Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0125)
| Name |
Baicalein
|
||||
|---|---|---|---|---|---|
| Synonyms |
baicalein; 491-67-8; 5,6,7-Trihydroxyflavone; Noroxylin; 5,6,7-trihydroxy-2-phenyl-4H-chromen-4-one; Biacalein; BaiKalein; 5,6,7-trihydroxy-2-phenylchromen-4-one; Baicelein; 5,6,7-trihydroxy-2-phenyl-chromen-4-one; MFCD00017459; NSC 661431; NSC-661431; CHEBI:2979; UNII-49QAH60606; NSC661431; 49QAH60606; 5,6,7-Trihydroxy-2-phenyl-4H-1-benzopyran-4-one; CHEMBL8260; 4H-1-Benzopyran-4-one, 5,6,7-trihydroxy-2-phenyl-; DTXSID2022389; 5,7-Trihydroxyflavone; BAICALEIN (USP-RS); BAICALEIN [USP-RS]; Sho-saiko-to; SMR000112462; SR-01000597499; Baicalein, 8; Baicalein,(S); 3WL; Baicalein, 14; Baicalein, 98%; Spectrum_000427; Tocris-1761; SpecPlus_000758; BAICALEIN [MI]; BAICALEIN [INCI]; Spectrum2_000466; Spectrum3_001608; Spectrum4_000537; Spectrum5_001418; BAICALEIN [WHO-DD]; Oprea1_765614; BSPBio_003215; KBioGR_001173; KBioSS_000907; MLS002473007; MLS006011756; BIDD:ER0121; DivK1c_006854; SCHEMBL139617; SPECTRUM1504002; SPBio_000572; DTXCID802389; GTPL5144; KBio1_001798; KBio2_000907; KBio2_003475; KBio2_006043; KBio3_002435; HMS1922O22; HMS2267F15; HMS3268C22; HMS3412F14; HMS3649O19; HMS3655P18; HMS3676F14; BCP14393; HY-N0196; TNP00121; BBL027840; BDBM50009001; CCG-38705; LMPK12111095; NSC729192; s2268; STL146746; AKOS005747014; AC-7991; CS-6159; NSC-729192; SDCCGMLS-0066744.P001; Baicalein, analytical reference material; SMP1_000037; NCGC00017236-01; NCGC00017236-02; NCGC00017236-03; NCGC00017236-04; NCGC00017236-05; NCGC00017236-06; NCGC00017236-07; NCGC00017236-08; NCGC00017236-10; NCGC00017236-11; NCGC00025282-01; NCGC00025282-02; NCGC00025282-03; NCGC00025282-04; NCGC00178204-01; AS-57923; PD132943; SY057137; 5,6,7-Trihydroxy-2-phenyl-chroman-4-one; FT-0622548; SW219229-1; T2721; EN300-303171; S00113; 5,7-Trihydroxy-2-phenyl-4H-1-benzopyran-4-one; A827663; Q-100550; Q2879363; SR-01000597499-1; SR-01000597499-3; SR-01000597499-4; SR-01000597499-6; W-202870; 5,6,7-Trihydroxy-2-phenyl-(4H)-1-benzopyran-4-one; BRD-K72327355-001-02-2; BRD-K72327355-001-06-3; Z1824568302
Click to Show/Hide
|
||||
| Structure |
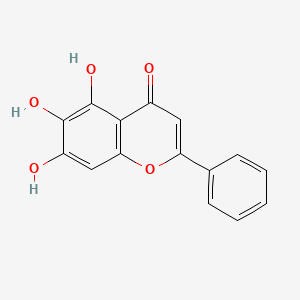 |
||||
| Formula |
C15H10O5
|
||||
| IUPAC Name |
5,6,7-trihydroxy-2-phenylchromen-4-one
|
||||
| Canonical SMILES |
C1=CC=C(C=C1)C2=CC(=O)C3=C(O2)C=C(C(=C3O)O)O
|
||||
| InChI |
InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H
|
||||
| InChIKey |
FXNFHKRTJBSTCS-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Degenerative arthritis | ICD-11: FA05 | |||
| Responsed Regulator | Kelch-like ECH-associated protein 1 (KEAP1) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCDs (Chondrocytes) | ||||
| In Vivo Model |
C57BL/6J (WT) mice (8 weeks old, male) were purchased from Nanjing Medical university, and AMPK-KO mice were purchased from Shanghai Model Organisms. They were used to create an OA model by destabilization of the medial meniscus surgery (DMM) (n = 6 per group). Briefly, after the mice were anaesthetized, a medial articular incision was made to expose the leftjoint cavity, and then the tibial collateral ligament was transected. Finally, the articular incision was closed. In the control group, only the joint cavity was opened. One week after surgery, 1 mg/kg baicalein (MCE, HY-N0196) per knee, 1 mg/kg ML385 (MCE, HY-100523) per knee, 1 mg/kg AICAR (MCE, HY-13417) per knee or 1 mg/kg of the ferroptosis inhibitor ferrostatin-1 (Fer-1, MCE, HY-100579) was injected into the joint cavity of the mice once a week. Meanwhile, saline was injected into the control group. Mice were sacrificed after surgery 10 weeks.
