Oral cavity Squamous cell carcinoma | Homo sapiens | CVCL_2458 | ||
| HCC827 cells | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| In Vivo Model |
The LSL-KrasG12D mouse model was obtained from the Jackson Laboratory (Sacramento, CA). Adeno-Cre (Genechem, Shanghai, China) was introduced into the trachea of mice at a dose of 1.25 x 1011 PFU in a total volume of 50 uL. Tumor tissues from 12-week post-infection mice were washed with cold PBS, cut into small pieces, and washed with DMEM/F12 (containing 1 x Glutamine, 10 mM HEPES, and antibiotics), digested with collagenase I and IV for 0.5-1 h at 37. After washing twice with DMEM/F12 and centrifugation (500 g, 5 min), the dissociated cells were seeded into growth factor-reduced matrigel (Corning, #356237) at 37 for 30 min.
Click to Show/Hide
|
||||
| Response Description | Manoalide (MA) induces ferroptosis by suppressing the NRF2-SLC7A11 axis and mitochondrial Ca2+overload induced-FTH1 pathways to promote the sensitivity of osimertinib-resistant lung cancer cells to osimertinib. | ||||
Ovarian cancer [ICD-11: 2C73]
| In total 4 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [101] | ||||
| Responsed Drug | Apatinib | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| Response Description | Apatinib combined with olaparib-induced ferroptosis via a p53-dependent manner in ovarian cancer. Further studies showed that apatinib combined with olaparib-induced ferroptosis by inhibiting the expression of Nrf2 and autophagy, thereby inhibiting the expression of GPX4. The Nrf2 activator RTA408 and the autophagy activator rapamycin rescued the combination drug-induced ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [102] | ||||
| Responsed Drug | Triptolide | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A2780/DDP cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_D619 | |
| In Vivo Model |
All female BALB/cnude mice(4-6 weeks old, 15-20 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). They were raised in specific pathogen-free conditions and allowed to access sterile water and food freely. A2780/DDP cell suspension (100 uL) with a density of 1 x 107 cells/mL was injected subcutaneously into the axilla of the mice. After observing the nude mice for a week, it was confirmed that subcutaneous A2780/DDP cells were inoculated successfully. Sterile saline (100 uL) was injected into the abdominal cavity of the nude mice in the control group for 14 days. The mice in the DDP treatment group were given DDP (4 mg/kg/day) intraperitoneally on the first and eighth days. TG (100 uL, 1 mg/kg) diluted with sterile physiological saline were injected into the abdominal cavity of the nude mice in the TG treatment group for 14 days. In addition, the nude mice in the TG + DDP treatment group were given TG (100 uL, 1 mg/kg) for 14 days and DDP (4 mg/kg/day) intraperitoneally on the first and eighth days.
Click to Show/Hide
|
||||
| Response Description | Tripterygium (TG) can effectively inhibit the proliferation of drug-resistant ovarian tumor cells A2780/DDP and increase the sensitivity to cisplatin chemotherapy both invitro and invivo. In terms of mechanism, TG induces ferroptosis by targeting the NRF2/GPX4 signal axis to weaken the antioxidant capacity of cancer cells. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Responsed Drug | Norcantharidin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Athymic nu/nu female mice aged 6-8 weeks (n = 9; mean weight, 20.21 ± 1.54 g) were purchased from the specific pathogen SPF (Beijing) Lab Animals Technology Co. Ltd. Mice were housed in a temperature- and humidity-controlled environment (20-24 , 45-55% humidity), with free access to food and water and in groups of three. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC ID: 17-3256) at Nantong University and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Nuclear factor erythroid 2-related factor 2 (NRF2), heme oxygenase 1 (HO-1), glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (xCT) expression levels were significantly decreased following norcantharidin (NCTD) treatment. Collectively, NCTD may represent a potent anticancer agent in ovarian cancer cells, and NCTD-induced ferroptotic cell death may be achieved by inhibiting the NRF2/HO-1/GPX4/xCT axis. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [101] | ||||
| Responsed Drug | Olaparib | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| Response Description | Apatinib combined with olaparib-induced ferroptosis via a p53-dependent manner in ovarian cancer. Further studies showed that apatinib combined with olaparib-induced ferroptosis by inhibiting the expression of Nrf2 and autophagy, thereby inhibiting the expression of GPX4. The Nrf2 activator RTA408 and the autophagy activator rapamycin rescued the combination drug-induced ferroptosis. | ||||
Bladder cancer [ICD-11: 2C94]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [104] | ||||
| Responsed Drug | Erianin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
RT4 cells | Bladder carcinoma | Homo sapiens | CVCL_0036 | |
| KU-19-19 cells | Bladder carcinoma | Homo sapiens | CVCL_1344 | ||
| In Vivo Model |
4-weeks-old female BALB/c nude mice aged were injected into 5 x 105 cells. Every 2 days mice weight and tumor size were assessed, and the tumor volume (V) was calculated with the formula: (maximum length) x (maximum width)2/2. Once tumors were found, the mice were stochastically divided into 2 groups: the control (PBS) group and the treatment (erianin 100 mg/kg) group. For 14 days, mice were injected intraperitoneally with drugs once a day, then puting the mice to death, after that tumors were taken for IHC (immunohistochemical) analysis.
Click to Show/Hide
|
||||
| Response Description | Erianin inhibited cell proliferation and triggered cell death in bladder cancer cells. Mechanistically, we showed NRF2 was a key determinant for erianin-triggered ferroptosis. NRF2 activation by TBHQ treatment protected against erianin-induced cell death and increased the expression of GPX4, ferritin, xCT and glutaminase. | ||||
Diabetes mellitus [ICD-11: 5A10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [105] | ||||
| Responsed Drug | Bilirubin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MIN6 cells | Insulinoma | Mus musculus | CVCL_0431 | |
| In Vivo Model |
BALB/c mouse are used as recipients and donors in the transplantation. Fifteen diabetic BALB/c mice were randomly divided into 5 groups (3 mice in each group). Then, 250 IEQ islets pretreated with or without bilirubin (20 uM) for 48 h were transplanted into the subrenal site of the diabetic mouse. Ferrin 1 (10 uM) and DFO (10 mM) pretreated islets were also transplanted for comparison. Following, the non-fasting glucose level and bodyweight was the mice were recorded daily.
Click to Show/Hide
|
||||
| Response Description | Bilirubin protects transplanted islets by inhibiting ferroptosis through multiple mechanisms, including ROS scavenging ability, iron-chelating property, and upregulation of Nrf2/HO-1 signaling pathway. Bilirubin could improve islet viability and function through inhibiting ferroptosis, which could be of clinic interest to apply bilirubin into the islet transplantation system. Islet transplantation is an attractive treatment for type 1 diabetic patients. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [106] | |||
| Responsed Drug | Gastrodin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
C6 cells | Malignant glioma | Rattus norvegicus | CVCL_0194 |
| Response Description | Gastrodin induced GPX4, Nrf2 and HO-1 expression to protect C6 cells from H2O2-induced ferroptosis. Gastrodin pretreatment effectively reduced H2O2-induced oxidative damage, indicating gastrodin is a potential antioxidant that reduced cytotoxic ROS. The role of gastrodin in ferroptosis presents a new perspective for understanding parkinson's disease. | |||
Alzheimer disease [ICD-11: 8A20]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [107] | ||||
| Responsed Drug | Forsythoside A | Investigative | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| Neuro-2a cells | Neuroblastoma | Mus musculus | CVCL_0470 | ||
| BV-2 cells | Normal | Mus musculus | CVCL_0182 | ||
| In Vivo Model |
All animal experiments were approved by the Animal Ethics Committee of Jilin University (permit No. SY201905013) and were conducted in compliance with the ARRIVE guidelines. Eight-month-old B6C3-Tg (APPswePSEN1dE9)/Nju double transgenic male mice (APP/PS1) (genotype: (Appswe) T, (Psen1) T) and age-matched wild-type (WT) (genotype: (Appswe) W, (Psen1) W) male mice were purchased from Nanjing Biomedical Research Institute of Nanjing University. All mice were individually housed at 24 with food and drinking water availablead libitum. After 1 week of adaption in the new environment, WT mice received oral administration of normal saline (10 mL/kg) and were designated as the control group (n = 12). APP/PS1 mice were randomly divided into two groups: the model group (n = 12) received oral administration of normal saline (10 mL/kg) and the agent-treated group (n = 12) received oral treatment with 30 mg/kg FA (L-012-171216, 98.83% purity, Chengdu Herbpurify Co., Ltd., Chengdu, China) beginning on day 8. After 30-day treatment, behavioral experiments were serially performed. The entire treatment protocol lasted for 42 days. Blood samples were collected from the caudal vein. After euthanasia via CO2 inhalation, organs including the brain, liver, spleen, and kidney were collected for further analysis.
Click to Show/Hide
|
||||
| Response Description | Forsythoside A treatment exerted anti-ferroptosis and anti-neuroinflammatory effects in erastin-stimulated HT22 cells, and the Nrf2/GPX4 axis played a key role in these effects. Collectively, these results demonstrate the protective effects of FA and highlight its therapeutic potential as a drug component for AD ( Alzheimer's disease) treatment. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [108] | ||||
| Responsed Drug | ginkgolide-B | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
Male 6-month-old senescence-resistant R1 (SAMR1) and SAMP8 mice (weight, 28-35 g) were purchased from Beijing SPF Biotechnology. First, mice were placed in the center of an empty testing arena (40 x 40 x 40 cm) and allowed to move freely for adaptation. Next, in the training stage, two similar objects were presented in the testing arena and mice were allowed to explore for 10 min, for 3 consecutive days. On day 4, one of the two familiar objects was replaced by a new object. The time of exploring a novel object or familiar object in 10 min was recorded.
Click to Show/Hide
|
||||
| Response Description | Ginkgolide B attenuated Alzheimer's disease (AD)-related cognitive impairment through the regulation of oxidative stress, neuroinflammation and ferroptosis, and that GB-induced protection in AD is dependent on the inhibition of ferroptosis. Furthermore, the involvement of Nrf2/GPX4 pathway-regulated ferroptosis in the GB-related protective effects on the AD mouse model. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [109] | ||||
| Responsed Drug | Salidroside | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CD8T cells (Mouse CD8+ T cells) | ||||
| In Vivo Model |
SAMP8 mice were employed as an AD model and were treated with salidroside for 12 weeks. Behavioral tests, immunohistochemistry, HE and Nissl staining, immunofluorescence, transmission electron microscopy, quantitative proteomics, bioinformatic analysis, flow cytometry, iron staining,western blotting, andmolecular dockingwere performed.
Click to Show/Hide
|
||||
| Response Description | Salidroside alleviates cognitive impairment and inhibits neuronal ferroptosis in Alzheimer's disease. The underlying mechanisms may involve the Nrf2/GPX4 axis activation and reduction in CD8T cells infiltration. | ||||
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [111] | ||||
| Responsed Drug | Curcumin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MDCK cells | Normal | Canis lupus familiaris | CVCL_0422 | |
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks old) were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China). Briefly, mice were anesthetized and placed in a prone position with head stabilization in a stereotaxic frame. A dental drill was then utilized to generate a 1 mm burr hole at 2.0 mm to the lateral right of the bregma and 3.5 mm deep of the brain. Next, acute ICH was induced by slowly injecting 0.1U of type IV collagenase into this hole.
Click to Show/Hide
|
||||
| Response Description | Curcumin in NPs (Cur-NPs) were shown to suppress erastin-induced ferroptosis in HT22 murine hippocampal cells. Cur-NPs effectively regulated the expression levels of HMOX1 and NFE2L2, which indicated that it might inhibit the ROS production through regulating the NRF2/HO-1 pathway. Cur-NPs served as an effective treatment for Intracerebral hemorrhage owing to their ability to inhibit ferroptosis. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 5 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [112] | ||||
| Responsed Drug | Edaravone | Approved | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
Seventy-three specific-pathogen-free (SPF)grade healthy male Sprague Dawley (SD) rats, weighing 240 ± 20 g, were purchased from Hunan Slake Jingda Experimental Animal Co., Ltd., China (animal certificate number SCXK (Xiang) 2013-0004). The animals were reared in an SPF animal laboratory, and the ambient temperature was maintained at 23 ± 1 . All protocols followed the ARRIVE guidelines in terms of study design, sample size, randomization, outcome measures, data analysis, experimental procedures, and reporting of results. This study was approved by the Animal Ethics Committee of the Hunan University of Chinese Medicine.
Click to Show/Hide
|
||||
| Response Description | Edaravone inhibits ferroptosis to attenuate cerebral ischemia-reperfusion injury, probably through the activation of the Nrf2/FPN pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [113] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Rats were randomly assigned to six groups: (1) the sham group, (2) the middle cerebral artery ischaemia-occlusion-reperfusion (MCAO/R) group, (3) the AST IV group, (4) the PNS group, (5) the combination group and (6) the combination + brusatol group. One hundred rats were used in the experiment, of which 9 died during surgery, 10 died of intracranial haemorrhage and brain injury and 63 rats were successfully modelled, for a final success rate of 76.8%. Each group included 9 rats. Behavioural testing was performed on 5 animals in each group. After behavioural testing, 3 rats were used for TTC staining and 6 were used for kit detection and western blot analysis. Existing studies have revealed the toxicological effects of the compatibility of astragalus and P. notoginseng. The dosage and method of AST IV (28 mg/kg) and PNS (80 mg/kg) alone or in combination have been previously determined and were administered intragastrically for three consecutive days (10 ml/kg each time), and the optimal administration times were 50, 26 and 2 h before model establishment.Brusatol (1 mg/kg) was administered intraperitoneally for 1 h prior to modelling. The sham group and the MCAO/R group were given the same amount of saline.
Click to Show/Hide
|
||||
| Response Description | Combining Astragaloside IV and Panax notoginseng saponins attenuates cerebral ischemia-reperfusion injury by activating Nrf2 to inhibit ferroptosis and inflammatory responses. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [113] | ||||
| Responsed Drug | Panax notoginseng saponins | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Rats were randomly assigned to six groups: (1) the sham group, (2) the middle cerebral artery ischaemia-occlusion-reperfusion (MCAO/R) group, (3) the AST IV group, (4) the PNS group, (5) the combination group and (6) the combination + brusatol group. One hundred rats were used in the experiment, of which 9 died during surgery, 10 died of intracranial haemorrhage and brain injury and 63 rats were successfully modelled, for a final success rate of 76.8%. Each group included 9 rats. Behavioural testing was performed on 5 animals in each group. After behavioural testing, 3 rats were used for TTC staining and 6 were used for kit detection and western blot analysis. Existing studies have revealed the toxicological effects of the compatibility of astragalus and P. notoginseng. The dosage and method of AST IV (28 mg/kg) and PNS (80 mg/kg) alone or in combination have been previously determined and were administered intragastrically for three consecutive days (10 ml/kg each time), and the optimal administration times were 50, 26 and 2 h before model establishment.Brusatol (1 mg/kg) was administered intraperitoneally for 1 h prior to modelling. The sham group and the MCAO/R group were given the same amount of saline.
Click to Show/Hide
|
||||
| Response Description | Combining Astragaloside IV and Panax notoginseng saponins attenuates cerebral ischemia-reperfusion injury by activating Nrf2 to inhibit ferroptosis and inflammatory responses. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [114] | ||||
| Responsed Drug | Propofol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Male C57BL/6 mice weighing 20-25 g each were obtained from the Animal Experimental Center of Yisi (Changchun, China). Mice were group-housed in a 12 h light/dark cycle (light between 08:00 and 20:00 h) in a temperature-controlled environment room (23-25 ). Mice had ad libitum access to food and water. All surgical procedures were carried out on animals anesthetized with sodium pentobarbital (30 mg/kg) via intraperitoneal injection. MCAO was achieved by inserting a silicone rubber-coated nylon monofilament into the internal carotid artery through the external carotid artery and temporary ligation of the right common carotid artery with a suture. After 45 min of ischemia, blood flow was restored by removing the filament and the suture, and the mice were allowed to recover for 24 h.
Click to Show/Hide
|
||||
| Response Description | Our data support a protective role of propofol against ferroptosis as a cause of cell death in mice with cerebral ischemia-reperfusion injury. Propofol protected against cerebral ischemia-reperfusion injury-induced ferroptosis partly by regulating the Nrf2/Gpx4 signaling pathway. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [115] | ||||
| Responsed Drug | Caryophyllene | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPAs (Rat primary astrocytes) | ||||
| In Vivo Model |
Rats were anesthetized withisoflurane(2-3% oxygen) and placed in asupine position. And theright common carotid artery (CCA),external carotid artery (ECA), andinternal carotid artery (ICA) were exposed in sequence and separated carefully. Then we ligated the CCA and the ECA in turn, and at the same time, we clamped the internal carotid artery with an arterial clamp. Finally, we inserted a silicone nylon monofilament from the CCA into themiddle cerebral arteryand temporarily fixed it. After 1.5 h ofischemia, the monofilament was taken out and the blood vessels were ligated at theincision. The neck wound was sutured with surgical sutures. Subsequent experiments were performed after 12 h ofreperfusion. In thesham operationrats, except for the absence of the monofilament, the sham operation rats underwent the same surgical procedures as the MCAO/R model rats.
Click to Show/Hide
|
||||
| Response Description | Our results indicated the critical role of ferroptosis in cerebral ischemia reperfusion injury. For the first time, we showed that the significant neuroprotective effects of b-Caryophyllene (BCP) in attenuating ischemic stroke injury are correlated with ferroptosis regulation, and its mechanism is associated with activation of the NRF2/HO-1 axis. | ||||
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [116] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
1% sodium pentobarbital (40 mg/kg) was administered to the rats intraperitoneally to anesthetize them before placing them in a brain stereotaxic device. An incision was created in the midline of the neck to expose the common internal and external carotid arteries. After ligating and cutting the external carotid artery on the left side, a 3-mm stump was exposed. We then perforated the carotid artery at the bifurcation of the middle and anterior cerebral arteries utilizing an 18-20-mm-long surgical filament (0.26 mm diameter; Beijing Cinontech Co. Ltd., China) was threaded through the external carotid artery stump into the internal carotid artery and left in situ for 120 min. After that, the filament was withdrawn to facilitate reperfusion. Rats in the sham surgery group received the identical procedure as the other rats but without filament insertion.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AS-IV) administration decreased the infarct volume, brain edema, neurological deficits, and inflammatory cytokines TNF-, interleukin-1 (IL-1), IL-6, and NF-B, increased the levels of SLC7A11 and glutathione peroxidase 4 (GPX4), decreased lipid reactive oxygen species (ROS) levels, and prevented neuronal ferroptosis. Meanwhile, AS-IV triggered the Nrf2/HO-1 signaling pathway and alleviated ferroptosis due to the induction of stroke. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [117] | ||||
| Responsed Drug | Kaempferol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mPCNs (Mouse primary cortical neurons) | ||||
| Response Description | Kaempferol provides protection from OGD/R-induced ferroptosis, at least in part, by activating Nrf2/SLC7A11/GPX4 signaling pathway. Therefore, pharmacological inhibition of ferroptosis may be an attractive therapeutic target for the treatment of ischemic stroke. | ||||
Nervous system disease [ICD-11: 8E7Z]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [118] | |||
| Responsed Drug | Ajudecunoid C | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Ajudecunoid C effectively prevented ferroptosis through scavenging free radical and activating NRF2-antioxidant response elements (AREs) pathway. This study reveals that ADC, as a new ferroptosis inhibitor, is a promising lead compound for the development of drugs against ferroptosis-related neurological diseases. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [119] | |||
| Responsed Drug | Gastrodin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Gastrodin (GAS) is a component of Gastrodia elata Blume, with strong antioxidant activity in neurodegenerative diseases. GAS increased the nuclear translocation of Nrf2, up-regulated the downstream HO-1 protein expression in HT-22 cells following treatment with glutamate. | |||
Extraocular muscles disorder [ICD-11: 9C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [120] | |||
| Responsed Drug | Artesunate | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hOFs (Human ocular fibroblasts) | |||
| Response Description | Expression of mitochondrial GPX4 but no other forms of GPX4 was decreased after artesunate treatment and that mitochondrial GPX4 overexpression rescued artesunate-induced lipid peroxidation and ferroptosis. Other cellular ferroptosis defense mechanisms, including cellular FSP1 and Nrf2, were also inhibited by artesunate. In conclusion, our study demonstrated that artesunate protects against fibrosis through abrogation of fibroblast activation and induction of mitochondria-dependent ferroptosis in ocular fibrosis, which may offer a potential treatment for ocular fibrosis. | |||
Cardiomyopathy [ICD-11: BC43]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [121] | ||||
| Responsed Drug | Berberine | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response Description | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [122] | ||||
| Responsed Drug | Curcumin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Two-month-old male New Zealand rabbits purchased from the Medical Experimental Animal Center of Bengbu Medical College were used as experimental subjects. Streptozotocin was dissolved in sterile saline and intraperitoneally injected into the rabbits at a dose of 80 mg/kg. The rabbits were allowed to eat freely after receiving the injection. The fasting blood glucose levels of the rabbits were monitored regularly. The diabetic rabbit model was considered successfully established when the fasting blood glucose level was measured as 11 mmol/L twice or 14 mmol/L once. Following successful modelling, grouping was performed as follows: blank control group (Con-Group), diabetic rabbit group (DM-Group), diabetic rabbit + every other day curcumin administration group (Qod-Group), and diabetic rabbit + daily administration group (Qd-Group).
Click to Show/Hide
|
||||
| Response Description | Curcumin can promote the nuclear translocation of Nrf2, increase the expression of oxidative scavenging factors, such as HO-1, reduce excessive Gpx4 loss, and inhibit glucose-induced ferroptosis in cardiomyocytes. This highlights a potentially new therapeutic route for investigation for the treatment diabetic cardiomyopathy. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [123] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
A total of 24 SD male rats weighing 200-210 g from the Vital River Laboratory Animal Technology Co., Ltd. were divided randomly into control, ADR, ADR+AsIV, and AsIV group (n = 6). AsIV was administered by gavage at a dose of 10 mg/kg/day over a period of five weeks. ADR was administered intraperitoneally once a week (30 mg/kg/week) for five weeks. Controls were administered saline intraperitoneally (i.p.) at the same dose as ADR and intragastrically at the same dose as AsIV.
Click to Show/Hide
|
||||
| Response Description | Adriamycin (ADR) was found to promote cardiac ferroptosis, whereas administration of Astragaloside IV (AsIV) attenuated the process via activating Nrf2 signaling pathway and the subsequent GPX4 expression increasing. These results suggest that AsIV might play a protective role against ADR-induced myocardial fibrosis. | ||||
Chronic obstructive pulmonary disease [ICD-11: CA22]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [124] | ||||
| Responsed Drug | Dihydroquercetin | Preclinical | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HBE1 cells | Normal | Homo sapiens | CVCL_0287 | |
| In Vivo Model |
Thirty-two male BALB/c mice (21-25 g, 6-8 weeks) were purchased from Hunan Slyke Jingda Laboratory Animal Co., Ltd. and kept in a clean unit at 23 ± 2 , 50% ± 10% relative humidity and 12 h rhythm of light and dark. Mice were randomly divided into four groups (n = 8 for each group): the control group, cigarette smoke-inducedCOPDgroup, COPD + low dose (50mg/kg/d)DHQgroup, and COPD + high dose (100 mg/kg/d) DHQ group. The mice in the control group were maintained in fresh air and given anintraperitoneal injectionof 0.3 ml/20 g phosphate-buffered saline (PBS) on Days 0, 11, and 23. The COPD mouse model was established as previously described. Mice in this group were exposed to cigarette smoke for 2 cycles per day (1 h per cycle), 6 days per week for 4 consecutive weeks in a sealed box with ventilation holes except for Days 0, 11, and 22, and over these 3 days, the mice were intraperitoneally injected with 0.3 ml/20g 100% CSE. Mice in the COPD+low-dose DHQ group and COPD+high-dose DHQ group were treated with cigarette smoke and 100% CSE as mentioned above and intraperitoneally injected with DHQ for 25 consecutive days except for Days 0, 11, and 22, while mice in the control and COPD groups were intraperitoneally injected with an equal volume of PBS except for Days 0, 11, and 22. All mice were sacrificed by intraperitoneal injection of 0.5 ml 3% chloral hydrateon the 29 th day of the experiment.
