Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10060)
| Target Name | Transferrin receptor protein 1 (TFRC) | ||||
|---|---|---|---|---|---|
| Synonyms |
T9; p90; CD_antigen=CD71
Click to Show/Hide
|
||||
| Gene Name | TFRC | ||||
| Sequence |
MMDQARSAFSNLFGGEPLSYTRFSLARQVDGDNSHVEMKLAVDEEENADNNTKANVTKPK
RCSGSICYGTIAVIVFFLIGFMIGYLGYCKGVEPKTECERLAGTESPVREEPGEDFPAAR RLYWDDLKRKLSEKLDSTDFTGTIKLLNENSYVPREAGSQKDENLALYVENQFREFKLSK VWRDQHFVKIQVKDSAQNSVIIVDKNGRLVYLVENPGGYVAYSKAATVTGKLVHANFGTK KDFEDLYTPVNGSIVIVRAGKITFAEKVANAESLNAIGVLIYMDQTKFPIVNAELSFFGH AHLGTGDPYTPGFPSFNHTQFPPSRSSGLPNIPVQTISRAAAEKLFGNMEGDCPSDWKTD STCRMVTSESKNVKLTVSNVLKEIKILNIFGVIKGFVEPDHYVVVGAQRDAWGPGAAKSG VGTALLLKLAQMFSDMVLKDGFQPSRSIIFASWSAGDFGSVGATEWLEGYLSSLHLKAFT YINLDKAVLGTSNFKVSASPLLYTLIEKTMQNVKHPVTGQFLYQDSNWASKVEKLTLDNA AFPFLAYSGIPAVSFCFCEDTDYPYLGTTMDTYKELIERIPELNKVARAAAEVAGQFVIK LTHDVELNLDYERYNSQLLSFVRDLNQYRADIKEMGLSLQWLYSARGDFFRATSRLTTDF GNAEKTDRFVMKKLNDRVMRVEYHFLSPYVSPKESPFRHVFWGSGSHTLPALLENLKLRK QNNGAFNETLFRNQLALATWTIQGAANALSGDVWDIDNEF Click to Show/Hide
|
||||
| Family | Peptidase M28 family | ||||
| Function |
Cellular uptake of iron occurs via receptor-mediated endocytosis of ligand-occupied transferrin receptor into specialized endosomes. Endosomal acidification leads to iron release. The apotransferrin-receptor complex is then recycled to the cell surface with a return to neutral pH and the concomitant loss of affinity of apotransferrin for its receptor. Transferrin receptor is necessary for development of erythrocytes and the nervous system. A second ligand, the heditary hemochromatosis protein HFE, competes for binding with transferrin for an overlapping C-terminal binding site. Positively regulates T and B cell proliferation through iron uptake. Acts as a lipid sensor that regulates mitochondrial fusion by regulating activation of the JNK pathway. When dietary levels of stearate (C18:0) are low, promotes activation of the JNK pathway, resulting in HUWE1-mediated ubiquitination and subsequent degradation of the mitofusin MFN2 and inhibition of mitochondrial fusion. When dietary levels of stearate (C18:0) are high, TFRC stearoylation inhibits activation of the JNK pathway and thus degradation of the mitofusin MFN2.
Click to Show/Hide
|
||||
| Gene ID | 7037 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
TFRC can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
N6-adenosine-methyltransferase non-catalytic subunit (METTL14)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Doxorubicin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Doxorubicin treatment resulted in the upregulation of methyltransferase-like 14 (METTL14), which catalyzes the m6A modification of the long non-coding RNA KCNQ1OT1, a miR-7-5p sponge. And miR-7-5p inhibits DOX-induced ferroptosis in cardiomyocytes by directly repressing TFRC. Inhibiting ferroptosis mediated by a METTL14/KCNQ1OT1/miR-7-5p positive feedback loop in cardiomyocytes could provide a new therapeutic approach to control DOX-induced cardiac injury. | ||||
KCNQ1OT1 (IncRNA)
Cardiomyopathy [ICD-11: BC43]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Doxorubicin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Doxorubicin treatment resulted in the upregulation of methyltransferase-like 14 (METTL14), which catalyzes the m6A modification of the long non-coding RNA KCNQ1OT1, a miR-7-5p sponge. And miR-7-5p inhibits DOX-induced ferroptosis in cardiomyocytes by directly repressing TFRC. Inhibiting ferroptosis mediated by a METTL14/KCNQ1OT1/miR-7-5p positive feedback loop in cardiomyocytes could provide a new therapeutic approach to control DOX-induced cardiac injury. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | The RNA-binding protein IGF2BP1 is associated with KCNQ1OT1 to increase its stability and robustly inhibit miR-7-5p activity. MiR-7-5p could effectively suppress METLL14 and TFRC expression. The study suggested a therapeutic strategy to alleviate doxorubicin (DOX)-induced cardiomyopathy. | ||||
hsa-miR-7-5p (miRNA)
Cardiomyopathy [ICD-11: BC43]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Doxorubicin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Doxorubicin treatment resulted in the upregulation of methyltransferase-like 14 (METTL14), which catalyzes the m6A modification of the long non-coding RNA KCNQ1OT1, a miR-7-5p sponge. And miR-7-5p inhibits DOX-induced ferroptosis in cardiomyocytes by directly repressing TFRC. Inhibiting ferroptosis mediated by a METTL14/KCNQ1OT1/miR-7-5p positive feedback loop in cardiomyocytes could provide a new therapeutic approach to control DOX-induced cardiac injury. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | The RNA-binding protein IGF2BP1 is associated with KCNQ1OT1 to increase its stability and robustly inhibit miR-7-5p activity. MiR-7-5p could effectively suppress METLL14 and TFRC expression. The study suggested a therapeutic strategy to alleviate doxorubicin (DOX)-induced cardiomyopathy. | ||||
Zinc finger protein basonuclin-1 (BNC1)
Primary ovarian insufficiency [ICD-11: GA30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
ES-2 cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Ovaries collected from 12-week-old mice were fixed in 2.5% glutaraldehyde at room temperature for 2 h and then at 4 overnight. The ovaries were washed with PBS three times for 10 min. Then, the ovaries were fixed with 1% osmic acid for 1 h and washed with PBS three times for 10 min each. The ovaries were fixed with 2% uranyl acetate for 30 min; dehydrated with 50%, 70%, 90% and 100% ethanol for 10 min each; and washed with 100% acetone twice for 15 min each.
Click to Show/Hide
|
||||
| Response Description | Pharmacologic inhibition of YAP signaling or ferroptosis significantly rescues Bnc mutation-induced primary ovarian insufficiency (POI). BNC1 directly regulates Nf2 expression. BNC1 deficiency downregulates NF2 expression, which reduces YAP phosphorylation and promote YAP nuclear accumulation. YAP activation upregulates Tfrc and Acsl4 expression. | ||||
YY1-associated protein 1 (YY1AP1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
mEFs (Mouse embryonic fibroblasts) | ||||
| NF639 (Mouse breast epithelial cells) | |||||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| BT-474 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0179 | ||
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | ||
| In Vivo Model |
Six- to eight-week-old female athymic nu/nu mice were purchased from Envigo (East Millstone, NJ, USA). For s.c. tumour models, mice were injected in the right flank with 1 x 107 shNT-GPX4 iKO MSTO-211H cells or shMerlin-GPX4 iKO MSTO-211H cells suspended in 150 uL Matrigel. Tumours were measured with callipers every 3 days. When tumours reached a mean volume of 100 mm3, mice with similarly sized tumours were grouped into four treatment groups. For control or knockout cohorts, mice were given intraperitoneal (i.p.) injections of 0.9% sterile saline or Doxycycline (100 mg/kg body weight) for two days. At the same time, mice were provided with either a normal diet or Doxycycline diet for control or knockout cohorts, respectively.
Click to Show/Hide
|
||||
| Response Description | In epithelial cells, such interactions mediated by E-cadherin suppress ferroptosis by activating the intracellular NF2 (also known as merlin) and Hippo signalling pathway in Breast adenocarcinoma. Antagonizing this signalling axis allows the proto-oncogenic transcriptional co-activator YAP to promote ferroptosis by upregulating several ferroptosis modulators, including ACSL4 and TFRC. | ||||
Ubiquitin carboxyl-terminal hydrolase 7 (USP7)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague-Dawley (SD) rats (250-300 g) were purchased from the Laboratory Animal Center, Xiangya School of Medicine, Central South University, China. Briefly, a left thoracotomy was carried out in the fourth intercostal space and the heart was exposed via opening the pericardium. The left coronary artery was around via a 4-0 silk suture and a snare was formed by passing both ends of the suture via a short polyethylene tubing. Blockage of the coronary artery was conducted via clamping the snare against the heart surface. Reperfusion was performed by release of the snare. The sham group conducted the same procedure but without ischemia (the snare was not tightened). To establish the I/R injury model, the rat hearts were subjected to 1 h-ischemia plus 3 h-reperfusion.