Click to Show/Hide
|
||||
| Response regulation | Baicalein alleviated osteoarthritis (OA) development by improving the activity of AMPKa/Nrf2/HO-1 signaling to inhibit chondrocyte ferroptosis, revealing baicalein to be a potential therapeutic strategy for OA. AMPKa preserved Nrf2 abundance in chondrocytes and promoted Nrf2 into nucleus by promoting Keap1 degradation | ||||
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Degenerative arthritis | ICD-11: FA05 | |||
| Responsed Regulator | Kelch-like ECH-associated protein 1 (KEAP1) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCDs (Chondrocytes) | ||||
| In Vivo Model |
C57BL/6J (WT) mice (8 weeks old, male) were purchased from Nanjing Medical university, and AMPK-KO mice were purchased from Shanghai Model Organisms. They were used to create an OA model by destabilization of the medial meniscus surgery (DMM) (n = 6 per group). Briefly, after the mice were anaesthetized, a medial articular incision was made to expose the leftjoint cavity, and then the tibial collateral ligament was transected. Finally, the articular incision was closed. In the control group, only the joint cavity was opened. One week after surgery, 1 mg/kg baicalein (MCE, HY-N0196) per knee, 1 mg/kg ML385 (MCE, HY-100523) per knee, 1 mg/kg AICAR (MCE, HY-13417) per knee or 1 mg/kg of the ferroptosis inhibitor ferrostatin-1 (Fer-1, MCE, HY-100579) was injected into the joint cavity of the mice once a week. Meanwhile, saline was injected into the control group. Mice were sacrificed after surgery 10 weeks.
Click to Show/Hide
|
||||
| Response regulation | Baicalein alleviated osteoarthritis (OA) development by improving the activity of AMPKa/Nrf2/HO-1 signaling to inhibit chondrocyte ferroptosis, revealing baicalein to be a potential therapeutic strategy for OA. AMPKa preserved Nrf2 abundance in chondrocytes and promoted Nrf2 into nucleus by promoting Keap1 degradation | ||||
Unspecific Target
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Responsed Regulator | Endoplasmic reticulum chaperone BiP (HSPA5) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rRHs (Rat right hemispheres) | ||||
| In Vivo Model |
Healthy male Sprague-Dawley rats (weighing 370 g-420 g) were purchased from the Laboratory Animal Center of Sun Yat-sen University. The rats were randomized into five groups. Rats in sham group (n = 6) underwent the same anesthetic and surgical procedures, excluding cardiac arrest and CPR. All of the remaining four groups were given the interventions within 10 minutes of ROSC. Rats in CPR group (n = 6) received an intraperitoneal injection of 0.9% saline (1 mL/kg). Rats in baicalein group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein at the same time, based on previous studies. Rats in tunicamycin group (n = 6) were intraperitoneally injected with tunicamycin (2 mg/kg body weight) (22-24). Rats in baicalein + tunicamycin group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein and 2 mg/kg (body weight) tunicamycin. All groups were given the same volume of normal saline solvent (2 mL/kg).