Click to Show/Hide
|
||||
| Response Description | Treatment with DHQ (Taxifolin) significantly reverses the ferroptosis induced by cigarette smoke both in vivo and in vitro via a Nrf2-dependent signaling pathway. These findings may provide novel therapeutic options for the treatment of chronic obstructive pulmonary disease (COPD) patients. | ||||
Hepatoblastoma [ICD-11: DB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [125] | ||||
| Responsed Drug | Glycyrrhizin | Phase 3 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
In total, 40 male specific- pathogen-free C57BL/6 mice (Hubei Animal Experimental Center) 6-8 weeks old. The mice were randomly divided into 5 groups: The normal group, model group, 15 mg/kg GLY group, 30 mg/kg GLY group and 60 mg/kg GLY group. Except for the normal group, the other four groups of mice were injected intraperitoneally with D-GalN (400 mg/kg) and LPS (100 ug/kg) to induce the ALF model. According to a previous study on GLY gavage doses, three doses of GLY (15, 30 and 60 mg/kg/day) intervention groups were used. A total of 24 mice were divided into three groups. Mice received gavage with different doses of GLY for 3 days before induction of the ALF model.
Click to Show/Hide
|
||||
| Response Description | The HMGB1 inhibitor glycyrrhizin (GLY) significantly reduced the degree of ferroptosis during acute liver failure (ALF) by inhibiting oxidative stress. Treatment with GLY reduced the degree of liver damage, the expression of HMGB1 was decreased, and the levels of Nrf2, HO1 and GPX4 were increased. | ||||
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [126] | ||||
| Responsed Drug | Ginkgolide B | Terminated | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| In Vivo Model |
Male 8-week-old C57/BL6 ApoE-/-mice of weight (22~25 g) were purchased from Changzhou Cavens experimental animal Co., Ltd (Jiangsu, China). After 5 weeks of feeding, HFD-fed mice were randomly assigned into 4 groups (n = 10) : HFD group (0.9 % sodium chloride by gavage), GB-L group (at a high dose of 20 mg kg-1d-1 GB in 0.9 % sodium chloride by gavage), GB-H group (at a high dose of 30 mg kg-1d-1 GB in 0.9 % sodium chloride by gavage), and Ato group (1.3 mg kg-1d-1 Ato in 0.9 % sodium chloride by gavage) as a positive control. The mice in ND group were given the same volume of 0.9 % sodium chloride.
Click to Show/Hide
|
||||
| Response Description | Ginkgolide B (GB), a main constituent of Ginkgo biloba extracts, reduces hepatic lipid accumulation and ameliorates nonalcoholic fatty liver disease (NAFLD) in obese mice. Remarkably, after Nrf2 interference, GB treatment significantly increased Nrf2 expression, indicating that GB exerted anti-ferroptosis effects by activation of Nrf2 pathway. | ||||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 7 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [127] | ||||
| Responsed Drug | Etomidate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rMTs (Rat myocardial tissues) | ||||
| In Vivo Model |
Male Sprague-Dawley rats (8 weeks, 180 g-210 g) were provided by the experimental animal center of Beijing Institute of Life Sciences. Rats were anesthetized with 2.5% sodium pentobarbital and fixed in supine position. The LAD was ligated using a 6-0 silk for a 30 min ischemic period. Ischemia was confirmed by discoloration of heart surface and ST elevation on the electrocardiogram (ECG) recording. After 30 min, the LAD ligation was released and the reperfusion was continued for 3 h, which was confirmed by the redness of the heart surface and the decrease in ST recorded by ECG. Rats in Sham group (n = 12) underwent the same surgical procedures, except that LAD was threaded but not ligated.
Click to Show/Hide
|
||||
| Response Description | Etomidate (Eto) attenuated MIRI-induced heart failure, pathological damage, myocardial fibrosis, andinflammation, which may be related to its inhibition onferroptosis. Mechanically, the protection of Eto in myocardial ischemia reperfusion (MIR) injury (MIRI) may be achieved by activating Nrf2 pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [128] | ||||
| Responsed Drug | Gossypol acetic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
A total of 55 adult male Sprague-Dawley rat (350-450 g) were anesthetized with urethane (1.5 g/kg, i.p.), then the hearts were perfused in a Langendorff system. After 30 min of stabilization, hearts were subjected to 30 min of global no-flow ischemia by stopping the perfusion. Reperfusion was followed with Krebs Henseleit (KH) buffer and GAA together for 2 h. A thermoregulated chamber kept the heart at 37 throughout the experiment. Control hearts were not subjected to I/R. The heart slices were sectioned at a thickness of 2 mm and stained with triphenyltetrazolium chloride (25 mg/100 mL) for 10 min and then fixed with 4% formaldehyde solution for 48 h to enhance color contrast.
Click to Show/Hide
|
||||
| Response Description | Gossypol acetic acid significantly attenuated myocardial infarct size, reduced lipid peroxidation, decreased the mRNA levels of the ferroptosis markers Ptgs2 and Acsl4, decreased the protein levels of ACSL4 and NRF2, and increased the protein levels of GPX4 in I/R-induced ex vivo rat hearts. Thus, GAA may play a cytoprotectant role in ferroptosis-induced cardiomyocyte death and myocardial ischemia/reperfusion-induced ferroptotic cell death. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [129] | ||||
| Responsed Drug | Histochrome | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mCMs (Mouse cardiomyocytes) | ||||
| In Vivo Model |
Male Fischer 344 rats (8 weeks old and 160 to 180 g; KOATECH, Pyeongtaek-si, Korea) were anesthetized by inhalation with 2% isoflurane and intubated using an 18-gauge intravenous catheter. The rats were mechanically ventilated with medical-grade oxygen. Surgery was performed on a 37 heating pad to prevent the body from getting cold. A left thoracotomy was performed after the chest was shaved to prevent contamination during surgery.
Click to Show/Hide
|
||||
| Response Description | Histochrome treatment significantly increased GPx4 and free GSH levels, but decreased Cox-2 level. HC treatment significantly decreased intracellular and mitochondrial ROS levels by upregulating the expression of Nrf2 and antioxidant genes. The substantial cardioprotective effects of HC against myocardia I/R injury by reducing ferroptosis-associated myocardial injury. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [130] | ||||
| Responsed Drug | Naringenin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
20 Sprague Dawley (SD) rats (6-8 weeks, 200-220 g) were acquired from the Second Clinical College of Guangzhou University of Traditional Chinese Medicine, and were weighed, coded, and randomly assigned to experimental groups. Rats were divided into Sham group, MI/R group, MI/R +NAR (low dose, 10 mg/kg/d) group, and MI/R +NAR (high dose, 50 mg/kg/d) group. For MI/R model, rats were anaesthetized by intraperitoneal injection of 1% pentobarbital sodium (60 mg/kg) and then received mechanical ventilation from an animal ventilator after endotracheal intubation.
Click to Show/Hide
|
||||
| Response Description | Naringenin alleviated MI/R-induced pathological damage, inflammation and lipid peroxidation in myocardial tissue of rats. NAR adjusted the NRF2 /System xc - /GPX4 axis and improved ferroptosis. In conclusion, NAR can alleviate myocardial ischemia-reperfusion injury by regulating the Nrf2/System xc-/GPX4 axis to inhibit ferroptosis. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [131] | ||||
| Responsed Drug | Salidroside | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | ||
| In Vivo Model |
Following endotracheal intubation, mice were ventilated with room air at a rate of 120 cycles/min and atidal volumeof 7 mL/kg (MiniVent, Harvard Apparatus, USA). To induce ischemia, mice underwent left thoracotomy, and the left pulmonary hilum was blocked for 60 min with a microvascular clamp. After ischemia, the coronary artery was reperfused for 120 min by removing the clamp. The mice were euthanized at the end of the experiment through CO2 asphyxiation and cervical dislocation. Next, bronchoalveolar lavage fluid (BALF), blood, and lung samples were collected for testing.
Click to Show/Hide
|
||||
| Response Description | Salidroside postconditioning attenuates ferroptosis-mediated lung ischemia-reperfusion injury by activating the Nrf2/SLC7A11 signaling axis. | ||||
| Experiment 6 Reporting the Ferroptosis-centered Disease Response of This Regulator | [132] | ||||
| Responsed Drug | Dimethyl fumarate | Approved | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
The mice were randomly divided into four groups of six: sham + vehicle, sham + DMF, IR + vehicle, and IR + DMF. The mice were supplemented with DMF at a concentration of 100 mg/kg or DMSO by daily oral gavage for a week before surgery, as previously reported. As stated in a prior study, the partial warm liver IRI model was developed. Briefly, the sham group only had free hepatic portal blood vessels after laparotomy, and the blood flow was not obstructed. As for the hepatic IR group, the blood supply to the left and mid-hepatic lobes was blocked, resulting in 70% mouse liver IRI for 90 min. The mice were put on a heated blanket after surgery in order to maintain body temperature and monitor vital signs. Blood supply was restored for 6 h. Died mice were eliminated for testing prior to sample collection. The mice were euthanized after the sample were obtained. The same experimenter carried out all surgeries.
Click to Show/Hide
|
||||
| Response Description | NRF2 knockdown notably decreased the expression of SLC7A11 and HO-1 and blocked the anti-ferroptosis effects of dimethyl fumarate (DMF). DMF inhibits ferroptosis by activating the NRF2/SLC7A11/HO-1 axis and exerts a protective effect against hepatic ischemia-reperfusion injury. | ||||
| Experiment 7 Reporting the Ferroptosis-centered Disease Response of This Regulator | [133] | ||||
| Responsed Drug | Iridin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
In vivo, the LIRI model was established as described earlier. All mice were anesthetized with pentobarbital administered intraperitoneally (50 mg/kg, Sigma-Aldrich, MO, USA). After endotracheal intubation, the mice were ventilated using a rodent ventilator (MiniVent, Harvard Apparatus, USA), with the title volume set to 7 ml/kg, the respiratory rate set to 120 times/min, and the inspiratory/expiratory ratio set to 1: 2. A noninvasive clamp was used to interrupt the left pulmonary hilum, causing lung ischemia. The clamp was released after 60 minutes of ischemia, and the left lung was reperfused for 120 minutes. Animals were euthanized via cervical dislocation at the end of the experiment. Following that, lung specimens and bronchoalveolar lavage fluid were harvested for analysis. All procedures except lung ischemia were performed on mice in the sham group.
Click to Show/Hide
|
||||
| Response Description | As a result, irisin postconditioning may protect against lung I/R damage by suppressing ferroptosis via the Nrf2/HO-1 signaling axis. | ||||
Acute pancreatitis [ICD-11: DC31]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [134] | ||||
| Responsed Drug | Ginsenoside Rg3 | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AR42J cells | Digestive system neoplasms | Rattus norvegicus | CVCL_0143 | |
| In Vivo Model |
Male C57BL/6 mice (8 weeks old; SPF; weighing 24-26 g, n = 16 in total) were purchased from SPF (Beijing) Biotechnology Co., Ltd. All mice were housed in an environmentally controlled room at a temperature ranging from 20 to 24 on a 12 h light/dark cycle and used in the experiments following an overnight fast with water, availablead libitum. All procedures followed the Principles of Laboratory Animal Care (NIH publication number 85Y23, revised in 1996), and the experimental protocol was approved by the Animal Care Committee, Nanjing Medical University (NMU-2021JK-085).
Click to Show/Hide
|
||||
| Response Description | Taken together, the present study, to the best of our knowledge, is the first to reveal a protective role for Ginsenoside Rg3 in mice with acute pancreatitis by suppressing oxidative stressrelated ferroptosis and the activation of the NRF2/HO1 pathway. | ||||
Ulcerative colitis [ICD-11: DD71]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [135] | ||||
| Responsed Drug | Tert-Butylhydroquinone | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HIEC-6 cells | Normal | Homo sapiens | CVCL_6C21 | |
| In Vivo Model |
Male C57/BL6 mice (eight weeks old, 18-20 g) were purchased from Chengdu Dossy Experimental Animals (China). All the mice were housed in plastic cages with free access to food and water at 25 with a 12 h light/dark cycle. The mice were randomly divided into four groups (n = 6 mice/group): the control group, 5-FU group, 5-FU + TBHQ group, and 5-FU + Fer-1 group. 50 mg/kg body weight 5-FU was intraperitoneally (i.p.) injected into the mice of the 5-FU, 5-FU + TBHQ and 5-FU + Fer-1 group per day for five days to induce intestinal mucositis. Starting on the same day, the mice in the 5-FU + TBHQ and 5-FU + Fer-1 group were treated with TBHQ (10 mg/kg body weight; in DMSO, i.p. injection) or Fer-1 (2.5 mol/kg body weight; in DMSO; i.p. injection) once daily for eight days (days 18). Starting on the same day, the mice of the 5-FU group were treated with equivalent volumes of dimethyl sulfoxide (DMSO) for eight days.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis was shown to be involved in 5-FU-induced intestinal mucositis, and Tertiary butylhydroquinone markedly hampered its activation. Mechanistically, TBHQ activated Nrf2 effectively and selective Nrf2 knockdown significantly reduced the anti-ferroptotic functions of TBHQ in 5-FU-treated HIECs. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [136] | ||||
| Responsed Drug | Astragalus polysaccharide | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | |
| In Vivo Model |
Six-eight-week-old male C57BL/6 mice weighing 19.74 ± 0.77 g were purchased from Shanghai Laboratory. To investigate the therapeutic effect of APS on DSS-induced colitis, mice were randomly divided into the following 5 groups (n = 5 each): control, DSS, DSS + APS (100 mg/kg), DSS + APS (200 mg/kg), and DSS + APS (300 mg/kg). The mice in the DSS + APS (100 mg/kg), DSS + APS (200 mg/kg), and DSS + APS (300 mg/kg) groups were intraperitoneally injected with 100, 200, and 300 mg/kg APS once a day, respectively from day 3 to day 10.
Click to Show/Hide
|
||||
| Response Description | The therapeutic effects of Astragalus polysaccharide on DSS-induced Ulcerative colitis by blocking ferroptosis in IECs. Furthermore, our results revealed that APS-mediated inhibition of ferroptosis was associated with the NRF2/HO-1 pathway. | ||||
Knee osteoarthritis [ICD-11: FA01]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [137] | ||||
| Responsed Drug | Biochanin A | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
Male mice were purchased from Guangzhou University of Chinese Medicine's Experimental Animal Center C57BL/6 mice (7-week-old, 20 g) (Guangzhou, China). After one week of adaptively feeding with chow meals and sterilized water, the animals were separated into five groups of ten mice randomly assigned to the negative control (NC); model, positive control (PC); model group; high dosage of BCA treatment (BCA-H) group; and low dosage of BCA treatment (BCA-L) group. The iron overload mice model was designed based on earlier research. Except for the NC group, mice were administered ID intraperitoneally (500 mg/kg) once a week for eight weeks. In the right knee joints, OA was induced with the initial injection of iron dextran two weeks after the injection by destabilizing the medial meniscus (DMM) using a microscope. After the operation, the positive control group was administered with NAC intragastrically (100 mg/kg) for eight weeks. BCA-H and BCA-L groups were administered 20 mg/kg and 40 mg/kg of BCA separately for eight weeks according to previous studies.
Click to Show/Hide
|
||||
| Response Description | Biochanin A (BCA) could directly reduce intracellular iron concentration by inhibiting TfR1 and promoting FPN but also target the Nrf2/system xc-/GPX4 signaling pathway to scavenge free radicals and prevent lipid peroxidation. The results of this research indicate that BCA regulates iron homeostasis during the progression of osteoarthritis, which can open a new field of treatment for knee osteoarthritis. | ||||
Intervertebral disc degeneration [ICD-11: FA80]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [138] | ||||
| Responsed Drug | Hesperidin | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| NF-kappa B signaling pathway | hsa04064 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hNPCs (Human nucleus pulposus cells) | ||||
| In Vivo Model |
Male C57BL/6 mice (10-12 weeks) were devoted to generate needle puncture-induced intervertebral disc degeneration model. For intervertebral disc degeneration (IVDD) treatment, hesperidin was administratedorally and the dose was calculated in reference to previous studies using themetrological conversion formula between human and mouse. The dosein mice is = 5.5 mg/kg x 70 kg x 0.0026/20g = 9.1 x 5.5 mg/kg = 50.05 mg/kg ~50 mg/kg.
Click to Show/Hide
|
||||
| Response Description | Hesperidin may protect HNP cells from degeneration by suppressing ferroptosis in an oxidative stress-dependent via enhancing the expression of Nrf2 and suppressing the NF-B pathway. The evidence will provide a possible basis for future targeted treatment for intervertebral disc degeneration. | ||||
Osteoporosis [ICD-11: FB83]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [139] | ||||
| Responsed Drug | Melatonin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MC3T3-E1 cells | Normal | Mus musculus | CVCL_0409 | |
| In Vivo Model |
Eight-week-old specific-pathogen-free Sprague Dawley rats weighing 220 ± 20 g were purchased from China Medical University, Department of Experimental Animals. A total of 60 rats were used to determine the targets of bone histomorphometry; 45 rats were used to establish a diabetic model, and remaining 15 rats were divided into a control group. The diabetic rats were divided into three groups (n = 15 each) treated with intraperitoneal injection of high-dose melatonin (50 mg/kg, HMT group), intraperitoneal injection of low-dose melatonin (10 mg/kg, LMT group), and a control T2DM group.
Click to Show/Hide
|
||||
| Response Description | High glucose induces ferroptosis via increased ROS/lipid peroxidation/glutathione depletion in type 2 diabetic osteoporosis. More importantly, melatonin significantly reduced the level of ferroptosis and improved the osteogenic capacity of MC3T3-E1 through activating the Nrf2/HO-1 pathway in vivo and in vitro. | ||||
Male infertility [ICD-11: GB04]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [140] | ||||
| Responsed Drug | Busulfan | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mTTs (Mouse testicular tissues) | ||||
| In Vivo Model |
Eight-week-old healthy ICR male mice, weighted 20-24 g, were provided by Experimental Animal Center of Nantong University (Nantong, China). For the first animal study, eight-week-old ICR male mice were randomly assigned to four groups: control, busulfan, busulfan plus Fer-1 and busulfan plus DFO groups (n = 6 per group). Mice were anesthetized and then given testicular injection of busulfan on both sides at the dose of 4 mg/kg body weight. The solution containing busulfan was directly injected from the scrotum into testicular transverse diameter. Fer-1 and DFO were administered by intraperitoneal injectionat concentrations of 1 mg/kg and 30 mg/kg respectively three times a week after busulfan injection. Four weeks later, the epididymal spermatozoa and testes from all mice were collected for assessment.
Click to Show/Hide
|
||||
| Response Description | Busulfan treatment induced spermatogenic cells ferroptosis by down-regulating nuclear factor-E2-related factor 2 (Nrf2) and glutathione peroxidase 4 (GPX4) expressions, and decreasing iron efflux through reduction of ferroportin 1 (FPN1) expression. Targeting ferroptosis serves as a potential strategy for prevention of busulfan-induced damage and male infertility. | ||||
Aristolochic acid nephropathy [ICD-11: GB55]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [141] | |||
| Responsed Drug | Aristololactam | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 |
| Response Description | Long-term administration of medicine-containing Aristolactam I (ALI) was reported to be related to aristolochic acid nephropathy (AAN), which was attributed to ALI-induced nephrotoxicity. ALI dose-dependently inhibited these protein contents of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), and glutathione peroxidase 4 (GPX4), which could be partly rescued by Tin-protoporphyrin IX (SnPP) and mitoTEMPO co-treatment. | |||
Acute kidney failure [ICD-11: GB60]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [142] | ||||
| Responsed Drug | Dioscin | Preclinical | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Six-week-old male Wistar rats (170-200 g) were obtained from Changsheng Biotechnology Co., Ltd. (Changchun, China), and all of them were fed under SPF-conditions. The rats were acclimatized to natural light/dark cycles at a controlled temperature of 22 + 2 with free access to food and water. The experiment was comprised of four groups: the C group (0.5% carboxymethyl cellulose sodium [CMC-Na], n = 6); the Dio group (dioscin-treated rats, n = 6); the CP group (cisplatin-treated mice, n = 6); and the Dio + CP group (dioscin plus cisplatin-treated rats, n = 6). Rats were gavaged with dioscin (60 mg/kg) for ten days, and cisplatin (10 mg/kg) was intraperitoneally injected once on the seventh day.
Click to Show/Hide
|
||||
| Response Description | Dioscin exerts a reno-protective effect by decreasing renal oxidative injury, apoptosis and ferroptosis through the Nrf2/HO-1 signaling pathway, providing a new insight into acute kidney injury prevention. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [143] | ||||
| Responsed Drug | Pachymic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mKTs (Mouse knee tissues) | ||||
| In Vivo Model |
A total of 30 C57BL/6 male mice (8-10 weeks; 20-25 g body weight) were purchased from Chongqing Medical University (Chongqing, China). The mice were anesthetized with 50-60 mg/kg of pentobarbital sodium (cat. no. P3761; Sigma-Aldrich; Merck KGaA) by intraperitoneal injection; the skin at the surgical area was wiped with 70% alcohol. The incision was positioned at the left and right sides of the spine (0.5 cm), and the incision length was 1-1.5 cm along the back. The kidneys were subsequently pulled out from the incision to expose the renal pedicle. A microaneurysm clip was used to clamp the pedicle to block the blood flow to the kidney and induce renal ischemia. Complete ischemia was indicated by a change in the color of the kidney from red to dark purple within a few seconds. After 40 min of ischemia, the microaneurysm clips were released to allow each kidney to start reperfusion, which was indicated by the change of the kidney color to red.
Click to Show/Hide
|
||||
| Response Description | Pachymic acid has a protective effect on ischemiareperfusion induced acute kidney injury in mice, which may be associated with the inhibition of ferroptosis in the kidneys through direct or indirect activation of NRF2, and upregulation of the expression of the downstream ferroptosis related proteins, GPX4, SLC7A11 and HO1. | ||||
Urinary system disease [ICD-11: GC2Z]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [144] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male SD rats (200 ± 10 g) were obtained from Beijing Vital River Company (Beijing, China). They were kept under 12-h light/dark cycles and allowed free access to food and water. Rats were randomly divided into CON, ADR, ADR + ASIV, and ASIV groups (n = 6). Rats in ADR and ADR + ASIV groups received four equal injections of ADR intraperitoneally (4 mg/kg) in 5 weeks. Rats in ASIV and ADR + ASIV groups intragastrically received ASIV (10 mg/kg, daily) for 5weeks, while rats in CON and ADR groups were administered the same dose of solvent as ADR. Finally, the rats were euthanized, and the bilateral kidneys were excised.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV increased the phosphorylation of Pi3K, Akt, and the expression of Nrf2 and glutathione peroxidase 4 compared to HK-2 cells stimulated by ADR. In conclusion, ferroptosis may involve in Adriamycin (ADR)-induced nephrotoxicity, and ASIV might protect nephrocytes against ADR-induced ferroptosis, perhaps via activations of the Pi3K/Akt and Nrf2 signaling pathways. | ||||
Cognition disorder [ICD-11: MB21]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [145] | ||||
| Responsed Drug | Echinatin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
rPHNs (Rat primary hippocampal neurons) | ||||
| In Vivo Model |
The Sprague-Dawley rats (male, 20-month-old, 550-700 g, n = 6 per group) were obtained from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijng, China). A total of 78 rats were used in animal experiments. Rats were allocated into the following five experimental groups: control, Sev, Sev + Ech (L), Sev + Ech (M), and Sev + Ech (H). Ech (Sigma-Aldrich; purity 98%) was given to rats by intraperitoneal injection as a single dose of 20 (L), 40 (M), or 80 mg/kg (H) at 1 h before Sev exposure. The injection volume of each rat was 5 mL. For control and Sev groups, an equal volume of vehicle was intraperitoneally injected into rats. Then, rats except for the control group were anaesthetised with 2% Sev (Sigma-Aldrich) for 5 h. The histological and biochemical analysis of the hippocampus was done 48 h later after the rats were sacrificed and the brains were removed.