Click to Show/Hide
|
||||
| Response Description | A novel pathway of USP7/p53/TfR1 has been identified in the ischemia/reperfusion (I/R)-treated rat hearts, where up-regulation of USP7 promotes ferrptosis via activation of the p53/TfR1 pathway. | ||||
Tribbles homolog 2 (TRIB2)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
BEL-7404 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6568 |
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | |
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | |
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| Response Description | The effects by which TrCP-mediated ubiquitination and followed degradation of TFRC to decline labile iron are critical for TRIB2 to desensitize liver cancer cells to ferroptosis. Appropriate reduction of TRIB2 function might be beneficial for patients bearing liver cancer because it will definitely sensitize ferroptosis-based therapy. | |||
Transcriptional coactivator YAP1 (YAP1)
Primary ovarian insufficiency [ICD-11: GA30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
ES-2 cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Ovaries collected from 12-week-old mice were fixed in 2.5% glutaraldehyde at room temperature for 2 h and then at 4 overnight. The ovaries were washed with PBS three times for 10 min. Then, the ovaries were fixed with 1% osmic acid for 1 h and washed with PBS three times for 10 min each. The ovaries were fixed with 2% uranyl acetate for 30 min; dehydrated with 50%, 70%, 90% and 100% ethanol for 10 min each; and washed with 100% acetone twice for 15 min each.
Click to Show/Hide
|
||||
| Response Description | Pharmacologic inhibition of YAP signaling or ferroptosis significantly rescues Bnc1 mutation-induced primary ovarian insufficiency (POI). BNC1 directly regulates Nf2 expression. BNC1 deficiency downregulates NF2 expression, which reduces YAP phosphorylation and promote YAP nuclear accumulation. YAP activation upregulates Tfrc and Acsl4 expression. | ||||
rno-miR-29a-3p (miRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rBMMSCs (Rat bone marrow mesenchymal stem cells) | ||||
| IAR 20 cells | Normal | Homo sapiens | CVCL_5296 | ||
| In Vivo Model |
Clean-grade male Sprague-Dawley (SD) rats were purchased from China Food and Drug Administration (Beijing, China). SD rats were fed a high-fat diet (Composition: 15% triglyceride, 15% sucrose, 10% egg yolk powder, 1% cholesterol, 0.2% bile salt, 58.8% basic feed) for 20 weeks. Hematoxylin and eosin (HE) and oil red O staining showed that the area of mixed macrovesicular steatosis was more than 60% under the microscope, indicating that a model of severe steatotic liver was established successfully. A 70% liver thermal ischemia model was established, continuously blocked for 80 min, and then, the ischemic liver was obtained 24 h after reperfusion.
Click to Show/Hide
|
||||
| Response Description | miR-29a-3p, which targets IREB2, is abundant in HO-1/BMMSC-exosomes and could decrease the IREB2 protein level. The reduced IREB2 level led to an increase in the level of FTH1 and decreased the level of TFR1 through posttranscriptional regulation, which ultimately reduced the level of intracellular Fe2+ and the production of lipid ROS and inhibited the occurrence of ferroptosis in SHP-HR. In conclusion, ferroptosis plays an important role in HO-1/BMMSC-mediated alleviation of steatotic hepatic ischemia-reperfusion injury. | ||||
RNA binding protein fox-1 homolog 2 (RBFOX2)
Corpus uteri cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
KLE cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 | |
| Ishikawa cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 | ||
| HEC-1-A cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_0293 | ||
| HEC-1-B cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_0294 | ||
| RL95-2 cells | Endometrial adenosquamous carcinoma | Homo sapiens | CVCL_0505 | ||
| In Vivo Model |
Female 4-week-old BALB/c-nu nude mice were purchased from Vital River (Beijing, China) and housed in a specific pathogen-free facility. EC cells were washed twice with serum-free medium and injected subcutaneously into nude mice (5 x 106 cells/site). Tumors were measured with calipers every four days for four weeks. The mice were sacrificed on day 32 after cell implantation, and the tumors were excised and weighed.
Click to Show/Hide
|
||||
| Response Description | CircRAPGEF5 interacts with RBFOX2, an important splicing regulator, to modulate the splicing of TFRC pre-mRNA. Importantly, circRAPGEF5 promotes exon-4 skipping of TFRC by sequestering RBFOX2, resulting in resistance to ferroptosis via the reduction of labile iron in endometrial cancer cells. | ||||
PVT1 (IncRNA)
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | PVT1 regulates ferroptosis through miR-214-mediated p53 and TFR1. The discovery of PVT1 and miR-214 as potential targets for I/R also implies that PVT1 and miR-214 play critical roles in ferroptosis, shedding new light on the mechanism of ferroptosis in acute ischemic stroke. | |||
Plasmanylethanolamine desaturase (PEDS1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| In Vivo Model |
Twenty BALB/c nude female mice (4- to 6-week-old) were purchased from Charles River Laboratories (Beijing, China) to establish tumorigenesis (5 mice in each). We injected MCF-7 or MDA-MB-231 cells (4 x 106) transfected with the stable knockdown and over-expressing TMEM189 into the flanks of mice to construct tumorigenesis models, respectively. Meanwhile, the sh-Con and empty vector were served as the control groups for each model.
Click to Show/Hide
|
||||
| Response Description | TMEM189 (PEDS1) could inhibit autophagy to mediate ferroptosis in breast cancer cells. Moreover, TMEM189 ablation strongly up-regulated LC3BII and transferrin receptor 1 (TfR1) expression levels in breast cancer cells, whereas down-regulated p62 and GPX4. | ||||
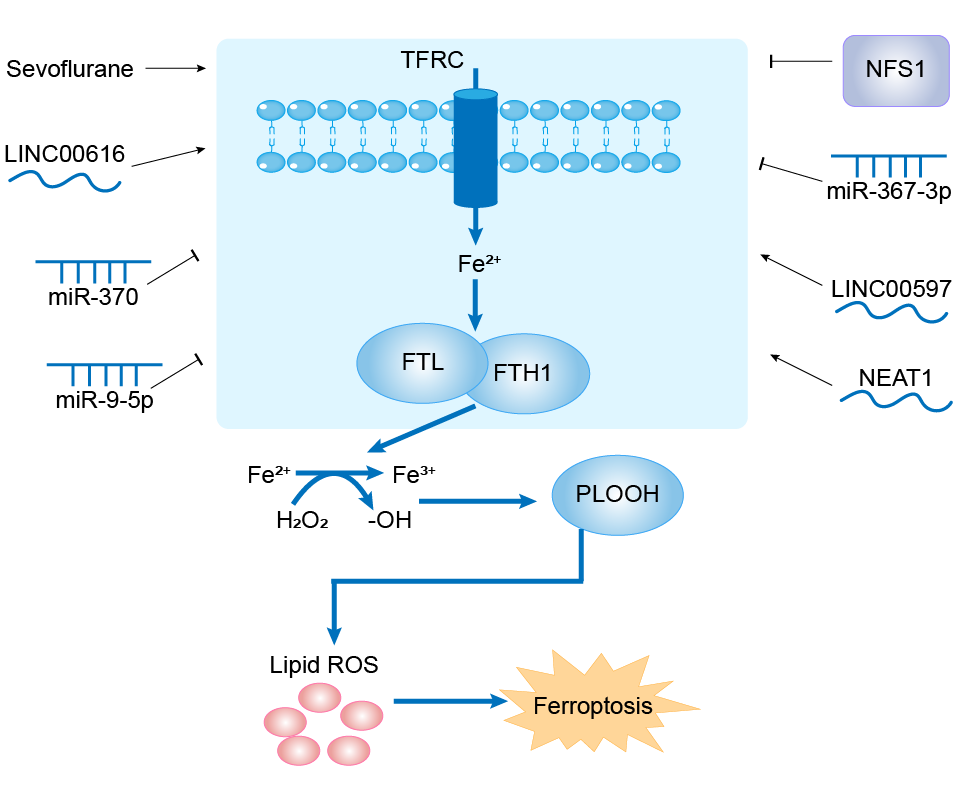
NEAT1 (IncRNA)
Sepsis [ICD-11: 1G40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
In Vitro Model |
bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Thirty specific-pathogen-free (SPF) male C57BL/6 rats at 8 weeks weighing 200-250 g were obtained from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). The rats were housed under room temperatures (25 ± 2 and 12-h light/dark cycle) and given water and food ad libitum before the trial. Then, they were randomly assigned into the control group (n = 10), model group (n = 10), and model + miR-9-5p angomir group (n = 10). All animal procedures were performed under the Care and Use of Laboratory Animals guidelines and approved by the Guangdong Academy of Medical Sciences. According to a previous study, sepsis was induced by the cecal ligation and puncture (CLP) method. Briefly, we anesthetized the rats with 5% chloral hydrate (0.6 ml/100 g body weight) and made a 1.5 cm midline incision on the anterior abdomen to expose the cecum. Then, the cecum was ligated at 30%, punctured twice with a No.4 surgical needle to extrude the fecal content. Finally, 1 ml of normal saline was used for resuscitation. The rats in miR-9-5p angomir group were injected with 100 ng/ul miR-9-5p angomir (RiboBio, Guangzhou, China) into caudal vein (model + miR-9-5p angomir). The rats in the control group experienced the same procedure without ligation and puncture.