Click to Show/Hide
|
||||
| Response regulation | Our research suggests that baicalein inhibited ferroptosis after ROSC by targeting ALOX15. Iron content, and MDA were reduced. More importantly, baicalein alleviated ER stress by inhibiting the expression of GRP78, ATF4, and CHOP. Baicalein is a potential drug to relieve brain injury after ROSC. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Responsed Regulator | Cyclic AMP-dependent transcription factor ATF-4 (ATF4) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rRHs (Rat right hemispheres) | ||||
| In Vivo Model |
Healthy male Sprague-Dawley rats (weighing 370 g-420 g) were purchased from the Laboratory Animal Center of Sun Yat-sen University. The rats were randomized into five groups. Rats in sham group (n = 6) underwent the same anesthetic and surgical procedures, excluding cardiac arrest and CPR. All of the remaining four groups were given the interventions within 10 minutes of ROSC. Rats in CPR group (n = 6) received an intraperitoneal injection of 0.9% saline (1 mL/kg). Rats in baicalein group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein at the same time, based on previous studies. Rats in tunicamycin group (n = 6) were intraperitoneally injected with tunicamycin (2 mg/kg body weight) (22-24). Rats in baicalein + tunicamycin group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein and 2 mg/kg (body weight) tunicamycin. All groups were given the same volume of normal saline solvent (2 mL/kg).
Click to Show/Hide
|
||||
| Response regulation | Our research suggests that baicalein inhibited ferroptosis after ROSC by targeting ALOX15. Iron content, and MDA were reduced. More importantly, baicalein alleviated ER stress by inhibiting the expression of GRP78, ATF4, and CHOP. Baicalein is a potential drug to relieve brain injury after ROSC. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Responsed Regulator | DNA damage-inducible transcript 3 protein (DDIT3) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rRHs (Rat right hemispheres) | ||||
| In Vivo Model |
Healthy male Sprague-Dawley rats (weighing 370 g-420 g) were purchased from the Laboratory Animal Center of Sun Yat-sen University. The rats were randomized into five groups. Rats in sham group (n = 6) underwent the same anesthetic and surgical procedures, excluding cardiac arrest and CPR. All of the remaining four groups were given the interventions within 10 minutes of ROSC. Rats in CPR group (n = 6) received an intraperitoneal injection of 0.9% saline (1 mL/kg). Rats in baicalein group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein at the same time, based on previous studies. Rats in tunicamycin group (n = 6) were intraperitoneally injected with tunicamycin (2 mg/kg body weight) (22-24). Rats in baicalein + tunicamycin group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein and 2 mg/kg (body weight) tunicamycin. All groups were given the same volume of normal saline solvent (2 mL/kg).
Click to Show/Hide
|
||||
| Response regulation | Our research suggests that baicalein inhibited ferroptosis after ROSC by targeting ALOX15. Iron content, and MDA were reduced. More importantly, baicalein alleviated ER stress by inhibiting the expression of GRP78, ATF4, and CHOP. Baicalein is a potential drug to relieve brain injury after ROSC. | ||||
Transferrin receptor protein 1 (TFRC)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | |||
| Target for Ferroptosis | Marker/Suppressor/Driver | |||
| Responsed Disease | Vitiligo | ICD-11: ED63 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hMCs (Human mesangial cells) | |||
| Response regulation | Baicalein up-regulated GPX4 and reduced TFR1 level in melanocytes treated with RSL3+FAC. Baicalein protected melanocytes against ferroptosis through up-regulating GPX4. Ferroptosis might be pervasive in the occurrence and development of vitiligo, and could be proposed as the potential therapeutic target. | |||
Prostaglandin G/H synthase 2 (PTGS2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Marker | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response regulation | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
Polyunsaturated fatty acid lipoxygenase ALOX15 (ALOX15)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
All adult male C57/BL6 mice weighing 18-22g were obtained from the Experimental Animal Center of Central South University, China. Animals were randomly divided into six groups as follows: 1) control group (n = 6) was given vehicle intracranial injection (PBS 5 ul); 2) FeCl3 group (n = 6) was given 5 ul 50 mM FeCl3; 3) and 4) baicalein groups were pretreated with baicalein 50 mg/kg (n = 6) and 100 mg/kg (n = 6) 30 min prior to FeCl3 administration, respectively; 5) ferroptosis inhibitor group (n = 6) was administered continuously with 10 mg/kg Lipo-1 3 d prior to FeCl3 administration; and 6) baicalein administration group (n = 6) was given 100 mg/kg baicalein once by intraperitoneal injection 30 min prior to 5 ul PBS administration.