Click to Show/Hide
|
||||
| Response Description | Echinatin (Ech) could mitigate Sev-induced apoptosis, oxidative stress, and ferroptosis in hippocampal neurons and hippocampus of rats by activating Nrf2 signalling. Moreover, Ech improved Sev-induced cognitive deficits in aged rats. These findings suggested that Ech may be developed as a neuroprotective agent to reduce postoperative cognitive dysfunction in the clinic. | ||||
Myocardial injury [ICD-11: NB31]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [146] | ||||
| Responsed Drug | Pyridoxine | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male c57BL/6 mice (8 weeks old) were purchased from Beijing Wei Tong Li Hua Experimental Animal Technology Co. Ltd. (Beijing, China). Mice were divided into control (n = 8), LPS (n = 9), and VitB6+LPS (n = 9) groups. Mice were pretreated with PBS or VitB6 for 6 h and then treated with LPS (4 mg/kg) for 24 h. Cardiac ultrasound was performed before sacrifice. Inhaled isoflurane was given to mice for volatile anesthesia and the chest hair was removed with a depilatory cream. Then, mice were fixed on the warmed imaging platform and wore with the coupling agent. The Vevo2100 imaging system, equipped with a 40-MHz high-frequency transducer (VisualSonics Inc., Toronto, Canada), was applied to perform non-invasive examinations. The M-mode echocardiogram at the parasternal long axis was used to obtain the ejection fraction (EF) of left ventricular and fractional shortening (FS).
Click to Show/Hide
|
||||
| Response Description | Vitamin B6 (VitB6) is a water-soluble vitamin and includes pyridoxine, pyridoxal, pyridoxamine, and their phosphorylated forms. VitB6 regulated the expression of LPS-induced apoptosis-related proteins and iron regulatory proteins. It mediated the expression of Nrf2, transcription factor NF-E2-related factor 2, which promoted the expression of antioxidant enzymes and restrained LPS-induced ferroptosis and apoptosis. Overall, VitB6 can be used on novel therapies to relieve LPS-induced myocardial injury. | ||||
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [147] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hT2AECs (Type II alveolar epithelial cells) | ||||
| In Vivo Model |
The animals were randomly assigned to six groups (7 mice in each) as follows: (I) Normal saline (NS) group, (II) Ast-IV 100 mg/kg (Ast) group, (III) PM2.5 group, (IV) Ast-IV 50 mg/kg + PM2.5 (Ast-L) group, (V) Ast 100 mg/kg + PM2.5 (Ast-H) group, and (VI) Ast-IV 100 mg/kg + erastin 20 mg/kg + PM2.5 (Era) group. Based on our previous results, this study adopted anintraperitoneal injection(i.p.) of Ast-IV (dissolved in normal saline containing 0.1% DMSO for preventive treatment. After all the mice were adaptively fed for 5 days, in the NS and PM2.5 groups, mice received the normal saline containing 0.1% DMSO viai.p.once a day for the next three consecutive days. Similar to the NS group, in the Ast, Ast-H, and Era groups, mice received Ast-IV (100 mg/kg) viai.p. Ast-L group received Ast-IV (50 mg/kg) viai.p. To evaluate the effect of Ast-IV on ferroptosis in PM2.5-induced lung injury, we used the ferroptosis agonist erastin to activate ferroptosisin vivo. In the Era group, mice received erastin (20 mg/kg, 10% DMSO + 40% PEG300 + 5%Tween80 + 45% normal saline) 30 min before each preventive treatment of Ast-IV.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (Ast-IV) reduced the lung wet-dry ratio and the levels of interleukin 6 (IL-6), tumor necrosis factor- (TNF-) and interleukin 1 (IL-1) in serum. Ast-IV could also improve the oxidative stress level in BALF, restore the GSH level in the lung tissue, and reduce the iron content in the lung tissue. Western blot outcomes revealed that Ast-IV regulated the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to protect PM2.5-mediated lung injury. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 6 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [148] | ||||
| Responsed Drug | Astaxanthin | Investigative | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Mice were randomly divided into four groups as follows (n = 5): (1) control, (2) APAP (MCE, Monmouth Junction, NJ, USA), (3) olive oil + APAP (oil + APAP), and (4) ASX (Energy Chemical, Shanghai, China) dissolved in olive oil + APAP (ASX + APAP). Astaxanthin was dissolved in olive oil to obtain a mixture of 20 mg/mL. Mice in groups 3 and 4 were given a dose of olive oil and a mixture of 5 mL/kgBW by gavage every day for 2 weeks. On day 15, mice in groups 2, 3, and 4 were given a peritoneal injection of 500 mg/kg APAP to induce liver injury. The mice were fasted for 12 h before the administration of APAP. Ten hours after APAP administration, blood and liver tissue were collected for further examination and analyses. Blood was centrifuged to obtain supernatants,which were stored at -80. Liver tissues were immediately removed from each animal, and homogenates were processed with formaldehyde and glutaraldehyde for protein and histological analysis.
Click to Show/Hide
|
||||
| Response Description | Astaxanthin reduced inflammation through the NF-B pathway, inhibited oxidative stress and ferroptosis, and increased autophagy through the Nrf2/HO-1 pathway, ameliorating acetaminophen-induced liver injury in vivo and in vitro. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [149] | ||||
| Responsed Drug | Bicyclol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
The mice were treated with intraperitoneal administration (i.p.) of oil (control group) or a mixture of CCl4 (50%) and oil (50%) at a dosage of 2 ml/kg body weight. In the bicyclol-treated group, mice accepted administration of 200 mg/kg (using 0.5% carboxymethyl cellulose as solvent) by gavage three times a day 1 h before CCl4 exposure, while other groups accepted vehicles of the equal volume. Fer-1 was prepared in DMSO (5 mg/kg), andi.p. injected into mice once 1 h before CCl4 exposure. The dosage of bicyclol was consistent with our previous work. The mice were then sacrificed to collect liver and serum samples after 24 or 48 h.
Click to Show/Hide
|
||||
| Response Description | Bicyclol exerted its hepatoprotection by preventing the aforesaid ferroptotic process. Furthermore, bicyclol alleviated erastin-induced cellular inviability, destruction, and lipid peroxidation in vitro. Knockdown of GPX4 diminished these protective activities against perturbations associated with ferroptosis in L-O2 hepatocytes. Additionally, Nrf2 silencing drastically reduced GPX4 levels, and further impeded the medicinal effects of bicyclol. In summary, positively regulating Nrf2-GPX4 axis by bicyclol can prevent ferroptosis in CCl4-induced acute liver injury in mice. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [150] | ||||
| Responsed Drug | Epigallocatechin Gallate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [151] | ||||
| Responsed Drug | Kaempferol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male BALB/c mice (8-week-old, 20-22 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). The experimental animals were fed adaptively for one week in the Experimental Animal Center of Guangdong Pharmaceutical University (Guangzhou, China). Feeding conditions were set at 26 , humidity 65% and a lightdark cycle for 12 hours. All animal experiments were performed following the Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Research Ethical Committee of Guangdong Pharmaceutical University (gdpulacspf2020007).
Click to Show/Hide
|
||||
| Response Description | Kaempferol (KA) activated the Nrf2 pathway and upregulated Gpx4 in mouse livers and L02 cells to inhibit ferroptosis induced by APAP. Finally, molecular docking indicated the potential interaction of KA with Keap1. Taken together, KA ameliorated oxidative stress and ferroptosis-mediated acetaminophen-induced liver injury by activating Nrf2 signaling. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Responsed Drug | Ulinastatin | Phase 3 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Ulinastatin plays a role in mitigation of APAP-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
| Experiment 6 Reporting the Ferroptosis-centered Disease Response of This Regulator | [152] | ||||
| Responsed Drug | Abietic acid | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
APAP-induced liver injury model was induced byintraperitoneal injection 300 mg/kg APAP. The mice of APAP + abietic acid (10, 20, 40 mg/kg) were given abietic acid by intraperitoneal injection 1 h before APAP treatment. The doses of abietic acid used in this study were based on previous studies. Twelve hours later, the mice were sacrificed after anesthesia with 1%pentobarbital (50 mg/kg) injected intraperitoneally and the samples were collected.
Click to Show/Hide
|
||||
| Response Description | APAP could increase malondialdehyde (MDA) and Fe2+ levels, and decrease ATP and glutathione (GSH) levels, as well as glutathione peroxidase 4 (GPX4) and xCT expression. However, these changes induced by APAP were prevented by abietic acid, indicating abietic acid could inhibit APAP-induced ferroptosis. Furthermore, abietic acid inhibited APAP-induced liver injury, NF-B activation and increased the expression of Nrf2 and HO-1. | ||||
Muscle injury [ICD-11: ND36]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [153] | |||
| Responsed Drug | Atorvastatin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hCMs (Human cardiomyocytes) | |||
| C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| HUVECs (Human umbilical vein endothelial cells) | ||||
| Response Description | Atorvastatin suppressed the Nrf2, which would, in turn, inhibit the expression of System xc-(SLC7A11)and GPX4 (especially the mitochondrial GPX4), leading to a severe damage to the antioxidant system of ferroptosis.The datas point toward ferroptosis as an essential molecular mechanism leading to statin-induced muscle damage. | |||
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [154] | |||
| Responsed Drug | Carnosic acid | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Ferroptosis is involved in the pathogenesis of spinal cord injury (SCI). Carnosic acid (CA) is a natural phenolic diterpene, which possesses diversiform activities. CA can inhibit ferroptosis in PC12 cells induced by erastin via activating Nrf2 pathway, indicating that CA could lead to neuroprotective effect by restraining the occurrence of ferroptosis. | |||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [158] | |||
| Responsed Drug | Berberine | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Berberine can inhibit erastin-induced ferroptosis in HT22 cells possibly by activating the Nrf2-HO-1/ GPX4 pathway. | |||
TGF-beta receptor type-1 (TGFBR1)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [155] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Necroptosis | hsa04217 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell necrosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 |
| Response Description | The activin receptor-like kinase (ALK) 4/5 (ACVR1B/ TGFBR1), also known as activin-transforming growth factor (TGF) receptor, is involved in stress-induced renal injury. Pharmacological inhibition of ALK4/5 signaling attenuated erastin-induced ferroptosis by hyperactivating Nrf2 signaling in HK-2 cells. | |||
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP2)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [156] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | ENPP2 overexpression causes upregulation of GPX4 in H9c2 cells. In erastin-induced ferroptosis of H9c2 cells, both NRF2 and ACSL4 are increased, whereas ENPP2 overexpression reduces their expression in erastin-treated H9c2 cells. | |||
E3 ubiquitin-protein ligase SIAH2 (SIAH2)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [157] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| Phoenix-Eco cells | Normal | Homo sapiens | CVCL_H717 | |
| i-MCF (Mouse primary cardiac fibroblasts) | ||||
| Response Description | NRF2 is a known SIAH2 target and master regulator of HO-1 expression. The increased vulnerability of SIAH2 knock-out cells to ferroptosis is probably due to several factors, including the increased expression of pro-ferroptotic HO-1 and the decreased expression of GPX4, a key factor for this iron-catalysed necrotic pathway. | |||
Activin receptor type-1B (ACVR1B)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [155] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Necroptosis | hsa04217 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell necrosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 |
| Response Description | The activin receptor-like kinase (ALK) 4/5 ( ACVR1B/TGFBR1), also known as activin-transforming growth factor (TGF) receptor, is involved in stress-induced renal injury. Pharmacological inhibition of ALK4/5 signaling attenuated erastin-induced ferroptosis by hyperactivating Nrf2 signaling in HK-2 cells. | |||
Vascular endothelial growth factor receptor 2 (KDR)
Apatinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female BALB/c nude mice (age, 4 weeks old) were purchased from Changzhou Cavens Experimental Animal Co., Ltd. (Changzhou, China).The gliomas from the nude mice were fixed in 10% paraformaldehyde at 4 for 12 h and then dehydrated in different concentrations of ethanol. The tumor tissues were permeabilized using xylene and embedded in paraffin. They were then sliced (0.5 um), rehydrated, and stained with HE at 4 for 10 min and sealed. For IHC assessment of Ki-67 in gliomas, the DAKO Envision system (Dako; Agilent Technologies, Inc.) was used.
Click to Show/Hide
|
||||
| Response Description | Apatinib could restrain proliferation of glioma cells through induction of ferroptosis via inhibiting the activation of VEGFR2/Nrf2/Keap1 pathway. Overexpression of Nrf2 could counteract the induction of ferroptosis by apatinib. | ||||
Ubiquitin carboxyl-terminal hydrolase 11 (USP11)
RSL3
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| H2122 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1531 | ||
| In Vivo Model |
After two weeks in house, the mice were subcutaneously injected with A549 cells (100 uL containing 5 x 106 cells/injection) and monitored for tumor cell xenografts to reach approximately 100 mm3. The mice were then divided into two groups (n = 5), the RSL3 treatment (100 mg/kg; dissolved in 5% dimethyl sulfoxide/corn oil; administrated intratumorally twice a day for one week) and control (5% dimethyl sulfoxide/corn oil only) groups.
Click to Show/Hide
|
||||
| Response Description | RSL3 was able to directly bind to USP11, a recently identified de-ubiquitinase of NRF2, and inactivate USP11 protein to induce NRF2 protein ubiquitination and degradation in KLK lung adenocarcinoma cells. | ||||
Tyrosine-protein kinase BTK (BTK)
Ibrutinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| LS513 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1386 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| SW1116 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0544 | ||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| HT-29 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0320 | ||
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| In Vivo Model |
Sixty mice were randomly divided into six groups, (1) the CRC model group (model), (2) mice with RSL3 treatment, (3) mice with Erastin treatment, (4) mice with Ibrutinib treatment, (5) mice with RSL3 and Ibrutinib treatment, and (6) Erastin and Ibrutinib group. Murine subcutaneous tumor model and xenograft tumor mouse model were established and please refer to supplemental method for details. For CRC model group, the mice were treated with PBS for two weeks. For RSL3 group, the mice were intraperitoneal injected with RSL3 (5 mg/kg daily) for two weeks. For Erastin group, the mice were intraperitoneal injected with Erastin (30 mg/kg, twice every other day) for two weeks. For Ibrutinib treatment group, mice were administered in drinking water at a concentration of 0.16 mg/ml for two weeks. Mice were also treated in combination with RSL and Ibrutinib or Erastin and Ibrutinib.
Click to Show/Hide
|
||||
| Response Description | Ibrutinib inhibited BTK, which prevented Nrf2 translocating to cell nucleus and the activation of the Nrf2 dependent antioxidant genes during oxidative stress conditions and eventually enhanced the sensitivity of Colorectal cancer (CRC) cells to ferroptosis. | ||||
Signal transducer and activator of transcription 3 (STAT3)
Peoniflorin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| In Vivo Model |
U251 cells (6 x 106) were inoculated into the flanks of 4-to 5-week-old athymic nude mice (Shanghai Laboratory Animal Company, Shanghai, China) subcutaneously to generate a subcutaneous xenograft tumor model. After 2 weeks, the tumor model was successfully constructed, the mice were treated single and combined with 100 mg/kg RSL3 (2 times/week) and 1.0 g/kg/days PF. Tumor volumes were measured every 4 days to draw the growth curve. Mice were sacrificed 4 weeks after cell injection. Tumor xenografts were collected, photographed, and weighed and the tumor apoptosis was analyzed by Tunel staining.
Click to Show/Hide
|
||||
| Response Description | Paeoniflorin (PF) can function as an antitumor agent for glioma treatment by targeting NEDD4L-dependent STAT3 ubiquitination as well as by regulating the Nrf2/GPX4 signaling axis, which might trigger ferroptosis. | ||||
Sestrin-2 (SESN2)
Empagliflozin
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Liver fibrosis [ICD-11: DB93] | ||||
| Pathway Response | Autophagy | hsa04140 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
After a one-week acclimatization period, rats were randomly divided into four experimental groups of six rats each. Group I (the control group) received saline intraperitoneally in the same manner as BLM injections, as well as 1% carboxymethyl cellulose (CMC) orally in the same manner as EMPA. Group II (the BLM-treated group) received BLM (15 mg/kg) intraperitoneally three times per week for four successive weeks in order to induce pulmonary fibrosis. Group III (the EMPA-treated group) received EMPA dissolved in 1% CMC orally via oral gavage at a dose of 10 mg/kg/day throughout the experimental period. Group IV (the combined EMPA and BLM-treated group) received EMPA (10 mg/kg) orally via oral gavage seven days before BLM administration and continued for four weeks after BLM injection.
Click to Show/Hide
|
||||
| Response Description | Empagliflozin has a promising protective effect against BLM-induced liver fibrosis in rats by enhancing autophagy and mitigating ferroptosis, inflammation, and ER stress via modulating the Sesn2/AMPK/Nrf2/HO-1 signaling pathway. | ||||
Serine/threonine-protein kinase TBK1 (TBK1)
Tiliroside
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
All animal studies were approved by the Committee on Ethics of Animal Experiments of Binzhou Medical University (approval no: BZMU-IACUC-2021-331, date: 09/10/2021). To generate the ectopic HCC mouse models, HepG2-luciferase cells (HepG2 cells transfected with luciferase gene) were suspended in serum-free media and matrigel (BD Biosciences) at a ratio of 1:1 v/v. A total of 2.5 x 106 HepG2-luciferase cells/100 ul were injected into the left axilla of mice. After reaching a tumor size of 100-150 mm3, all mice were randomly divided into four groups: control (vehicle, intraperitoneal [i.p.]), tiliroside (20 mg/kg,i.p.), sorafenib (30 mg/kg,i.p.), or combination treatment (tiliroside and sorafenib,i.p.). All treatments were administered every 3 d, and the length and width of tumor were measured every 4 d. The formula tumor volume = (length x width2)/2 was used to calculate the tumor volume. Body weight was recorded every 7 d, and the morphology of the tumor was photographed using animal in vivo imaging technology (IVIS Spectrum; PerkinElmer) before the day of sacrifice. The mice were sacrificed 40 d after administration, and the tumors were dissected and weighed. The major organs and xenograft tumors were fixed with 4% paraformaldehyde.
Click to Show/Hide
|
||||
| Response Description | Tiliroside directly binds to TBK1 and inhibits its activity, which inhibits the phosphorylation of Ser349 on p62. Consequently, this decreases the affinity of p62 for Keap1, promotes ubiquitination and degradation of Nrf2 and ferroptosis, and eventually increases the sensitivity of hepatocellular carcinoma cells to sorafenib. | ||||
Sequestosome-1 (SQSTM1)
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hepa 1-6 cells | Hepatocellular carcinoma | Mus musculus | CVCL_0327 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 1 x 106 Hepa16 cells in control shRNA or NRF2 knockdown cells in 200 ul phosphate buffered saline were injected subcutaneously to the right of the dorsal midline in C57BL/6 mice. Once the tumors reached 80-100 mm3 at day seven, mice were randomly allocated into groups and treated with erastin (30 mg/kg intraperitoneal injection [i.p.], twice every other day) and sorafenib (10 mg/kg i.p., once every other day) for two weeks.
Click to Show/Hide
|
||||
| Response Description | Upon exposure to ferroptosis-inducing compounds (e.g., erastin, sorafenib, and buthionine sulfoximine), p62 (SQSTM1) expression prevented NRF2 degradation and enhanced subsequent NRF2 nuclear accumulation through inactivation of Kelch-like ECH-associated protein 1. The status of NRF2 is a key factor that determines the therapeutic response to ferroptosis-targeted therapies in hepatocellular carcinoma cells. | ||||
Astragaloside IV
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Rats were randomly divided into the Sham, middle cerebral artery occlusion-reperfusion (MCAO/R), and MCAO/R + AST IV (28 mg/kg) groups. The MCAO/R + AST IV group was intragastrically injected with 10 mL/kg AST IV at 50, 26, and 2 h before modelling (Xiao et al., 2021). The Sham and MCAO/R groups received equal amounts of normal saline. As described previously, the modified Longa method (Longa et al., 1989) was used to establish the MCAO/R model. After anaesthesia with 2%sodium pentobarbital, the left common carotid artery(CCA), the external carotid artery(ECA), and the internal carotid artery(ICA) were isolated. The distal end of the ECA was ligated, a small incision was made at the stump of the ECA, and a suture (Batch number: 2636A2, Beijing Seinong Technology Co., Ltd., Beijing, China; head-end diameter: 0.36 ± 0.02 mm) was inserted into the ICA from the ECA through the bifurcation of the CCA. To achieve cerebral ischaemia, the head-end was used to block blood flow in the middle cerebral artery until the intracranial segment of the ICA was inserted. The suture was removed after 2 h, and follow-up experiments were performed 24 h after reperfusion. In the Sham group, the CCA, ECA, and ICA were exposed and separated, but no sutures were inserted. Penicillin powder was used to fight infection after operation.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AST IV) increased the P62 (SQSTM1) and Nrf2 levels and decreased the Keap1 levels. P62 silencing reduced the effects of AST IV on the P62/Keap1/Nrf2 pathway and ferroptosis. Our findings suggest that AST IV mitigates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via activation of the P62/Keap1/Nrf2 pathway. | ||||
Guizhi Fuling Capsule
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Endometrial hyperplasia [ICD-11: GA16] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | mUTs (Mouse uterine tissues) | ||||
| In Vivo Model |
Female C57BL/6 mice (8-week-old) were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). Fifteen mice were randomly divided into three groups: Olive oil group, Estradiol group and Estradiol + IKE group. The Estradiol group was subcutaneously injected estradiol (50 ug/kg/day), Estradiol + IKE group was subcutaneously injected estradiol and intraperitoneally injected IKE (50 mg/kg) for 21 days, while the Olive oil group received the same volume of olive oil. In the experiment of exploring the improvement of GFC to EH, twenty mice were randomly divided into four groups: Olive oil group, Estradiol group, 75 mg/kg GFC group and 150 mg/kg GFC group. Except for Olive oil group, mice were subcutaneously daily injected with estradiol (50 ug/kg/day) for 21 days, while the Olive oil group received the same volume of olive oil. 75 mg/kg GFC group and 150 mg/kg GFC group were treated with GFC intragastrical administration.
Click to Show/Hide
|
||||
| Response Description | Guizhi Fuling Capsule (GFC) may attenuate estrogen-induced endometrial hyperplasia in mice through triggering ferroptosis via inhibiting p62 (SQSTM1)-Keap1-NRF2 pathway. GFC might act as a promising traditional Chinese medicine to treat endometrial hyperplasia. | ||||
Entacapone
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks; 20-25 g) were purchased from LINGCHANG BIOTECH (China). Mice were divided into four groups: (i) sham, (ii) I/R, (iii) I/R+entacapone, and (iv) I/R + Fer-1. Entacapone (15 mg/kg bodyweight) was dissolved in sodium carboxymethyl cellulose (0.5%) and administered (i.g.) to mice. Mice in the sham group were administered (i.g.) an equal volume of solvent. Fer-1 was dissolved in 5% dimethyl sulfoxide + 30% polyethylene glycol-400 + 60% saline and injected (i.p.). Mice were treated three times per day for 3 days in advance. Before I/R, mice were fasted for 12 h and anesthetized (1% pentobarbital sodium, i.p.). The abdomen was exposed and bilateral renal pedicles were clamped to induce renal I/R. After 25 min, the arterial clamps were removed. A body temperature of 37 was maintained throughout the procedure. The sham group underwent the same procedure except for clamping of the renal pedicle. Mice were killed 24 h after reperfusion, and kidney and blood samples were collected for experimentation.