Click to Show/Hide
|
||||
| Response Description | NEAT1 functions as a ceRNA for miR-9-5p to facilitate TFRC and GOT1 expression. Overexpression of NEAT1 enhanced ferroptosis stress in bEnd.3 cells. Increased miR-9-5p alleviated sepsis-induced ferroptosis by suppressing the expression of TFRC and GOT1 both in vivo and in vitro. | ||||
N-myc proto-oncogene protein (MYCN)
Neuroblastoma [ICD-11: 2D50]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SH-EP cells | Neuroblastoma | Homo sapiens | CVCL_0524 |
| SK-N-AS cells | Neuroblastoma | Homo sapiens | CVCL_1700 | |
| SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| Response Description | Pediatric neuroblastoma (NB) cells harboring MYCN amplification are prone to undergo ferroptosis conferred by TFRC upregulation, suggesting that GPX4-targeting ferroptosis inducers or TFRC agonists can be potential strategies in treating MYCN-amplified NB. | |||
Metalloproteinase inhibitor 1 (TIMP1)
Cardiac hypertrophy [ICD-11: BC45]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hNCs (Neutrophils cells) | ||||
| In Vivo Model |
The rats were randomly divided into five groups as follows: (1) sham group: the rats underwent sham operation and received vehicle (PBS, caudal vein injection). (2) Model group: the rats were subjected to AAC and received vehicle (PBS, caudal vein injection). (3-5) The treatment groups: the rats were subjected to AAC and after 24 h treated with si-AAB, si-AAB + MSN, or NM + si-AAB + MSN (50 nM siRNA dose per rat every 2 days for 4 weeks, caudal vein injection).
Click to Show/Hide
|
||||
| Response Description | LncRNA AAB was upregulated in the hearts of cardiac hypertrophy rats as well as in the Ang II-induced CMECs. Importantly, we found that lncRNA AAB sponged and sequestered miR-30b-5p to induce the imbalance of MMP9/ TIMP1, which enhanced the activation of transferrin receptor 1 (TFR-1) and then eventually led to the ferroptosis of CMECs. | ||||
Merlin (NF2)
Primary ovarian insufficiency [ICD-11: GA30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
ES-2 cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Ovaries collected from 12-week-old mice were fixed in 2.5% glutaraldehyde at room temperature for 2 h and then at 4 overnight. The ovaries were washed with PBS three times for 10 min. Then, the ovaries were fixed with 1% osmic acid for 1 h and washed with PBS three times for 10 min each. The ovaries were fixed with 2% uranyl acetate for 30 min; dehydrated with 50%, 70%, 90% and 100% ethanol for 10 min each; and washed with 100% acetone twice for 15 min each.
Click to Show/Hide
|
||||
| Response Description | Pharmacologic inhibition of YAP signaling or ferroptosis significantly rescues Bnc1 mutation-induced primary ovarian insufficiency (POI). BNC1 directly regulates Nf2 expression. BNC1 deficiency downregulates NF2 expression, which reduces YAP phosphorylation and promote YAP nuclear accumulation. YAP activation upregulates Tfrc and Acsl4 expression. | ||||
Matrix metalloproteinase-9 (MMP9)
Cardiac hypertrophy [ICD-11: BC45]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hNCs (Neutrophils cells) | ||||
| In Vivo Model |
The rats were randomly divided into five groups as follows: (1) sham group: the rats underwent sham operation and received vehicle (PBS, caudal vein injection). (2) Model group: the rats were subjected to AAC and received vehicle (PBS, caudal vein injection). (3-5) The treatment groups: the rats were subjected to AAC and after 24 h treated with si-AAB, si-AAB + MSN, or NM + si-AAB + MSN (50 nM siRNA dose per rat every 2 days for 4 weeks, caudal vein injection).
Click to Show/Hide
|
||||
| Response Description | LncRNA AAB was upregulated in the hearts of cardiac hypertrophy rats as well as in the Ang II-induced CMECs. Importantly, we found that lncRNA AAB sponged and sequestered miR-30b-5p to induce the imbalance of MMP9/TIMP1, which enhanced the activation of transferrin receptor 1 (TFR-1) and then eventually led to the ferroptosis of CMECs. | ||||
lncRNA?AAB (IncRNA)
Cardiac hypertrophy [ICD-11: BC45]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hNCs (Neutrophils cells) | ||||
| rCMECs (Rat cardiac microvascular endothelial cells) | |||||
| In Vivo Model |
The rats were randomly divided into five groups as follows: (1) sham group: the rats underwent sham operation and received vehicle (PBS, caudal vein injection). (2) Model group: the rats were subjected to AAC and received vehicle (PBS, caudal vein injection). (3-5) The treatment groups: the rats were subjected to AAC and after 24 h treated with si-AAB, si-AAB + MSN, or NM + si-AAB + MSN (50 nM siRNA dose per rat every 2 days for 4 weeks, caudal vein injection).
Click to Show/Hide
|
||||
| Response Description | LncRNA AAB was upregulated in the hearts of cardiac hypertrophy rats as well as in the Ang II-induced CMECs. Importantly, we found that lncRNA AAB sponged and sequestered miR-30b-5p to induce the imbalance of MMP9/TIMP1, which enhanced the activation of transferrin receptor 1 (TFR-1) and then eventually led to the ferroptosis of CMECs. | ||||
LINC00616 (IncRNA)
Periodontitis [ICD-11: DA0C]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hPLSCs (Periodontal ligament stem cells) | |||
| Response Description | Inhibition of LINC00616 promoted cell viability and suppressed ferroptosis of PDLSCs. miR-370 was verified to be a target of LINC00616. Additionally, miR-370 targeting the transferrin receptor protein and upregulated transferrin receptor (TFRC) abolished the effects of overexpressed miR-370 on cell viability and ferroptosis of PDLSCs. Therefore, LINC00616 knockdown may be a promising therapeutic strategy for periodontitis. | |||
LINC00597 (IncRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| Response Description | This study indicated that cinobufotalin induced ferroptosis to suppress lung cancer cell growth by lncRNA LINC00597/hsa-miR-367-3p/TFRC pathway via resibufogenin might provide novel therapeutic targets for lung cancer therapy. | |||
hsa-miR-9-5p (miRNA)
Sepsis [ICD-11: 1G40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
In Vitro Model |
bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Thirty specific-pathogen-free (SPF) male C57BL/6 rats at 8 weeks weighing 200-250 g were obtained from the Experimental Animal Center of Sun Yat-sen University (Guangzhou, China). The rats were housed under room temperatures (25 ± 2 and 12-h light/dark cycle) and given water and food ad libitum before the trial. Then, they were randomly assigned into the control group (n = 10), model group (n = 10), and model + miR-9-5p angomir group (n = 10). All animal procedures were performed under the Care and Use of Laboratory Animals guidelines and approved by the Guangdong Academy of Medical Sciences. According to a previous study, sepsis was induced by the cecal ligation and puncture (CLP) method. Briefly, we anesthetized the rats with 5% chloral hydrate (0.6 ml/100 g body weight) and made a 1.5 cm midline incision on the anterior abdomen to expose the cecum. Then, the cecum was ligated at 30%, punctured twice with a No.4 surgical needle to extrude the fecal content. Finally, 1 ml of normal saline was used for resuscitation. The rats in miR-9-5p angomir group were injected with 100 ng/ul miR-9-5p angomir (RiboBio, Guangzhou, China) into caudal vein (model + miR-9-5p angomir). The rats in the control group experienced the same procedure without ligation and puncture.
