Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10055)
| Target Name | Cystine/glutamate transporter (SLC7A11) | ||||
|---|---|---|---|---|---|
| Synonyms |
Amino acid transport system xc-; Calcium channel blocker resistance protein CCBR1; Solute carrier family 7 member 11; xCT
Click to Show/Hide
|
||||
| Gene Name | SLC7A11 | ||||
| Sequence |
MVRKPVVSTISKGGYLQGNVNGRLPSLGNKEPPGQEKVQLKRKVTLLRGVSIIIGTIIGA
GIFISPKGVLQNTGSVGMSLTIWTVCGVLSLFGALSYAELGTTIKKSGGHYTYILEVFGP LPAFVRVWVELLIIRPAATAVISLAFGRYILEPFFIQCEIPELAIKLITAVGITVVMVLN SMSVSWSARIQIFLTFCKLTAILIIIVPGVMQLIKGQTQNFKDAFSGRDSSITRLPLAFY YGMYAYAGWFYLNFVTEEVENPEKTIPLAICISMAIVTIGYVLTNVAYFTTINAEELLLS NAVAVTFSERLLGNFSLAVPIFVALSCFGSMNGGVFAVSRLFYVASREGHLPEILSMIHV RKHTPLPAVIVLHPLTMIMLFSGDLDSLLNFLSFARWLFIGLAVAGLIYLRYKCPDMHRP FKVPLFIPALFSFTCLFMVALSLYSDPFSTGIGFVITLTGVPAYYLFIIWDKKPRWFRIM SEKITRTLQIILEVVPEEDKL Click to Show/Hide
|
||||
| Family | L-type amino acid transporter family | ||||
| Function |
Heterodimer with SLC3A2, that functions as an antiporter by mediating the exchange of extracellular anionic L-cystine and intracellular L-glutamate across the cellular plasma membrane. Provides L-cystine for the maintenance of the redox balance between extracellular L- cystine and L-cysteine and for the maintenance of the intracellular levels of glutathione that is essential for cells protection from oxidative stress. The transport is sodium-independent, electroneutral with a stoichiometry of 1:1, and is drove by the high intracellular concentration of L-glutamate and the intracellular reduction of L-cystine . In addition, mediates the import of L-kynurenine leading to anti-ferroptotic signaling propagation required to maintain L-cystine and glutathione homeostasis. Moreover, mediates N-acetyl-L-cysteine uptake into the placenta leading to subsequently down-regulation of pathways associated with oxidative stress, inflammation and apoptosis. In vitro can also transport L-aspartate. May participate in astrocyte and meningeal cell proliferation during development and can provide neuroprotection by promoting glutathione synthesis and delivery from non-neuronal cells such as astrocytes and meningeal cells to immature neurons. Controls the production of pheomelanin pigment directly.
Click to Show/Hide
|
||||
| Gene ID | 23657 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
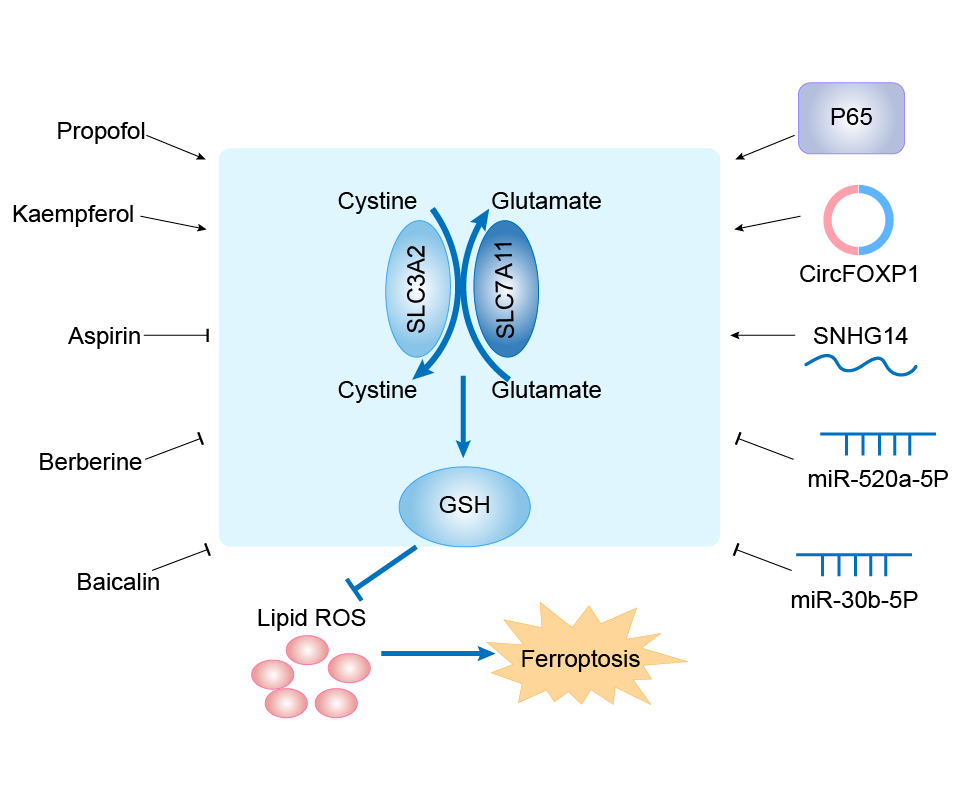
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
SLC7A11 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Ubiquitin-fold modifier 1 (UFM1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Metformin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| HCC1937 cells | Breast ductal carcinoma | Homo sapiens | CVCL_0290 | ||
| Bcap37 cells | Breast carcinoma | Homo sapiens | CVCL_0164 | ||
| HBL-100 cells | Normal | Homo sapiens | CVCL_4362 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| In Vivo Model |
T47D xenografts were established in 5-week-old nude mice (Shanghai SLAC Laboratory Animal Corporation) by inoculating 1 x 107 cells mixed with Matrigel (BD Biosciences) at 1:1 ratio (volume) into the abdominal mammary fat pad. When the tumor reached 50-100 mm3, the mice were assigned randomly into different treatment groups (DMSO, Metformin, SAS, and Metformin + SAS groups). Metformin (200 mg/kg/day) was provided in drinking water. Sulfasalazine was dissolved in dimethyl sulfoxide (DMSO), diluted in PBS, and then intraperitoneally injected into mice at a dose of 250 mg/kg once a day.
Click to Show/Hide
|
||||
| Response Description | Metformin reduces the protein stability of SLC7A11, which is a critical ferroptosis regulator, by inhibiting its UFMylation process. Furthermore, metformin combined with sulfasalazine, the system xc-inhibitor, can work in a synergistic manner to induce ferroptosis and inhibit the proliferation of breast cancer cells. | ||||
Ubiquitin thioesterase OTUB1 (OTUB1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Solasonine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| CFPAC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_1119 | ||
| In Vivo Model |
For xenograft assays, we subcutaneously injected 1 x 106 PANC-1 and CFPAC-1 into the right side of each male nude mouse (n = 6). Tumor volumes (length x width2 x 0.5) were measured at specified time points. For one treatment cycle in a week (starting from week 1 to week 5), solasonine (40 or 80 mg/kg, oral administration, 2 times) were given. A total of five treatment cycles were conducted in this experiment.
Click to Show/Hide
|
||||
| Response Description | Solasonine is involved in ferroptosis by suppressing TFAP2A-mediated transcriptional upregulation of OTUB1, thereby activating ubiquitinated degradation of SLC7A11 and promoting pancreatic cancer cell ferroptosis. | ||||
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [36] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BT-474 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0179 | |
| SK-BR-3 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | ||
| In Vivo Model |
A total of 24 NOD/SCID mice were purchased from Model Animal Research Center and grown under specific-pathogen-free condition. Mice were randomly divided into three groups (n = 8 per group), BT474-Tr cells were inoculated orthotopically onto the abdominal mammary fat pad. After 1 week, mice were treated with erastin (15 mg/kg intraperitoneal, twice every other day). Erastin was dissolved in 5% DMSO + corn oil (C8267, Sigma). To better dissolve erastin, we warmed the tube at 37 water bath and shook it gently. At the end of the sixth week, all mice were sacrificed, tumor tissues were collected and weighed.
Click to Show/Hide
|
||||
| Response Description | Knockdown of circ-BGN inhibited breast cancer cell viability and notably restored its sensitivity to trastuzumab. Further, we found that circ-BGN could directly bind to OTUB1 and SLC7A11, enhancing OTUB1-mediated SLC7A11 deubiquitination and thereby inhibiting ferroptosis. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [37] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mPHs (Mouse primary hepatocytes) | ||||
| In Vivo Model |
ALI induction was performed in 6-8-week-old age-matched C57BL/6J male mice (n = 10-12 per group) by intraperitoneal injection of 3 mL/kg CCl4 in coconut oil. Control and negative control mice were injected with PBS and coconut oil, respectively. At 6 h after injection of CCl4, mice were divided into three groups: CCl4 group, injected with 100 uL PBS (supplemented with 2% mouse serum) through a tail vein; CCl4 + MSC group, injected with 5 x 105 MSCs suspended in 100 uL PBS (supplemented with 2% mouse serum) through a tail vein; CCl4 + Fer-1 group, intraperitoneally injected with ferrostatin-1 (Fer-1, a ferroptosis inhibitor, 2.5 umol/kg body weight). Erastin, intraperitoneal injection of erastin (a ferroptosis inducer, 30 mg/kg body weight) twice every other day, and then the mice were divided into two groups (n = 10-12 per group): Erastin group, injected with 100 uL PBS (supplemented with 2% mouse serum) through a tail vein; Erastin + MSC group, injected with 5 x 105 MSCs suspended in 100 uL PBS (supplemented with 2% mouse serum) through the tail vein.