Click to Show/Hide
|
||||
| Response Description | Entacapone upregulates p62 (SQSTM1) expression and affects the p62-KEAP1-NRF2 pathway, thereby upregulating nuclear translocation of NRF2. This action results in increased expression of the downstream SLC7A11, and significant suppression of oxidative stress and ferroptosis. Entacapone may serve as a novel strategy to improve treatment of, and recovery from, ischemia/reperfusion-induced acute kidney injury (I/R-AKI). | ||||
Baicalin
[Terminated]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
C57BL/6 mice at 6-8 weeks were intraperitoneally injected with D-GalN/LPS (1772-03-8/L2880, Sigma-Aldrich, USA) at a dose of 700 mg/kg and 10 ug/kg, respectively. The constructed D-GaIN/LPS-induced ALI model mice were named the model group, and the normal mice injected with phosphate-buffered saline (PBS) were named the blank group. After 1 h of LPS/D-GalN treatment, Exo and Ba-Exo (150 ug/mice) were injected into the tail vein of the mice in the Exo and Ba-Exo groups, respectively. Mice were sacrificed via anesthesia overdose 12 h after the intervention. Half of the liver tissue was fixed in paraformaldehyde, while the other half was frozen at 80 . Peripheral blood serum was stored at -80 .
Click to Show/Hide
|
||||
| Response Description | Baicalin-pretreated MSCs (Ba-Exo) exerts a protective effect on liver function and activates the Keap1-NRF2 pathway via P62 (SQSTM1), thereby inhibiting ROS production and lipid peroxide-induced ferroptosis. Therefore, baicalin pretreatment is an effective and promising approach in optimizing the therapeutic efficacy of Exo in acute liver injury (ALI). | ||||
RAC-alpha serine/threonine-protein kinase (AKT1)
Fraxetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Acute myocardial infarction [ICD-11: BA41] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Wistar rats (200-250 g) were obtained from Slac Laboratory Animal Center (Shanghai, China) and kept in cages. The rats were anesthetized with 1% pentobarbital and then lied on its back. Thereafter, the left precordial area of the rats were shaved and disinfected, followed by trachea intubation for artificial ventilation. After the left thoracotomy, the heart was fully exposed and the left coronary artery (LAD) was ligated with a 6-0 prolene suture at 2-3 mm from its origin between the pulmonary artery conus and the left atrial appendage. After 30 min, the suture was gently removed to allow reperfusion for 2 h.
Click to Show/Hide
|
||||
| Response Description | Fraxetin activated phosphorylation of AKT and Nrf2 nuclear accumulation in Myocardial infarction in vivoandin vitromodels. Moreover, Fra reduced the activity of serum LDH, the accumulation of iron and the MDA level, and increased GSH and glutathione peroxidase 4 (GPX4) in rats with MI. | ||||
Roxadustat
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Kidney injury [ICD-11: NB92] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mRTs (Mouse renal tissues) | ||||
| In Vivo Model |
C57BL/6 male mice, 6 to 8 weeks old, were purchased from Liaoning Changsheng Biotechnology Co. (Liaoning, China). The animal experiment was conducted in three parts. In the first part, mice were randomly divided into 4 groups (n = 12/group): (1) control group that received an intraperitoneal injection of saline, (2) FG-4592 group that received intraperitoneal injection of FG-4592 once (10 mg/kg, dissolved in DMSO at 50 mg/ml and then further diluted in sterile phosphate-buffered saline to 1 mg/ml), (3) FA group that received intraperitoneal injection of a single dose of FA (250 mg/kg, dissolved in 0.3 M sodium bicarbonate), and (4) FA + FG-4592 group that received FG-4592 two days prior to FA single-dose injection. Kidney specimens and blood samples were collected on the second day (n = 6/group) and the fourteenth day (n = 6/group) after FA injection for further examination. In the second part, mice were treated with a ferroptosis inhibitor (Fer-1). In the third part, mice were treated with a PI3K inhibitor (wortmannin).
Click to Show/Hide
|
||||
| Response Description | Roxadustat (FG-4592) pretreatment is achieved mainly by decreasing ferroptosis at the early stage of FA-induced kidney injury via Akt/GSK-3-mediated Nrf2 activation, which retards the fibrosis progression. | ||||
lipoxin A4
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [14] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Spinal cord injury [ICD-11: ND51] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPSCNs (Mouse primary spinal cord neurons) | ||||
| In Vivo Model |
Pregnant C57BL/6 mouse was purchased from Laboratory Animal Center of Xinxiang Medical University. pregnant mouse was anesthetized with CO2 and sacrificed by cervical dislocation at embryonic day 15. All embryos were separated from pregnant mouse under aseptic conditions. Under dissection microscope, each embryo was quickly killed by cervical dislocation, and the spinal cord was isolated. The membrane of the spinal cord and dorsal root ganglion was removed from the spinal cord applying microforceps. Subsequently, the spinal cord was quickly cut into small pieces (1 mm3) using ultrafine microscissors.
Click to Show/Hide
|
||||
| Response Description | Lipoxin A4 (LXA4) enhanced the protein expression of p-AKT, nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and haem-oxygenase-1 (HO-1) in primary spinal cord neurons. LXA4 exerted a neuroprotective effect in Erastin-induced ferroptosis of primary spinal cord neurons by activating the Akt/Nrf2/HO-1 signaling pathway. Thus, LXA4 may be a potential therapeutic agent for spinal cord injury (SCI). | ||||
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA)
Thioctic acid
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [15] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | a-Lipoic acid (a-LA) suppressed cell viability decline and mitigated ferroptosis in an MPP-induced PC12 cell model of parkinson's disease (PD) via activating the PI3K/Akt/Nrf2 pathway. These results discovered a novel a-LA-based therapy for PD patients, and activating the PI3K/Akt/Nrf2 pathway might be developed as a promising therapeutic approach for PD. | |||
Peroxisome proliferator-activated receptor gamma (PPARG)
Pioglitazone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rPNCs (Rat primary nerve cells) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
The rats underwent surgery using an ultraclean table and were fixed in a stereotaxic frame. The scalp was opened to expose the anterior brain region. A dental drill was used to drill a 1-mm-diameter hole in the skull surface. Blood (100 ul) was collected from the rat tail vein and injected into the rat striatum with a microsyringe (stereotaxic coordinates; 2 mm lateral to the midline, 0.2 mm posterior to bregma, and 5.5 mm deep below the skull). First, 60 ul of autogenous blood were injected at a rate of 2 ul/min, and the next 40 ul of blood were injected at 5 ul/min. Finally, the needle was left for 10 min before being removed.
Click to Show/Hide
|
||||
| Response Description | Pioglitazone (PDZ), a PPAR agonist, promotes Gpx4 expression through the interaction between PPAR and the Nrf2 pathway, inhibits ferroptosis of neurons after intracerebral hemorrhage (ICH), and promotes the recovery of neural function. | ||||
NAD-dependent protein deacylase sirtuin-6 (SIRT6)
Isoorientin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [17] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
The A549/DDP tumor cells were subcutaneously injected (2 x 106 cells/mL) into BALB/c-nu mice under aseptic conditions. 6 days later, the average diameter of the tumor reaches 0.5 cm, and the mice were randomly divided into the 1 mg/kg DDP group and the 1 mg/kg DDP + 25mg/kg IO group. Six mice from each group were intraperitoneally administered medications every 2 days for a total of 10 doses each.
Click to Show/Hide
|
||||
| Response Description | Isoorientin (IO) can promote ferroptosis and reverse drug resistance in lung cancer through the SIRT6/Nrf2/GPX4 signaling pathway, thus offering a theoretical basis for its potential clinical application. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Iridin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [18] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Sepsis [ICD-11: 1G40] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
All animals were purchased from the Animal Experimental Center of Wuhan University (ABLS-III Laboratory). C57BL/6 male mice weighing 20-25 g were used for this study. HK-2 cells were seeded into 96-well plates (5 x 105 cells/well) and cultured for 24 h until 80% confluence. Subsequently, we have added LPS (10 ug/ml) into the cultured cells for 22 h to establish the cell model of LPS-induced AKI.
Click to Show/Hide
|
||||
| Response Description | Sepsis-associated acute kidney injury induced ferroptosis by increasing iron and lipid peroxidation. Irisin effectively suppressed ferroptosis and alleviated SA-AKI and improved the mitochondria functionviainduction of the SIRT1/Nrf2 signal axis. | ||||
Edaravone
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Depressive disorder [ICD-11: 6A70] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male C57BL/6J mice (aged 7-8 weeks) and retired male CD-1 mice (aged 16-20 weeks) were obtained from the Experimental Animal Centre of Chongqing Medical University (Chongqing, China). The experimental animals were housed in cages under a 12 h light/12 h dark cycle (lights on at 8:00 a.m.), 60 ± 5% humidity, and a temperature of 23 ± 1 with access to water and food freely. All experimental procedures were conducted in accordance with the Ethics Committee of Chongqing Medical University. EDA was purchased from Sigma-Aldrich (St. Louis, USA) and was dissolved in Vehicle (NaCl, 0.9%) at a dosage of 10 mg/kg. EX527 (a Sirt1 inhibitor) and ML385 (a Nrf2 inhibitor) were obtained from MedChemExpress (New Jersey, USA).
Click to Show/Hide
|
||||
| Response Description | The inflammation and oxidative stress (OS) have been considered crucial components of the pathogenesis of depression. Edaravone possesses potent antidepressant and anxiolytic properties through Sirt1/Nrf2/HO-1/Gpx4 axis and Gpx4-mediated ferroptosis may play a key role in this effect. | ||||
Quercetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from Gempharmatech Co., Ltd (Changzhou, China). All mice were housed in cages with standard laboratory conditions: a consistent temperature of 24 , a 12 h light/dark cycle, and free access to water and food. The mice were randomized into four groups: 1) the KA group (n = 6), injected intraperitoneally with 20 mg/kg KA, as described in a previous study; while 2) the control group (n = 6), injected intraperitoneally with an equal volume of PBS; 3) the KA + QCT group (n = 6): this group was givenintragastric administrationof 50 mg/kg of QCT once daily for 21 days before KA injection based on the literature; and 4) the KA+ferrostatin1 (Fer-1) group (n = 6), injected intraperitoneally with a well-known ferroptosis inhibitor (3 mg/kg Fer-1) for 21 days before KA administration, as described in a previous study.
Click to Show/Hide
|
||||
| Response Description | The association between the Nrf2-mediated ferroptosis pathway and seizures in a clinical setting. Quercetin effectively protects against seizure-induced neuron death in vivo and in vitro and alleviates cognitive function impairment via the SIRT1/Nrf2/SLC7A11/GPX4 pathway. | ||||
Astragaloside IV
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [21] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Retinopathy [ICD-11: 9B71] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Astragaloside IV (AS-IV) inhibited miR-138-5p expression, subsequently increasing Sirt1/Nrf2 activity and cellular antioxidant capacity to alleviate ferroptosis, resulting decreased cell death, which potentially inhibits the diabetic retinopathy pathological process. | |||
Icariin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [22] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Supraventricular tachycardia [ICD-11: BC81] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response Description | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Ulinastatin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Ulinastatin plays a role in mitigation of Acetaminophen (APAP)-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1)
Micafungin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [24] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
The surgical procedure for establishing the myocardial I/R injury rat model was carried out as we did before. Briefly, a left thoracotomy was performed in the fourth intercostal space and the heart was exposed via opening thepericardium. The left coronary artery was surrounded with a 4-0 silk suture and a snare was formed by passing both ends of the suture via a short polyethylene tubing. Blockage of the coronary artery was conducted via clamping the snare against the heart surface. Reperfusion was performed by release of the snare. The sham group conducted the same procedure but without ischemia (the snare was not tightened). To establish the I/R injury model, the rat hearts were subjected to 1 h-ischemia plus 3 h-reperfusion. At the end, the blood and hearts were collected for assay of the creatine kinase(CK) activity and infarct size to determine the success of I/R injury model. To explore the role of MALT1 in myocardial I/R injury the underlying mechanisms, three sets of experiment were performed.
Click to Show/Hide
|
||||
| Response Description | The inhibition of MALT1 can reduce ischemia/reperfusion-induced myocardial ferroptosis through enhancing the Nrf2/SLC7A11 pathway; and MALT1 may be used as a potential target to seek novel or existing drugs (such as micafungin) for treating myocardial infarction. | ||||
Mitogen-activated protein kinase 14 (MAPK14)
Andrographis
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [25] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Multiple myeloma [ICD-11: 2A83] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 |
| U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 | |
| AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| Response Description | Andrographolide (Andro) may block the Nrf2/HO-1 signaling pathway by activating P38 ( MAPK14), thereby inducing ferroptosis. Moreover, inhibition of P38 expression rescued Andro-induced cell death, changes in the level of Nrf2 and HO-1 expression, Fe2+ and lipid peroxidation. Taken together, our findings suggest that Andro induces ferroptosis in Multiple myeloma (MM) cells via the P38/Nrf2/HO-1 pathway, providing a potential preventative and therapeutic approach for MM. | |||
Cetuximab
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [26] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
The DLD-1 cell suspension (4 x 106 cells/200 ul) was injected subcutaneously into the right dorsal flank of 5-week-old male BALB/c nude mice (Charles River, China). The mice were randomly divided into four groups (5 mice/group): 1) the control group, 2) the RSL3 group, 3) the cetuximab group, and 4) the RSL3 + cetuximab group. Both RSL3 (5 mg/kg) and cetuximab (13 mg/kg) were administered by intraperitoneal injection in a volume of 100 ul once per day. The tumour volume was calculated as 0.5 x length x width2. After 17 days of treatment, the mice were sacrificed, and the tumours were removed. Then, tumour tissue obtained from the different treated groups was subjected to western blotting and immunohistochemical experiments.
Click to Show/Hide
|
||||
| Response Description | Our work reveals that cetuximab enhances the cytotoxic effect of RSL3 on KRAS mutant Colorectal cancer (CRC) cells and that cetuximab enhances RSL3-induced ferroptosis by inhibiting the Nrf2/HO-1 axis through the activation of p38 MAPK. | ||||
LINC01134 (IncRNA)
Oxaliplatin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [27] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response Description | LINC01134 was positively correlated with GPX4 or Nrf2, demonstrating the clinical significance of LINC01134, Nrf2 and GPX4 in OXA resistance of hepatocellular carcinoma. Silenced LINC01134 enhances Oxaliplatin sensitivity by facilitating ferroptosis through GPX4 in hepatocarcinoma. | |||
Krueppel-like factor 15 (KLF15)
Elabela
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [28] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | rAFs (Rat adventitial fibroblasts) | |||
| Response Description | KLF15 siRNA impeded the beneficial roles of elabela (ELA) in DOX-pretreated rat aortic AFs by suppressing the Nrf2/SLC7A11/GPX4 signaling. In conclusion, ELA prevents DOX-triggered promotion of cytotoxicity, and exerts anti-oxidative and anti-ferroptotic effects in rat aortic AFs via activation of the KLF15/GPX4 signaling, indicating a promising therapeutic value of ELA in antagonizing DOX-mediated cardiovascular abnormality and disorders. | |||
Kelch-like ECH-associated protein 1 (KEAP1)
Apatinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female BALB/c nude mice (age, 4 weeks old) were purchased from Changzhou Cavens Experimental Animal Co., Ltd. (Changzhou, China).The gliomas from the nude mice were fixed in 10% paraformaldehyde at 4 for 12 h and then dehydrated in different concentrations of ethanol. The tumor tissues were permeabilized using xylene and embedded in paraffin. They were then sliced (0.5 um), rehydrated, and stained with HE at 4 for 10 min and sealed. For IHC assessment of Ki-67 in gliomas, the DAKO Envision system (Dako; Agilent Technologies, Inc.) was used.
Click to Show/Hide
|
||||
| Response Description | Apatinib could restrain proliferation of glioma cells through induction of ferroptosis via inhibiting the activation of VEGFR2/Nrf2/ Keap1 pathway. Overexpression of Nrf2 could counteract the induction of ferroptosis by apatinib. | ||||
Withaferin A
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [29] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Pathways in cancer | hsa05200 | ||
| Ferroptosis | hsa04216 | |||
| Cell adhesion molecules | hsa04514 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell invasion | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| SNU-449 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | |
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| Response Description | Withaferin A may attenuate the metastatic potential and sorafenib resistance by regulating Keap1/Nrf2-associated EMT and ferroptosis. Thus, Withaferin A may serve as a promising agent for Hepatocellular carcinoma therapy, especially for advanced hepatocellular carcinoma. | |||
Tiliroside
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
All animal studies were approved by the Committee on Ethics of Animal Experiments of Binzhou Medical University (approval no: BZMU-IACUC-2021-331, date: 09/10/2021). To generate the ectopic HCC mouse models, HepG2-luciferase cells (HepG2 cells transfected with luciferase gene) were suspended in serum-free media and matrigel (BD Biosciences) at a ratio of 1:1 v/v. A total of 2.5 x 106 HepG2-luciferase cells/100 ul were injected into the left axilla of mice. After reaching a tumor size of 100-150 mm3, all mice were randomly divided into four groups: control (vehicle, intraperitoneal [i.p.]), tiliroside (20 mg/kg,i.p.), sorafenib (30 mg/kg,i.p.), or combination treatment (tiliroside and sorafenib,i.p.). All treatments were administered every 3 d, and the length and width of tumor were measured every 4 d. The formula tumor volume = (length x width2)/2 was used to calculate the tumor volume. Body weight was recorded every 7 d, and the morphology of the tumor was photographed using animal in vivo imaging technology (IVIS Spectrum; PerkinElmer) before the day of sacrifice. The mice were sacrificed 40 d after administration, and the tumors were dissected and weighed. The major organs and xenograft tumors were fixed with 4% paraformaldehyde.
Click to Show/Hide
|
||||
| Response Description | Tiliroside directly binds to TBK1 and inhibits its activity, which inhibits the phosphorylation of Ser349 on p62. Consequently, this decreases the affinity of p62 for Keap1, promotes ubiquitination and degradation of Nrf2 and ferroptosis, and eventually increases the sensitivity of hepatocellular carcinoma cells to sorafenib. | ||||
Nobiletin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [30] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Melanoma [ICD-11: 2C30] | |||
| Pathway Response | Pathways in cancer | hsa05200 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | SK-MEL-28 cells | Cutaneous melanoma | Homo sapiens | CVCL_0526 |
| Response Description | Nobiletin could induce ferroptosis by regulating the GSK3B-mediated Keap1/Nrf2/HO-1 signalling pathway in human melanoma cells. Hence, nobiletin stands as a promising drug candidate for melanoma treatment with development prospects. | |||
Gastrodin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [31] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Vascular dementia [ICD-11: 6D81] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male Sprague-Dawley rats (weight 260 ± 20 g; Guizhou Medical University Experimental Animal Center; Certificate No. SCXK2018-0001; Grant No. 2200483) were reared in a specific pathogen-free environment with 12 h light/dark cycle and 55% ± 10% humidity at a temperature of 20~25 , were provided with sufficient feed and sterile drinking water and fasted for 6 h before and after surgery. All animal experiments were performed in accordance with the Declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Gastrodin (GAS) inhibited ferroptosis in hippocampal neurons by activating the Nrf2/ Keap1-GPx4 signaling pathway, suggesting its possible application as a functional food for improving vascular dementia by inhibiting ferroptosis. | ||||
L. lactis MG1363-pMG36e-GLP-1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [32] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Colon tissues (Mouse colon tissues) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
Fifty male C57BL/6 mice provided by Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China) resided in an animal house (temperature 26 ± 1 , humidity 50 ± 10%), in which the light was on for 12 h and off for 12 h. Mice were acclimatised for 1 week and allowed water and animal food with no limitations. Then, all mice were stochastically divided into 5 groups using random number tables available online (https://www.random-online.com/, accessed on 26 December 2021), including: (1) C group, a control group treated with normal saline for 7 consecutive days (n = 10); (2) M group, a model group with intraperitoneal injection of 20 mg/kg/day MPTP (Sigma-Aldrich, Taufkirchen, Germany, M0896) for 7 consecutive days (n = 10); (3) L group, treated with MPTP and 0.4 mg/kg/day liraglutide for 7 consecutive days (n = 10); (4) R group, treated with MPTP and 109 colony-forming unit (CFU) L. lactis MG1363 for 7 consecutive days via gavage (n = 10); (5) RG group, treated with MPTP and 109 CFUL. lactis MG1363-pMG36e-GLP-1 for 7 consecutive days via gavage (n = 10). All animals survived treatment and all animal experiments were administered from 9:00 to 12:00 in the morning to reduce systematic errors.
Click to Show/Hide
|
||||
| Response Description | L. lactis MG1363-pMG36e-GLP-1 exerts neurotrophic effects via activating the Keap1/Nrf2/GPX4 signalling pathway to down-regulate ACSL4 and up-regulate FSP1 to suppress ferroptosis. These results indicated that the neurotrophic effects of the next-generation probiotics L. lactis MG1363-pMG36e-GLP-1 against MPTP-induced Parkinsonism are mediated by modulating oxidative stress, inhibiting ferroptosis, and redressing dysbiosis. | ||||
Astragaloside IV
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Subarachnoid Hemorrhage [ICD-11: 8B01] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
SAH model was constructed by applying endovascular perforation in the rats, according to the protocol introduced in a previous study (Wei et al., 2020), except for slight modifications. Briefly, after performing intraperitoneal anesthesia with 40 mg/kg sodium pentobarbital, the right common carotid, external and internal carotid arteries of the rats were exposed and isolated. The right external carotid artery was ligated, and a 4-0 single-strand nylon thread was used to insert the right internal carotid artery through the stump of the external carotid artery and the bifurcation of the common carotid artery. When resistance is felt when the suture enters the intracranial segment, proceed approximately 3 mm to penetrate internal carotid artery at the bifurcation of middle cerebral artery. The suture was held in this position for 10 s and was then withdrawn. The rats in the Sham group went through an identical procedure, without the suture at the point of resistance. Throughout the experiment, the body temperature of the rats was sustained at around 37 by using a thermal blanket. After the wounds were sutured, the rats were placed in a separate cage and neurological function was closely observed.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AS-IV) triggered Nrf2/HO-1 signaling pathway and alleviated ferroptosis due to the induction of subarachnoid hemorrhage (SAH). The Nrf2 inhibitor ML385 blocked the beneficial effects of neuroprotection. Ferroptosis is profoundly implicated in facilitating EBI in SAH, and that AS-IV thwarts the process of ferroptosis in SAH by activating Nrf2/HO-1 pathway. The liberation of Nrf2 from Keap1, its cytoplasmic repressor will provoke Nrf2 accumulation in the nucleus. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Rats were randomly divided into the Sham, middle cerebral artery occlusion-reperfusion (MCAO/R), and MCAO/R + AST IV (28 mg/kg) groups. The MCAO/R + AST IV group was intragastrically injected with 10 mL/kg AST IV at 50, 26, and 2 h before modelling (Xiao et al., 2021). The Sham and MCAO/R groups received equal amounts of normal saline. As described previously, the modified Longa method (Longa et al., 1989) was used to establish the MCAO/R model. After anaesthesia with 2%sodium pentobarbital, the left common carotid artery(CCA), the external carotid artery(ECA), and the internal carotid artery(ICA) were isolated. The distal end of the ECA was ligated, a small incision was made at the stump of the ECA, and a suture (Batch number: 2636A2, Beijing Seinong Technology Co., Ltd., Beijing, China; head-end diameter: 0.36 ± 0.02 mm) was inserted into the ICA from the ECA through the bifurcation of the CCA. To achieve cerebral ischaemia, the head-end was used to block blood flow in the middle cerebral artery until the intracranial segment of the ICA was inserted. The suture was removed after 2 h, and follow-up experiments were performed 24 h after reperfusion. In the Sham group, the CCA, ECA, and ICA were exposed and separated, but no sutures were inserted. Penicillin powder was used to fight infection after operation.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AST IV) increased the P62 (SQSTM1) and Nrf2 levels and decreased the Keap1 levels. P62 silencing reduced the effects of AST IV on the P62/ Keap1/Nrf2 pathway and ferroptosis. Our findings suggest that AST IV mitigates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via activation of the P62/ Keap1/Nrf2 pathway. | ||||
Dehydroabietic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [34] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Nonalcoholic fatty liver disease [ICD-11: DB92] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Pathways in cancer | hsa05200 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
The male C57BL/6J mice (6-8 weeks, Beijing Vital River Laboratory Animal Technology Co., Ltd., China) were exposed to 12 h of light and darkness at temperature (22 ± 2 ), humidity (55%) with free access to water and food. All the mice were acclimated for 1 week before the experiment, then the mice were fed normal chow diet (NCD) and high-fat diet (HFD, D12492) for 12 weeks. The HFD group was divided into 3 groups (HFD, low dose of DA (DA-L, 10 mg/kg/d), high dose of DA (DA-H, 20 mg/kg/d),n = 8)). DA was administered by gavage for 9 weeks, and 0.5% CMC-Na was administered by NCD and HFD.