Click to Show/Hide
|
||||
| Response Description | NEAT1 functions as a ceRNA for miR-9-5p to facilitate TFRC and GOT1 expression. Overexpression of NEAT1 enhanced ferroptosis stress in bEnd.3 cells. Increased miR-9-5p alleviated sepsis-induced ferroptosis by suppressing the expression of TFRC and GOT1 both in vivo and in vitro. | ||||
hsa-miR-370-3p (miRNA)
Periodontitis [ICD-11: DA0C]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hPLSCs (Periodontal ligament stem cells) | |||
| Response Description | Inhibition of LINC00616 promoted cell viability and suppressed ferroptosis of PDLSCs. miR-370 was verified to be a target of LINC00616. Additionally, miR-370 targeting the transferrin receptor protein and upregulated transferrin receptor (TFRC) abolished the effects of overexpressed miR-370 on cell viability and ferroptosis of PDLSCs. Therefore, LINC00616 knockdown may be a promising therapeutic strategy for periodontitis. | |||
hsa-miR-367-3p (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| Response Description | This study indicated that cinobufotalin induced ferroptosis to suppress lung cancer cell growth by lncRNA LINC00597/hsa-miR-367-3p/TFRC pathway via resibufogenin might provide novel therapeutic targets for lung cancer therapy. | |||
hsa-miR-30b-5p (miRNA)
Cardiac hypertrophy [ICD-11: BC45]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hNCs (Neutrophils cells) | ||||
| rCMECs (Rat cardiac microvascular endothelial cells) | |||||
| In Vivo Model |
The rats were randomly divided into five groups as follows: (1) sham group: the rats underwent sham operation and received vehicle (PBS, caudal vein injection). (2) Model group: the rats were subjected to AAC and received vehicle (PBS, caudal vein injection). (3-5) The treatment groups: the rats were subjected to AAC and after 24 h treated with si-AAB, si-AAB + MSN, or NM + si-AAB + MSN (50 nM siRNA dose per rat every 2 days for 4 weeks, caudal vein injection).
Click to Show/Hide
|
||||
| Response Description | LncRNA AAB was upregulated in the hearts of cardiac hypertrophy rats as well as in the Ang II-induced CMECs. Importantly, we found that lncRNA AAB sponged and sequestered miR-30b-5p to induce the imbalance of MMP9/TIMP1, which enhanced the activation of transferrin receptor 1 (TFR-1) and then eventually led to the ferroptosis of CMECs. | ||||
hsa-mir-222 (Precursor RNA)
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Male C57BL/6 mice (6-8-weeks old; Vitalriver, Beijing, China) were employed. Briefly, 200 ul AAV8-HBV-1.2 was introduced through the tail vein. The exosomes (10 ug) derived from HBV-infected LO2 cells were dissolved in PBS (50 ul) and introduced through the tail vein 2 h after AAV8-HBV-1.2 injection. Mice were anesthetized by inhalation with 3% isoflurane and sacrificed by cervical dislocation after 4 weeks to collect livers for hematoxylin-eosin (HE) and Masson's Trichrome staining and measurement of liver injury as previously described.
Click to Show/Hide
|
||||
| Response Description | Exosomes derived from HBV-infected LO2 cells promote LX-2 cell activation and liver fibrosis in mouse Exosomal miR-222 derived from HBV-infected LO2 cells promotes LX-2 cell activation TFRC is a target of miR-222 and inhibits LX-2 cell activation induced by miR-222 miR-222 promotes LX-2 cell activation through inhibiting TFRC-induced ferroptosis. | ||||
hsa-mir-214 (Precursor RNA)
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | PVT1 regulates ferroptosis through miR-214-mediated p53 and TFR1. The discovery of PVT1 and miR-214 as potential targets for I/R also implies that PVT1 and miR-214 play critical roles in ferroptosis, shedding new light on the mechanism of ferroptosis in acute ischemic stroke. | |||
hsa-miR-200b-3p (miRNA)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Downregulation of circ-PSEN1 ameliorates HG-induced ferroptosis in ARPE19 cells via miR-200b-3p/CFL2 and may be a novel therapeutic target for diabetic retinopathy. Enhancement of CFL2 suppressed the mRNA and protein expression of GPX4 and SLC7A11. However, TFR1 expression was promoted. | |||
Histone-lysine N-methyltransferase EZH2 (EZH2)
Diffuse large B-cell lymphoma [ICD-11: 2A81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Karpas-422 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1325 | |
| U-2932 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1896 | ||
| WILL-2 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1901 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal experiments were approved by the Institutional Animal Care and Use Committee of Shanghai Institute of Materia Medica and performed in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care. Tumors were measured twice weekly and volumes were calculated using the formula TV=length x width2 x 1/2.
Click to Show/Hide
|
||||
| Response Description | EZH2 inhibition upregulated-TfR-1 dysregulation leads to drug resistance in EZH2 WT diffuse large B-cell lymphoma (DLBCL). On the other hand, EZH2i impaired the occurrence of ferroptosis by upregulating the heat shock protein family A (Hsp70) member 5 (HSPA5) and stabilizing glutathione peroxidase 4 (GPX4), a ferroptosis suppressor. | ||||
High mobility group protein B1 (HMGB1)
Myeloid leukaemia [ICD-11: 2B33]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
In Vitro Model |
HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| NB4 cells | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | ||
| KG-1 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0374 | ||
| U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | ||
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| In Vivo Model |
Seven- to eight-week male NOD/SCID (non-obese diabetic/severe combined immunodeficient) mice that weighed about 20 g were purchased from Xiangya Medical College Animal Laboratory (Changsha, China). Indicated HL-60/NRASQ61L cells were subcutaneously injected into the dorsal flanks right of the midline in NOD/SCID mice (weight, approximately 20 g). At day seven, mice were intraperitoneal injected with erastin (20 mg/kg i.v., three times a week) for two weeks. Erastin was dissolved in the vehicle (2% DMSO and 98% PBS) and prepared by Ultrasonic Cleaner (Fisher Scientific, Hampton, NH). A final volume of 300 uL of erastin was applied via intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | HMGB1 could be a potential drug target for therapeutic interventions in leukemia.Importantly, these data were further supported by our in vivo experiment, in which xenografts formed by HMGB1 knockdown HL-60/NRASQ61L cells had lower PTGS2 and TfR1 expression than that in control mice. | ||||
Glutaredoxin-related protein 5, mitochondrial (GLRX5)
Head neck squamous cell carcinoma [ICD-11: 2D60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AMC-HN-3 cells | Laryngeal squamous cell carcinoma | Homo sapiens | CVCL_5961 | |
| HN3R (Human head and neck squamous cell carcinoma cell) | |||||
| HN4 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_IS30 | ||
| HN4R (Human head and neck squamous cell carcinoma cell) | |||||
| In Vivo Model |
Five-week-old athymic BALB/c male nude mice (nu/nu) were purchased from Central Lab Animal Inc. (Seoul, Republic of Korea). HN4R cells with vector control or shGLRX5 were subcutaneously injected into the bilateral flank of nude mice. From the day when gross nodules were detected in tumor implants, mice were subjected to different treatments: vehicle or SAS (250 mg/kg daily per intraperitoneal route). Each group included seven mice.
Click to Show/Hide
|
||||
| Response Description | The suppression of GLRX5 activated the IRE-binding activity of IRP and canonical iron-starvation responsive proteins (increased TfR, decreased FTH), resulting in increased intracellular free iron. The data suggest that inhibition of GLRX5 predisposes therapy-resistant head and neck cancer (HNC) cells to ferroptosis. | ||||
Fanconi anemia group D2 protein (FANCD2)
Bone marrow injury [ICD-11: PK81]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
mBMSCs (Mouse bone marrow stromal cells) | |||
| Response Description | Bone marrow injury remains a serious concern in traditional cancer treatment. FANCD2 regulated genes and/or expression of proteins involved in iron metabolism (e.g., FTH1, TF, TFRC, HAMP, HSPB1, SLC40A1, and STEAP3) and lipid peroxidation (e.g., GPX4). | |||
Cysteine desulfurase (NFS1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MCF10DCIS cells | Normal | Homo sapiens | CVCL_5552 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| SW900 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_1731 | ||
| NCI-H196 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1509 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| NCI-H2170 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_1535 | ||
| NCI-H647 cells | Lung adenosquamous carcinoma | Homo sapiens | CVCL_1574 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| NCI-H838 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | ||
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | ||
| NCI-H322 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1556 | ||
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | ||
| In Vivo Model |
Tumours were initiated in 4-8-week-old female NOD. CB17 Scid/J mice. Orthotopically in the mouse mammary gland, by implantation of 500,000 cells in 25 ul 33% Matrigel into the fourth mouse mammary fat pad; subcutaneously, by injection of 500,000 cells in 100 ul 33% Matrigel into the left or right flank of the mouse; via tail vein by injection of 500,000 cells in 150 ul RPMI into the mouse tail vein; and via intratracheal instillation by instilling 200,000 cells in 50 ul 2 mM EDTA as described. Cancer cells were transduced with viral shRNAs, selected for 3 days with puromycin, and allowed to recover for one day before introduction into mice. For experiments comparing subcutaneous and lung tumour formation, shRNA transduced cells were prepared at the same time and injected on the same day. Animals were imaged by IVIS (Perkin Elmer) 15 min following injection subcutaneously into the neck scruff with XenoLight d-Luciferin (165 mg per kg body weight, Perkin Elmer). Average luminescence was quantified per mouse from equal sized bins covering the mouse thorax. For experiments in which tumour growth was measured upon drug treatment, MDA-MB-231 cells, implanted as described above, were allowed to form palpable tumours (~4 mm diameter) and mice were sorted into treatment groups as described below. PEG-Cyst(e)inase was delivered via intraperitoneal injection at 50 mg per kg body weight every 3 days, SSA was delivered by daily intraperitoneal injection at 250 mg per kg body weight, and BSO was delivered in the drinking water at 20 mM with 5 mg ml-1 sucralose.