Click to Show/Hide
|
||||
| Response Description | MSC-Exo protected against CCl4-induced acute liver injury (ALI) through inhibiting hepatocyte ferroptosis via restoring the SLC7A11 protein level. Additionally, the exosome-induced recovery of SLC7A11 protein was accompanied by upregulations of CD44 and OTUB1. | ||||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [145] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HEK293 cells | Normal | Homo sapiens | CVCL_0045 | |
| SK-N-BE(2)-C cells | Neuroblastoma | Homo sapiens | CVCL_0529 | ||
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| SK-RC-42 cells | Renal cell carcinoma | Homo sapiens | CVCL_6192 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| T24 cells | Bladder carcinoma | Homo sapiens | CVCL_0554 | ||
| UM-UC-3 cells | Bladder carcinoma | Homo sapiens | CVCL_1783 | ||
| SW780 cells | Bladder carcinoma | Homo sapiens | CVCL_1728 | ||
| In Vivo Model |
5.0 x 106 cells were mixed with Matrigel (BD Biosciences) at 1:1 ratio (v/v) and injected subcutaneously into seven-week old nude mice (NU/NU; Charles River). Mice were fed with regular chow. After nine weeks, the mice were killed and the tumors were weighed and recorded.
Click to Show/Hide
|
||||
| Response Description | Overexpression of the cancer stem cell marker CD44 enhanced the stability of SLC7A11 by promoting the interaction between SLC7A11 and OTUB1; depletion of CD44 partially abrogated this interaction. CD44 expression suppressed ferroptosis in cancer cells in an OTUB1-dependent manner. | ||||
Tumor necrosis factor alpha-induced protein 3 (TNFAIP3)
Lung cancer [ICD-11: 2C25]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Erastin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Cisplatin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
Transcription factor AP-2-alpha (TFAP2A)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Solasonine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| CFPAC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_1119 | ||
| In Vivo Model |
For xenograft assays, we subcutaneously injected 1 x 106 PANC-1 and CFPAC-1 into the right side of each male nude mouse (n = 6). Tumor volumes (length x width2 x 0.5) were measured at specified time points. For one treatment cycle in a week (starting from week 1 to week 5), solasonine (40 or 80 mg/kg, oral administration, 2 times) were given. A total of five treatment cycles were conducted in this experiment.
Click to Show/Hide
|
||||
| Response Description | Solasonine is involved in ferroptosis by suppressing TFAP2A-mediated transcriptional upregulation of OTUB1, thereby activating ubiquitinated degradation of SLC7A11 and promoting pancreatic cancer cell ferroptosis. | ||||
Signal transducer and activator of transcription 3 (STAT3)
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Bavachin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | |||
Head neck squamous cell carcinoma [ICD-11: 2D60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [51] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ferroptosis | hsa04216 | ||||
| JAK-STAT signaling pathway | hsa04630 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HN4 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_IS30 | |
| CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | ||
| In Vivo Model |
Four-week-old male BALB/c-nu mice were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). About 4 x 106 CAL27 cells were stably transfected with lentivirus. After administration of 2 ug/mL puromycin for three days, transfection efficiency was confirmed by western blotting. Approximately 2 x 106 transfected cells were subcutaneously injected into flanks. For the drug-administration study, 20 mg/kg erastin (S7242, Selleck Chemicals) were administrated intraperitoneally twice every other day. Approximately 20 uL IL-6 (10 ug/mL, PeproTech, USA) were given intratumorally twice every other day.
Click to Show/Hide
|
||||
| Response Description | The study demonstrate the critical role of IL-6-induced ferroptosis resistance during head and neck squamous cell carcinoma carcinogenesis. The IL-6/ STAT3/xCT (encoded by SLC7A11) axis acts as a novel mechanism driving tumor progression and thus may potentially be utilized as a target for tumor prevention and therapy. | ||||
rno-miR-23a-3p (miRNA)
Supraventricular tachycardia [ICD-11: BC81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | GW4869 | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| rCFs (Rat cardiac fibroblasts) | |||||
| In Vivo Model |
Eighteen beagles, randomly divided into three groups, both sexes and an average age of 1 year, weighing 7.5 ± 1.5 kg, were used for the study as follows: Sham group (n = 6), Pacing group (n = 6), and GW4869 + Pacing group (n = 6). Each beagle canine was given an intramuscular injection of 25 mg/kg ketamine sulfate before being premedicated with pentobarbital sodium (30 mg/kg, intravenous injection) and ventilated with room air by a respirator (MAO01746, Harvard Apparatus Holliston, United States). Venous access was established to supply saline (50-100 mL/h) or pentobarbital sodium (2.5 mg/kg/h).
Click to Show/Hide
|
||||
| Response Description | The exosome inhibitor GW4869 reduced ferroptosis, fibrosis, and inflammation and improved histological and electrophysiological remodeling. Pacing-CF-exos highly expressed miR-23a-3p by informatics analysis and experimental verification. Inhibitor- miR-23a-3p protected h9c2 cells from ferroptosis accompanying with upregulation of SLC7A11. The development of atrial fibrillation (AF) in a persistent direction could be prevented by intervening with exosomal miRNAs to reduce oxidative stress injury and ferroptosis. | ||||
RNA-binding motif, single-stranded-interacting protein 1 (RBMS1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Nortriptyline hydrochloride | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Doxorubicin (Dox)- inducible RBMS1 knockdown stable cells (3 x 106 ) were injected subcutaneously into the abdomen side of 6-week-old BALB/c nude mice (Vital River). Mice were fed either with sucrose water or sucrose water containing 0.1% doxycycline hyclate. H1299 vector, 4 H1299 pLKO.1 RBMS1 and H1299 pLKO.1 RBMS1/SLC7A11 cells (2.5 x 106 ) were injected subcutaneously into the abdomen side of 6-week-old BALB/c nude mice(Vital River). The xenograft tumour formation was monitored using callipers every 3 days.
Click to Show/Hide
|
||||
| Response Description | RBMS1 ablation inhibited the translation of SLC7A11, reduced SLC7A11-mediated cystine uptake, and promoted ferroptosis. Nortriptyline hydrochloride decreased the level of RBMS1, thereby promoting ferroptosis. Importantly, RBMS1 depletion or inhibition by nortriptyline hydrochloride sensitized radioresistant lung cancer cells to radiotherapy. | ||||
RAF proto-oncogene serine/threonine-protein kinase (RAF1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Tetraarsenic tetrasulfide | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| Response Description | On H23 cells treated with realgar, the expression of GPX4, SCL7A11 decreased while ACSL4 expression increased; this effect could also be amplified by Sorafenib. In conclusion, the present study indicated that realgar may induce ferroptosis by regulating the Raf, and hence plays a role in antiKRAS mutant lung cancer. | |||
Protein FAM98A (FAM98A)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Metformin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| HCT 15 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0292 | ||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| FHC cells | Normal | Homo sapiens | CVCL_3688 | ||
| In Vivo Model |
A total 1 x 106 SW620-vector or SW620-FAM98A cells were suspended in 100 ul PBS and injected s.c. into the back of 4- to 6-week-old male BALB/cnude mice (Laboratory Animal Unit, Southern Medical University, China). The sizes of the resulting tumors were measured weekly. Tumor volumes were calculated as follows: total tumor volume (mm3) = (Length x Width2)/2, where Length is the longest length. When the tumor sizes reached about 200 mm3, nude mice in the four groups were given PBS or 5-FU treatment (30 mg/kg, intraperitoneal injection, twice a week), respectively. Nude mice were maintained in a barrier facility in racks filtered with a high-efficiency particulate air filter.
Click to Show/Hide
|
||||
| Response Description | The expressions of FAM98A and SLC7A11 were also downregulated after metformin treatment. And FAM98A is predominantly expressed in the colorectal cancer tissues and high FAM98A expression is usually accompanied by the high expression of SLC7A11, which usually means ferroptosis resistance. | ||||
NF-kappa-B inhibitor alpha (NFKBIA)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | RSL3 | Investigative | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female B-NDG mice (4-6 weeks old, 16-20 g) were purchased from Biocytogen (Biocytogen Jiangsu Co., Ltd., Jiangsu, China) and housed under specific pathogen-free conditions. 5 x 106 U87 cells were resuspended in 200 uL PBS buffer and then inoculated into the left hind limb of each mouse. Once tumor volumes reached >=50 mm3, the mice were randomly divided into four groups (n = 5): the control, RSL3-only, BAY-only, and RSL3 plus BAY groups. Chemicals were administered through intratumor injection (100 mg/kg for RSL3 and 1 mg/kg for BAY 11-7082) biweekly for two weeks.
Click to Show/Hide
|
||||
| Response Description | NF-kB pathway activation is vital for RSL3-induced ferroptosis in glioblastoma cells both in vitro and in vivo. Furthermore, RNAi-mediated GPX4 silencing cannot trigger ferroptosis in glioblastoma cells unless the NF-kB pathway is activated simultaneously. Finally, NF-kB pathway activation promotes ferroptosis by downregulating the expression of ATF4 and SLC7A11. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Quercetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from Gempharmatech Co., Ltd (Changzhou, China). All mice were housed in cages with standard laboratory conditions: a consistent temperature of 24 , a 12 h light/dark cycle, and free access to water and food. The mice were randomized into four groups: 1) the KA group (n = 6), injected intraperitoneally with 20 mg/kg KA, as described in a previous study; while 2) the control group (n = 6), injected intraperitoneally with an equal volume of PBS; 3) the KA + QCT group (n = 6): this group was givenintragastric administrationof 50 mg/kg of QCT once daily for 21 days before KA injection based on the literature; and 4) the KA+ferrostatin1 (Fer-1) group (n = 6), injected intraperitoneally with a well-known ferroptosis inhibitor (3 mg/kg Fer-1) for 21 days before KA administration, as described in a previous study.
Click to Show/Hide
|
||||
| Response Description | The association between the Nrf2-mediated ferroptosis pathway and seizures in a clinical setting. Quercetin effectively protects against seizure-induced neuron death in vivo and in vitro and alleviates cognitive function impairment via the SIRT1/Nrf2/SLC7A11/GPX4 pathway. | ||||
Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Micafungin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
The surgical procedure for establishing the myocardial I/R injury rat model was carried out as we did before. Briefly, a left thoracotomy was performed in the fourth intercostal space and the heart was exposed via opening thepericardium. The left coronary artery was surrounded with a 4-0 silk suture and a snare was formed by passing both ends of the suture via a short polyethylene tubing. Blockage of the coronary artery was conducted via clamping the snare against the heart surface. Reperfusion was performed by release of the snare. The sham group conducted the same procedure but without ischemia (the snare was not tightened). To establish the I/R injury model, the rat hearts were subjected to 1 h-ischemia plus 3 h-reperfusion. At the end, the blood and hearts were collected for assay of the creatine kinase(CK) activity and infarct size to determine the success of I/R injury model. To explore the role of MALT1 in myocardial I/R injury the underlying mechanisms, three sets of experiment were performed.