Click to Show/Hide
|
||||
| Response regulation | Baicalein, as a naturel bioactive compound, could ameliorate behavioral seizures and play a key neuroprotective role in FeCl3-induced posttraumatic epileptic seizures through inhibiting ferroptosis and its neuroprotection might be related to suppression of 12-LOX/15-LOX. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rRHs (Rat right hemispheres) | ||||
| In Vivo Model |
Healthy male Sprague-Dawley rats (weighing 370 g-420 g) were purchased from the Laboratory Animal Center of Sun Yat-sen University. The rats were randomized into five groups. Rats in sham group (n = 6) underwent the same anesthetic and surgical procedures, excluding cardiac arrest and CPR. All of the remaining four groups were given the interventions within 10 minutes of ROSC. Rats in CPR group (n = 6) received an intraperitoneal injection of 0.9% saline (1 mL/kg). Rats in baicalein group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein at the same time, based on previous studies. Rats in tunicamycin group (n = 6) were intraperitoneally injected with tunicamycin (2 mg/kg body weight) (22-24). Rats in baicalein + tunicamycin group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein and 2 mg/kg (body weight) tunicamycin. All groups were given the same volume of normal saline solvent (2 mL/kg).
Click to Show/Hide
|
||||
| Response regulation | Baicalein inhibited ferroptosis after ROSC by targeting ALOX15. Iron content, and MDA were reduced. More importantly, baicalein alleviated ER stress by inhibiting the expression of GRP78, ATF4, and CHOP. Baicalein is a potential drug to relieve brain injury after ROSC. | ||||
Polyunsaturated fatty acid lipoxygenase ALOX12 (ALOX12)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
All adult male C57/BL6 mice weighing 18-22g were obtained from the Experimental Animal Center of Central South University, China. Animals were randomly divided into six groups as follows: 1) control group (n = 6) was given vehicle intracranial injection (PBS 5 ul); 2) FeCl3 group (n = 6) was given 5 ul 50 mM FeCl3; 3) and 4) baicalein groups were pretreated with baicalein 50 mg/kg (n = 6) and 100 mg/kg (n = 6) 30 min prior to FeCl3 administration, respectively; 5) ferroptosis inhibitor group (n = 6) was administered continuously with 10 mg/kg Lipo-1 3 d prior to FeCl3 administration; and 6) baicalein administration group (n = 6) was given 100 mg/kg baicalein once by intraperitoneal injection 30 min prior to 5 ul PBS administration.
Click to Show/Hide
|
||||
| Response regulation | Baicalein, as a naturel bioactive compound, could ameliorate behavioral seizures and play a key neuroprotective role in FeCl3-induced posttraumatic epileptic seizures through inhibiting ferroptosis and its neuroprotection might be related to suppression of 12-LOX/15-LOX. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 4 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [6] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cerebral ischemia | ICD-11: 8B10 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
The mice (23-25 g, 8-10 weeks old) were subjected to transientmiddle cerebral artery occlusion (tMCAO) to induce cerebral ischemia as previously described protocol . Briefly, mice were anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg) and subcutaneous injection of meloxicam (10mg/kg) during tMCAO operation. Monofilament with a silicon coating on the tip and a diameter of 0.12 mm (A5-122, Beijing Cinontech Co. Ltd., China) was inserted into the ICA from CCA to occlude the middle cerebral artery (MCA) for 1.5 h. The suture was then removed to restore blood flow for another 22.5 h reperfusion. Sham control mice were subjected to similar surgical operations without MCA occlusion. Specifically, the monofilament was inserted only 5 mm above the carotid bifurcation and withdrew immediately in the Sham group.