Click to Show/Hide
|
||||
| Response Description | NFS1 suppression induced TFRC expression and repressed FTH1 and cytoplasmic aconitase activity. Suppression of NFS1 cooperates with inhibition of cysteine transport to trigger ferroptosis in vitro and slow tumour growth. Therefore, lung adenocarcinomas select for expression of a pathway that confers resistance to high oxygen tension and protects cells from undergoing ferroptosis in response to oxidative damage. | ||||
Cofilin-2 (CFL2)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Downregulation of circ-PSEN1 ameliorates HG-induced ferroptosis in ARPE19 cells via miR-200b-3p/ CFL2 and may be a novel therapeutic target for diabetic retinopathy. Enhancement of CFL2 suppressed the mRNA and protein expression of GPX4 and SLC7A11. However, TFR1 expression was promoted. | |||
CircRAPGEF5 (circRNA)
Corpus uteri cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
KLE cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 | |
| Ishikawa cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 | ||
| HEC-1-A cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_0293 | ||
| HEC-1-B cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_0294 | ||
| RL95-2 cells | Endometrial adenosquamous carcinoma | Homo sapiens | CVCL_0505 | ||
| In Vivo Model |
Female 4-week-old BALB/c-nu nude mice were purchased from Vital River (Beijing, China) and housed in a specific pathogen-free facility. EC cells were washed twice with serum-free medium and injected subcutaneously into nude mice (5 x 106 cells/site). Tumors were measured with calipers every four days for four weeks. The mice were sacrificed on day 32 after cell implantation, and the tumors were excised and weighed.
Click to Show/Hide
|
||||
| Response Description | CircRAPGEF5 interacts with RBFOX2, an important splicing regulator, to modulate the splicing of TFRC pre-mRNA. Importantly, circRAPGEF5 promotes exon-4 skipping of TFRC by sequestering RBFOX2, resulting in resistance to ferroptosis via the reduction of labile iron in endometrial cancer cells. | ||||
Circ-PSEN1 (circRNA)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
ARPE-19 cells | Normal | Homo sapiens | CVCL_0145 |
| Response Description | Downregulation of circ-PSEN1 ameliorates HG-induced ferroptosis in ARPE19 cells via miR-200b-3p/CFL2 and may be a novel therapeutic target for diabetic retinopathy. Enhancement of CFL2 suppressed the mRNA and protein expression of GPX4 and SLC7A11. However, TFR1 expression was promoted. | |||
Cellular tumor antigen p53 (TP53)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague-Dawley (SD) rats (250-300 g) were purchased from the Laboratory Animal Center, Xiangya School of Medicine, Central South University, China. Briefly, a left thoracotomy was carried out in the fourth intercostal space and the heart was exposed via opening the pericardium. The left coronary artery was around via a 4-0 silk suture and a snare was formed by passing both ends of the suture via a short polyethylene tubing. Blockage of the coronary artery was conducted via clamping the snare against the heart surface. Reperfusion was performed by release of the snare. The sham group conducted the same procedure but without ischemia (the snare was not tightened). To establish the I/R injury model, the rat hearts were subjected to 1 h-ischemia plus 3 h-reperfusion.
Click to Show/Hide
|
||||
| Response Description | A novel pathway of USP7/ p53/TfR1 has been identified in the ischemia/reperfusion (I/R)-treated rat hearts, where up-regulation of USP7 promotes ferrptosis via activation of the p53/TfR1 pathway. | ||||
Unspecific Regulator
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | |||
| Responsed Drug | Bavachin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | |||
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | ||||
| Responsed Drug | Polyphyllin B | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
NUGC-3 cells | Gastric carcinoma | Homo sapiens | CVCL_1612 | |
| MKN-1 cells | Gastric carcinoma | Homo sapiens | CVCL_1415 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| In Vivo Model |
The nude mice were raised in our laboratory for a week before the experiment. Then, 5 x 106 MKN-1 cells were subcutaneously injected to establish the subcutaneous xenograft tumour model in nude mice. When the maximum diameter of the xenograft tumours grew steadily to 1 cm, they were dissected completely and cut into 1 mm3 tissue fragments. Then, the tissue fragment was inserted into the surface of the serosa on the greater curvature of the stomach. Different doses of PB (2.5 mg/kg or 5.0 mg/kg) were given by intraperitoneal injection once a day for 3 weeks. The control group was given the same volume of vehicle. The positive control group was given 5-Fu at the dose of 10 mg/kg. The body weight and tumour size of nude mice were recorded. Mice were administered fluorescein substrate (150 mg/kg) intraperitoneally for in vivo imaging twice a week on a Xenogen IVIS 200 imaging system (Caliper Life Sciences, USA). The tumour inhibition rate was analysed using LT Living Image 4.3 Software.
Click to Show/Hide
|
||||
| Response Description | We identified a novel GPx4 inhibitor, polyphyllin B (PB), which can induce ferroptosis by down-regulating GPx4 expression in gastric cancer cells. It has also been shown to inhibit cell proliferation, suppress invasion and migration, induce apoptosis, and block the cell cycle progression in GC cellsin vitro. Then, immunofluorescence and western blotting assay confirmed that PB can regulate the expression of LC3B, TFR1, NOCA4 and FTH1in vitro, which suggested that suggest that PB may increase the level of Fe2+by transporting Fe3+into the cell by TFR1 and promoting NCOA4-dependent iron autophagy. | ||||
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [25] | ||||
| Responsed Drug | Pt3R5G | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| NCM460 cells | Normal | Homo sapiens | CVCL_0460 | ||
| In Vivo Model |
Five-week-old male BALB/c-nude mice from Central Laboratory of Animal, Xi'an Jiaotong University Health Science Center were housed 4 per cage under controlled temperature (23 ± 2 ), a 12 h/12 h light/dark cycle with ad libitum access to food and water and specific pathogen-free conditions. Twelve BALB/c-nude mice were randomly divided into three groups (control, 25 mg/kg, 50 mg/kg). 1 x 106 RKO cells were subcutaneously injected into either side of the same mice dorsal flanks. After 14 days, animals then received Pt3R5G (25 mg/kg, 50 mg/kg) byintraperitoneal injectionfor 15 days. The weight of mouse and tumor nodules sizer were measured every 3 days for 29 days.
Click to Show/Hide
|
||||
| Response Description | Pt3R5G significantly down-regulated SLC7A11 expression and up-regulated TFR1 in RKO cells. Pt3R5G inhibits cell proliferation through inducing ferroptosis by down-regulating SLC7A11 in colon cancer. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | |||
| Responsed Drug | Atractylodin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hccm (Human hepatocellular carcinoma cells) | ||||
| Response Description | Atractylodin can inhibit the proliferation, migration, and invasion of Huh7 and Hccm liver cancer cells, and induce cell apoptosis and cell cycle arrest. In addition, atractylodin may induce ferroptosis in hepatocellular carcinoma cells by inhibiting the expression of GPX4 and FTL proteins, and up-regulating the expression of ACSL4 and TFR1 proteins. | |||
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [27] | |||
| Responsed Drug | Sulfasalazine | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | |
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| Response Description | Sulfasalazine (SAS) upregulated TFRC and DMT1. Knockdown of the ER increased TFRC expression in breast cancer cells. In conclusion SAS could trigger ferroptosis in breast cancer cells, especially in cells with low ER expression. | |||
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [28] | |||
| Responsed Drug | Tetrachlorobenzoquinone | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Tetrachlorobenzoquinone (TCBQ)-induced ferroptosis occurred as a result of iron accumulation and inhibition of GPX4 expression. Mechanistically, TCBQ promotes the iron import into cells by improving the expression of TF and TFR1, and the complex of TF and TFR1 is internalized by endocytosis in Adrenal gland pheochromocytoma. | |||
Alzheimer disease [ICD-11: 8A20]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [29] | ||||
| Responsed Drug | Thioctic acid | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
The P301S transgenic mice [B6C3-Tg (Prnp-MAPT*P301S) PS19 Vle/J], originally obtained from the Jackson laboratory (Bar Harbor, ME, USA), were used as a model of tauopathy. The female mice at the age of 5 months were randomly allocated to three treatment groups (7 mice/group) corresponding to vehicle control, 3 mg/kg LA (T5625, Sigma, St. Louis, MO; the dosage was calculated everyday based on weight), and 10 mg/kg LA. LA was administered by intraperitoneal injection once per day (no injection was administered one day every three days), and vehicle control mice received physiological saline.