Click to Show/Hide
|
||||
| Response Description | The inhibition of MALT1 can reduce ischemia/reperfusion-induced myocardial ferroptosis through enhancing the Nrf2/SLC7A11 pathway; and MALT1 may be used as a potential target to seek novel or existing drugs (such as micafungin) for treating myocardial infarction. | ||||
Mothers against decapentaplegic homolog 3 (SMAD3)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Formononetin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mPRTECs (Mouse primary renal tubular epithelial cells) | ||||
| In Vivo Model |
For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium(30 mg/kg). Then, UUO surgery orsham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10% DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10% DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO. For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg). Then, UUO surgery or sham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10 % DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10 % DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO.
Click to Show/Hide
|
||||
| Response Description | Formononetin (FN) alleviates chronic kidney disease (CKD) by impeding ferroptosis-associated fibrosis by suppressing the Smad3/ATF3/SLC7A11 signaling and could serve as a candidate therapeutic drug for CKD. In addition, FN also promoted the separation of the Nrf2/Keap1 complex and enhanced Nrf2 nuclear accumulation. | ||||
Mitogen-activated protein kinase 8 (MAPK8)
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Seratrodast | Discontinued in Phase 3 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Drugs were dissolved in vehicle (0.1% DMSO + 20% PEG 300 + 0.5% CMC-Na + ddH2O). Mice in Control and PTZ groups were administered for five days with an equivalent volume of vehicle. PTZ-induced seizure model was done for the subsequent 1 h after the last administration of drugs. We performed a preliminary doseresponse trial, the dose of 60 mg/kg was established as being sufficient to trigger seizures with lower mortality and chosen as the optimal dose. One mouse in PTZ group was dead due to a severe seizure. At the end of the experiment, the mice were anesthetized or euthanized. For histopathological studies, the mice were anesthetized and intracardially perfused with 0.9% saline, followed by 0.4% paraformaldehyde for fixation of the brain. For immunoblot analysis, the hippocampus was rapidly isolated.
Click to Show/Hide
|
||||
| Response Description | Seratrodast could reduce lipid ROS production, regulate the system xc-/glutathione (GSH)/glutathione peroxidase 4 (GPX4) axis, and inhibit JNK (MAPK8) phosphorylation and p53 expression. JNK can directly or indirectly modulate the expression and activation of p53, which could regulate ferroptosis through inhibition of SLC7A11 transcription. Seratrodast increased the latency of seizures and reduced seizure duration in pentylenetetrazole-induced seizures. | ||||
Mitogen-activated protein kinase 1 (MAPK1)
Corpus uteri cancer [ICD-11: 2C76]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Simvastatin | Investigative | ||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
Ishikawa cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 |
| Response Description | Simvastatin has the potential to be a targeted drug for endometrial cancer (EC) treatment. Besides, the inhibition to the RAS/MAPK signaling pathway allows simvastatin to induce ferroptosis through up-regulating the level of ROS, MDA, Fe2+, and TRF1 (TF) and reducing the level of GSH, SLC7A11, and FPN in cells. | |||
LINC00618 (IncRNA)
Myeloid leukaemia [ICD-11: 2B33]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Vincristine | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 |
| K-562 cells | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | |
| Response Description | LINC00618, is reduced in human leukemia and strongly increased by vincristine (VCR) treatment. Furthermore, LINC00618 promotes apoptosis by increasing the levels of BCL2-Associated X (BAX) and cleavage of caspase-3. LINC00618 also accelerates ferroptosis by increasing the levels of lipid reactive oxygen species (ROS) and iron, two surrogate markers of ferroptosis, and decreasing the expression of solute carrier family 7 member 11 (SLC7A11). | |||
Krueppel-like factor 4 (KLF4)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Polyphyllin III | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| HBL-100 cells | Normal | Homo sapiens | CVCL_4362 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| MDA-MB-453 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | ||
| In Vivo Model |
MDA-MB-231 xenografts were established in 5 week-old BALB/C nude mice (Shanghai SLAC Laboratory Animal Corporation) by inoculating 1 x 106 cells mixed with Matrigel (BD Biosciences) at a 1:1 ratio into the abdominal mammary fat pad. When the tumor reached 50-100 mm3, the mice were assigned randomly into different treatment groups (DMSO, PPIII, SAS, and PPIII + SAS groups), and each group consisted of 5 mice. PPIII (5 mg/kg/day) and SAS (200 mg/kg/day) were dissolved in dimethyl sulfoxide (DMSO), diluted in PBS, and then intraperitoneally injected into mice at a dose of 10 ml/kg/d once a day.
Click to Show/Hide
|
||||
| Response Description | Polyphyllin III, which is a major saponin extracted fromParis polyphyllarhizomes, exerted its proliferation-inhibitory effect on MDA-MB-231 triple-negative breast cancer cells mainly through ACSL4-mediated lipid peroxidation elevation and ferroptosis induction. Polyphyllin III treatment also induced KLF4-mediated protective upregulation of xCT(SLC7A11), which is the negative regulator of ferroptosis. | ||||
Krueppel-like factor 15 (KLF15)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Elabela | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
rAFs (Rat adventitial fibroblasts) | |||
| Response Description | KLF15 siRNA impeded the beneficial roles of elabela (ELA) in DOX-pretreated rat aortic AFs by suppressing the Nrf2/SLC7A11/GPX4 signaling. In conclusion, ELA prevents DOX-triggered promotion of cytotoxicity, and exerts anti-oxidative and anti-ferroptotic effects in rat aortic AFs via activation of the KLF15/GPX4 signaling, indicating a promising therapeutic value of ELA in antagonizing DOX-mediated cardiovascular abnormality and disorders. | |||
hsa-miR-489-3p (miRNA)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Levobupivacaine | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | |
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| In Vivo Model |
Ten SCID nude mice aged 6-8 weeks were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), and subcutaneously injected with SGC7901 cells (5 x 106 cells per mouse) in left back. One week after feeding, the mice were randomly divided into two groups, the control and treatment group. For the next 25 days, the mice in treatment group were injected with erastin (15 mg/kg intraperitoneally) or co-treated with 40 mol/kg body weight of levobupivacaine once a day. The body weight and tumor size were measured every 3 days.
Click to Show/Hide
|
||||
| Response Description | Levobupivacaine-upregulated miR-489-3p enhanced ferroptosis of gastric cancer cells by targeting SLC7A11. MiR-489-3p was involved in levobupivacaine-induced ferroptosis of gastric cancer cells. Levobupivacaine/miR-489-3p/SLC7A11 axis attenuates gastric cancer cell proliferationin vitro. | ||||
hsa-miR-382-5p (miRNA)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Lidocaine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| In Vivo Model |
SPF-level male nude mice aged 56weeks and weighted around 20 g were purchased from Vitalriver (China). All mice were maintained in a 12-hour circadian rhythm, and had free access to water and food. Cancer cells were subcutaneously injected into the right flank of mice. Lidocaine was administrated to mice at a dose of 1.5 mg per kg injected through the vail tails. For control group, the mice were treated with saline. Tumor volume and mice body weight were monitored every 5 days.
Click to Show/Hide
|
||||
| Response Description | The ovarian and breast cancer cell proliferation was suppressed while cell apoptosis was induced by lidocaine in vitro. Lidocaine attenuated invasion and migration of ovarian and breast cancer cells as well. Regarding the mechanism, lidocaine downregulated solute carrier family 7 member 11 (SLC7A11) expression by enhancing microRNA-382-5p (miR-382-5p) in the cells. | ||||
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Lidocaine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| In Vivo Model |
SPF-level male nude mice aged 56weeks and weighted around 20 g were purchased from Vitalriver (China). All mice were maintained in a 12-hour circadian rhythm, and had free access to water and food. Cancer cells were subcutaneously injected into the right flank of mice. Lidocaine was administrated to mice at a dose of 1.5 mg per kg injected through the vail tails. For control group, the mice were treated with saline. Tumor volume and mice body weight were monitored every 5 days.
Click to Show/Hide
|
||||
| Response Description | The ovarian and breast cancer cell proliferation was suppressed while cell apoptosis was induced by lidocaine in vitro. Lidocaine attenuated invasion and migration of ovarian and breast cancer cells as well. Regarding the mechanism, lidocaine downregulated solute carrier family 7 member 11 (SLC7A11) expression by enhancing microRNA-382-5p (miR-382-5p) in the cells. | ||||
hsa-miR-122-5p (miRNA)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Isorhynchophylline | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| p53 signaling pathway | hsa04115 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (SD rats, weighing 250-300 g) aged 11-12 weeks were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All 96 rats were randomly divided into four groups of 24 rats each: Sham group, Sham + IRN (30 mg/Kg) group, ICH group, and ICH + IRN (30 mg/Kg) group. The rats in sham group were injected with PBS solution, and the Sham + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection) after the sham operation. After ICH, the rats in ICH group were injected with PBS solution, and the ICH + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection).
Click to Show/Hide
|
||||
| Response Description | Isorhynchophylline (IRN) decreased ferroptosis and lipid ROS level, upregulated the expression of miR-122-5p and SLC7A11 mRNA, and inhibited TP53 expression. In conclusion, IRN protects neurocyte from intracerebral hemorrhage (ICH)-induced ferroptosis via miR-122-5p/TP53/SLC7A11 pathway, which may provide a potential therapeutic mechanism for ICH. | ||||
Gap junction alpha-1 protein (GJA1)
Acute kidney failure [ICD-11: GB60]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | GAP 27 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Glutathione metabolism | hsa00480 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Thirty-two male C57BL/6 mice (20 ± 2) g (Beijing Weitonglihua Experimental Animal Technology Co., Ltd.) were bred in individually ventilated cages (IVC) at SPF conditions, kept on a 12 h light/dark cycle, relative humidity conditions (40-70%) and controlled temperature (24 ± 2 ). After one-week acclimation mice were divided randomly into four groups: control group, cisplatin group (20 mg/kg cisplatin dissolved in saline), cisplatin + Fer-1 group (5 mg/kg Fer-1 dissolved in DMSO), and cisplatin + gap27 group (35 ug/kg gap27 dissolved in DMSO). There were eight animals in each group and 20 mg/kg cisplatin was given to each animal once by intraperitoneal injection except mice in the control group. Fer-1 and gap27 was administered 1 h before the injection of cisplatin.