Click to Show/Hide
|
||||
| Response regulation | Baicalein inhibited the ferroptosis by regulating on the expression levels of GPX4, ACSL4 and ACSL3 in OGD/R cells, tMCAO mice and RSL3-stimulated HT22 cells. Our findings demonstrated that baicalein reversed the cerebral ischemia-reperfusion injury via anti-ferroptosis, which was regulated by GPX4/ACSL4/ACSL3 axis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response regulation | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Vitiligo | ICD-11: ED63 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hMCs (Human mesangial cells) | ||||
| Response regulation | Baicalein up-regulated GPX4 and reduced TFR1 level in melanocytes treated with RSL3+FAC. Baicalein protected melanocytes against ferroptosis through up-regulating GPX4. Ferroptosis might be pervasive in the occurrence and development of vitiligo, and could be proposed as the potential therapeutic target. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Drug Act on This Target | [7] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Endometriosis | ICD-11: GA10 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| hMPs (Human macrophages) | |||||
| Response regulation | Baicalein, a potential anti-ferroptosis compound, increased GPX4 expression, significantly inhibited ferroptosis, and restored phagocytosis of THP-1 cells in vitro. Collectively, our study reveals that ferroptosis triggered by high iron in cyst fluid promotes the development of endometriosis by impairing macrophage phagocytosis and producing more angiogenic cytokines (e.g., IL8 and VEGFA). | ||||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [6] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Cerebral ischemia | ICD-11: 8B10 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
The mice (23-25 g, 8-10 weeks old) were subjected to transientmiddle cerebral artery occlusion (tMCAO) to induce cerebral ischemia as previously described protocol . Briefly, mice were anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg) and subcutaneous injection of meloxicam (10mg/kg) during tMCAO operation. Monofilament with a silicon coating on the tip and a diameter of 0.12 mm (A5-122, Beijing Cinontech Co. Ltd., China) was inserted into the ICA from CCA to occlude the middle cerebral artery (MCA) for 1.5 h. The suture was then removed to restore blood flow for another 22.5 h reperfusion. Sham control mice were subjected to similar surgical operations without MCA occlusion. Specifically, the monofilament was inserted only 5 mm above the carotid bifurcation and withdrew immediately in the Sham group.
Click to Show/Hide
|
||||
| Response regulation | Baicalein inhibited the ferroptosis by regulating on the expression levels of GPX4, ACSL4 and ACSL3 in OGD/R cells, tMCAO mice and RSL3-stimulated HT22 cells. Our findings demonstrated that baicalein reversed the cerebral ischemia-reperfusion injury via anti-ferroptosis, which was regulated by GPX4/ACSL4/ACSL3 axis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response regulation | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
Fatty acid CoA ligase Acsl3 (ACSL3)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [6] | ||||
| Target for Ferroptosis | Driver/Suppressor | ||||
| Responsed Disease | Cerebral ischemia | ICD-11: 8B10 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
The mice (23-25 g, 8-10 weeks old) were subjected to transientmiddle cerebral artery occlusion (tMCAO) to induce cerebral ischemia as previously described protocol . Briefly, mice were anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg) and subcutaneous injection of meloxicam (10mg/kg) during tMCAO operation. Monofilament with a silicon coating on the tip and a diameter of 0.12 mm (A5-122, Beijing Cinontech Co. Ltd., China) was inserted into the ICA from CCA to occlude the middle cerebral artery (MCA) for 1.5 h. The suture was then removed to restore blood flow for another 22.5 h reperfusion. Sham control mice were subjected to similar surgical operations without MCA occlusion. Specifically, the monofilament was inserted only 5 mm above the carotid bifurcation and withdrew immediately in the Sham group.
Click to Show/Hide
|
||||
| Response regulation | Baicalein inhibited the ferroptosis by regulating on the expression levels of GPX4, ACSL4 and ACSL3 in OGD/R cells, tMCAO mice and RSL3-stimulated HT22 cells. Our findings demonstrated that baicalein reversed the cerebral ischemia-reperfusion injury via anti-ferroptosis, which was regulated by GPX4/ACSL4/ACSL3 axis. | ||||
References