Click to Show/Hide
|
||||
| Response Description | Alzheimer's disease (AD) is the most common neurodegenerative disease and is characterized by neurofibrillary tangles (NFTs) composed of Tau protein. a-Lipoic acid (LA) plays a role in inhibiting Tau hyperphosphorylation and neuronal loss, including ferroptosis. After LA administration, TFR expression level was downregulated while Fpn1 level was upregulated, thereby reducing the iron overload. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [30] | ||||
| Responsed Drug | Chrysin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [31] | ||||
| Responsed Drug | Canagliflozin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57BL/6J mice aged 6-8 weeks with weights of 18-20 g were obtained from the Slack Laboratory Animal Co., Ltd. (Shanghai, China). Mice were allowed to acclimatize in the laboratory environment for 1 week before the beginning of the experiment. DCM model establishment: The mice were given a single intraperitoneal injection of 150 mg/kg 1% streptozotocin (STZ, V900890, Sigma, USA, dissolved in 0.1 mol/L sodium citrate buffer, pH = 4.4 - 4.6). Mouse blood from the tail vein was collected in each group of the model mice and tested by glucose meter (Accu-Chek Performa test strips, Roche, Accu-Chek Performa Combo, Roche, USA) on day 3, 5 and 7 after injection.
Click to Show/Hide
|
||||
| Response Description | Canagliflozin (Cana) promotes upregulation of SLC7A11 and downregulation of TfR1 and FTN-H, which protect the cardiomyocytes from ferroptosis. These finding suggests that Cana inhibit ferroptosis by balancing cardiac iron homeostasis and promoting the system Xc/GSH/GPX4 axis in diabetic cardiomyopathy. | ||||
Cardiovascular diseases [ICD-11: BE2Z]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [32] | ||||
| Responsed Drug | Sesamin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
Forty specific pathogen-free normal Sprague Dawley (SD) rats (7 weeks old and 251-275 g in weight) were supplied by Charles River Laboratories. The SD rats were randomly allocated into five groups (n = 8). In the PM2.5 exposure group, the rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times. In the saline control group, the SD rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and intratracheally instilled with 0.9% saline (1 mL per kg b.w.) every other day for a total of three times. In the Ses pretreatment groups, the SD rats were gavaged with low (L-Ses, 40 mg per kg b.w), medium (M-Ses, 80 mg per kg b.w.), and high (H-Ses, 160 mg per kg b.w.) doses of Ses. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times.
Click to Show/Hide
|
||||
| Response Description | Sesamin pretreatment upregulated the expression levels of GPX4, SLC7A11, TFRC, and FPN1 and inhibited the expression levels of FTH1 and FTL. Ses pretreatment could ameliorate PM2.5-induced cardiovascular injuries perhaps by inhibiting ferroptosis. | ||||
Pulmonary fibrosis [ICD-11: CB03]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [33] | ||||
| Responsed Drug | Bleomycin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response Description | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of ACSL4 but similarly decreased those of FSP1. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [34] | ||||
| Responsed Drug | Cyanidin-3-glucoside | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Adult male Sprague Dawley (SD) rats weighing 260-280 g were purchased from Qinglongshan Animal Farm (Nanjing, China). After a week of adaptation, the rats were randomly assigned into five groups (n = 8): (1) sham group, rats receiving saline gavage and sham surgery were used as control group; (2) I/R model group, rats receiving saline gavage and left anterior descending (LAD) ligation surgery were used as the model group; (3) C3G-10 group, I/R model plus intraperitoneal injection of 10 mg/kg C3G; (4) C3G-20 group, I/R model plus intraperitoneal injection of 20 mg/kg C3G; and (5) DIL group, I/R model plus oral administration of 20 mg/kg diltiazem. C3G and DIL were dissolved in DMSO and then diluted with saline so that the DMSO concentration was less than 0.1% (v/v).
Click to Show/Hide
|
||||
| Response Description | The administration of Cyanidin-3-glucoside (C3G) reduced the infarction area, mitigated pathological alterations, inhibited ST segment elevation, and attenuated oxidative stress and ferroptosis-related protein expression. C3G also suppressed the expressions of USP19, Beclin1, NCOA4, and LC3II/LC3I. In addition, treatment with C3G relieved oxidative stress, downregulated LC3II/LC3I, reduced autophagosome number, downregulated TfR1 expression, and upregulated the expressions of FTH1 and GPX4 in OGD/R-induced H9c2 cells. Taken together, C3G could be a potential agent to protect myocardium from myocardial ischemia-reperfusion (IR) injury. | ||||
Vitiligo [ICD-11: ED63]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [35] | |||
| Responsed Drug | Baicalein | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hMCs (Human mesangial cells) | |||
| Response Description | Baicalein up-regulated GPX4 and reduced TFR1 level in melanocytes treated with RSL3+FAC. Baicalein protected melanocytes against ferroptosis through up-regulating GPX4. Ferroptosis might be pervasive in the occurrence and development of vitiligo, and could be proposed as the potential therapeutic target. | |||
Knee osteoarthritis [ICD-11: FA01]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [36] | ||||
| Responsed Drug | Biochanin A | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
Male mice were purchased from Guangzhou University of Chinese Medicine's Experimental Animal Center C57BL/6 mice (7-week-old, 20 g) (Guangzhou, China). After one week of adaptively feeding with chow meals and sterilized water, the animals were separated into five groups of ten mice randomly assigned to the negative control (NC); model, positive control (PC); model group; high dosage of BCA treatment (BCA-H) group; and low dosage of BCA treatment (BCA-L) group. The iron overload mice model was designed based on earlier research. Except for the NC group, mice were administered ID intraperitoneally (500 mg/kg) once a week for eight weeks. In the right knee joints, OA was induced with the initial injection of iron dextran two weeks after the injection by destabilizing the medial meniscus (DMM) using a microscope. After the operation, the positive control group was administered with NAC intragastrically (100 mg/kg) for eight weeks. BCA-H and BCA-L groups were administered 20 mg/kg and 40 mg/kg of BCA separately for eight weeks according to previous studies.
Click to Show/Hide
|
||||
| Response Description | Biochanin A (BCA) could directly reduce intracellular iron concentration by inhibiting TfR1 and promoting FPN but also target the Nrf2/system xc-/GPX4 signaling pathway to scavenge free radicals and prevent lipid peroxidation. The results of this research indicate that BCA regulates iron homeostasis during the progression of osteoarthritis, which can open a new field of treatment for knee osteoarthritis. | ||||
Traumatic brain injury [ICD-11: NA07]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [37] | ||||
| Responsed Drug | Ruxolitinib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Adult male C57BL/6J mice (6-8 weeks, weighting 20-25 g) were used for all experiments. Mice were housed in pairs in a cage with access to food and water ad libitum. Mice were anesthetized with 4% chloral hydrate (0.4 mg/g) and mounted in a stereotaxic system (David Kopf Instruments, Tujunga, California). A midline skin incision was performed on the scalp to expose the skull, and a 5-mm craniotomy was made lateral to the sagittal suture and centered between bregma and lambda. The skull cap was then removed carefully to avoid destroying the dura mater.
Click to Show/Hide
|
||||
| Response Description | Ruxolitinib exerts neuroprotection via repressing ferroptosis in a mouse model of traumatic brain injury. Ruxo significantly inhibited the expressions of COX2 and TfR1. In addition, Ruxo also reversed the lower expression of GPX4 caused by Traumatic brain injury. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [38] | ||||
| Responsed Drug | Sevoflurane | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Sixty-four male Sprague-Dawley rats (2 weeks), weighing 20-30 g. All animals were brought from the Institute of Medical Laboratory Animals at the Chinese Academy of Medical Sciences and were kept in the same unit in a temperature-controlled environment [(22 ± 1) ]. The rats were fasted for 12 h before the experiment and drank water freely. After being numbered according to body weight, the rats were randomly divided into four groups using the random number table. The number of rats in each group was 16. The experimental groups were as follows: sham-operated group (S group, n = 16), the model group receiving HIR (HIR group, n = 16), sevoflurane group treated (HIR + Sev group, n = 16), and desferrioxamine treated group [deferoxamine (HIR + Sev + DFO) group, n = 16]. In HIR+Sev and HIR + Sev + DFO groups, rats were placed in an anesthetizing chamber and exposed to 3.6% sevoflurane (Cayman, 23996, USA) with complete oxygen for 2 h, and sham and HIR group rats were conducted with the same procedure without sevoflurane exposure. DFO (100 mg/kg, MCE, HY-B0988, China) was administered continuously daily for 6 days before surgery in the HIR + Sev + DFO group. Other groups were given equal amounts of saline.