Click to Show/Hide
|
||||
| Response Description | Downregulation of Cx43 expression by gap27 reduced acute kidney injury in the animal model by inhibiting cisplatin-induced ferroptosis. Therefore, our results indicated that downregulation of Cx43 can inhibit ferroptosis by restoring the level of SLC7A11 in the system xctransporter and alleviate cisplatin-induced acute kidney injury. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Thirty-two male C57BL/6 mice (20 ± 2) g (Beijing Weitonglihua Experimental Animal Technology Co., Ltd.) were bred in individually ventilated cages (IVC) at SPF conditions. After one-week acclimation mice were divided randomly into four groups: control group, cisplatin group (20 mg/kg cisplatin dissolved in saline), cisplatin + Fer-1 group (5 mg/kg Fer-1 dissolved in DMSO), and cisplatin + gap27 group (35 ug/kg gap27 dissolved in DMSO). There were eight animals in each group and 20 mg/kg cisplatin was given to each animal once by intraperitoneal injection except mice in the control group. Fer-1 and gap27 was administered 1 h before the injection of cisplatin.
Click to Show/Hide
|
||||
| Response Description | Downregulation of Cx43 ( GJA1) can inhibit ferroptosis by restoring the level of SLC7A11 in the system xctransporter and alleviate cisplatin-induced acute kidney injury. And downregulating Cx43 not only inhibits ferroptosis, but also inhibits apoptosis. | ||||
Endothelial PAS domain-containing protein 1 (EPAS1)
Degenerative arthritis [ICD-11: FA05]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | D-Mannose | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
C57BL/6 J mice (8 weeks old, female) were purchased from Dossy Experimental Animal Limited Company (Chengdu, China). For surgery, mice were anaesthetized with pentobarbital sodium (100 mg/kg, injected intraperitoneally) and subjected to unilateral ACLT procedures. 28 The sham group received a skin incision and suturing without patellar dislocation or ligament transection. For virus injection, mice were intraarticularly injected with 1 x 109 pfu (8 ul) of mock or AdEpas1 virus after one week of surgery. For Fer1 (MCE, Monmouth Junction, HY100579) injection, mice were intraarticularly injected with 1 mg/kg Fer1 or with vehicle two weeks after surgery, the injection was repeated once a week.
Click to Show/Hide
|
||||
| Response Description | D-mannose alleviates osteoarthritis (OA) progression by suppressing HIF-2a-mediated chondrocyte sensitivity to ferroptosis. Overexpression of HIF-2a in chondrocytes by Ad- Epas1 intra-articular injection abolished the chondroprotective effect of D-mannose during OA progression and eliminated the role of D-mannose as a ferroptosis suppressor. Also, the RNA and protein levels of the two key ferroptosis suppressors, Gpx4 and Slc7a11, were increased in Dmannosetreated chondrocytes. | ||||
Cyclic GMP-AMP synthase (CGAS)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Niraparib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cytosolic DNA-sensing pathway | hsa04623 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| MC-38 cells | Colon adenocarcinoma | Homo sapiens | CVCL_B288 | ||
| In Vivo Model |
Six-week-old male BALB/c athymic nude mice were purchased from the Experimental Animal Center of Peking (Beijing, China). Stable cells (5 x 106) were seeded into the right flanks of the mice. After the xenografts had grown to 200 mm3, saline as a vehicle or sorafenib (30 mg/kg) was administered by gavage every day, and the mice were euthanized by the cervical dislocation method five weeks later. Before sacrifice, the tumor sizes and body weights were measured twice per week. The tumor volume (V) was calculated as follows: (L x W2)/2 (length, L, and width, W). The xenografts were excised and further assessed.
Click to Show/Hide
|
||||
| Response Description | Niraparib, a widely used PARPi, augmented cGAS-mediated ferroptosis and immune activation. In colorectal cancer models, cGAS signaling exerts tumor control via ATF3SLC7A11GPX4-mediated ferroptosis and IFNCD8 T cell-mediated antitumor immune response. | ||||
Cyclic AMP-dependent transcription factor ATF-3 (ATF3)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Brucine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U118 cells | Astrocytoma | Homo sapiens | CVCL_0633 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| In Vivo Model |
The athymic BALB/c nude mice (4 weeks; 20-22 g; Beijing Vital River Laboratory Animal Technology Company, China) were housed in a specific pathogen-free environment under a 12-h lightdark cycle with free access to food and water. The animals were allowed to acclimatize to their surroundings for 3 days. U87 cells (1 x 106) in the logarithmic growth phase in 100 uL PBS were subcutaneously injected into the right flank. Therapeutic experiments were started when the tumor reached around 150 mm3 after about 10 days. Mice were allocated to receive intraperitoneal injections of vehicle (control group, n = 6) or 40 mg/kg bodyweight (n = 6) in the same volume (50 uL) once a day for 13 times.
Click to Show/Hide
|
||||
| Response Description | Brucine inhibited glioma cell growth in vitro and in vivo, and brucine induced ATF3 upregulation and translocation into nuclei via activation of ER stress. ATF3 promoted brucine-induced H2O2 accumulation via upregulating NOX4 and SOD1 to generate H2O2 on one hand, and downregulating catalase and xCT (SLC7A11) to prevent H2O2 degradation on the other hand. | ||||
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Formononetin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mPRTECs (Mouse primary renal tubular epithelial cells) | ||||
| In Vivo Model |
For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium(30 mg/kg). Then, UUO surgery orsham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10% DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10% DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO. For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg). Then, UUO surgery or sham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10 % DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10 % DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO.
Click to Show/Hide
|
||||
| Response Description | Formononetin (FN) alleviates chronic kidney disease (CKD) by impeding ferroptosis-associated fibrosis by suppressing the Smad3/ATF3/SLC7A11 signaling and could serve as a candidate therapeutic drug for CKD. In addition, FN also promoted the separation of the Nrf2/Keap1 complex and enhanced Nrf2 nuclear accumulation. | ||||
Cellular tumor antigen p53 (TP53)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [25] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Pseudolaric acid B | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-87MG cells | Glioblastoma | Homo sapiens | CVCL_0022 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| SHG-44 cells | Astrocytoma | Homo sapiens | CVCL_6728 | ||
| In Vivo Model |
Twenty athymic BALB/c nude mice (aged 4 weeks, weight 20-22 g, from Shanghai laboratory animal Center, Shanghai, China) were housed in a specific pathogen-free environment. A total of 1 x 106 logarithmically growing C6 cells in 100 uL of PBS were subcutaneously injected into the right flank of each mouse. Therapeutic experiments were started when the tumor reached about 150 mm3 after about 7 days. The mice were allocated to receive intraperitoneal injections of vehicle (control group, n = 5/group), PAB at the dosage of 10 mg/kg body weight (n = 10/group) and 20 mg/kg body weight (n = 10/group) in the same volume 50 uL once a days for 8 times.
Click to Show/Hide
|
||||
| Response Description | Pseudolaric acid B (PAB) improved intracellular iron by upregulation of transferrin receptor. The increased iron activated Nox4, which resulted in overproduction of H2O2and lipid peroxides. Moreover, PAB depleted intracellular GSH via p53-mediated xCT (SLC7A11) pathway, which further exacerbated accumulation of H2O2and lipid peroxides. Thus, PAB triggers ferroptosis in glioma cells and is a potential medicine for glioma treatment. | ||||
T-cell lymphoma [ICD-11: 2B01]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Kayadiol | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
YT cells | Natural killer cell lymphoblastic leukemia | Homo sapiens | CVCL_1797 |
| hPBLs (Human peripheral blood lymphocytes) | ||||
| Response Description | Kayadiol decreased the expression of SLC7A11 and GPX4, the negative regulatory proteins for ferroptosis. And p53 was the key mediator of kayadiol-induced ferroptosis by SLC7A11/GPX4 axis through p53 knockout experiments. Kayadiol can serve as an effective alternative in the treatment of NK/T cell lymphoma. | |||
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Bavachin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | |||
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [27] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Tanshinone IIA | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| NCI-N87 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1603 | ||
| In Vivo Model |
All mice were housed under a setting of 12-h light/dark cycle at 22 ± 1, 55% humidity and fed with water and food provided at regular time. During the entire maintenance period, all mice were permitted free cage activity without joint immobilization. The initial body weights of the mice were between 20 and 23 grams. After subcutaneous injection of 2 x 106 BGC-823 gastric cancer cells into the back of NOD-SCID mice, the mice were treated with or without Tan IIA (50 mg/kg) or Tan IIA in combination with Fer-1 (50 mg/kg). Tan IIA was diluted in DMSO:Methanol:Hydroxypropyl-b-cydodextrin (HP-b-CD) = 1:1:1. Fer-1 was also dissolved in DMSO:Methanol:HP-b-CD. Seven days after BGC-823 gastric cancer cells injection, intraperitoneal injection with Tan IIA was carried out every other day followed by killing at day 22 of tumor cell inoculation. All mice were killed by dislocation of the cervical vertebrae. Before killing, the tumor volume was measured every 3 days. All experiments were carried out using six mice each group in three independent experiments of a time-dependent manner with three time points.