Click to Show/Hide
|
||||
| Response Description | Dehydroabietic acid (DA) inhibited ferroptosis and increased the expression of key genes such as ferroptosis suppressor protein 1 (FSP1) in vitro and vivo. In all, DA may bind with Keap1, activate Nrf2-ARE, induce its target gene expression, inhibit ROS accumulation and lipid peroxidation, and reduce HFD-induced nonalcoholic fatty liver disease (NAFLD). | ||||
Baicalein
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [35] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Degenerative arthritis [ICD-11: FA05] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCDs (Chondrocytes) | ||||
| In Vivo Model |
C57BL/6J (WT) mice (8 weeks old, male) were purchased from Nanjing Medical university, and AMPK-KO mice were purchased from Shanghai Model Organisms. They were used to create an OA model by destabilization of the medial meniscus surgery (DMM) (n = 6 per group). Briefly, after the mice were anaesthetized, a medial articular incision was made to expose the leftjoint cavity, and then the tibial collateral ligament was transected. Finally, the articular incision was closed. In the control group, only the joint cavity was opened. One week after surgery, 1 mg/kg baicalein (MCE, HY-N0196) per knee, 1 mg/kg ML385 (MCE, HY-100523) per knee, 1 mg/kg AICAR (MCE, HY-13417) per knee or 1 mg/kg of the ferroptosis inhibitor ferrostatin-1 (Fer-1, MCE, HY-100579) was injected into the joint cavity of the mice once a week. Meanwhile, saline was injected into the control group. Mice were sacrificed after surgery 10 weeks.
Click to Show/Hide
|
||||
| Response Description | Baicalein alleviated osteoarthritis (OA) development by improving the activity of AMPKa/Nrf2/HO-1 signaling to inhibit chondrocyte ferroptosis, revealing baicalein to be a potential therapeutic strategy for OA. AMPKa preserved Nrf2 abundance in chondrocytes and promoted Nrf2 into nucleus by promoting Keap1 degradation | ||||
Guizhi Fuling Capsule
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Endometrial hyperplasia [ICD-11: GA16] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | mUTs (Mouse uterine tissues) | ||||
| In Vivo Model |
Female C57BL/6 mice (8-week-old) were purchased from Model Animal Research Center of Nanjing University (Nanjing, China). Fifteen mice were randomly divided into three groups: Olive oil group, Estradiol group and Estradiol + IKE group. The Estradiol group was subcutaneously injected estradiol (50 ug/kg/day), Estradiol + IKE group was subcutaneously injected estradiol and intraperitoneally injected IKE (50 mg/kg) for 21 days, while the Olive oil group received the same volume of olive oil. In the experiment of exploring the improvement of GFC to EH, twenty mice were randomly divided into four groups: Olive oil group, Estradiol group, 75 mg/kg GFC group and 150 mg/kg GFC group. Except for Olive oil group, mice were subcutaneously daily injected with estradiol (50 ug/kg/day) for 21 days, while the Olive oil group received the same volume of olive oil. 75 mg/kg GFC group and 150 mg/kg GFC group were treated with GFC intragastrical administration.
Click to Show/Hide
|
||||
| Response Description | Guizhi Fuling Capsule (GFC) may attenuate estrogen-induced endometrial hyperplasia in mice through triggering ferroptosis via inhibiting p62 (SQSTM1)- Keap1-NRF2 pathway. GFC might act as a promising traditional Chinese medicine to treat endometrial hyperplasia. | ||||
Entacapone
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks; 20-25 g) were purchased from LINGCHANG BIOTECH (China). Mice were divided into four groups: (i) sham, (ii) I/R, (iii) I/R+entacapone, and (iv) I/R + Fer-1. Entacapone (15 mg/kg bodyweight) was dissolved in sodium carboxymethyl cellulose (0.5%) and administered (i.g.) to mice. Mice in the sham group were administered (i.g.) an equal volume of solvent. Fer-1 was dissolved in 5% dimethyl sulfoxide + 30% polyethylene glycol-400 + 60% saline and injected (i.p.). Mice were treated three times per day for 3 days in advance. Before I/R, mice were fasted for 12 h and anesthetized (1% pentobarbital sodium, i.p.). The abdomen was exposed and bilateral renal pedicles were clamped to induce renal I/R. After 25 min, the arterial clamps were removed. A body temperature of 37 was maintained throughout the procedure. The sham group underwent the same procedure except for clamping of the renal pedicle. Mice were killed 24 h after reperfusion, and kidney and blood samples were collected for experimentation.
Click to Show/Hide
|
||||
| Response Description | Entacapone upregulates p62 (SQSTM1) expression and affects the p62- KEAP1-NRF2 pathway, thereby upregulating nuclear translocation of NRF2. This action results in increased expression of the downstream SLC7A11, and significant suppression of oxidative stress and ferroptosis. Entacapone may serve as a novel strategy to improve treatment of, and recovery from, ischemia/reperfusion-induced acute kidney injury (I/R-AKI). | ||||
Formononetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [36] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPRTECs (Mouse primary renal tubular epithelial cells) | ||||
| In Vivo Model |
For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium(30 mg/kg). Then, UUO surgery orsham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10% DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10% DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO. For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg). Then, UUO surgery or sham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10 % DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10 % DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO.
Click to Show/Hide
|
||||
| Response Description | Formononetin (FN) alleviates chronic kidney disease (CKD) by impeding ferroptosis-associated fibrosis by suppressing the Smad3/ATF3/SLC7A11 signaling and could serve as a candidate therapeutic drug for CKD. In addition, FN also promoted the separation of the Nrf2/ Keap1 complex and enhanced Nrf2 nuclear accumulation. | ||||
Astaxanthin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [37] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | |
| In Vivo Model |
6-week-Babl/c female mice were randomized to the following three groups of seven mice each: vehicle group, LPS group, Astaxanthin plus LPS group. Astaxanthin plus LPS group mice were pretreated with astaxanthin (20 mg/kg) byi.v injectionfor daily for 7 consecutive days. Astaxanthin was dissolved in 2%DMSO (vol/vol), 40% PEG-400 (vol/vol), 2% Tween 80 (vol/vol), and 56% PBS (vol/vol). On the last day, the mice were intraperitoneally injected with 5 mg/kg LPS or normal saline 2 h after the injection of astaxanthin. After 6 h of LPS stimulation, mice were euthanized to collect the BALF, and lung tissue samples. BALF was collected three times through a tracheal cannula with autoclaved normal saline, instilled up to a total volume of 1.8 ml.
Click to Show/Hide
|
||||
| Response Description | Astaxanthin protected LPS-induced cell inflammation and acute lung injury (ALI) in mice by inhibiting ferroptosis, and its effect was achieved through Keap1-Nrf2/HO-1 pathway. Therefore, our study indicates that ferroptosis will become a new target for the treatment of ALI, and astaxanthin is a potential drug for the treatment of ALI. | ||||
Panaxydol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [38] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Pathways in cancer | hsa05200 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
Specific pathogen-free (SPF) male C57BL/6 mice (6-8 weeks old, 20-24 g body weight) were purchased from the Experimental Animal Center, Anhui Medical University (Hefei, China). All mice were randomly divided into five groups (8 mice every group): control group, LPS group, PX+LPS group (administered 20 mg/kg PX), Fe+LPS group (administered 15 mg/kg Fe-citrate (III)), and PX+Fe+LPS group (administered 20 mg/kg PX and 15 mg/kg Fe-citrate (III)). Fe-citrate (III) was dissolved in stroke-physiological saline solution (SPSS). PX was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich), and further diluted in SPSS. Intravenous injection of Fe or/and intraperitoneal injection of PX were performed from day 0 to day 2. At 1 h after the final Fe and PX treatment, the mice were anesthetized with 30 mg/kg of pentobarbital sodium (Beijing Chemical Co., China) and then LPS (10 ug/mouse; InvivoGen, San Diego, CA, USA) or SPSS was injected into the trachea.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis mediated inflammation in LPS-treated BEAS-2B cells, and panaxydol (PX) might ameliorate LPS-induced inflammation via inhibiting ferroptosis. PX attenuates ferroptosis against LPS-induced acute lung injury via Keap1-Nrf2/HO-1 pathway, and is a promising novel therapeutic candidate for acute lung injury. | ||||
Clausenamide
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [39] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Pathways in cancer | hsa05200 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HepaRG cells | Hepatocellular carcinoma | Homo sapiens | CVCL_9720 | |
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | ||
| In Vivo Model |
Male C57BL/6 mice aged 8-10 weeks were purchased from Guangdong Experimental Animal Center (Guangzhou, China). The animals were maintained on a 12 h light-dark cycle in a regulated temperature and humidity environment for 1 week before drug administration. (+)-CLA (50 mg/kg/day, i.g.) or fer-1 (2.5 umol/kg/day, i.p.)were administered for 7 consecutive days. To induce liver injury, mice were injected with erastin (100 mg/kg/day, i.p., twice a day) on both the 6th and 7th day, or a single dose of APAP (600 mg/kg/day, i.p.) on the 7th day after overnight food deprivation. The serum and livers were obtained for analysis.
Click to Show/Hide
|
||||
| Response Description | (+)-clausenamide ((+)-CLA) specifically reacted with the Cys-151 residue of Keap1, which blocked Nrf2 ubiquitylation and resulted in an increased Nrf2 stability. Thus, (+)-CLA protects against acetaminophen-induced hepatotoxicity via inhibiting ferroptosis and activating the Keap1/Nrf2 pathway in a Cys-151-dependent manner. | ||||
Baicalin
[Terminated]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
C57BL/6 mice at 6-8 weeks were intraperitoneally injected with D-GalN/LPS (1772-03-8/L2880, Sigma-Aldrich, USA) at a dose of 700 mg/kg and 10 ug/kg, respectively. The constructed D-GaIN/LPS-induced ALI model mice were named the model group, and the normal mice injected with phosphate-buffered saline (PBS) were named the blank group. After 1 h of LPS/D-GalN treatment, Exo and Ba-Exo (150 ug/mice) were injected into the tail vein of the mice in the Exo and Ba-Exo groups, respectively. Mice were sacrificed via anesthesia overdose 12 h after the intervention. Half of the liver tissue was fixed in paraformaldehyde, while the other half was frozen at 80 . Peripheral blood serum was stored at -80 .
Click to Show/Hide
|
||||
| Response Description | Baicalin-pretreated MSCs (Ba-Exo) exerts a protective effect on liver function and activates the Keap1-NRF2 pathway via P62 (SQSTM1), thereby inhibiting ROS production and lipid peroxide-induced ferroptosis. Therefore, baicalin pretreatment is an effective and promising approach in optimizing the therapeutic efficacy of Exo in acute liver injury (ALI). | ||||
Xiaojianzhong
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [40] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hGCs (Gastric cells) | ||||
| In Vivo Model |
C57BL/6 mice (Male, 6-8 weeks old, 20 g± 2 g) were purchased from Chengdu Yaokang Biotechnology Co., Ltd. (Chengdu, China). All animals were housed in the animal room of Shaanxi University ofTraditional Chinese Medicine, at a temperature of 22 ± 2 and a humidity of 40% ± 5%, alternating between light and dark. In the study, the mice were randomly divided into six groups (n = 10 in each group): the blank group, model group,XJZ high dose group, XJZ medium dose group, XJZ low dose group, and positive control (omeprazole) group. The mice in the model group were given Aspirin (300 mg/kg) via gavage for 14 days; the mice in the XJZ high dose group, XJZ medium dose group, and XJZ low dose group were given aspirin (300 mg/kg) by gavage in the morning and three different concentrations (12 g/kg, 6 g/kg, or 3 g/kg) of XJZ decoction by gavage in the afternoon; the mice in the positive control group were given aspirin (300 mg/kg) by gavage in the morning andomeprazole(20 mg/kg) by gavage in the afternoon. After the model was successfully constructed, the mice were anesthetized with isoflurane and gastric tissues were extracted for analysis.
Click to Show/Hide
|
||||
| Response Description | Xiaojianzhong (XJZ) significantly counteracted aspirin-induced gastric mucosal injury and inhibited oxidative stress and ferroptosis in mice. Upon examining SQSTM1/p62(p62)/ Kelch-like ECH-associated protein 1 (Keap1)/Nuclear Factor erythroid 2-Related Factor 2 (Nrf2), a well-known signaling pathway involved in the regulation of oxidative stress and ferroptosis, we found that its activation was significantly inhibited by aspirin treatment and that this signaling pathway was activated after XJZ intervention. | ||||
Calcitriol
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [70] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Health [ICD-11: N.A.] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Pathways in cancer | hsa05200 | |||
| NF-kappa B signaling pathway | hsa04064 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | ZFL cells | Normal | Danio rerio | CVCL_3276 |
| Response Description | This study confirmed the protective effect of calcitriol on RSL3-induced ferroptosis in zebrafish liver cells, and reported for the first time that calcitriol inhibits ferroptosis in fish cells by regulating the Keap1/Nrf2/GPx4 axis and NF-kB/hepcidin axis. | |||
hsa-miR-138-5p (miRNA)
Astragaloside IV
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [21] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Retinopathy [ICD-11: 9B71] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Astragaloside IV (AS-IV) inhibited miR-138-5p expression, subsequently increasing Sirt1/Nrf2 activity and cellular antioxidant capacity to alleviate ferroptosis, resulting decreased cell death, which potentially inhibits the diabetic retinopathy pathological process. | |||
High mobility group protein B1 (HMGB1)
D-Glucose
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [41] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| NF-kappa B signaling pathway | hsa04064 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | SV40 MES 13 cells | Normal | Mus musculus | CVCL_5368 |
| Response Description | Erastin and high glucose both induced ferroptosis in mesangial cells. Suppression of HMGB1 restored cellular proliferation, prevented ROS and LDH generation, decreased ACSL4, PTGS2, and NOX1, and increased GPX4 levels in mesangial cells. Furthermore, nuclear factor E2-related factor 2 (Nrf2) was decreased in diabetic nephropathy (DN) patients and high glucose-mediated translocation of HMGB1 in mesangial cells. | |||
Glycogen synthase kinase-3 beta (GSK3B)
Nobiletin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [30] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Melanoma [ICD-11: 2C30] | |||
| Pathway Response | Pathways in cancer | hsa05200 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | SK-MEL-28 cells | Cutaneous melanoma | Homo sapiens | CVCL_0526 |
| Response Description | Nobiletin could induce ferroptosis by regulating the GSK3B-mediated Keap1/Nrf2/HO-1 signalling pathway in human melanoma cells. Hence, nobiletin stands as a promising drug candidate for melanoma treatment with development prospects. | |||
Fibronectin type III domain-containing protein 5 (FNDC5)
Iridin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [42] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Acute myocardial infarction [ICD-11: BA41] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hCMs (Human cardiomyocytes) | |||
| Response Description | Myocardial infarction is characterized by cardiomyocyte death and mitochondrial dysfunction induced by ischemia. FNDC5 overexpression and/or irisin administration elevated cell viability, decreased ferroptosis, and reversed mitochondrial impairments induced by hypoxia. Mechanistically, FNDC5/irisin reduced ferroptosis and reversed mitochondrial impairments by Nrf2/HO-1 axis in hypoxic cardiomyocytes. | |||
E3 ubiquitin-protein ligase NEDD4-like (NEDD4L)
Peoniflorin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| In Vivo Model |
U251 cells (6 x 106) were inoculated into the flanks of 4-to 5-week-old athymic nude mice (Shanghai Laboratory Animal Company, Shanghai, China) subcutaneously to generate a subcutaneous xenograft tumor model. After 2 weeks, the tumor model was successfully constructed, the mice were treated single and combined with 100 mg/kg RSL3 (2 times/week) and 1.0 g/kg/days PF. Tumor volumes were measured every 4 days to draw the growth curve. Mice were sacrificed 4 weeks after cell injection. Tumor xenografts were collected, photographed, and weighed and the tumor apoptosis was analyzed by Tunel staining.
Click to Show/Hide
|
||||
| Response Description | Paeoniflorin (PF) can function as an antitumor agent for glioma treatment by targeting NEDD4L-dependent STAT3 ubiquitination as well as by regulating the Nrf2/GPX4 signaling axis, which might trigger ferroptosis. | ||||
CircOMA1 (circRNA)
Cabergoline
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [43] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Prolactinoma [ICD-11: 2F37] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1)
Ascorbic Acid
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [44] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | PaTu 8988t cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1847 | |
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| mEFs (Mouse embryonic fibroblasts) | |||||
| Panc02 cells | Pancreatic ductal adenocarcinoma | Mus musculus | CVCL_D627 | ||
| In Vivo Model |
All animal experiments were approved by the Ethics Committee of Jiangsu University. To investigate the role of the combination of erastin and vitamin C in inducing ferroptosis, Panc02 cells (1 x 105 cells/site) were transfected and subcutaneously injected into 4-week-old C57BL/6 mice to generate xenografts. When the tumors reached a volume of 50-100 mm3, the mice were randomly divided into four groups (five mice per group) and treated with DMSO (control), imidazole ketone erastin (IKE, MedChemExpress), vitamin C, or a combination of erastin and vitamin C. Mice were treated with 80 ul (400M) erastin by intratumoral injection and/or 4 g/kg vitamin C by intraperitoneal injection every 2 days.
Click to Show/Hide
|
||||
| Response Description | The combination of erastin and vitamin C mainly increases the levels of ferrous iron through the AMPK/NRF2/HMOX1 signaling pathway. Cotreatment with erastin and vitamin C also exhibited a synergistic effect in a pancreatic cancer xenograft model in mice. | ||||
zero-valent-iron nanoparticle
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [45] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| mTOR signaling pathway | hsa04150 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| A9 cells | Lung carcinoma | Mus musculus | CVCL_S007 | ||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| IMR-90 cells | Normal | Homo sapiens | CVCL_0347 | ||
| In Vivo Model |
5-6-week-old BALB/c nude mice (ZVI@Ag treatment) or NOD/SCID mice (ZVI@CMC treatment) were subcutaneously implanted with 1 x 106 H460 cells. For A549 xenograft model of immunodeficient mouse and spontaneous lung metastasis model, 5-6-week-old NOD/SCID mice were subcutaneously implanted with 5 x 106 A549 cells. For experimental lung metastasis model, H460 cells (1 x 106 cells/200 uL) were resuspended in serum-free medium and injected intravenously (i.v.) into tail-vein of NOD/SCID mice. For subcutaneous model of immunocompetent mouse, LLC cells (5 x 105) were injected into both flank of 6-week-old C57BL/6 mice.
Click to Show/Hide
|
||||
| Response Description | Zero-valent-iron nanoparticle (ZVI-NP) triggered ferroptosis selectively in lung cancer cells by suppressing NRF2-mediated cytoprotection program, which was attributed to the ZVI-NP-induced disruption of PRKAA1 (AMPK)/mTOR signaling and activation of GSK3/-TrCP-dependent degradation system. | ||||
Unspecific Regulator
Ferulic acid
[Patented]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [81] | ||||
| Responsed Disease | Sepsis [ICD-11: 1G40] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
Briefly, female BALB/c mice (6-8 weeks, weighing 20-25 g, purchased from Hunan SJA Laboratory Animal Co., Ltd., Changsha, Hunan, China) were raised in specific pathogen-free conditions under controlled temperature (23-25 ) and humidity (40-80%) as well as a 12 h dark/light cycle for 1 week of acclimation. They were fed a standard chow diet and waterad libitum. Mice were anaesthetised with 2% isoflurane inhalation and underwent moderate caecal ligation and puncture in accordance with a previously reported protocol (Rittirsch etal.2009). Meanwhile, mice in the control groups were subjected to a sham operation. Buprenorphin (0.05 mg/kg) was injected for postoperative analgesia, and all mice were placed in cages immediately after the surgical procedures with free access to water and food (Rittirsch etal. 2009).
Click to Show/Hide
|
||||
| Response Description | Collectively, our data highlighted the alleviatory role of ferulic acid in sepsis-induced ALI by activating the Nrf2/HO-1 pathway and inhibiting ferroptosis, offering a new basis for sepsis treatment. | ||||
Iridin
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [82] | ||||
| Responsed Disease | Sepsis [ICD-11: 1G40] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Eight-week-old wild-type (WT) and Nrf2-knockout (Nrf2-/-) littermate male mice on a C57BL/6J background were purchased from Cyagen (Suzhou, China.) and maintained at the Centre for Animals of Wuhan University (Wuhan, China). Before the experiment, the mice were separated and given light and dark cycles for 12 h, 22 ± 0.5 temperature, 60 ± 10% humidity, and free accessed to food and water for at least 1 week. Mice were randomly distributed into sham, CLP, CLP + Irisin (Ir group) and CLP + Irisin + Era (Ir + Era group) groups.
Click to Show/Hide
|
||||
| Response Description | In conclusion, irisin could ameliorate inflammatory microenvironment in sepsis-associated encephalopathy by suppressing hippocampus ferroptosis via the Nrf2/GPX4 signaling pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [133] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
In vivo, the LIRI model was established as described earlier. All mice were anesthetized with pentobarbital administered intraperitoneally (50 mg/kg, Sigma-Aldrich, MO, USA). After endotracheal intubation, the mice were ventilated using a rodent ventilator (MiniVent, Harvard Apparatus, USA), with the title volume set to 7 ml/kg, the respiratory rate set to 120 times/min, and the inspiratory/expiratory ratio set to 1: 2. A noninvasive clamp was used to interrupt the left pulmonary hilum, causing lung ischemia. The clamp was released after 60 minutes of ischemia, and the left lung was reperfused for 120 minutes. Animals were euthanized via cervical dislocation at the end of the experiment. Following that, lung specimens and bronchoalveolar lavage fluid were harvested for analysis. All procedures except lung ischemia were performed on mice in the sham group.
Click to Show/Hide
|
||||
| Response Description | As a result, irisin postconditioning may protect against lung I/R damage by suppressing ferroptosis via the Nrf2/HO-1 signaling axis. | ||||
MCTR1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [83] | ||||
| Responsed Disease | Sepsis [ICD-11: 1G40] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice aged 8-12 weeks, weighing about 25 g, were purchased from Shanghai Experimental Animal Center of China. The mice were anesthetized with isoflurane. The abdomen area was disinfected after shaving. A 1 cm midline incision was made on the lower abdomen skin to expose the cecum. A 5-0 silk thread was used to ligate the midpoint between the distal pole and the bottom of the cecum. A 21 g needle was used to penetrate the cecum twice from the mesenteric toward the antimesenteric direction. After the wound was sutured, 0.25% ropivacaine was injected subcutaneously for postoperative analgesia. The mice were immediately resuscitated by injecting prewarmed normal saline 1 ml subcutaneously.
