Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0170)
| Name |
Canagliflozin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Canagliflozin; 842133-18-0; Invokana; Canagliflozin anhydrous; TA-7284; JNJ-28431754; JNJ 24831754ZAE; canagliflozin hemihydrate; Canagliflozin [INN]; Canagliflozin hydrate; (2S,3R,4R,5S,6R)-2-(3-((5-(4-FLUOROPHENYL)THIOPHEN-2-YL)METHYL)-4-METHYLPHENYL)-6-(HYDROXYMETHYL)TETRAHYDRO-2H-PYRAN-3,4,5-TRIOL; TA 7284; 1-(Glucopyranosyl)-4-methyl-3-(5-(4-fluorophenyl)-2-thienylmethyl)benzene; JNJ 28431754; CHEBI:73274; 6S49DGR869; (2S,3R,4R,5S,6R)-2-[3-[[5-(4-fluorophenyl)thiophen-2-yl]methyl]-4-methylphenyl]-6-(hydroxymethyl)oxane-3,4,5-triol; (1S)-1,5-Anhydro-1-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4-methylphenyl)-D-glucitol; (1S)-1,5-anhydro-1-(3-{[5-(4-fluorophenyl)-2-thienyl]methyl}-4-methylphenyl)-D-glucitol; (1S)-1,5-Anhydro-1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-D-glucitol; D-Glucitol,1,5-anhydro-1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-, (1S)-; (1s)-1,5-anhydro-1-c-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4-methylphenyl)-d-glucitol; (2S,3R,4R,5S,6R)-2-(3-{[5-(4-fluorophenyl)thiophen-2-yl]methyl}-4-methylphenyl)-6-(hydroxymethyl)oxane-3,4,5-triol; UNII-6S49DGR869; JNJ 24831754AAA; JNJ 24831754; CANAGLIFLOZIN [MI]; MLS006011126; SCHEMBL157162; CANAGLIFLOZIN [WHO-DD]; GTPL4582; CHEMBL2048484; HSDB 8284; AMY3291; XTNGUQKDFGDXSJ-ZXGKGEBGSA-N; BCPP000303; DTXSID601004469; JNJ 28431754AAA; BDBM50386885; MFCD18251436; s2760; AKOS025401827; BCP9000477; CCG-229581; CS-0522; DB08907; KS-1443; NCGC00346691-02; (1S)-1,5-Anhydro-1-c-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-D-glucitol; AC-26303; HY-10451; SMR004702906; SW219119-1; A25050; EN300-6733492; J-500391; Q5030940; Z2235801995; (1S)-1,5-anhydro-1-(3-{[5-(4-fluorophenyl)-2-thienyl]methyl}-4-methyl-phenyl)-D-glucitol; (1S)-1,5-ANHYDRO-1-C-(3-((5-(4-FLUOROPHENYL)THIOPHEN-2-YL)METHYL)-4-METHYLPHENYL)-D- GLUCITOL; D-Glucitol, 1,5-anhydro-1-C-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4- methylphenyl)-, (1S)-; D-glucitol, 1,5-anhydro-1-c-(3-((5-(4-fluorophenyl)-2-thienyl)methyl)-4- methylphenyl)-, (1s)-; D-GLUCITOL, 1,5-ANHYDRO-1-C-(3-((5-(4-FLUOROPHENYL)-2-THIENYL)METHYL)-4-METHYLPHENYL)-; JNJ24831754ZAE; TA 7284;(2S,3R,4R,5S,6R)-2-(3-((5-(4-fluorophenyl)thiophen-2-yl)methyl)-4-methylphenyl)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol
Click to Show/Hide
|
||||
| Structure |
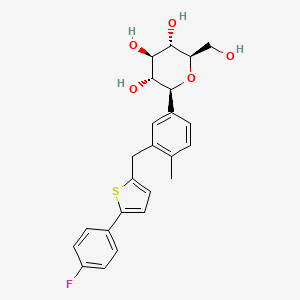 |
||||
| Formula |
C24H25FO5S
|
||||
| IUPAC Name |
(2S,3R,4R,5S,6R)-2-[3-[[5-(4-fluorophenyl)thiophen-2-yl]methyl]-4-methylphenyl]-6-(hydroxymethyl)oxane-3,4,5-triol
|
||||
| Canonical SMILES |
CC1=C(C=C(C=C1)C2C(C(C(C(O2)CO)O)O)O)CC3=CC=C(S3)C4=CC=C(C=C4)F
|
||||
| InChI |
InChI=1S/C24H25FO5S/c1-13-2-3-15(24-23(29)22(28)21(27)19(12-26)30-24)10-16(13)11-18-8-9-20(31-18)14-4-6-17(25)7-5-14/h2-10,19,21-24,26-29H,11-12H2,1H3/t19-,21-,22+,23-,24+/m1/s1
|
||||
| InChIKey |