Click to Show/Hide
|
||||
| Response Description | Tanshinone IIA increased lipid peroxidation and up-regulated Ptgs2 and Chac1 expression, two markers of ferroptosis. In addition, Tan IIA also up-regulated p53 expression and down-regulated xCT (SLC7A11) expression. Therefore, Tan IIA could suppress the proliferation of gastric cancer via inducing p53 upregulation-mediated ferroptosis. | ||||
Melanoma [ICD-11: 2C30]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [28] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Gambogenic Acid | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell adhesion molecules | hsa04514 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 |
| A2058 cells | Amelanotic melanoma | Homo sapiens | CVCL_1059 | |
| Response Description | Gambogenic acid (GNA) significantly inhibited the invasion, migration and EMT in melanoma cells, and these cells exhibited small mitochondrial wrinkling (an important feature of ferroptosis). GNA upregulated the expression of p53, solute carrier family 7 member 11 (SLC7A11) and glutathione peroxidase 4 (GPX4) in the model cells, contributing to the mechanisms underlying GNA-induced ferroptosis. | |||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [29] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Cyperquat | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | As a classic drug employed inin vitromodels of Parkinson's disease, 1-methyl-4-phenylpyridinium (MPP) can induce senescence in PC12 cells. The expression of the ferroptosis-related proteins ASCL4 was upregulated and FTH1 was downregulated, which promoted accumulation of lipid peroxides and eventually led to ferroptosis. By rescuing MPP-induced ferroptosis, cell senescence could be inhibited, and its molecular mechanism was related to a p53/SLC7A11/GPX4 signaling pathway. | |||
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Seratrodast | Discontinued in Phase 3 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Drugs were dissolved in vehicle (0.1% DMSO + 20% PEG 300 + 0.5% CMC-Na + ddH2O). Mice in Control and PTZ groups were administered for five days with an equivalent volume of vehicle. PTZ-induced seizure model was done for the subsequent 1 h after the last administration of drugs. We performed a preliminary doseresponse trial, the dose of 60 mg/kg was established as being sufficient to trigger seizures with lower mortality and chosen as the optimal dose. One mouse in PTZ group was dead due to a severe seizure. At the end of the experiment, the mice were anesthetized or euthanized. For histopathological studies, the mice were anesthetized and intracardially perfused with 0.9% saline, followed by 0.4% paraformaldehyde for fixation of the brain. For immunoblot analysis, the hippocampus was rapidly isolated.
Click to Show/Hide
|
||||
| Response Description | Seratrodast could reduce lipid ROS production, regulate the system xc-/glutathione (GSH)/glutathione peroxidase 4 (GPX4) axis, and inhibit JNK (MAPK8) phosphorylation and p53 expression. JNK can directly or indirectly modulate the expression and activation of p53, which could regulate ferroptosis through inhibition of SLC7A11 transcription. Seratrodast increased the latency of seizures and reduced seizure duration in pentylenetetrazole-induced seizures in Epilepsy. | ||||
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Isorhynchophylline | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| p53 signaling pathway | hsa04115 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (SD rats, weighing 250-300 g) aged 11-12 weeks were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All 96 rats were randomly divided into four groups of 24 rats each: Sham group, Sham + IRN (30 mg/Kg) group, ICH group, and ICH + IRN (30 mg/Kg) group. The rats in sham group were injected with PBS solution, and the Sham + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection) after the sham operation. After ICH, the rats in ICH group were injected with PBS solution, and the ICH + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection).
Click to Show/Hide
|
||||
| Response Description | Isorhynchophylline (IRN) decreased ferroptosis and lipid ROS level, upregulated the expression of miR-122-5p and SLC7A11 mRNA, and inhibited TP53 expression. In conclusion, IRN protects neurocyte from intracerebral hemorrhage (ICH)-induced ferroptosis via miR-122-5p/TP53/SLC7A11 pathway, which may provide a potential therapeutic mechanism for ICH. | ||||
Sarcopenia [ICD-11: FB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [30] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Ferric citrate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| In Vivo Model |
The 8-week- and 40-week-old male SAMP8 mice were purchased from the model animal research center of Zhishan Institute of Healthcare Research Co., Ltd. (Beijing, China). All the mice were kept in an SPF grade animal facility at 24 with a relative humidity of 50%-60%, and in a light/dark cycle of 12 h/12 h.
Click to Show/Hide
|
||||
| Response Description | Ferric citrate induced ferroptosis in C2C12 cells, as well as impaired their differentiation from myoblasts to myotubes. Iron overload upregulated the expression of P53, which subsequently repressed the protein level of Slc7a11 (solute carrier family 7, member 11), a known ferroptosis-related gene. Targeting iron accumulation and ferroptosis might be a therapeutic strategy for treating sarcopenia. | ||||
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [50] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| HBE1 cells | Normal | Homo sapiens | CVCL_0287 | ||
| In Vivo Model |
For the models of CS and LPS exposure, mice were anesthetized and intratracheally instilled with CS suspensions (3 mg/50 ul) or LPS (1 mg/kg). For the models of CS + Ferr-1/DFO, mice were intraperitoneally injected with Ferr-1 (1.25 umol/kg) or intranasal instilled with DFO (10 mg/kg) for 7 consecutive days after CS instillation. For the models of LPS + Ferr-1/DFO, mice were pretreated with Ferr-1 or DFO for 2 consecutive days and then intratracheally instilled with LPS. Mice were sacrificed 24 h after LPS instillation. For the X-ray exposure model, mice were exposed to ionizing radiation (IR) at 20 Gy, which was delivered at the dose rate of 2 Gy/min and a source skin distance of 51 cm by an X-ray generator (Model X-RAD320iX; Precision X-Ray, Inc., North Branford, CT, USA), and sacrificed 3 days after radiation.
Click to Show/Hide
|
||||
| Response Description | STAT6 negatively regulates ferroptosis through competitively binding with CBP, which inhibits P53 acetylation and transcriptionally restores SLC7A11 expression. Finally, pulmonary-specific STAT6 overexpression decreased the ferroptosis and attenuated CS and LPS induced acute lung injury. | ||||
CD44 antigen (CD44)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [31] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Sodium butyrate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
FHC cells | Normal | Homo sapiens | CVCL_3688 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| In Vivo Model |
Six-week-old male C57BL/6J mice were purchased from the Medical Laboratory Animal Center of Guangdong Province (Foshan, China). Forty-five C57BL/6L mice were randomized into 4 groups after 1 week of adaptive feeding: (1) Control group (n = 9); (2) AOM/DSS group (n = 12); (3) AOM/DSS + NaB (orally) group (n = 12); 4) AOM/DSS + NaB (intraperitoneal injection) group (n = 12). The Control group received an intraperitoneal injection of saline solution beginning on day 1, and received sterile drinking water throughout the study. Other three groups received an intraperitoneal injection of 10 mg/kg AOM (Sigma Aldrich) beginning on day 1, and received drinking water containing 2.5% DSS at the second and eighth weeks (2% DSS in the fifth week). Besides, 0.1 M NaB (Sigma Aldrich) was given in drinking water during the whole experiment process in AOM/DSS + NaB (p.o.) group, while AOM/DSS + NaB (i.p.) group was injected intraperitoneally (IP) with 1 g/kg NaB per day.
Click to Show/Hide
|
||||
| Response Description | Sodium butyrate (NaB) induces ferroptosis in colorectal cancer cells through the CD44/SLC7A11 signaling pathway and has synergistic effects with Erastin. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [37] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mPHs (Mouse primary hepatocytes) | ||||
| In Vivo Model |
ALI induction was performed in 6-8-week-old age-matched C57BL/6J male mice (n = 10-12 per group) by intraperitoneal injection of 3 mL/kg CCl4 in coconut oil. Control and negative control mice were injected with PBS and coconut oil, respectively. At 6 h after injection of CCl4, mice were divided into three groups: CCl4 group, injected with 100 uL PBS (supplemented with 2% mouse serum) through a tail vein; CCl4 + MSC group, injected with 5 x 105 MSCs suspended in 100 uL PBS (supplemented with 2% mouse serum) through a tail vein; CCl4 + Fer-1 group, intraperitoneally injected with ferrostatin-1 (Fer-1, a ferroptosis inhibitor, 2.5 umol/kg body weight). Erastin, intraperitoneal injection of erastin (a ferroptosis inducer, 30 mg/kg body weight) twice every other day, and then the mice were divided into two groups (n = 10-12 per group): Erastin group, injected with 100 uL PBS (supplemented with 2% mouse serum) through a tail vein; Erastin + MSC group, injected with 5 x 105 MSCs suspended in 100 uL PBS (supplemented with 2% mouse serum) through the tail vein.
Click to Show/Hide
|
||||
| Response Description | MSC-Exo protected against CCl4-induced acute liver injury (ALI) through inhibiting hepatocyte ferroptosis via restoring the SLC7A11 protein level. Additionally, the exosome-induced recovery of SLC7A11 protein was accompanied by upregulations of CD44 and OTUB1. | ||||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [145] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HEK293 cells | Normal | Homo sapiens | CVCL_0045 | |
| SK-N-BE(2)-C cells | Neuroblastoma | Homo sapiens | CVCL_0529 | ||
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| SK-RC-42 cells | Renal cell carcinoma | Homo sapiens | CVCL_6192 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| T24 cells | Bladder carcinoma | Homo sapiens | CVCL_0554 | ||
| UM-UC-3 cells | Bladder carcinoma | Homo sapiens | CVCL_1783 | ||
| SW780 cells | Bladder carcinoma | Homo sapiens | CVCL_1728 | ||
| In Vivo Model |
5.0 x 106 cells were mixed with Matrigel (BD Biosciences) at 1:1 ratio (v/v) and injected subcutaneously into seven-week old nude mice (NU/NU; Charles River). Mice were fed with regular chow. After nine weeks, the mice were killed and the tumors were weighed and recorded.
Click to Show/Hide
|
||||
| Response Description | Overexpression of the cancer stem cell marker CD44 enhanced the stability of SLC7A11 by promoting the interaction between SLC7A11 and OTUB1; depletion of CD44 partially abrogated this interaction. CD44 expression suppressed ferroptosis in cancer cells in an OTUB1-dependent manner. | ||||
Bromodomain-containing protein 4 (BRD4)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [32] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | JQ1 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| In Vivo Model |
Female athymic BALB/c nude mice (4-6-week old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Approximately 1 x 107 cells (A549) in 200 uL of serum-free medium and Matrigel solution were injected directly into the right axilla of each mouse. Tumor growth was measured with calipers every 3 days.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis was induced under (+)-JQ1 treatment and BRD4 knockdown, indicating that (+)-JQ1 induces ferroptosis via BRD4 inhibition in breast adenocarcinoma. In addition, expression of the ferroptosis-associated genes GPX4, SLC7A11, and SLC3A2 was downregulated under (+)-JQ1 treatment. Moreover, JQ1 treatment and BRD4 knockdown led to decreased FTH1 expression. | ||||
5'-AMP-activated protein kinase catalytic subunit alpha-2 (PRKAA2)
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Sulfasalazine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 HCT116, CX-1, or HT1080 cells in 100 ul phosphate buffered saline (PBS; Thermo Fisher Scientific, AM9625) were injected subcutaneously right of the dorsal midline in athymic nude immunodeficient mice (six- to eight-week-old, female). To generate orthotopic tumors, 1 x 106 KPC cells in 10 ul PBS were surgically implanted into the pancreases of immunocompetent C57BL/6J mice (six- to eight-week-old, female).