Click to Show/Hide
|
||||
| Response Description | MCTR1 effectively alleviates sepsis-associated acute kidney injury, at least in part, by inhibiting ferroptosis. Mechanistically, MCTR1 activates the Nrf2, which may contribute to the inhibition of ferroptosis. | ||||
Ibuprofen
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [84] | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
In the intracranial glioma model, U87MG cells (5 x 105) were intracerebrally injected into the left side (bregma: 1 mm; lateral: 2 mm; ventral: 3 mm) of the brains of nude mice. Two weeks after tumor cell transplantation, mouse brains were scanned to detect tumor formation using a 3.0-T scanner (GE Signa HD MRI Systems). Then, mice were divided randomly into two groups (n = 6/group) and treated with vehicle control (PBS), oribuprofen (20 mg/kg), in 100 ul of PBS given i.p. 1x/day, 5 days/week.
Click to Show/Hide
|
||||
| Response Description | Ibuprofen could induce ferroptosis of glioblastoma cells via downregulation of Nrf2 signaling pathway and is a potential drug for glioma treatment. All the data suggested that Nrf2 could regulate the expression of GPX4 and SLC7A11 in glioma cells. | ||||
Polygonatum cyrtonemaHua Polysaccharides
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [85] | |||
| Responsed Disease | Central nervous system cancer [ICD-11: 2A02] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | BV-2 cells | Normal | Mus musculus | CVCL_0182 |
| Response Description | Subsequent studies have revealed that Polygonatum cyrtonemaHua Polysaccharides (PCP) alleviates ferroptosis in microglia due to protein levels of ERASTIN/RSL3 inhibitor SLC7A11/GPX4 by activating the NRF2/HO-1 signaling pathway. PCP has the development potential as a new drug candidate for treating CNS diseases. | |||
All-trans retinoic acid derivative
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [86] | ||||
| Responsed Disease | Acute myeloid leukaemia [ICD-11: 2A60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | NB4 cells | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | |
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | ||
| U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | ||
| In Vivo Model |
Six- to seven-week-old female NCG mice were obtained from the Nanjing model animal research institute (Nanjing, China). Mice were raised in individually ventilated cages of Anhui Medical University (Hefei, China). Then, an injection of NB4 cells (5 x 106/100 ul) suspended in chilled Matrigel and PBS (1:1) was given into the right flank of each mouse. For each experiment, the animals (n = 12) were randomly allocated into the two groups and treated with solvent or 10 mg/kg ATPR (intraperitoneal injection, once every other day) for 7 days.
Click to Show/Hide
|
||||
| Response Description | ATPR, a novel all-trans retinoic acid (ATRA) derivative, has been extensively developed to show superior anticancer effect than ATRA in acute myeloid leukemia (AML). ATPR-induced ferroptosis was regulated by autophagy via iron homeostasis, especially Nrf2. | ||||
Triptolide
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [87] | ||||
| Responsed Disease | Myeloid leukaemia [ICD-11: 2B33] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | K-562 cells | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | |
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| Response Description | Triptolide inhibited Nrf2 expression and induced leukemia cell ferroptosis, as evidenced by increased ROS levels and lipid oxidation as well as decreased glutathione peroxidase 4 expression. Therefore, it is plausible that triptolide can promote leukemia cell sensitivity to DOX via downregulation of Nrf2 expression. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [102] | ||||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A2780/DDP cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_D619 | |
| In Vivo Model |
All female BALB/cnude mice(4-6 weeks old, 15-20 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). They were raised in specific pathogen-free conditions and allowed to access sterile water and food freely. A2780/DDP cell suspension (100 uL) with a density of 1 x 107 cells/mL was injected subcutaneously into the axilla of the mice. After observing the nude mice for a week, it was confirmed that subcutaneous A2780/DDP cells were inoculated successfully. Sterile saline (100 uL) was injected into the abdominal cavity of the nude mice in the control group for 14 days. The mice in the DDP treatment group were given DDP (4 mg/kg/day) intraperitoneally on the first and eighth days. TG (100 uL, 1 mg/kg) diluted with sterile physiological saline were injected into the abdominal cavity of the nude mice in the TG treatment group for 14 days. In addition, the nude mice in the TG + DDP treatment group were given TG (100 uL, 1 mg/kg) for 14 days and DDP (4 mg/kg/day) intraperitoneally on the first and eighth days.
Click to Show/Hide
|
||||
| Response Description | Tripterygium (TG) can effectively inhibit the proliferation of drug-resistant ovarian tumor cells A2780/DDP and increase the sensitivity to cisplatin chemotherapy both invitro and invivo. In terms of mechanism, TG induces ferroptosis by targeting the NRF2/GPX4 signal axis to weaken the antioxidant capacity of cancer cells. | ||||
Baicalin
[Terminated]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [88] | ||||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| 143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | ||
| hBMMSCs (Human bone marrow mesenchymal stem cells) | |||||
| In Vivo Model |
A total of 24 BALB/c-nude mice (4-5 weeks old) were purchased and MG63 cells were injected into the right tibial bone marrow cavity of mice in a volume of 1 x 106/100 ul. When the tumor volume was visible, all animals were randomly divided into four groups (n = 6): the control (10% DMSO + 40% PEG300 + 5% Tween-80 + 45% Saline) group, the baicalin (200 mg/kg/day) group, the Fer-1 (0.8 mg/kg/day) group and Fer-1 + baicalin group. The baicalin and Fer-1 were intraperitoneally administered every day for two consecutive weeks and tumor sizes were measured every two days.
Click to Show/Hide
|
||||
| Response Description | By promoting the Fe accumulation, ROS formation, MDA production and suppressing the ratio of GSH/GSSG, baicalin was found to trigger ferroptosis in Osteosarcoma and ferroptosis inhibitor ferrostatin-1 (Fer-1) successfully reversed these suppressive effects, indicating that ferroptosis participated in the baicalin mediated anti-OS activity. Mechanistically, baicalin physically interacted with Nrf2, a critical regulator of ferroptosis, and influenced its stability via inducing ubiquitin degradation, which suppressed the Nrf2 downstream targets GPX4 and xCT expression, and led to stimulating ferroptosis. | ||||
Carnosic acid
[Investigative]
| In total 2 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [89] | |||
| Responsed Disease | Oral squamous cell carcinoma [ICD-11: 2B6E] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Response Description | The current findings highlight that carnosic acid may re-sensitize cisplatin-resistant cells to cisplatin by inducing ferroptosis, which involves the inactivation of Nrf2/HO-1/xCT pathway. Hence, this research may support a promising therapeutic approach to overcome chemoresistance in Oral squamous cell carcinoma. | |||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [154] | |||
| Responsed Disease | Spinal cord injury [ICD-11: ND51] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Ferroptosis is involved in the pathogenesis of spinal cord injury (SCI). Carnosic acid (CA) is a natural phenolic diterpene, which possesses diversiform activities. CA can inhibit ferroptosis in PC12 cells induced by erastin via activating Nrf2 pathway, indicating that CA could lead to neuroprotective effect by restraining the occurrence of ferroptosis. | |||
Polyphyllin I
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [90] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| In Vivo Model |
A subcutaneous gastric tumor model was established by subcutaneously injecting 1 x 106 AGS cells or 2 x 106 MKN-45 cells near the right axilla of mice. Seven days after tumor cell inoculation, mice received daily i. p. Injection of PPI (3 mg/kg, dissolved in 1% DMSO + 5% PEG300 + 5% Tween 80 + 89% deionized water), as described previously, or the control solution with the same solvent. Mice were weighed at day 15, while tumor volumes were measured every 3 days and calculated using a formula: length x width2/2.
Click to Show/Hide
|
||||
| Response Description | For the first time, our results have demonstrated that Polyphyllin I exerts its antitumor activity on the gastric cancer by, at least partially, inducing cancer cell ferroptosisviaregulating NRF2/FTH1 pathway. | ||||
Propofol
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [91] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response Description | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [114] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male C57BL/6 mice weighing 20-25 g each were obtained from the Animal Experimental Center of Yisi (Changchun, China). Mice were group-housed in a 12 h light/dark cycle (light between 08:00 and 20:00 h) in a temperature-controlled environment room (23-25 ). Mice had ad libitum access to food and water. All surgical procedures were carried out on animals anesthetized with sodium pentobarbital (30 mg/kg) via intraperitoneal injection. MCAO was achieved by inserting a silicone rubber-coated nylon monofilament into the internal carotid artery through the external carotid artery and temporary ligation of the right common carotid artery with a suture. After 45 min of ischemia, blood flow was restored by removing the filament and the suture, and the mice were allowed to recover for 24 h.
Click to Show/Hide
|
||||
| Response Description | Our data support a protective role of propofol against ferroptosis as a cause of cell death in mice with cerebral ischemia-reperfusion injury. Propofol protected against cerebral ischemia-reperfusion injury-induced ferroptosis partly by regulating the Nrf2/Gpx4 signaling pathway. | ||||
Oxaliplatin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [92] | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ |
| Response Description | Oxaliplatin promoted ferroptosis and oxidative stress in colorectal cancer cells by inhibiting the Nrf2 signaling pathway. Treatment with oxaliplatin enhanced the effects of erastin on CRC cells by promoting ferroptosis and oxidative stress and inhibiting cell viability. | |||
tagitinin C
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [93] | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| Response Description | Tagitinin C induces ferroptosis in colorectal cancer cells and has synergistic effect together with erastin. Mechanistically, tagitinin C induces ferroptosis through ER stress-mediated activation of PERK-Nrf2-HO-1 signaling pathway. | |||
Itaconic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [94] | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 cells in 100 ul PBS were injected subcutaneously into the right of the dorsal midline in 6- to 8-week-old femaleathymic nude mice(n = 6 mice/group). After the tumor reached 60-80 mm3 on day 7, the mice were randomly grouped and then given intraperitoneal injections with itaconic acid (50 mg/kg, once every other day) at day 7 for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Itaconic acid-induced expression and activation of NFE2L2 serves as a defense mechanism to limit ferroptosis by producing antioxidant genes. Consequently, impaired NCOA4 expression prevented, whereas a disrupted NFE2L2 pathway enhanced, sensitivity to itaconic acid-induced ferroptosis in pancreatic cancer cells. | ||||
Wogonin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [95] | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| AsPC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0152 | ||
| HPDE6-C7 cells | Normal | Homo sapiens | CVCL_0P38 | ||
| In Vivo Model |
Female BALB/c nude mice (5 weeks old) were procured from Hangzhou Ziyuan Laboratory Animal Technology Co., Ltd (Zhejiang, China) and given 5 days to acclimate to their surroundings. PANC-1 cells (1 x 107) in 100 uL PBS at the logarithmic growth phase were administered to mice subcutaneously in the left flank. The mice were treated with indicated treatments after nearly 10 days when the tumour size was approximately 1,000 mm3. In the control group, mice (n = 5) received intraperitoneal injections of the vehicle. In the treatment group, the mice (n = 5) were administered 50 uL of 60 mg/kg body weight of wogonin once a day for 12 days. A slide calliper size was used to measure the tumour size. The equation for calculating tumour volume is as follows: tumour volume = AB2/2, wherein A is the length, and B is the width of the tumour. The mice were sacrificed the next day after the treatment procedure was complete by cervical dislocation. The tumour tissues were harvested and snap-frozen using liquid nitrogen for subsequent analyses.
Click to Show/Hide
|
||||
| Response Description | Wogonin could significantly reduces pancreatic cancer cell proliferation and induce ferroptosisviathe Nrf2/GPX4 axis. Therefore, wogonin could be potentially used for treating patients with pancreatic cancer. | ||||
Camptothecin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [96] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response Description | Sorafenib is a potent inducer of ferroptosis used to manage hepatocellular carcinoma (HCC). Nrf2 inhibition by Camptothecin improves sorafenib's sensitivity and reduces sorafenib's resistance via the augmentation of sorafenib's ferroptosis action. | |||
Erastin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [97] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
A total of 60 BALB/c-nu/nu nude mice (male; age, 4-6 weeks; weight, 16-22 g) were obtained from the Shanghai Laboratory Animal Co., Ltd. N5CP cells (5 x 106) were suspended in 200 ul DMEM and Matrigel mixture at a ratio of 1:1. Subsequently, the mixture was injected subcutaneously into the upper right flank of 20 nude mice. After 10 days, the mice were randomly divided into four groups and were treated with CDDP (5 mg/kg/2 days), erastin (10 mg/kg/2 days), sorafenib (10 mg/kg/2 days) or PBS by intraperitoneal injection. Two days after the third injection, the mice were sacrificed and tumours were carefully removed. For the combination experiment, CDDP (1 mg/kg) and erastin (5 mg/kg) or sorafenib (3 mg/kg) were also injected three times.
Click to Show/Hide
|
||||
| Response Description | The potential mechanism by which sorafenib and erastin induced ferroptosis in cisplatin (CDDP)-resistant non-small cell lung cancer (NSCLC) cells may be associated with inhibition of the expression of the Nrf2 downstream target gene xCT. | ||||
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [97] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
A total of 60 BALB/c-nu/nu nude mice (male; age, 4-6 weeks; weight, 16-22 g) were obtained from the Shanghai Laboratory Animal Co., Ltd. N5CP cells (5 x 106) were suspended in 200 ul DMEM and Matrigel mixture at a ratio of 1:1. Subsequently, the mixture was injected subcutaneously into the upper right flank of 20 nude mice. After 10 days, the mice were randomly divided into four groups and were treated with CDDP (5 mg/kg/2 days), erastin (10 mg/kg/2 days), sorafenib (10 mg/kg/2 days) or PBS by intraperitoneal injection. Two days after the third injection, the mice were sacrificed and tumours were carefully removed. For the combination experiment, CDDP (1 mg/kg) and erastin (5 mg/kg) or sorafenib (3 mg/kg) were also injected three times.
Click to Show/Hide
|
||||
| Response Description | The potential mechanism by which sorafenib and erastin induced ferroptosis in cisplatin (CDDP)-resistant non-small cell lung cancer (NSCLC) cells may be associated with inhibition of the expression of the Nrf2 downstream target gene xCT. | ||||
Ginkgetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [98] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SPC-A1 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | ||
| In Vivo Model |
Briefly, when tumours on transplanted nude mice reached around 100 mm3, the mice were randomized divided into eight groups: control, ginkgetin, DDP, ginkgetin + DDP, UAMC 3203, ginkgetin + UAMC 3203, DDP + UAMC 3203, ginkgetin + DDP + UAMC 3203. Both DDP (3 mg/kg) and ginkgetin (30 mg/kg) were administered by intraperitoneal injection, with 2 - 3 times per week and once per day, respectively. UAMC 3203 (10 mg/kg) was administered 5 days/week by intraperitoneally injection. Tumour size and body weight were measured 3 times per week. After dosing 31 days, the nude mice were sacrificed, and tumours were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | The induction of ferroptosis mediated by ginkgetin was further confirmed by the decreased expression of SLC7A11 and GPX4, and a decreased GSH/GSSG ratio. Simultaneously, ginkgetin disrupted redox hemostasis in DDP-treated cells, as demonstrated by the enhanced ROS formation and inactivation of the Nrf2/HO-1 axis. Ginkgetin also enhanced DDP-induced mitochondrial membrane potential (MMP) loss and apoptosis in cultured non-small cell lung cancer (NSCLC) cells. | ||||
S-3'-hydroxy-7', 2', 4'-trimethoxyisoxane
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [99] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
When tumor volumes in xenograft nude mice reached an average of roughly 100 mm3, the mice were randomly divided into 3 groups of 6 mice each: control, ShtIX, and ShtIX + Fer-1. The treated group received ShtIX or ShtIX combined with Fer-1 injections into the tail vein of the mice every three days for 7 times, whereas the control group received saline. Every four days, the volume and weight of the tumors were measured. As soon as the test was completed, the nude mice were slaughtered, and the tumor tissues were retrieved. The in vivo experiments were approved by the Animal Care and Use Committee of Hainan Medical College and following the animal rules.
Click to Show/Hide
|
||||
| Response Description | S-3'-hydroxy-7', 2', 4'-trimethoxyisoxane (ShtIX) caused ferroptosis in Non-small cell lung cancer (NSCLC) cells, and inhibiting the Nrf2/HO-1 pathway can considerably exacerbate the effect of ShtIX-induced ferroptosis. | ||||
Manoalide
[Phase 2]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [100] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| H157 cells | Oral cavity Squamous cell carcinoma | Homo sapiens | CVCL_2458 | ||
| HCC827 cells | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| In Vivo Model |
The LSL-KrasG12D mouse model was obtained from the Jackson Laboratory (Sacramento, CA). Adeno-Cre (Genechem, Shanghai, China) was introduced into the trachea of mice at a dose of 1.25 x 1011 PFU in a total volume of 50 uL. Tumor tissues from 12-week post-infection mice were washed with cold PBS, cut into small pieces, and washed with DMEM/F12 (containing 1 x Glutamine, 10 mM HEPES, and antibiotics), digested with collagenase I and IV for 0.5-1 h at 37. After washing twice with DMEM/F12 and centrifugation (500 g, 5 min), the dissociated cells were seeded into growth factor-reduced matrigel (Corning, #356237) at 37 for 30 min.
Click to Show/Hide
|
||||
| Response Description | Manoalide (MA) induces ferroptosis by suppressing the NRF2-SLC7A11 axis and mitochondrial Ca2+overload induced-FTH1 pathways to promote the sensitivity of osimertinib-resistant lung cancer cells to osimertinib. | ||||
Apatinib
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [101] | |||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | |
| Response Description | Apatinib combined with olaparib-induced ferroptosis via a p53-dependent manner in ovarian cancer. Further studies showed that apatinib combined with olaparib-induced ferroptosis by inhibiting the expression of Nrf2 and autophagy, thereby inhibiting the expression of GPX4. The Nrf2 activator RTA408 and the autophagy activator rapamycin rescued the combination drug-induced ferroptosis. | |||
Norcantharidin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [103] | ||||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Athymic nu/nu female mice aged 6-8 weeks (n = 9; mean weight, 20.21 ± 1.54 g) were purchased from the specific pathogen SPF (Beijing) Lab Animals Technology Co. Ltd. Mice were housed in a temperature- and humidity-controlled environment (20-24 , 45-55% humidity), with free access to food and water and in groups of three. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC ID: 17-3256) at Nantong University and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Nuclear factor erythroid 2-related factor 2 (NRF2), heme oxygenase 1 (HO-1), glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (xCT) expression levels were significantly decreased following norcantharidin (NCTD) treatment. Collectively, NCTD may represent a potent anticancer agent in ovarian cancer cells, and NCTD-induced ferroptotic cell death may be achieved by inhibiting the NRF2/HO-1/GPX4/xCT axis. | ||||
Olaparib
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [101] | |||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | |
| Response Description | Apatinib combined with olaparib-induced ferroptosis via a p53-dependent manner in ovarian cancer. Further studies showed that apatinib combined with olaparib-induced ferroptosis by inhibiting the expression of Nrf2 and autophagy, thereby inhibiting the expression of GPX4. The Nrf2 activator RTA408 and the autophagy activator rapamycin rescued the combination drug-induced ferroptosis. | |||
Erianin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [104] | ||||
| Responsed Disease | Bladder cancer [ICD-11: 2C94] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | RT4 cells | Bladder carcinoma | Homo sapiens | CVCL_0036 | |
| KU-19-19 cells | Bladder carcinoma | Homo sapiens | CVCL_1344 | ||
| In Vivo Model |
4-weeks-old female BALB/c nude mice aged were injected into 5 x 105 cells. Every 2 days mice weight and tumor size were assessed, and the tumor volume (V) was calculated with the formula: (maximum length) x (maximum width)2/2. Once tumors were found, the mice were stochastically divided into 2 groups: the control (PBS) group and the treatment (erianin 100 mg/kg) group. For 14 days, mice were injected intraperitoneally with drugs once a day, then puting the mice to death, after that tumors were taken for IHC (immunohistochemical) analysis.
Click to Show/Hide
|
||||
| Response Description | Erianin inhibited cell proliferation and triggered cell death in bladder cancer cells. Mechanistically, we showed NRF2 was a key determinant for erianin-triggered ferroptosis. NRF2 activation by TBHQ treatment protected against erianin-induced cell death and increased the expression of GPX4, ferritin, xCT and glutaminase. | ||||
Bilirubin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [105] | ||||
| Responsed Disease | Diabetes mellitus [ICD-11: 5A10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MIN6 cells | Insulinoma | Mus musculus | CVCL_0431 | |
| In Vivo Model |
BALB/c mouse are used as recipients and donors in the transplantation. Fifteen diabetic BALB/c mice were randomly divided into 5 groups (3 mice in each group). Then, 250 IEQ islets pretreated with or without bilirubin (20 uM) for 48 h were transplanted into the subrenal site of the diabetic mouse. Ferrin 1 (10 uM) and DFO (10 mM) pretreated islets were also transplanted for comparison. Following, the non-fasting glucose level and bodyweight was the mice were recorded daily.
Click to Show/Hide
|
||||
| Response Description | Bilirubin protects transplanted islets by inhibiting ferroptosis through multiple mechanisms, including ROS scavenging ability, iron-chelating property, and upregulation of Nrf2/HO-1 signaling pathway. Bilirubin could improve islet viability and function through inhibiting ferroptosis, which could be of clinic interest to apply bilirubin into the islet transplantation system. Islet transplantation is an attractive treatment for type 1 diabetic patients. | ||||
Gastrodin
[Investigative]
| In total 2 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [106] | |||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | C6 cells | Malignant glioma | Rattus norvegicus | CVCL_0194 |
| Response Description | Gastrodin induced GPX4, Nrf2 and HO-1 expression to protect C6 cells from H2O2-induced ferroptosis. Gastrodin pretreatment effectively reduced H2O2-induced oxidative damage, indicating gastrodin is a potential antioxidant that reduced cytotoxic ROS. The role of gastrodin in ferroptosis presents a new perspective for understanding parkinson's disease. | |||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [119] | |||
| Responsed Disease | Nervous system disease [ICD-11: 8E7Z] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Gastrodin (GAS) is a component of Gastrodia elata Blume, with strong antioxidant activity in neurodegenerative diseases. GAS increased the nuclear translocation of Nrf2, up-regulated the downstream HO-1 protein expression in HT-22 cells following treatment with glutamate. | |||
Forsythoside A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [107] | ||||
| Responsed Disease | Alzheimer disease [ICD-11: 8A20] | ||||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| Neuro-2a cells | Neuroblastoma | Mus musculus | CVCL_0470 | ||
| BV-2 cells | Normal | Mus musculus | CVCL_0182 | ||
| In Vivo Model |
All animal experiments were approved by the Animal Ethics Committee of Jilin University (permit No. SY201905013) and were conducted in compliance with the ARRIVE guidelines. Eight-month-old B6C3-Tg (APPswePSEN1dE9)/Nju double transgenic male mice (APP/PS1) (genotype: (Appswe) T, (Psen1) T) and age-matched wild-type (WT) (genotype: (Appswe) W, (Psen1) W) male mice were purchased from Nanjing Biomedical Research Institute of Nanjing University. All mice were individually housed at 24 with food and drinking water availablead libitum. After 1 week of adaption in the new environment, WT mice received oral administration of normal saline (10 mL/kg) and were designated as the control group (n = 12). APP/PS1 mice were randomly divided into two groups: the model group (n = 12) received oral administration of normal saline (10 mL/kg) and the agent-treated group (n = 12) received oral treatment with 30 mg/kg FA (L-012-171216, 98.83% purity, Chengdu Herbpurify Co., Ltd., Chengdu, China) beginning on day 8. After 30-day treatment, behavioral experiments were serially performed. The entire treatment protocol lasted for 42 days. Blood samples were collected from the caudal vein. After euthanasia via CO2 inhalation, organs including the brain, liver, spleen, and kidney were collected for further analysis.