Click to Show/Hide
|
||||
| Response Description | TFRC levels and ACSL4 levels were elevated after sevoflurane administration, suggesting that ferroptosis occurs in whole-brain regions of young rats after HIR and that sevoflurane aggravates the extent of ferroptosis. The results suggest that ferroptosis may mediate sevoflurane-aggravated young rats' brain injury induced by liver transplantation. | ||||
N6-adenosine-methyltransferase non-catalytic subunit (METTL14)
Doxorubicin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Doxorubicin treatment resulted in the upregulation of methyltransferase-like 14 (METTL14), which catalyzes the m6A modification of the long non-coding RNA KCNQ1OT1, a miR-7-5p sponge. And miR-7-5p inhibits DOX-induced ferroptosis in cardiomyocytes by directly repressing TFRC. Inhibiting ferroptosis mediated by a METTL14/KCNQ1OT1/miR-7-5p positive feedback loop in cardiomyocytes could provide a new therapeutic approach to control DOX-induced cardiac injury. | ||||
KCNQ1OT1 (IncRNA)
Doxorubicin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Doxorubicin treatment resulted in the upregulation of methyltransferase-like 14 (METTL14), which catalyzes the m6A modification of the long non-coding RNA KCNQ1OT1, a miR-7-5p sponge. And miR-7-5p inhibits DOX-induced ferroptosis in cardiomyocytes by directly repressing TFRC. Inhibiting ferroptosis mediated by a METTL14/KCNQ1OT1/miR-7-5p positive feedback loop in cardiomyocytes could provide a new therapeutic approach to control DOX-induced cardiac injury. | ||||
hsa-miR-7-5p (miRNA)
Doxorubicin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 | |
| In Vivo Model |
Male Sprague-Dawley rats (6-8 weeks old; weighed from 210 to 230 g) were purchased from HFK Bioscience Co. Ltd. Rats were randomly assigned to four groups (n = 6 per group). The first was the control group, which were treated daily with 0.5 ml of 0.9% saline by intraperitoneal injection for 14 days, and there were three DOX model groups, which were treated three times weekly with 2.5 mg/kg of DOX by intraperitoneal injection for 14 weeks. At day 14, mice in the DOX model groups were infected through an intramyocardial injection of either control shNC or shMettl14 (1 x 109 titer) at three distinct locations in the left ventricular free wall three times a week for 2 weeks, and they were treated daily with 30 mg/kg of ferroptosis inducer erastin (MedChemExpress, USA) through intragastric administration or vehicle control (Saline) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Doxorubicin treatment resulted in the upregulation of methyltransferase-like 14 (METTL14), which catalyzes the m6A modification of the long non-coding RNA KCNQ1OT1, a miR-7-5p sponge. And miR-7-5p inhibits DOX-induced ferroptosis in cardiomyocytes by directly repressing TFRC. Inhibiting ferroptosis mediated by a METTL14/KCNQ1OT1/miR-7-5p positive feedback loop in cardiomyocytes could provide a new therapeutic approach to control DOX-induced cardiac injury. | ||||
Unspecific Regulator
Bavachin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | |||
Polyphyllin B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [24] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | NUGC-3 cells | Gastric carcinoma | Homo sapiens | CVCL_1612 | |
| MKN-1 cells | Gastric carcinoma | Homo sapiens | CVCL_1415 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| In Vivo Model |
The nude mice were raised in our laboratory for a week before the experiment. Then, 5 x 106 MKN-1 cells were subcutaneously injected to establish the subcutaneous xenograft tumour model in nude mice. When the maximum diameter of the xenograft tumours grew steadily to 1 cm, they were dissected completely and cut into 1 mm3 tissue fragments. Then, the tissue fragment was inserted into the surface of the serosa on the greater curvature of the stomach. Different doses of PB (2.5 mg/kg or 5.0 mg/kg) were given by intraperitoneal injection once a day for 3 weeks. The control group was given the same volume of vehicle. The positive control group was given 5-Fu at the dose of 10 mg/kg. The body weight and tumour size of nude mice were recorded. Mice were administered fluorescein substrate (150 mg/kg) intraperitoneally for in vivo imaging twice a week on a Xenogen IVIS 200 imaging system (Caliper Life Sciences, USA). The tumour inhibition rate was analysed using LT Living Image 4.3 Software.
Click to Show/Hide
|
||||
| Response Description | We identified a novel GPx4 inhibitor, polyphyllin B (PB), which can induce ferroptosis by down-regulating GPx4 expression in gastric cancer cells. It has also been shown to inhibit cell proliferation, suppress invasion and migration, induce apoptosis, and block the cell cycle progression in GC cellsin vitro. Then, immunofluorescence and western blotting assay confirmed that PB can regulate the expression of LC3B, TFR1, NOCA4 and FTH1in vitro, which suggested that suggest that PB may increase the level of Fe2+by transporting Fe3+into the cell by TFR1 and promoting NCOA4-dependent iron autophagy. | ||||
Pt3R5G
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [25] | ||||
| Responsed Disease | Colon cancer [ICD-11: 2B90] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| NCM460 cells | Normal | Homo sapiens | CVCL_0460 | ||
| In Vivo Model |
Five-week-old male BALB/c-nude mice from Central Laboratory of Animal, Xi'an Jiaotong University Health Science Center were housed 4 per cage under controlled temperature (23 ± 2 ), a 12 h/12 h light/dark cycle with ad libitum access to food and water and specific pathogen-free conditions. Twelve BALB/c-nude mice were randomly divided into three groups (control, 25 mg/kg, 50 mg/kg). 1 x 106 RKO cells were subcutaneously injected into either side of the same mice dorsal flanks. After 14 days, animals then received Pt3R5G (25 mg/kg, 50 mg/kg) byintraperitoneal injectionfor 15 days. The weight of mouse and tumor nodules sizer were measured every 3 days for 29 days.
Click to Show/Hide
|
||||
| Response Description | Pt3R5G significantly down-regulated SLC7A11 expression and up-regulated TFR1 in RKO cells. Pt3R5G inhibits cell proliferation through inducing ferroptosis by down-regulating SLC7A11 in colon cancer. | ||||
Atractylodin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [26] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
| In Vitro Model | Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hccm (Human hepatocellular carcinoma cells) | ||||
| Response Description | Atractylodin can inhibit the proliferation, migration, and invasion of Huh7 and Hccm liver cancer cells, and induce cell apoptosis and cell cycle arrest. In addition, atractylodin may induce ferroptosis in hepatocellular carcinoma cells by inhibiting the expression of GPX4 and FTL proteins, and up-regulating the expression of ACSL4 and TFR1 proteins. | |||
Sulfasalazine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [27] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | |
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| Response Description | Sulfasalazine (SAS) upregulated TFRC and DMT1. Knockdown of the ER increased TFRC expression in breast cancer cells. In conclusion SAS could trigger ferroptosis in breast cancer cells, especially in cells with low ER expression. | |||
Tetrachlorobenzoquinone
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [28] | |||
| Responsed Disease | Hereditary Leiomyomatosis [ICD-11: 2C90] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | Tetrachlorobenzoquinone (TCBQ)-induced ferroptosis occurred as a result of iron accumulation and inhibition of GPX4 expression. Mechanistically, TCBQ promotes the iron import into cells by improving the expression of TF and TFR1, and the complex of TF and TFR1 is internalized by endocytosis in Adrenal gland pheochromocytoma. | |||
Thioctic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [29] | ||||
| Responsed Disease | Alzheimer disease [ICD-11: 8A20] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
The P301S transgenic mice [B6C3-Tg (Prnp-MAPT*P301S) PS19 Vle/J], originally obtained from the Jackson laboratory (Bar Harbor, ME, USA), were used as a model of tauopathy. The female mice at the age of 5 months were randomly allocated to three treatment groups (7 mice/group) corresponding to vehicle control, 3 mg/kg LA (T5625, Sigma, St. Louis, MO; the dosage was calculated everyday based on weight), and 10 mg/kg LA. LA was administered by intraperitoneal injection once per day (no injection was administered one day every three days), and vehicle control mice received physiological saline.