Click to Show/Hide
|
||||
| Response Description | BECN1 plays a novel role in lipid peroxidation that could be exploited to improve anticancer therapy by the induction of ferroptosis. Mechanistically, phosphorylation of BECN1 at Ser90/93/96 by PRKAA/ AMPK contributes to the formation of a BECN1-SLC7A11 complex and system Xc-inhibition. Knockdown of BECN1 by shRNA inhibits ferroptosis induced by system X-c- inhibitors (e.g., erastin, sulfasalazine, and sorafenib) in Colon carcinoma. | ||||
5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1)
Multiple myeloma [ICD-11: 2A83]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [34] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Fingolimod | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| AMPK signaling pathway | hsa04152 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 |
| RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 | |
| Response Description | Multiple myeloma (MM) is the second hematological plasma cell malignany and sensitive to fingolimod (FTY720), a novel immunosuppressant. Fingolimod (FTY720) can activate PP2A subunit C and dephosphorylate AMPKa, and then inhibit the expression of SLC7A11 and GPX4. In conclusion, FTY720 induces ferroptosis and autophagy through the PP2A/AMPK pathway. | |||
Uc.339 (IncRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [35] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| 16HBE14o- cells | Normal | Homo sapiens | CVCL_0112 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| In Vivo Model |
6-8 weeks mice were divided into 4 groups randomly. Mice were injected with 2.5 x 105 wild type LLC cells, Uc.339 OE-LLC cells, with or without miR-339 inhibitors, respectively, through the lateral tail vain. The mice were killed after 4 weeks by carbon dioxide asphyxiation followed by cervical dislocation to ensure death. The lungs were removed, rinsed with PBS, and the number of metastatic foci on the lung surface was counted. The pulmonary lobes were subsequently kept in 4% paraformaldehyde for later paraffin embedding and hematoxylin and eosin staining.
Click to Show/Hide
|
||||
| Response Description | LncRNA Uc.339 competitively binds to pri-miR-339 and inhibits the production of mature miR-339. At the same time, it is the first to clarify the reason for the negative correlation between miR-339 and SLC7A11 expression in lung cancer, and for the first verification that the inhibition of miR-339 led to increased expression of SLC7A11 and weakens ferroptosis, which constituted an important carcinogenesis mechanism for lung adenocarcinoma metastasis. | ||||
Tripartite motif-containing protein 26 (TRIM26)
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [38] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
LX-2 cells | Normal | Homo sapiens | CVCL_5792 | |
| In Vivo Model |
A total of 24 C57BL/6 mice were obtained from the Sippr-BK laboratory animal Co., Ltd. (Shanghai, China). They were randomly divided into four groups: Group I, Vehicle (control); Group II, CCl4; Group III, CCL4 + Vector; Group IV, CCL4 + oeTRIM26. Liver fibrosis was induced in Groups II - IV, by intraperitoneally injecting 50% carbon tetrachloride (CCl4) in corn oil (0.1 mL/100 g body weight) over 8 weeks (3 times/week); control mice were injected with corn oil only. Then, recombinant adenovirus Vector or oeTRIM26 (5 x 109 pfu/mouse, 0.5 mL) was injected into the mice of Group III or IV, respectively, through the tail vein.
Click to Show/Hide
|
||||
| Response Description | TRIM26 promotes HSCs ferroptosis to suppress liver fibrosis through mediating the ubiquitination of SLC7A11. The TRIM26-targeted SLC7A11 suppression can be a novel therapeutic strategy for liver fibrosis. | ||||
Transforming growth factor beta-1 proprotein (TGFB1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [39] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| SNU-387 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0250 | |
| SNU-449 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | |
| SNU475 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0497 | |
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| HuH-6 cells | Hepatoblastoma | Homo sapiens | CVCL_4381 | |
| Response Description | TGF-B1 represses xCT (SLC7A11) expression via Smad3 activation and enhances lipid peroxidation in hepatocellular carcinoma cells with an early TGF-B1 signature, which would benefit from the targeting of GPX4. | |||
Transcriptional coactivator YAP1 (YAP1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [40] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| SNU-398 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0077 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| HLE cells | Hepatocellular carcinoma | Homo sapiens | CVCL_1281 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| In Vivo Model |
SNU398-parental cells, SNU398-shLuc, or SNU398-shYAP/TAZ cells (106 in 100 ul PBS) were implanted into the left flanks of immunodeficient NOD/SCID; common receptor-/-(NSG) mice. When tumors were palpable, Sorafenib (LC Laboratories, S-8502) was applied at 20 mg/kg daily via gavage, SSA (Sulfasalazine, Sigma, S0883) was given at 120 mg/kg daily by intraperitoneal injection, 20 mM BSO (Lbuthionine-sulfoximine, Sigma, B2515) was given in the drinking water for 3 weeks.
Click to Show/Hide
|
||||
| Response Description | In a TEAD-dependent manner, YAP/TAZ induce the expression of SLC7A11, a key transporter maintaining intracellular glutathione homeostasis, thus enabling hepatocellular carcinoma cells to overcome Sorafenib-induced ferroptosis. At the same time, YAP/TAZ sustain the protein stability, nuclear localization, and transcriptional activity of ATF4 which in turn cooperates to induce SLC7A11 expression. | ||||
Transcription factor Sp1 (SP1)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [42] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MPC-5 cells | Normal | Mus musculus | CVCL_AS87 | |
| In Vivo Model |
Male C57BL/6 mice (6-8 weeks old, 20-25 g) were obtained from KCI BioTech (Jiangsu, China. Mice were intraperitoneally injected 50 mg/kg/day of STZ for 5 straight days to generate DN mouse. At 3 days post injection, glucose levels were measured from tail blood, and blood glucose level more than 16.4 mmol/L indicated that DN models was established.
Click to Show/Hide
|
||||
| Response Description | Prdx6 overexpression also eliminated ferroptosis caused by HG, which was reflected in the suppression of iron accumulation and the increase in SLC7A11 and GPX4 expression. Moreover, Sp1 could bind to the three Sp1 response elements in the Prdx6 promoter, thereby directly regulating the transcriptional activation of Prdx6 in podocytes. Further, Prdx6 overexpression attenuated renal injuries in streptozotocin-induced diabetic nephropathy mice. | ||||
Transcription factor SOX-2 (SOX2)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [43] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hTCs (Human tumour cells) | ||||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| In Vivo Model |
Mice were housed in the SIBCB animal facility under SPF conditions with a 12 hours light/dark cycle at room temperature. For Erastin treatment, we sequentially dissolved Erastin in 5% DMSO, 30% PEG300, 5% Tween80 and ddH2O according to the manufacturers instructions, it should be noted that the solvent needed to be added from left to right, after the dissolution was completely clear, added the next reagent, 40 mg/kg Erastin was intraperitoneal injected, every other day for 2 weeks. For IKE treatment, 50 mg/kg IKE was intraperitoneal injected, once a day for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Tumors with high SOX2 expression were more resistant to ferroptosis, and SLC7A11 expression was positively correlated with SOX2 in both mouse and human lung cancer tissue. This study uncovers a SOX2-SLC7A11 regulatory axis that confers resistance to ferroptosis in lung cancer stem-like cells. | ||||
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [44] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mKTs (Mouse knee tissues) | ||||
| HK-2 (Human renal glomerular endothelial cells) | |||||
| In Vivo Model |
All C57BL/6 mice (5-6 weeks, 18-20 g) were obtained from Animal Testing Center of Qinglongshan (Nanjing, Suzhou, China). DN mice were fed with a high-fat diet (HFD) for 12 weeks and then injected with STZ (30 mg/kg of streptozotocin; Sigma-Aldrich, St Louis, MO, USA) i.p. for 7 consecutive days. Blood glucose levels of mice were measured using 16.7 mmol/l after one week of the final injection. Then, mice were killed under anesthesia and their kidneys taken for analysis after induction of STZ at 4 months.
Click to Show/Hide
|
||||
| Response Description | Circular RNA circ ASAP2 decreased inflammation and ferroptosis in diabetic nephropathy through SOX2/ SLC7A11 by miR-770-5p. Importantly, this research indicated that circ ASAP2 might act as a target for improving the role of ferroptosis in diabetes nephropathy. | ||||
Transcription factor AP-2 gamma (TFAP2C)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [45] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | ||
| In Vivo Model |
PC3 and PC3/DR cells (5 x 106 cells) were subcutaneously injected into each flank of six-week-old male BALB/c nude mice (HFK Biotech, China). When the tumor volume reached 100 mm3, the mice were treated with Dimethyl Sulfoxide (DMSO) alone, DTX (5 mg/kg body weight, every two days) with DMSO or erastin (20 mg/kg body weight in 20 ul DMSO plus 130 ul corn oil, daily) by intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Docetaxel (DTX)-resistant prostate cancer cells develop tolerance toward ferroptosis and that lncRNAPCAT1 promotes chemoresistance by blocking DTX-induced ferroptosis. Mechanistic studies indicated that PCAT1 activates the expression of SLC7A11 by interacting with c-Myc and sponging with miR-25-3p. In addition, TFAP2C activates PCAT1 expression to reduce ferroptosis susceptibility and enhance chemoresistance. | ||||
Toll-like receptor 4 (TLR4)
Preeclampsia [ICD-11: JA24]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [46] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hPTs (Human placental tissues) | |||
| Response Description | Panx1 and TLR4 are suggested to induce ferroptosis in Preeclampsia via SLC7A11-mediated signaling pathways, offering a novel perspective on PE pathogenesis and novel diagnostic tools for Preeclampsia. | |||
Telomerase reverse transcriptase (TERT)
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [47] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
We received 60 C57BL/6J mice and 48 Nrf2 knockout (Nrf2-/-) mice of the same genetic background from the RIKEN Bio-Resource Centre via the National BioResource Project, MEXT, Japan. Intestinal ischemia was simulated by clamping the superior mesenteric artery following the intraperitoneal administration of 50 mg/kg sodium pentobarbital. Forty-five minutes later, the intestine was allowed to re-perfuse for 3 h.