Click to Show/Hide
|
||||
| Response Description | Forsythoside A treatment exerted anti-ferroptosis and anti-neuroinflammatory effects in erastin-stimulated HT22 cells, and the Nrf2/GPX4 axis played a key role in these effects. Collectively, these results demonstrate the protective effects of FA and highlight its therapeutic potential as a drug component for AD ( Alzheimer's disease) treatment. | ||||
ginkgolide-B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [108] | ||||
| Responsed Disease | Alzheimer disease [ICD-11: 8A20] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHTs (Mouse hippocampus tissues) | ||||
| In Vivo Model |
Male 6-month-old senescence-resistant R1 (SAMR1) and SAMP8 mice (weight, 28-35 g) were purchased from Beijing SPF Biotechnology. First, mice were placed in the center of an empty testing arena (40 x 40 x 40 cm) and allowed to move freely for adaptation. Next, in the training stage, two similar objects were presented in the testing arena and mice were allowed to explore for 10 min, for 3 consecutive days. On day 4, one of the two familiar objects was replaced by a new object. The time of exploring a novel object or familiar object in 10 min was recorded.
Click to Show/Hide
|
||||
| Response Description | Ginkgolide B attenuated Alzheimer's disease (AD)-related cognitive impairment through the regulation of oxidative stress, neuroinflammation and ferroptosis, and that GB-induced protection in AD is dependent on the inhibition of ferroptosis. Furthermore, the involvement of Nrf2/GPX4 pathway-regulated ferroptosis in the GB-related protective effects on the AD mouse model. | ||||
Salidroside
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [109] | ||||
| Responsed Disease | Alzheimer disease [ICD-11: 8A20] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CD8T cells (Mouse CD8+ T cells) | ||||
| In Vivo Model |
SAMP8 mice were employed as an AD model and were treated with salidroside for 12 weeks. Behavioral tests, immunohistochemistry, HE and Nissl staining, immunofluorescence, transmission electron microscopy, quantitative proteomics, bioinformatic analysis, flow cytometry, iron staining,western blotting, andmolecular dockingwere performed.
Click to Show/Hide
|
||||
| Response Description | Salidroside alleviates cognitive impairment and inhibits neuronal ferroptosis in Alzheimer's disease. The underlying mechanisms may involve the Nrf2/GPX4 axis activation and reduction in CD8T cells infiltration. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [131] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | ||
| In Vivo Model |
Following endotracheal intubation, mice were ventilated with room air at a rate of 120 cycles/min and atidal volumeof 7 mL/kg (MiniVent, Harvard Apparatus, USA). To induce ischemia, mice underwent left thoracotomy, and the left pulmonary hilum was blocked for 60 min with a microvascular clamp. After ischemia, the coronary artery was reperfused for 120 min by removing the clamp. The mice were euthanized at the end of the experiment through CO2 asphyxiation and cervical dislocation. Next, bronchoalveolar lavage fluid (BALF), blood, and lung samples were collected for testing.
Click to Show/Hide
|
||||
| Response Description | Salidroside postconditioning attenuates ferroptosis-mediated lung ischemia-reperfusion injury by activating the Nrf2/SLC7A11 signaling axis. | ||||
Curcumin
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [111] | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MDCK cells | Normal | Canis lupus familiaris | CVCL_0422 | |
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks old) were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China). Briefly, mice were anesthetized and placed in a prone position with head stabilization in a stereotaxic frame. A dental drill was then utilized to generate a 1 mm burr hole at 2.0 mm to the lateral right of the bregma and 3.5 mm deep of the brain. Next, acute ICH was induced by slowly injecting 0.1U of type IV collagenase into this hole.
Click to Show/Hide
|
||||
| Response Description | Curcumin in NPs (Cur-NPs) were shown to suppress erastin-induced ferroptosis in HT22 murine hippocampal cells. Cur-NPs effectively regulated the expression levels of HMOX1 and NFE2L2, which indicated that it might inhibit the ROS production through regulating the NRF2/HO-1 pathway. Cur-NPs served as an effective treatment for Intracerebral hemorrhage owing to their ability to inhibit ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [122] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Two-month-old male New Zealand rabbits purchased from the Medical Experimental Animal Center of Bengbu Medical College were used as experimental subjects. Streptozotocin was dissolved in sterile saline and intraperitoneally injected into the rabbits at a dose of 80 mg/kg. The rabbits were allowed to eat freely after receiving the injection. The fasting blood glucose levels of the rabbits were monitored regularly. The diabetic rabbit model was considered successfully established when the fasting blood glucose level was measured as 11 mmol/L twice or 14 mmol/L once. Following successful modelling, grouping was performed as follows: blank control group (Con-Group), diabetic rabbit group (DM-Group), diabetic rabbit + every other day curcumin administration group (Qod-Group), and diabetic rabbit + daily administration group (Qd-Group).
Click to Show/Hide
|
||||
| Response Description | Curcumin can promote the nuclear translocation of Nrf2, increase the expression of oxidative scavenging factors, such as HO-1, reduce excessive Gpx4 loss, and inhibit glucose-induced ferroptosis in cardiomyocytes. This highlights a potentially new therapeutic route for investigation for the treatment diabetic cardiomyopathy. | ||||
Edaravone
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [112] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
Seventy-three specific-pathogen-free (SPF)grade healthy male Sprague Dawley (SD) rats, weighing 240 ± 20 g, were purchased from Hunan Slake Jingda Experimental Animal Co., Ltd., China (animal certificate number SCXK (Xiang) 2013-0004). The animals were reared in an SPF animal laboratory, and the ambient temperature was maintained at 23 ± 1 . All protocols followed the ARRIVE guidelines in terms of study design, sample size, randomization, outcome measures, data analysis, experimental procedures, and reporting of results. This study was approved by the Animal Ethics Committee of the Hunan University of Chinese Medicine.
Click to Show/Hide
|
||||
| Response Description | Edaravone inhibits ferroptosis to attenuate cerebral ischemia-reperfusion injury, probably through the activation of the Nrf2/FPN pathway. | ||||
Astragaloside IV
[Investigative]
| In total 5 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [113] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Rats were randomly assigned to six groups: (1) the sham group, (2) the middle cerebral artery ischaemia-occlusion-reperfusion (MCAO/R) group, (3) the AST IV group, (4) the PNS group, (5) the combination group and (6) the combination + brusatol group. One hundred rats were used in the experiment, of which 9 died during surgery, 10 died of intracranial haemorrhage and brain injury and 63 rats were successfully modelled, for a final success rate of 76.8%. Each group included 9 rats. Behavioural testing was performed on 5 animals in each group. After behavioural testing, 3 rats were used for TTC staining and 6 were used for kit detection and western blot analysis. Existing studies have revealed the toxicological effects of the compatibility of astragalus and P. notoginseng. The dosage and method of AST IV (28 mg/kg) and PNS (80 mg/kg) alone or in combination have been previously determined and were administered intragastrically for three consecutive days (10 ml/kg each time), and the optimal administration times were 50, 26 and 2 h before model establishment.Brusatol (1 mg/kg) was administered intraperitoneally for 1 h prior to modelling. The sham group and the MCAO/R group were given the same amount of saline.
Click to Show/Hide
|
||||
| Response Description | Combining Astragaloside IV and Panax notoginseng saponins attenuates cerebral ischemia-reperfusion injury by activating Nrf2 to inhibit ferroptosis and inflammatory responses. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [116] | ||||
| Responsed Disease | Cerebral ischaemic stroke [ICD-11: 8B11] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
1% sodium pentobarbital (40 mg/kg) was administered to the rats intraperitoneally to anesthetize them before placing them in a brain stereotaxic device. An incision was created in the midline of the neck to expose the common internal and external carotid arteries. After ligating and cutting the external carotid artery on the left side, a 3-mm stump was exposed. We then perforated the carotid artery at the bifurcation of the middle and anterior cerebral arteries utilizing an 18-20-mm-long surgical filament (0.26 mm diameter; Beijing Cinontech Co. Ltd., China) was threaded through the external carotid artery stump into the internal carotid artery and left in situ for 120 min. After that, the filament was withdrawn to facilitate reperfusion. Rats in the sham surgery group received the identical procedure as the other rats but without filament insertion.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (AS-IV) administration decreased the infarct volume, brain edema, neurological deficits, and inflammatory cytokines TNF-, interleukin-1 (IL-1), IL-6, and NF-B, increased the levels of SLC7A11 and glutathione peroxidase 4 (GPX4), decreased lipid reactive oxygen species (ROS) levels, and prevented neuronal ferroptosis. Meanwhile, AS-IV triggered the Nrf2/HO-1 signaling pathway and alleviated ferroptosis due to the induction of stroke. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Response of This Regulator | [123] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
A total of 24 SD male rats weighing 200-210 g from the Vital River Laboratory Animal Technology Co., Ltd. were divided randomly into control, ADR, ADR+AsIV, and AsIV group (n = 6). AsIV was administered by gavage at a dose of 10 mg/kg/day over a period of five weeks. ADR was administered intraperitoneally once a week (30 mg/kg/week) for five weeks. Controls were administered saline intraperitoneally (i.p.) at the same dose as ADR and intragastrically at the same dose as AsIV.
Click to Show/Hide
|
||||
| Response Description | Adriamycin (ADR) was found to promote cardiac ferroptosis, whereas administration of Astragaloside IV (AsIV) attenuated the process via activating Nrf2 signaling pathway and the subsequent GPX4 expression increasing. These results suggest that AsIV might play a protective role against ADR-induced myocardial fibrosis. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Drug Response of This Regulator | [144] | ||||
| Responsed Disease | Urinary system disease [ICD-11: GC2Z] | ||||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male SD rats (200 ± 10 g) were obtained from Beijing Vital River Company (Beijing, China). They were kept under 12-h light/dark cycles and allowed free access to food and water. Rats were randomly divided into CON, ADR, ADR + ASIV, and ASIV groups (n = 6). Rats in ADR and ADR + ASIV groups received four equal injections of ADR intraperitoneally (4 mg/kg) in 5 weeks. Rats in ASIV and ADR + ASIV groups intragastrically received ASIV (10 mg/kg, daily) for 5weeks, while rats in CON and ADR groups were administered the same dose of solvent as ADR. Finally, the rats were euthanized, and the bilateral kidneys were excised.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV increased the phosphorylation of Pi3K, Akt, and the expression of Nrf2 and glutathione peroxidase 4 compared to HK-2 cells stimulated by ADR. In conclusion, ferroptosis may involve in Adriamycin (ADR)-induced nephrotoxicity, and ASIV might protect nephrocytes against ADR-induced ferroptosis, perhaps via activations of the Pi3K/Akt and Nrf2 signaling pathways. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Drug Response of This Regulator | [147] | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hT2AECs (Type II alveolar epithelial cells) | ||||
| In Vivo Model |
The animals were randomly assigned to six groups (7 mice in each) as follows: (I) Normal saline (NS) group, (II) Ast-IV 100 mg/kg (Ast) group, (III) PM2.5 group, (IV) Ast-IV 50 mg/kg + PM2.5 (Ast-L) group, (V) Ast 100 mg/kg + PM2.5 (Ast-H) group, and (VI) Ast-IV 100 mg/kg + erastin 20 mg/kg + PM2.5 (Era) group. Based on our previous results, this study adopted anintraperitoneal injection(i.p.) of Ast-IV (dissolved in normal saline containing 0.1% DMSO for preventive treatment. After all the mice were adaptively fed for 5 days, in the NS and PM2.5 groups, mice received the normal saline containing 0.1% DMSO viai.p.once a day for the next three consecutive days. Similar to the NS group, in the Ast, Ast-H, and Era groups, mice received Ast-IV (100 mg/kg) viai.p. Ast-L group received Ast-IV (50 mg/kg) viai.p. To evaluate the effect of Ast-IV on ferroptosis in PM2.5-induced lung injury, we used the ferroptosis agonist erastin to activate ferroptosisin vivo. In the Era group, mice received erastin (20 mg/kg, 10% DMSO + 40% PEG300 + 5%Tween80 + 45% normal saline) 30 min before each preventive treatment of Ast-IV.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (Ast-IV) reduced the lung wet-dry ratio and the levels of interleukin 6 (IL-6), tumor necrosis factor- (TNF-) and interleukin 1 (IL-1) in serum. Ast-IV could also improve the oxidative stress level in BALF, restore the GSH level in the lung tissue, and reduce the iron content in the lung tissue. Western blot outcomes revealed that Ast-IV regulated the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to protect PM2.5-mediated lung injury. | ||||
Panax notoginseng saponins
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [113] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Rats were randomly assigned to six groups: (1) the sham group, (2) the middle cerebral artery ischaemia-occlusion-reperfusion (MCAO/R) group, (3) the AST IV group, (4) the PNS group, (5) the combination group and (6) the combination + brusatol group. One hundred rats were used in the experiment, of which 9 died during surgery, 10 died of intracranial haemorrhage and brain injury and 63 rats were successfully modelled, for a final success rate of 76.8%. Each group included 9 rats. Behavioural testing was performed on 5 animals in each group. After behavioural testing, 3 rats were used for TTC staining and 6 were used for kit detection and western blot analysis. Existing studies have revealed the toxicological effects of the compatibility of astragalus and P. notoginseng. The dosage and method of AST IV (28 mg/kg) and PNS (80 mg/kg) alone or in combination have been previously determined and were administered intragastrically for three consecutive days (10 ml/kg each time), and the optimal administration times were 50, 26 and 2 h before model establishment.Brusatol (1 mg/kg) was administered intraperitoneally for 1 h prior to modelling. The sham group and the MCAO/R group were given the same amount of saline.
Click to Show/Hide
|
||||
| Response Description | Combining Astragaloside IV and Panax notoginseng saponins attenuates cerebral ischemia-reperfusion injury by activating Nrf2 to inhibit ferroptosis and inflammatory responses. | ||||
Caryophyllene
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [115] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rPAs (Rat primary astrocytes) | ||||
| In Vivo Model |
Rats were anesthetized withisoflurane(2-3% oxygen) and placed in asupine position. And theright common carotid artery (CCA),external carotid artery (ECA), andinternal carotid artery (ICA) were exposed in sequence and separated carefully. Then we ligated the CCA and the ECA in turn, and at the same time, we clamped the internal carotid artery with an arterial clamp. Finally, we inserted a silicone nylon monofilament from the CCA into themiddle cerebral arteryand temporarily fixed it. After 1.5 h ofischemia, the monofilament was taken out and the blood vessels were ligated at theincision. The neck wound was sutured with surgical sutures. Subsequent experiments were performed after 12 h ofreperfusion. In thesham operationrats, except for the absence of the monofilament, the sham operation rats underwent the same surgical procedures as the MCAO/R model rats.
Click to Show/Hide
|
||||
| Response Description | Our results indicated the critical role of ferroptosis in cerebral ischemia reperfusion injury. For the first time, we showed that the significant neuroprotective effects of b-Caryophyllene (BCP) in attenuating ischemic stroke injury are correlated with ferroptosis regulation, and its mechanism is associated with activation of the NRF2/HO-1 axis. | ||||
Kaempferol
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [117] | ||||
| Responsed Disease | Cerebral ischaemic stroke [ICD-11: 8B11] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPCNs (Mouse primary cortical neurons) | ||||
| Response Description | Kaempferol provides protection from OGD/R-induced ferroptosis, at least in part, by activating Nrf2/SLC7A11/GPX4 signaling pathway. Therefore, pharmacological inhibition of ferroptosis may be an attractive therapeutic target for the treatment of ischemic stroke. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [151] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male BALB/c mice (8-week-old, 20-22 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China). The experimental animals were fed adaptively for one week in the Experimental Animal Center of Guangdong Pharmaceutical University (Guangzhou, China). Feeding conditions were set at 26 , humidity 65% and a lightdark cycle for 12 hours. All animal experiments were performed following the Guide for the Care and Use of Laboratory Animals, and the procedures were approved by the Research Ethical Committee of Guangdong Pharmaceutical University (gdpulacspf2020007).
Click to Show/Hide
|
||||
| Response Description | Kaempferol (KA) activated the Nrf2 pathway and upregulated Gpx4 in mouse livers and L02 cells to inhibit ferroptosis induced by APAP. Finally, molecular docking indicated the potential interaction of KA with Keap1. Taken together, KA ameliorated oxidative stress and ferroptosis-mediated acetaminophen-induced liver injury by activating Nrf2 signaling. | ||||
Ajudecunoid C
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [118] | |||
| Responsed Disease | Nervous system disease [ICD-11: 8E7Z] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Ajudecunoid C effectively prevented ferroptosis through scavenging free radical and activating NRF2-antioxidant response elements (AREs) pathway. This study reveals that ADC, as a new ferroptosis inhibitor, is a promising lead compound for the development of drugs against ferroptosis-related neurological diseases. | |||
Artesunate
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [120] | |||
| Responsed Disease | Extraocular muscles disorder [ICD-11: 9C82] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hOFs (Human ocular fibroblasts) | |||
| Response Description | Expression of mitochondrial GPX4 but no other forms of GPX4 was decreased after artesunate treatment and that mitochondrial GPX4 overexpression rescued artesunate-induced lipid peroxidation and ferroptosis. Other cellular ferroptosis defense mechanisms, including cellular FSP1 and Nrf2, were also inhibited by artesunate. In conclusion, our study demonstrated that artesunate protects against fibrosis through abrogation of fibroblast activation and induction of mitochondria-dependent ferroptosis in ocular fibrosis, which may offer a potential treatment for ocular fibrosis. | |||
Berberine
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [121] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response Description | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [158] | ||||
| Responsed Disease | Health [ICD-11: N.A.] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| Response Description | Berberine can inhibit erastin-induced ferroptosis in HT22 cells possibly by activating the Nrf2-HO-1/ GPX4 pathway. | ||||
Dihydroquercetin
[Preclinical]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [124] | ||||
| Responsed Disease | Chronic obstructive pulmonary disease [ICD-11: CA22] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HBE1 cells | Normal | Homo sapiens | CVCL_0287 | |
| In Vivo Model |
Thirty-two male BALB/c mice (21-25 g, 6-8 weeks) were purchased from Hunan Slyke Jingda Laboratory Animal Co., Ltd. and kept in a clean unit at 23 ± 2 , 50% ± 10% relative humidity and 12 h rhythm of light and dark. Mice were randomly divided into four groups (n = 8 for each group): the control group, cigarette smoke-inducedCOPDgroup, COPD + low dose (50mg/kg/d)DHQgroup, and COPD + high dose (100 mg/kg/d) DHQ group. The mice in the control group were maintained in fresh air and given anintraperitoneal injectionof 0.3 ml/20 g phosphate-buffered saline (PBS) on Days 0, 11, and 23. The COPD mouse model was established as previously described. Mice in this group were exposed to cigarette smoke for 2 cycles per day (1 h per cycle), 6 days per week for 4 consecutive weeks in a sealed box with ventilation holes except for Days 0, 11, and 22, and over these 3 days, the mice were intraperitoneally injected with 0.3 ml/20g 100% CSE. Mice in the COPD+low-dose DHQ group and COPD+high-dose DHQ group were treated with cigarette smoke and 100% CSE as mentioned above and intraperitoneally injected with DHQ for 25 consecutive days except for Days 0, 11, and 22, while mice in the control and COPD groups were intraperitoneally injected with an equal volume of PBS except for Days 0, 11, and 22. All mice were sacrificed by intraperitoneal injection of 0.5 ml 3% chloral hydrateon the 29 th day of the experiment.
Click to Show/Hide
|
||||
| Response Description | Treatment with DHQ (Taxifolin) significantly reverses the ferroptosis induced by cigarette smoke both in vivo and in vitro via a Nrf2-dependent signaling pathway. These findings may provide novel therapeutic options for the treatment of chronic obstructive pulmonary disease (COPD) patients. | ||||
Glycyrrhizin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [125] | ||||
| Responsed Disease | Hepatoblastoma [ICD-11: DB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
In total, 40 male specific- pathogen-free C57BL/6 mice (Hubei Animal Experimental Center) 6-8 weeks old. The mice were randomly divided into 5 groups: The normal group, model group, 15 mg/kg GLY group, 30 mg/kg GLY group and 60 mg/kg GLY group. Except for the normal group, the other four groups of mice were injected intraperitoneally with D-GalN (400 mg/kg) and LPS (100 ug/kg) to induce the ALF model. According to a previous study on GLY gavage doses, three doses of GLY (15, 30 and 60 mg/kg/day) intervention groups were used. A total of 24 mice were divided into three groups. Mice received gavage with different doses of GLY for 3 days before induction of the ALF model.
Click to Show/Hide
|
||||
| Response Description | The HMGB1 inhibitor glycyrrhizin (GLY) significantly reduced the degree of ferroptosis during acute liver failure (ALF) by inhibiting oxidative stress. Treatment with GLY reduced the degree of liver damage, the expression of HMGB1 was decreased, and the levels of Nrf2, HO1 and GPX4 were increased. | ||||
Ginkgolide B
[Terminated]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [126] | ||||
| Responsed Disease | Nonalcoholic fatty liver disease [ICD-11: DB92] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| In Vivo Model |
Male 8-week-old C57/BL6 ApoE-/-mice of weight (22~25 g) were purchased from Changzhou Cavens experimental animal Co., Ltd (Jiangsu, China). After 5 weeks of feeding, HFD-fed mice were randomly assigned into 4 groups (n = 10) : HFD group (0.9 % sodium chloride by gavage), GB-L group (at a high dose of 20 mg kg-1d-1 GB in 0.9 % sodium chloride by gavage), GB-H group (at a high dose of 30 mg kg-1d-1 GB in 0.9 % sodium chloride by gavage), and Ato group (1.3 mg kg-1d-1 Ato in 0.9 % sodium chloride by gavage) as a positive control. The mice in ND group were given the same volume of 0.9 % sodium chloride.
Click to Show/Hide
|
||||
| Response Description | Ginkgolide B (GB), a main constituent of Ginkgo biloba extracts, reduces hepatic lipid accumulation and ameliorates nonalcoholic fatty liver disease (NAFLD) in obese mice. Remarkably, after Nrf2 interference, GB treatment significantly increased Nrf2 expression, indicating that GB exerted anti-ferroptosis effects by activation of Nrf2 pathway. | ||||
Etomidate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [127] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rMTs (Rat myocardial tissues) | ||||
| In Vivo Model |
Male Sprague-Dawley rats (8 weeks, 180 g-210 g) were provided by the experimental animal center of Beijing Institute of Life Sciences. Rats were anesthetized with 2.5% sodium pentobarbital and fixed in supine position. The LAD was ligated using a 6-0 silk for a 30 min ischemic period. Ischemia was confirmed by discoloration of heart surface and ST elevation on the electrocardiogram (ECG) recording. After 30 min, the LAD ligation was released and the reperfusion was continued for 3 h, which was confirmed by the redness of the heart surface and the decrease in ST recorded by ECG. Rats in Sham group (n = 12) underwent the same surgical procedures, except that LAD was threaded but not ligated.