Click to Show/Hide
|
||||
| Response Description | Alzheimer's disease (AD) is the most common neurodegenerative disease and is characterized by neurofibrillary tangles (NFTs) composed of Tau protein. a-Lipoic acid (LA) plays a role in inhibiting Tau hyperphosphorylation and neuronal loss, including ferroptosis. After LA administration, TFR expression level was downregulated while Fpn1 level was upregulated, thereby reducing the iron overload. | ||||
Chrysin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [30] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
Canagliflozin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [31] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57BL/6J mice aged 6-8 weeks with weights of 18-20 g were obtained from the Slack Laboratory Animal Co., Ltd. (Shanghai, China). Mice were allowed to acclimatize in the laboratory environment for 1 week before the beginning of the experiment. DCM model establishment: The mice were given a single intraperitoneal injection of 150 mg/kg 1% streptozotocin (STZ, V900890, Sigma, USA, dissolved in 0.1 mol/L sodium citrate buffer, pH = 4.4 - 4.6). Mouse blood from the tail vein was collected in each group of the model mice and tested by glucose meter (Accu-Chek Performa test strips, Roche, Accu-Chek Performa Combo, Roche, USA) on day 3, 5 and 7 after injection.
Click to Show/Hide
|
||||
| Response Description | Canagliflozin (Cana) promotes upregulation of SLC7A11 and downregulation of TfR1 and FTN-H, which protect the cardiomyocytes from ferroptosis. These finding suggests that Cana inhibit ferroptosis by balancing cardiac iron homeostasis and promoting the system Xc/GSH/GPX4 axis in diabetic cardiomyopathy. | ||||
Sesamin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [32] | ||||
| Responsed Disease | Cardiovascular diseases [ICD-11: BE2Z] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
Forty specific pathogen-free normal Sprague Dawley (SD) rats (7 weeks old and 251-275 g in weight) were supplied by Charles River Laboratories. The SD rats were randomly allocated into five groups (n = 8). In the PM2.5 exposure group, the rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times. In the saline control group, the SD rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and intratracheally instilled with 0.9% saline (1 mL per kg b.w.) every other day for a total of three times. In the Ses pretreatment groups, the SD rats were gavaged with low (L-Ses, 40 mg per kg b.w), medium (M-Ses, 80 mg per kg b.w.), and high (H-Ses, 160 mg per kg b.w.) doses of Ses. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times.
Click to Show/Hide
|
||||
| Response Description | Sesamin pretreatment upregulated the expression levels of GPX4, SLC7A11, TFRC, and FPN1 and inhibited the expression levels of FTH1 and FTL. Ses pretreatment could ameliorate PM2.5-induced cardiovascular injuries perhaps by inhibiting ferroptosis. | ||||
Bleomycin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [33] | ||||
| Responsed Disease | Pulmonary fibrosis [ICD-11: CB03] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response Description | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of ACSL4 but similarly decreased those of FSP1. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
Cyanidin-3-glucoside
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [34] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Adult male Sprague Dawley (SD) rats weighing 260-280 g were purchased from Qinglongshan Animal Farm (Nanjing, China). After a week of adaptation, the rats were randomly assigned into five groups (n = 8): (1) sham group, rats receiving saline gavage and sham surgery were used as control group; (2) I/R model group, rats receiving saline gavage and left anterior descending (LAD) ligation surgery were used as the model group; (3) C3G-10 group, I/R model plus intraperitoneal injection of 10 mg/kg C3G; (4) C3G-20 group, I/R model plus intraperitoneal injection of 20 mg/kg C3G; and (5) DIL group, I/R model plus oral administration of 20 mg/kg diltiazem. C3G and DIL were dissolved in DMSO and then diluted with saline so that the DMSO concentration was less than 0.1% (v/v).
Click to Show/Hide
|
||||
| Response Description | The administration of Cyanidin-3-glucoside (C3G) reduced the infarction area, mitigated pathological alterations, inhibited ST segment elevation, and attenuated oxidative stress and ferroptosis-related protein expression. C3G also suppressed the expressions of USP19, Beclin1, NCOA4, and LC3II/LC3I. In addition, treatment with C3G relieved oxidative stress, downregulated LC3II/LC3I, reduced autophagosome number, downregulated TfR1 expression, and upregulated the expressions of FTH1 and GPX4 in OGD/R-induced H9c2 cells. Taken together, C3G could be a potential agent to protect myocardium from myocardial ischemia-reperfusion (IR) injury. | ||||
Baicalein
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [35] | |||
| Responsed Disease | Vitiligo [ICD-11: ED63] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hMCs (Human mesangial cells) | |||
| Response Description | Baicalein up-regulated GPX4 and reduced TFR1 level in melanocytes treated with RSL3+FAC. Baicalein protected melanocytes against ferroptosis through up-regulating GPX4. Ferroptosis might be pervasive in the occurrence and development of vitiligo, and could be proposed as the potential therapeutic target. | |||
Biochanin A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [36] | ||||
| Responsed Disease | Knee osteoarthritis [ICD-11: FA01] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCDs (Chondrocytes) | ||||
| In Vivo Model |
Male mice were purchased from Guangzhou University of Chinese Medicine's Experimental Animal Center C57BL/6 mice (7-week-old, 20 g) (Guangzhou, China). After one week of adaptively feeding with chow meals and sterilized water, the animals were separated into five groups of ten mice randomly assigned to the negative control (NC); model, positive control (PC); model group; high dosage of BCA treatment (BCA-H) group; and low dosage of BCA treatment (BCA-L) group. The iron overload mice model was designed based on earlier research. Except for the NC group, mice were administered ID intraperitoneally (500 mg/kg) once a week for eight weeks. In the right knee joints, OA was induced with the initial injection of iron dextran two weeks after the injection by destabilizing the medial meniscus (DMM) using a microscope. After the operation, the positive control group was administered with NAC intragastrically (100 mg/kg) for eight weeks. BCA-H and BCA-L groups were administered 20 mg/kg and 40 mg/kg of BCA separately for eight weeks according to previous studies.
Click to Show/Hide
|
||||
| Response Description | Biochanin A (BCA) could directly reduce intracellular iron concentration by inhibiting TfR1 and promoting FPN but also target the Nrf2/system xc-/GPX4 signaling pathway to scavenge free radicals and prevent lipid peroxidation. The results of this research indicate that BCA regulates iron homeostasis during the progression of osteoarthritis, which can open a new field of treatment for knee osteoarthritis. | ||||
Ruxolitinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [37] | ||||
| Responsed Disease | Traumatic brain injury [ICD-11: NA07] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Adult male C57BL/6J mice (6-8 weeks, weighting 20-25 g) were used for all experiments. Mice were housed in pairs in a cage with access to food and water ad libitum. Mice were anesthetized with 4% chloral hydrate (0.4 mg/g) and mounted in a stereotaxic system (David Kopf Instruments, Tujunga, California). A midline skin incision was performed on the scalp to expose the skull, and a 5-mm craniotomy was made lateral to the sagittal suture and centered between bregma and lambda. The skull cap was then removed carefully to avoid destroying the dura mater.
Click to Show/Hide
|
||||
| Response Description | Ruxolitinib exerts neuroprotection via repressing ferroptosis in a mouse model of traumatic brain injury. Ruxo significantly inhibited the expressions of COX2 and TfR1. In addition, Ruxo also reversed the lower expression of GPX4 caused by Traumatic brain injury. | ||||
Sevoflurane
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [38] | ||||
| Responsed Disease | Traumatic brain injury [ICD-11: NA07] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Sixty-four male Sprague-Dawley rats (2 weeks), weighing 20-30 g. All animals were brought from the Institute of Medical Laboratory Animals at the Chinese Academy of Medical Sciences and were kept in the same unit in a temperature-controlled environment [(22 ± 1) ]. The rats were fasted for 12 h before the experiment and drank water freely. After being numbered according to body weight, the rats were randomly divided into four groups using the random number table. The number of rats in each group was 16. The experimental groups were as follows: sham-operated group (S group, n = 16), the model group receiving HIR (HIR group, n = 16), sevoflurane group treated (HIR + Sev group, n = 16), and desferrioxamine treated group [deferoxamine (HIR + Sev + DFO) group, n = 16]. In HIR+Sev and HIR + Sev + DFO groups, rats were placed in an anesthetizing chamber and exposed to 3.6% sevoflurane (Cayman, 23996, USA) with complete oxygen for 2 h, and sham and HIR group rats were conducted with the same procedure without sevoflurane exposure. DFO (100 mg/kg, MCE, HY-B0988, China) was administered continuously daily for 6 days before surgery in the HIR + Sev + DFO group. Other groups were given equal amounts of saline.
Click to Show/Hide
|
||||
| Response Description | TFRC levels and ACSL4 levels were elevated after sevoflurane administration, suggesting that ferroptosis occurs in whole-brain regions of young rats after HIR and that sevoflurane aggravates the extent of ferroptosis. The results suggest that ferroptosis may mediate sevoflurane-aggravated young rats' brain injury induced by liver transplantation. | ||||
References