Click to Show/Hide
|
||||
| Response Description | Nrf2 can negatively regulate ferroptosis via modulation of TERT and SLC7A11 levels. Overexpression of TERT (OETERT) alleviates ferroptosis via modulation of SLC7A11. The conclusion from this study brings insight into new candidates that can be targeted in future ischemia/reperfusion-induced acute lung injury (IIR-ALI) therapy. | ||||
Tafazzin (TAFAZZIN)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [40] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| SNU-398 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0077 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| HLE cells | Hepatocellular carcinoma | Homo sapiens | CVCL_1281 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| In Vivo Model |
SNU398-parental cells, SNU398-shLuc, or SNU398-shYAP/TAZ cells (106 in 100 ul PBS) were implanted into the left flanks of immunodeficient NOD/SCID; common receptor-/-(NSG) mice. When tumors were palpable, Sorafenib (LC Laboratories, S-8502) was applied at 20 mg/kg daily via gavage, SSA (Sulfasalazine, Sigma, S0883) was given at 120 mg/kg daily by intraperitoneal injection, 20 mM BSO (Lbuthionine-sulfoximine, Sigma, B2515) was given in the drinking water for 3 weeks.
Click to Show/Hide
|
||||
| Response Description | In a TEAD-dependent manner, YAP/TAZ induce the expression of SLC7A11, a key transporter maintaining intracellular glutathione homeostasis, thus enabling hepatocellular carcinoma cells to overcome Sorafenib-induced ferroptosis. At the same time, YAP/TAZ sustain the protein stability, nuclear localization, and transcriptional activity of ATF4 which in turn cooperates to induce SLC7A11 expression. | ||||
SNHG14 (IncRNA)
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [48] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SJSA-1 cells | Osteosarcoma | Homo sapiens | CVCL_1697 |
| Response Description | LncRNA SNHG14 targeted and down-regulated the expression of miR-206, further affecting the common ferroptosis inhibitor SLC7A11, and preventing NR-SJSA1 cells from undergoing ferroptosis. In conclusion, our findings highlight the involvement of lncRNA SNHG14 in ferroptosis and chemotherapy resistance of nutlin3a-resistant NR-SJSA1 cells, thus shedding new insight on how to overcome drug resistance in osteosarcoma cells and improve treatment efficacy. | |||
SLC16A1-AS1 (IncRNA)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [49] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 |
| Response Description | SLC16A1-AS1 served as a sponge of miR-143-3p, and knockdown SLC16A1-AS1 significantly increased the enrichment of miR-143-3p. And then, SLC7A11 was identified as the target protein of miR-143-3p, and overexpression miR-143-3p remarkably inhibited the expression of SLC7A11. And silencing lncRNA SLC16A1-AS1 can induce ferroptosis through miR-143-3p/SLC7A11 signaling in renal cell carcinoma. | |||
Signal transducer and activator of transcription 6 (STAT6)
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [50] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| HBE1 cells | Normal | Homo sapiens | CVCL_0287 | ||
| In Vivo Model |
For the models of CS and LPS exposure, mice were anesthetized and intratracheally instilled with CS suspensions (3 mg/50 ul) or LPS (1 mg/kg). For the models of CS + Ferr-1/DFO, mice were intraperitoneally injected with Ferr-1 (1.25 umol/kg) or intranasal instilled with DFO (10 mg/kg) for 7 consecutive days after CS instillation. For the models of LPS + Ferr-1/DFO, mice were pretreated with Ferr-1 or DFO for 2 consecutive days and then intratracheally instilled with LPS. Mice were sacrificed 24 h after LPS instillation. For the X-ray exposure model, mice were exposed to ionizing radiation (IR) at 20 Gy, which was delivered at the dose rate of 2 Gy/min and a source skin distance of 51 cm by an X-ray generator (Model X-RAD320iX; Precision X-Ray, Inc., North Branford, CT, USA), and sacrificed 3 days after radiation.
Click to Show/Hide
|
||||
| Response Description | STAT6 negatively regulates ferroptosis through competitively binding with CBP, which inhibits P53 acetylation and transcriptionally restores SLC7A11 expression. Finally, pulmonary-specific STAT6 overexpression decreased the ferroptosis and attenuated CS and LPS induced acute lung injury. | ||||
Sentrin-specific protease 1 (SENP1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
SEMA5A-IT1 (IncRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [52] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 |
| Response Description | SEMA5A-IT1 overexpression upregulated the expression of BCL2 and SLC7A11 through sponging miR-143-3p, thereby protecting cardiomyocytes against apoptotic and ferroptosis cell death. In conclusion, we propose that SEMA5A-IT1, which is transported to cardiomyocytes through circulating sEVs, is an important regulatory molecule that protects cardiomyocytes from ischemia-reperfusion injury. | |||
RNA-binding protein PNO1 (PNO1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [53] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Autophagy | hsa04140 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| In Vivo Model |
Xenograft mouse model experiments were used male BALB/c nude mice (4 weeks old) purchased from SPF Biotechnology (Beijing, China). Each mouse was injected 5 x 106 tumor cells at the volume of 100 uL into the subcutaneous tissue. The tumor volume and weight of the mice was observed every 2 days. Mice were monitored daily and the tumor volume calculated according to the equation volume = length x width2 x 1/2.
Click to Show/Hide
|
||||
| Response Description | PNO1 inhibits autophagy-mediated ferroptosis via GSH metabolic reprogramming as demonstrated above. We also demonstrated that PNO1 inhibition repressed SLC7A11 through p53 to promote ferroptosis. These observations suggested that sh-PNO1 could be a new target in hepatocellular carcinoma therapy. | ||||
Protein mono-ADP-ribosyltransferase PARP9 (PARP9)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP9 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP9 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP8 (PARP8)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP8 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP8 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP6 (PARP6)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP6 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP6 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP4 (PARP4)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP4 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP4 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP3 (PARP3)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP3 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP3 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP16
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP16 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP16 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP15
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP15 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP15 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP14
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP14 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP14 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP12
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP12 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP12 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP11 (PARP11)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP11 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP11 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Protein mono-ADP-ribosyltransferase PARP10 (PARP10)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP10 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP10 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Poly [ADP-ribose] polymerase 2 (PARP2)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP2 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP2 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
Poly [ADP-ribose] polymerase 1 (PARP1)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
HEY cells | Ovarian carcinoma | Homo sapiens | CVCL_0297 | |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female 4- to 6-week-old BALB/c nude mice were purchased from SLA Laboratory Animal (Changsha, China) and housed in a specific pathogen-free facility. 2 x 106 A2780 or 1 x 106 HEY cells were injected subcutaneously into mice to grow tumors up to approximately 100 mm3. Mice were then intraperitoneally injected olaparib (100 mg/kg) or/and liproxstatin-1 (10 mg/kg, A2780) or/and sulfasalazine (250 mg/kg, HEY) until the endpoint indicated in the corresponding figures.
Click to Show/Hide
|
||||
| Response Description | Mechanistically, pharmacological inhibition or genetic deletion of PARP1 downregulates the expression of cystine transporter SLC7A11 in a p53-dependent manner. Consequently, decreased glutathione biosynthesis caused by SLC7A11 repression promotes lipid peroxidation and ferroptosis. Pharmacologic inhibition of PARP1 is the primary therapeutic strategy for BRCA mutant ovarian cancer. | ||||
PMAN (IncRNA)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [55] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| In Vivo Model |
For animal models of gastric subserosal injection, we collected MGC-803 cell lines (5 x 10 cells) that were infected by lentivirus with or without PMAN-OE, and suspended in 40 ul serum-free medium (50% Matrigel). After that, nude mice (six mice per group) were anesthetized by intraperitoneal injection of 100 ul of pentobarbital (1%). After disinfection, the abdominal cavity was opened to expose the greater curvature of the stomach. The tumor suspension (40 ul) was implanted under the serosa of the greater curvature of the stomach of the nude mice through an insulin needle.
Click to Show/Hide
|
||||
| Response Description | HIF-1 could act as a protective factor against ferroptosis in gastric cancer (GC) cells. HIF-1 activates PMAN at the transcriptional level, which greatly improves the output of ELAVL1 in the cytoplasm. ELAVL1 directly combines with the AREs of SLC7A11 mRNA 3-UTR and improves the stability ofSLC7A11mRNA, thereby increasing the expression of SLC7A11 and reducing the accumulation of ROS and iron in ferroptosis, ultimately promoting the proliferation and development of tumor cells. | ||||
Platelet-derived growth factor receptor alpha (PDGFRA)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [56] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
HEB (Human glial cells) | ||||
| SF126 cells | Glioblastoma | Homo sapiens | CVCL_1688 | ||
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
4-week-old female BALB/c nude mice were acquired from the National Laboratory Animal Center (Shanghai, China). Overall, 10 mice (n = 5 each group) were implanted with U251 cells stably knockdown circCDK14 by lentiviruses carrying sh-circCDK14 (Wanleibio, China), or control U251 cells with lentiviruses carrying sh-NC (Wanleibio, China). 5 x 106 cells were resuspended in phosphatebuffered saline (100 ul) and Matrigel substrate (100 ul) and injected into the right flank of nude mice. Tumor volume was documented once 7 days after implantation.