Click to Show/Hide
|
||||
| Response Description | Etomidate (Eto) attenuated MIRI-induced heart failure, pathological damage, myocardial fibrosis, andinflammation, which may be related to its inhibition onferroptosis. Mechanically, the protection of Eto in myocardial ischemia reperfusion (MIR) injury (MIRI) may be achieved by activating Nrf2 pathway. | ||||
Gossypol acetic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [128] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
A total of 55 adult male Sprague-Dawley rat (350-450 g) were anesthetized with urethane (1.5 g/kg, i.p.), then the hearts were perfused in a Langendorff system. After 30 min of stabilization, hearts were subjected to 30 min of global no-flow ischemia by stopping the perfusion. Reperfusion was followed with Krebs Henseleit (KH) buffer and GAA together for 2 h. A thermoregulated chamber kept the heart at 37 throughout the experiment. Control hearts were not subjected to I/R. The heart slices were sectioned at a thickness of 2 mm and stained with triphenyltetrazolium chloride (25 mg/100 mL) for 10 min and then fixed with 4% formaldehyde solution for 48 h to enhance color contrast.
Click to Show/Hide
|
||||
| Response Description | Gossypol acetic acid significantly attenuated myocardial infarct size, reduced lipid peroxidation, decreased the mRNA levels of the ferroptosis markers Ptgs2 and Acsl4, decreased the protein levels of ACSL4 and NRF2, and increased the protein levels of GPX4 in I/R-induced ex vivo rat hearts. Thus, GAA may play a cytoprotectant role in ferroptosis-induced cardiomyocyte death and myocardial ischemia/reperfusion-induced ferroptotic cell death. | ||||
Histochrome
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [129] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mCMs (Mouse cardiomyocytes) | ||||
| In Vivo Model |
Male Fischer 344 rats (8 weeks old and 160 to 180 g; KOATECH, Pyeongtaek-si, Korea) were anesthetized by inhalation with 2% isoflurane and intubated using an 18-gauge intravenous catheter. The rats were mechanically ventilated with medical-grade oxygen. Surgery was performed on a 37 heating pad to prevent the body from getting cold. A left thoracotomy was performed after the chest was shaved to prevent contamination during surgery.
Click to Show/Hide
|
||||
| Response Description | Histochrome treatment significantly increased GPx4 and free GSH levels, but decreased Cox-2 level. HC treatment significantly decreased intracellular and mitochondrial ROS levels by upregulating the expression of Nrf2 and antioxidant genes. The substantial cardioprotective effects of HC against myocardia I/R injury by reducing ferroptosis-associated myocardial injury. | ||||
Naringenin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [130] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
20 Sprague Dawley (SD) rats (6-8 weeks, 200-220 g) were acquired from the Second Clinical College of Guangzhou University of Traditional Chinese Medicine, and were weighed, coded, and randomly assigned to experimental groups. Rats were divided into Sham group, MI/R group, MI/R +NAR (low dose, 10 mg/kg/d) group, and MI/R +NAR (high dose, 50 mg/kg/d) group. For MI/R model, rats were anaesthetized by intraperitoneal injection of 1% pentobarbital sodium (60 mg/kg) and then received mechanical ventilation from an animal ventilator after endotracheal intubation.
Click to Show/Hide
|
||||
| Response Description | Naringenin alleviated MI/R-induced pathological damage, inflammation and lipid peroxidation in myocardial tissue of rats. NAR adjusted the NRF2 /System xc - /GPX4 axis and improved ferroptosis. In conclusion, NAR can alleviate myocardial ischemia-reperfusion injury by regulating the Nrf2/System xc-/GPX4 axis to inhibit ferroptosis. | ||||
Dimethyl fumarate
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [132] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
The mice were randomly divided into four groups of six: sham + vehicle, sham + DMF, IR + vehicle, and IR + DMF. The mice were supplemented with DMF at a concentration of 100 mg/kg or DMSO by daily oral gavage for a week before surgery, as previously reported. As stated in a prior study, the partial warm liver IRI model was developed. Briefly, the sham group only had free hepatic portal blood vessels after laparotomy, and the blood flow was not obstructed. As for the hepatic IR group, the blood supply to the left and mid-hepatic lobes was blocked, resulting in 70% mouse liver IRI for 90 min. The mice were put on a heated blanket after surgery in order to maintain body temperature and monitor vital signs. Blood supply was restored for 6 h. Died mice were eliminated for testing prior to sample collection. The mice were euthanized after the sample were obtained. The same experimenter carried out all surgeries.
Click to Show/Hide
|
||||
| Response Description | NRF2 knockdown notably decreased the expression of SLC7A11 and HO-1 and blocked the anti-ferroptosis effects of dimethyl fumarate (DMF). DMF inhibits ferroptosis by activating the NRF2/SLC7A11/HO-1 axis and exerts a protective effect against hepatic ischemia-reperfusion injury. | ||||
Ginsenoside Rg3
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [134] | ||||
| Responsed Disease | Acute pancreatitis [ICD-11: DC31] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AR42J cells | Digestive system neoplasms | Rattus norvegicus | CVCL_0143 | |
| In Vivo Model |
Male C57BL/6 mice (8 weeks old; SPF; weighing 24-26 g, n = 16 in total) were purchased from SPF (Beijing) Biotechnology Co., Ltd. All mice were housed in an environmentally controlled room at a temperature ranging from 20 to 24 on a 12 h light/dark cycle and used in the experiments following an overnight fast with water, availablead libitum. All procedures followed the Principles of Laboratory Animal Care (NIH publication number 85Y23, revised in 1996), and the experimental protocol was approved by the Animal Care Committee, Nanjing Medical University (NMU-2021JK-085).
Click to Show/Hide
|
||||
| Response Description | Taken together, the present study, to the best of our knowledge, is the first to reveal a protective role for Ginsenoside Rg3 in mice with acute pancreatitis by suppressing oxidative stressrelated ferroptosis and the activation of the NRF2/HO1 pathway. | ||||
Tert-Butylhydroquinone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [135] | ||||
| Responsed Disease | Ulcerative colitis [ICD-11: DD71] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HIEC-6 cells | Normal | Homo sapiens | CVCL_6C21 | |
| In Vivo Model |
Male C57/BL6 mice (eight weeks old, 18-20 g) were purchased from Chengdu Dossy Experimental Animals (China). All the mice were housed in plastic cages with free access to food and water at 25 with a 12 h light/dark cycle. The mice were randomly divided into four groups (n = 6 mice/group): the control group, 5-FU group, 5-FU + TBHQ group, and 5-FU + Fer-1 group. 50 mg/kg body weight 5-FU was intraperitoneally (i.p.) injected into the mice of the 5-FU, 5-FU + TBHQ and 5-FU + Fer-1 group per day for five days to induce intestinal mucositis. Starting on the same day, the mice in the 5-FU + TBHQ and 5-FU + Fer-1 group were treated with TBHQ (10 mg/kg body weight; in DMSO, i.p. injection) or Fer-1 (2.5 mol/kg body weight; in DMSO; i.p. injection) once daily for eight days (days 18). Starting on the same day, the mice of the 5-FU group were treated with equivalent volumes of dimethyl sulfoxide (DMSO) for eight days.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis was shown to be involved in 5-FU-induced intestinal mucositis, and Tertiary butylhydroquinone markedly hampered its activation. Mechanistically, TBHQ activated Nrf2 effectively and selective Nrf2 knockdown significantly reduced the anti-ferroptotic functions of TBHQ in 5-FU-treated HIECs. | ||||
Astragalus polysaccharide
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [136] | ||||
| Responsed Disease | Ulcerative colitis [ICD-11: DD71] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | |
| In Vivo Model |
Six-eight-week-old male C57BL/6 mice weighing 19.74 ± 0.77 g were purchased from Shanghai Laboratory. To investigate the therapeutic effect of APS on DSS-induced colitis, mice were randomly divided into the following 5 groups (n = 5 each): control, DSS, DSS + APS (100 mg/kg), DSS + APS (200 mg/kg), and DSS + APS (300 mg/kg). The mice in the DSS + APS (100 mg/kg), DSS + APS (200 mg/kg), and DSS + APS (300 mg/kg) groups were intraperitoneally injected with 100, 200, and 300 mg/kg APS once a day, respectively from day 3 to day 10.
Click to Show/Hide
|
||||
| Response Description | The therapeutic effects of Astragalus polysaccharide on DSS-induced Ulcerative colitis by blocking ferroptosis in IECs. Furthermore, our results revealed that APS-mediated inhibition of ferroptosis was associated with the NRF2/HO-1 pathway. | ||||
Biochanin A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [137] | ||||
| Responsed Disease | Knee osteoarthritis [ICD-11: FA01] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCDs (Chondrocytes) | ||||
| In Vivo Model |
Male mice were purchased from Guangzhou University of Chinese Medicine's Experimental Animal Center C57BL/6 mice (7-week-old, 20 g) (Guangzhou, China). After one week of adaptively feeding with chow meals and sterilized water, the animals were separated into five groups of ten mice randomly assigned to the negative control (NC); model, positive control (PC); model group; high dosage of BCA treatment (BCA-H) group; and low dosage of BCA treatment (BCA-L) group. The iron overload mice model was designed based on earlier research. Except for the NC group, mice were administered ID intraperitoneally (500 mg/kg) once a week for eight weeks. In the right knee joints, OA was induced with the initial injection of iron dextran two weeks after the injection by destabilizing the medial meniscus (DMM) using a microscope. After the operation, the positive control group was administered with NAC intragastrically (100 mg/kg) for eight weeks. BCA-H and BCA-L groups were administered 20 mg/kg and 40 mg/kg of BCA separately for eight weeks according to previous studies.
Click to Show/Hide
|
||||
| Response Description | Biochanin A (BCA) could directly reduce intracellular iron concentration by inhibiting TfR1 and promoting FPN but also target the Nrf2/system xc-/GPX4 signaling pathway to scavenge free radicals and prevent lipid peroxidation. The results of this research indicate that BCA regulates iron homeostasis during the progression of osteoarthritis, which can open a new field of treatment for knee osteoarthritis. | ||||
Hesperidin
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [138] | ||||
| Responsed Disease | Intervertebral disc degeneration [ICD-11: FA80] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| NF-kappa B signaling pathway | hsa04064 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hNPCs (Human nucleus pulposus cells) | ||||
| In Vivo Model |
Male C57BL/6 mice (10-12 weeks) were devoted to generate needle puncture-induced intervertebral disc degeneration model. For intervertebral disc degeneration (IVDD) treatment, hesperidin was administratedorally and the dose was calculated in reference to previous studies using themetrological conversion formula between human and mouse. The dosein mice is = 5.5 mg/kg x 70 kg x 0.0026/20g = 9.1 x 5.5 mg/kg = 50.05 mg/kg ~50 mg/kg.
Click to Show/Hide
|
||||
| Response Description | Hesperidin may protect HNP cells from degeneration by suppressing ferroptosis in an oxidative stress-dependent via enhancing the expression of Nrf2 and suppressing the NF-B pathway. The evidence will provide a possible basis for future targeted treatment for intervertebral disc degeneration. | ||||
Melatonin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [139] | ||||
| Responsed Disease | Osteoporosis [ICD-11: FB83] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MC3T3-E1 cells | Normal | Mus musculus | CVCL_0409 | |
| In Vivo Model |
Eight-week-old specific-pathogen-free Sprague Dawley rats weighing 220 ± 20 g were purchased from China Medical University, Department of Experimental Animals. A total of 60 rats were used to determine the targets of bone histomorphometry; 45 rats were used to establish a diabetic model, and remaining 15 rats were divided into a control group. The diabetic rats were divided into three groups (n = 15 each) treated with intraperitoneal injection of high-dose melatonin (50 mg/kg, HMT group), intraperitoneal injection of low-dose melatonin (10 mg/kg, LMT group), and a control T2DM group.
Click to Show/Hide
|
||||
| Response Description | High glucose induces ferroptosis via increased ROS/lipid peroxidation/glutathione depletion in type 2 diabetic osteoporosis. More importantly, melatonin significantly reduced the level of ferroptosis and improved the osteogenic capacity of MC3T3-E1 through activating the Nrf2/HO-1 pathway in vivo and in vitro. | ||||
Busulfan
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [140] | ||||
| Responsed Disease | Male infertility [ICD-11: GB04] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mTTs (Mouse testicular tissues) | ||||
| In Vivo Model |
Eight-week-old healthy ICR male mice, weighted 20-24 g, were provided by Experimental Animal Center of Nantong University (Nantong, China). For the first animal study, eight-week-old ICR male mice were randomly assigned to four groups: control, busulfan, busulfan plus Fer-1 and busulfan plus DFO groups (n = 6 per group). Mice were anesthetized and then given testicular injection of busulfan on both sides at the dose of 4 mg/kg body weight. The solution containing busulfan was directly injected from the scrotum into testicular transverse diameter. Fer-1 and DFO were administered by intraperitoneal injectionat concentrations of 1 mg/kg and 30 mg/kg respectively three times a week after busulfan injection. Four weeks later, the epididymal spermatozoa and testes from all mice were collected for assessment.
Click to Show/Hide
|
||||
| Response Description | Busulfan treatment induced spermatogenic cells ferroptosis by down-regulating nuclear factor-E2-related factor 2 (Nrf2) and glutathione peroxidase 4 (GPX4) expressions, and decreasing iron efflux through reduction of ferroportin 1 (FPN1) expression. Targeting ferroptosis serves as a potential strategy for prevention of busulfan-induced damage and male infertility. | ||||
Aristololactam
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [141] | |||
| Responsed Disease | Aristolochic acid nephropathy [ICD-11: GB55] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 |
| Response Description | Long-term administration of medicine-containing Aristolactam I (ALI) was reported to be related to aristolochic acid nephropathy (AAN), which was attributed to ALI-induced nephrotoxicity. ALI dose-dependently inhibited these protein contents of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), and glutathione peroxidase 4 (GPX4), which could be partly rescued by Tin-protoporphyrin IX (SnPP) and mitoTEMPO co-treatment. | |||
Dioscin
[Preclinical]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [142] | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Six-week-old male Wistar rats (170-200 g) were obtained from Changsheng Biotechnology Co., Ltd. (Changchun, China), and all of them were fed under SPF-conditions. The rats were acclimatized to natural light/dark cycles at a controlled temperature of 22 + 2 with free access to food and water. The experiment was comprised of four groups: the C group (0.5% carboxymethyl cellulose sodium [CMC-Na], n = 6); the Dio group (dioscin-treated rats, n = 6); the CP group (cisplatin-treated mice, n = 6); and the Dio + CP group (dioscin plus cisplatin-treated rats, n = 6). Rats were gavaged with dioscin (60 mg/kg) for ten days, and cisplatin (10 mg/kg) was intraperitoneally injected once on the seventh day.
Click to Show/Hide
|
||||
| Response Description | Dioscin exerts a reno-protective effect by decreasing renal oxidative injury, apoptosis and ferroptosis through the Nrf2/HO-1 signaling pathway, providing a new insight into acute kidney injury prevention. | ||||
Pachymic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [143] | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mKTs (Mouse knee tissues) | ||||
| In Vivo Model |
A total of 30 C57BL/6 male mice (8-10 weeks; 20-25 g body weight) were purchased from Chongqing Medical University (Chongqing, China). The mice were anesthetized with 50-60 mg/kg of pentobarbital sodium (cat. no. P3761; Sigma-Aldrich; Merck KGaA) by intraperitoneal injection; the skin at the surgical area was wiped with 70% alcohol. The incision was positioned at the left and right sides of the spine (0.5 cm), and the incision length was 1-1.5 cm along the back. The kidneys were subsequently pulled out from the incision to expose the renal pedicle. A microaneurysm clip was used to clamp the pedicle to block the blood flow to the kidney and induce renal ischemia. Complete ischemia was indicated by a change in the color of the kidney from red to dark purple within a few seconds. After 40 min of ischemia, the microaneurysm clips were released to allow each kidney to start reperfusion, which was indicated by the change of the kidney color to red.
Click to Show/Hide
|
||||
| Response Description | Pachymic acid has a protective effect on ischemiareperfusion induced acute kidney injury in mice, which may be associated with the inhibition of ferroptosis in the kidneys through direct or indirect activation of NRF2, and upregulation of the expression of the downstream ferroptosis related proteins, GPX4, SLC7A11 and HO1. | ||||
Echinatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [145] | ||||
| Responsed Disease | Cognition disorder [ICD-11: MB21] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | rPHNs (Rat primary hippocampal neurons) | ||||
| In Vivo Model |
The Sprague-Dawley rats (male, 20-month-old, 550-700 g, n = 6 per group) were obtained from the Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijng, China). A total of 78 rats were used in animal experiments. Rats were allocated into the following five experimental groups: control, Sev, Sev + Ech (L), Sev + Ech (M), and Sev + Ech (H). Ech (Sigma-Aldrich; purity 98%) was given to rats by intraperitoneal injection as a single dose of 20 (L), 40 (M), or 80 mg/kg (H) at 1 h before Sev exposure. The injection volume of each rat was 5 mL. For control and Sev groups, an equal volume of vehicle was intraperitoneally injected into rats. Then, rats except for the control group were anaesthetised with 2% Sev (Sigma-Aldrich) for 5 h. The histological and biochemical analysis of the hippocampus was done 48 h later after the rats were sacrificed and the brains were removed.
Click to Show/Hide
|
||||
| Response Description | Echinatin (Ech) could mitigate Sev-induced apoptosis, oxidative stress, and ferroptosis in hippocampal neurons and hippocampus of rats by activating Nrf2 signalling. Moreover, Ech improved Sev-induced cognitive deficits in aged rats. These findings suggested that Ech may be developed as a neuroprotective agent to reduce postoperative cognitive dysfunction in the clinic. | ||||
Pyridoxine
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [146] | ||||
| Responsed Disease | Myocardial injury [ICD-11: NB31] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male c57BL/6 mice (8 weeks old) were purchased from Beijing Wei Tong Li Hua Experimental Animal Technology Co. Ltd. (Beijing, China). Mice were divided into control (n = 8), LPS (n = 9), and VitB6+LPS (n = 9) groups. Mice were pretreated with PBS or VitB6 for 6 h and then treated with LPS (4 mg/kg) for 24 h. Cardiac ultrasound was performed before sacrifice. Inhaled isoflurane was given to mice for volatile anesthesia and the chest hair was removed with a depilatory cream. Then, mice were fixed on the warmed imaging platform and wore with the coupling agent. The Vevo2100 imaging system, equipped with a 40-MHz high-frequency transducer (VisualSonics Inc., Toronto, Canada), was applied to perform non-invasive examinations. The M-mode echocardiogram at the parasternal long axis was used to obtain the ejection fraction (EF) of left ventricular and fractional shortening (FS).
Click to Show/Hide
|
||||
| Response Description | Vitamin B6 (VitB6) is a water-soluble vitamin and includes pyridoxine, pyridoxal, pyridoxamine, and their phosphorylated forms. VitB6 regulated the expression of LPS-induced apoptosis-related proteins and iron regulatory proteins. It mediated the expression of Nrf2, transcription factor NF-E2-related factor 2, which promoted the expression of antioxidant enzymes and restrained LPS-induced ferroptosis and apoptosis. Overall, VitB6 can be used on novel therapies to relieve LPS-induced myocardial injury. | ||||
Astaxanthin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [148] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Mice were randomly divided into four groups as follows (n = 5): (1) control, (2) APAP (MCE, Monmouth Junction, NJ, USA), (3) olive oil + APAP (oil + APAP), and (4) ASX (Energy Chemical, Shanghai, China) dissolved in olive oil + APAP (ASX + APAP). Astaxanthin was dissolved in olive oil to obtain a mixture of 20 mg/mL. Mice in groups 3 and 4 were given a dose of olive oil and a mixture of 5 mL/kgBW by gavage every day for 2 weeks. On day 15, mice in groups 2, 3, and 4 were given a peritoneal injection of 500 mg/kg APAP to induce liver injury. The mice were fasted for 12 h before the administration of APAP. Ten hours after APAP administration, blood and liver tissue were collected for further examination and analyses. Blood was centrifuged to obtain supernatants,which were stored at -80. Liver tissues were immediately removed from each animal, and homogenates were processed with formaldehyde and glutaraldehyde for protein and histological analysis.
Click to Show/Hide
|
||||
| Response Description | Astaxanthin reduced inflammation through the NF-B pathway, inhibited oxidative stress and ferroptosis, and increased autophagy through the Nrf2/HO-1 pathway, ameliorating acetaminophen-induced liver injury in vivo and in vitro. | ||||
Bicyclol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [149] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
The mice were treated with intraperitoneal administration (i.p.) of oil (control group) or a mixture of CCl4 (50%) and oil (50%) at a dosage of 2 ml/kg body weight. In the bicyclol-treated group, mice accepted administration of 200 mg/kg (using 0.5% carboxymethyl cellulose as solvent) by gavage three times a day 1 h before CCl4 exposure, while other groups accepted vehicles of the equal volume. Fer-1 was prepared in DMSO (5 mg/kg), andi.p. injected into mice once 1 h before CCl4 exposure. The dosage of bicyclol was consistent with our previous work. The mice were then sacrificed to collect liver and serum samples after 24 or 48 h.
Click to Show/Hide
|
||||
| Response Description | Bicyclol exerted its hepatoprotection by preventing the aforesaid ferroptotic process. Furthermore, bicyclol alleviated erastin-induced cellular inviability, destruction, and lipid peroxidation in vitro. Knockdown of GPX4 diminished these protective activities against perturbations associated with ferroptosis in L-O2 hepatocytes. Additionally, Nrf2 silencing drastically reduced GPX4 levels, and further impeded the medicinal effects of bicyclol. In summary, positively regulating Nrf2-GPX4 axis by bicyclol can prevent ferroptosis in CCl4-induced acute liver injury in mice. | ||||
Epigallocatechin Gallate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [150] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
Ulinastatin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice were from the Experimental Animal Center of Xian Jiaotong University. The animal experiment procedures were performed in accordance with the Guide of Laboratory Animal Care and Use from the United States National Institution of Health and were approved by the Laboratory Animal Care Committee (LACC) of Xian Jiaotong University, China (No. XJTULAC2017-207). Mice were initially housed for 7 days to adjust to the environment. The experimental design included five groups (n = 10 per group): the control group included the saline control (0.9% saline) group, and the test groups included APAP, APAP + UTI (5 x 104 units/kg and 1 x 105 units/kg), APAP + Fer-1 (10 mg/kg), and APAP + Res (50 mg/kg) treatments administered by tail vein or intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Ulinastatin plays a role in mitigation of APAP-induced acute liver injury by inhibiting ferroptosis-induced lipid peroxide accumulation, and the effect of UT1 was mediated by the NRF2/HO-1 pathway and SIRT1 expression. | ||||
Abietic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [152] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
APAP-induced liver injury model was induced byintraperitoneal injection 300 mg/kg APAP. The mice of APAP + abietic acid (10, 20, 40 mg/kg) were given abietic acid by intraperitoneal injection 1 h before APAP treatment. The doses of abietic acid used in this study were based on previous studies. Twelve hours later, the mice were sacrificed after anesthesia with 1%pentobarbital (50 mg/kg) injected intraperitoneally and the samples were collected.
Click to Show/Hide
|
||||
| Response Description | APAP could increase malondialdehyde (MDA) and Fe2+ levels, and decrease ATP and glutathione (GSH) levels, as well as glutathione peroxidase 4 (GPX4) and xCT expression. However, these changes induced by APAP were prevented by abietic acid, indicating abietic acid could inhibit APAP-induced ferroptosis. Furthermore, abietic acid inhibited APAP-induced liver injury, NF-B activation and increased the expression of Nrf2 and HO-1. | ||||
Atorvastatin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [153] | |||
| Responsed Disease | Muscle injury [ICD-11: ND36] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hCMs (Human cardiomyocytes) | |||
| C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| HUVECs (Human umbilical vein endothelial cells) | ||||
| Response Description | Atorvastatin suppressed the Nrf2, which would, in turn, inhibit the expression of System xc-(SLC7A11)and GPX4 (especially the mitochondrial GPX4), leading to a severe damage to the antioxidant system of ferroptosis.The datas point toward ferroptosis as an essential molecular mechanism leading to statin-induced muscle damage. | |||
References