XTNGUQKDFGDXSJ-ZXGKGEBGSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Transferrin receptor protein 1 (TFRC)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57BL/6J mice aged 6-8 weeks with weights of 18-20 g were obtained from the Slack Laboratory Animal Co., Ltd. (Shanghai, China). Mice were allowed to acclimatize in the laboratory environment for 1 week before the beginning of the experiment. DCM model establishment: The mice were given a single intraperitoneal injection of 150 mg/kg 1% streptozotocin (STZ, V900890, Sigma, USA, dissolved in 0.1 mol/L sodium citrate buffer, pH = 4.4 - 4.6). Mouse blood from the tail vein was collected in each group of the model mice and tested by glucose meter (Accu-Chek Performa test strips, Roche, Accu-Chek Performa Combo, Roche, USA) on day 3, 5 and 7 after injection.
Click to Show/Hide
|
||||
| Response regulation | Canagliflozin (Cana) promotes upregulation of SLC7A11 and downregulation of TfR1 and FTN-H, which protect the cardiomyocytes from ferroptosis. These finding suggests that Cana inhibit ferroptosis by balancing cardiac iron homeostasis and promoting the system Xc/GSH/GPX4 axis in diabetic cardiomyopathy. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57BL/6J mice aged 6-8 weeks with weights of 18-20 g were obtained from the Slack Laboratory Animal Co., Ltd. (Shanghai, China). Mice were allowed to acclimatize in the laboratory environment for 1 week before the beginning of the experiment. DCM model establishment: The mice were given a single intraperitoneal injection of 150 mg/kg 1% streptozotocin (STZ, V900890, Sigma, USA, dissolved in 0.1 mol/L sodium citrate buffer, pH = 4.4 - 4.6). Mouse blood from the tail vein was collected in each group of the model mice and tested by glucose meter (Accu-Chek Performa test strips, Roche, Accu-Chek Performa Combo, Roche, USA) on day 3, 5 and 7 after injection.
Click to Show/Hide
|
||||
| Response regulation | Canagliflozin (Cana) promotes upregulation of SLC7A11 and downregulation of TfR1 and FTN-H, which protect the cardiomyocytes from ferroptosis. These finding suggests that Cana inhibit ferroptosis by balancing cardiac iron homeostasis and promoting the system Xc/GSH/GPX4 axis in diabetic cardiomyopathy. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57BL/6J mice aged 6-8 weeks with weights of 18-20 g were obtained from the Slack Laboratory Animal Co., Ltd. (Shanghai, China). Mice were allowed to acclimatize in the laboratory environment for 1 week before the beginning of the experiment. DCM model establishment: The mice were given a single intraperitoneal injection of 150 mg/kg 1% streptozotocin (STZ, V900890, Sigma, USA, dissolved in 0.1 mol/L sodium citrate buffer, pH = 4.4 - 4.6). Mouse blood from the tail vein was collected in each group of the model mice and tested by glucose meter (Accu-Chek Performa test strips, Roche, Accu-Chek Performa Combo, Roche, USA) on day 3, 5 and 7 after injection.
Click to Show/Hide
|
||||
| Response regulation | Canagliflozin (Cana) promotes upregulation of SLC7A11 and downregulation of TfR1 and FTN-H, which protect the cardiomyocytes from ferroptosis. These finding suggests that Cana inhibit ferroptosis by balancing cardiac iron homeostasis and promoting the system Xc/GSH/GPX4 axis in diabetic cardiomyopathy. | ||||