Click to Show/Hide
|
||||
| Response Description | CircCDK14 promotes the migration, invasion and proliferation of glioma cells in vitroas well as tumorigenesisin vivo. An evaluation of the underlying mechanism revealed that circCDK14 sponged miR-3938 to upregulate oncogenic gene PDGFRA expression. After knockdown of circCDK14 in glioma cells, protein levels of SLC7A11 and GPX4 decreased significantly and Fp became more sensitivity. | ||||
Phosphatidylglycerophosphatase and protein-tyrosine phosphatase 1 (PTPMT1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [57] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 |
| Response Description | PTPMT1 is upregulated in pancreatic cancer and PTPMT1 inhibits ferroptosis by suppressing the expression of ACSL4 and upregulating SLC7A11 in Panc-1 cells, suggesting PTPMT1 might be a potential prognosis biomarker and therapeutic target in PDAC. | |||
Peroxiredoxin-6 (PRDX6)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [42] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MPC-5 cells | Normal | Mus musculus | CVCL_AS87 | |
| In Vivo Model |
Male C57BL/6 mice (6-8 weeks old, 20-25 g) were obtained from KCI BioTech (Jiangsu, China. Mice were intraperitoneally injected 50 mg/kg/day of STZ for 5 straight days to generate DN mouse. At 3 days post injection, glucose levels were measured from tail blood, and blood glucose level more than 16.4 mmol/L indicated that DN models was established.
Click to Show/Hide
|
||||
| Response Description | Prdx6 overexpression also eliminated ferroptosis caused by HG, which was reflected in the suppression of iron accumulation and the increase in SLC7A11 and GPX4 expression. Moreover, Sp1 could bind to the three Sp1 response elements in the Prdx6 promoter, thereby directly regulating the transcriptional activation of Prdx6 in podocytes. Further, Prdx6 overexpression attenuated renal injuries in streptozotocin-induced diabetic nephropathy mice. | ||||
PCAT1 (IncRNA)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [45] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | ||
| In Vivo Model |
PC3 and PC3/DR cells (5 x 106 cells) were subcutaneously injected into each flank of six-week-old male BALB/c nude mice (HFK Biotech, China). When the tumor volume reached 100 mm3, the mice were treated with Dimethyl Sulfoxide (DMSO) alone, DTX (5 mg/kg body weight, every two days) with DMSO or erastin (20 mg/kg body weight in 20 ul DMSO plus 130 ul corn oil, daily) by intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | DTX-resistant prostate cancer cells develop tolerance toward ferroptosis and that lncRNA PCAT1 promotes chemoresistance by blocking DTX-induced ferroptosis. Mechanistic studies indicated that PCAT1 activates the expression of SLC7A11 by interacting with c-Myc and sponging with miR-25-3p. In addition, TFAP2C activates PCAT1 expression to reduce ferroptosis susceptibility and enhance chemoresistance. | ||||
Pannexin-1 (PANX1)
Preeclampsia [ICD-11: JA24]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [46] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hPTs (Human placental tissues) | |||
| Response Description | Panx1 and TLR4 are suggested to induce ferroptosis in Preeclampsia via SLC7A11-mediated signaling pathways, offering a novel perspective on PE pathogenesis and novel diagnostic tools for Preeclampsia. | |||
OIP5-AS1 (IncRNA)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [58] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| DU145 cells | Prostate carcinoma | Homo sapiens | CVCL_0105 | ||
| In Vivo Model |
A total of 2 x 106 PC3 and PC3/Cd cells were subcutaneously injected into the right flanks of 4-week-old male Balb/c nude mice. Tumor burdens were closely monitored by tumor volumes. When the largest tumors reached a size of 1.0 cm3, all mice were sacrificed due to ethical considerations. Moreover, the final tumor weight was also recorded.
Click to Show/Hide
|
||||
| Response Description | OIP5-AS1 served as an endogenous sponge of miR-128-3p to regulate the expression of SLC7A11, a surrogate marker of ferroptosis. Moreover, miR-128-3p decreased cell viability by enhancing ferroptosis. Taken together, lncRNA OIP5-AS1 promotes prostate cancer progression and ferroptosis resistance through miR-128-3p/SLC7A11 signaling. | ||||
Nuclear receptor subfamily 1 group D member 2 (NR1D2)
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [59] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MRTEpiC (Mouse renal tubular epithelial cells) | ||||
| mRTECs (Mouse renal tubular epithelial cells) | |||||
| M4100-57 (Mouse renal tubular epithelial cells) | |||||
| In Vivo Model |
Gene knockout (Rev-erb-a-/-,Rev-erb-b-/-and icDKO) mice and wild-type littermates were treated with folic acid (i.p., 100 mg/kg, once daily for seven consecutive days) at ZT6 or ZT18 to induce acute kidney injury.
Click to Show/Hide
|
||||
| Response Description | Rev-erb-b (NR1D2) promoted ferroptosis by repressing the transcription of Slc7a11 and HO1 (two ferroptosis-inhibitory genes) via direct binding to a RORE cis-element. Targeted inhibition of Rev-erb-b limits ferroptosis to ameliorate folic acid-induced acute kidney injury in mice. | ||||
Nuclear receptor subfamily 1 group D member 1 (NR1D1)
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [59] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MRTEpiC (Mouse renal tubular epithelial cells) | ||||
| mRTECs (Mouse renal tubular epithelial cells) | |||||
| M4100-57 (Mouse renal tubular epithelial cells) | |||||
| In Vivo Model |
Gene knockout (Rev-erb-a-/-,Rev-erb-b-/-and icDKO) mice and wild-type littermates were treated with folic acid (i.p., 100 mg/kg, once daily for seven consecutive days) at ZT6 or ZT18 to induce acute kidney injury.
Click to Show/Hide
|
||||
| Response Description | Rev-erb-a (NR1D1) promoted ferroptosis by repressing the transcription of Slc7a11 and HO1 (two ferroptosis-inhibitory genes) via direct binding to a RORE cis-element. Targeted inhibition of Rev-erb-a limits ferroptosis to ameliorate folic acid-induced acute kidney injury in mice. | ||||
Neurogenic locus notch homolog protein 2 (NOTCH2)
Hemangioma [ICD-11: 2E81]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [60] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Notch signaling pathway | hsa04330 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hHemECs (Human hemangioma endothelial cells) | |||
| Response Description | Knockdown of long non-coding RNA MEG8 inhibited the proliferation and induced the ferroptosis of hemangioma endothelial cells by regulating miR-497-5p/ NOTCH2 axis. Importantly, silencing MEG8 significantly decreased the expressions of SLC7A11 and GPX4 both in mRNA and protein level and had no effect on the level of AIFM2. | |||
N6-adenosine-methyltransferase non-catalytic subunit (METTL14)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [61] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Glutamate metabolism | hsa00250 | ||||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| MHCC97-H cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_4972 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
The BALB/C nude mice were obtained from Shanghai SLAC Laboratory Animal Co., Ltd. 5 x 105 stable SLC7A11-knockdown HCCLM3 cells or SLC7A11-vector cells were injected subcutaneously into BALB/C nude mice. After 5 weeks, mice were killed, and tumour photograph was detected with photography. In the other animal work, 5 x 105 stable METTL14-vector HCCLM3 cells, SLC7A11-Overexpression cells or SLC7A11-R298P cells were injected subcutaneously into BALB/C nude mice.
Click to Show/Hide
|
||||
| Response Description | METTL14 induced m6A modification at 5'UTR of SLC7A11 mRNA, which in turn underwent degradation relied on the YTHDF2-dependent pathway. Importantly, ectopic expression of SLC7A11 strongly blocked METTL14-induced tumour-suppressive effect in hypoxic hepatocellular carcinoma. | ||||
Myc proto-oncogene protein (MYC)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [45] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | ||
| In Vivo Model |
PC3 and PC3/DR cells (5 x 106 cells) were subcutaneously injected into each flank of six-week-old male BALB/c nude mice (HFK Biotech, China). When the tumor volume reached 100 mm3, the mice were treated with Dimethyl Sulfoxide (DMSO) alone, DTX (5 mg/kg body weight, every two days) with DMSO or erastin (20 mg/kg body weight in 20 ul DMSO plus 130 ul corn oil, daily) by intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Docetaxel (DTX)-resistant prostate cancer cells develop tolerance toward ferroptosis and that lncRNAPCAT1 promotes chemoresistance by blocking DTX-induced ferroptosis. Mechanistic studies indicated that PCAT1 activates the expression of SLC7A11 by interacting with c-Myc and sponging with miR-25-3p. In addition, TFAP2C activates PCAT1 expression to reduce ferroptosis susceptibility and enhance chemoresistance. | ||||
Mucin-1 (MUC1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [62] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MDA-MB-468 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0419 |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| Response Description | MUC1-C binds directly with CD44v and in turn promotes stability of xCT (SLC7A11) in the cell membrane in breast adenocarcinoma. The interaction between 2MUC1-C and xCT is further supported by the demonstration that targeting xCT with silencing or the inhibitor sulfasalazine suppresses MUC1 gene transcription by increasing histone and DNA methylation on the MUC1 promoter. | |||
mmu-miR-378a-3p (miRNA)
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [63] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| TCMK-1 cells | Normal | Mus musculus | CVCL_2772 | ||
| In Vivo Model |
Male Sprague-Dawley (SD) rats (5 weeks old, weighting 180-220 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. SD rats were anesthetized with an intraperitoneal (i.p.) injection of pentobarbital sodium (25 mg/kg) and placed on a surgical thermostator. Then the rats were subjected to an abdominal incision, and the right kidney was carefully liberated from surrounding tissue, and nephrectomy was performed. The left kidney was exposed after a midline incision, and the renal artery was clamped with non-traumatic clamps for 45 min, followed by restoring of the renal blood flow. The kidneys were harvested and the serum was collected 48 h after the surgery. The rats in sham group were subjected to an abdominal incision without clamping the renal artery.
Click to Show/Hide
|
||||
| Response Description | MiR-182-5p and miR-378a-3p induced ferroptosis in cells. And miR-182-5p and miR-378a-3p regulated the expression of GPX4 and SLC7A11 negatively by directly binding to the 3'UTR of GPX4 and SLC7A11 mRNA. In vivo study showed that silencing miR-182-5p and miR-378a-3p alleviated the I/R-induced kidney injury in rats. | ||||
MIT domain-containing protein 1 (MITD1)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [64] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Hippo signaling pathway | hsa04390 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 |
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | |
| ACHN cells | Papillary renal cell carcinoma | Homo sapiens | CVCL_1067 | |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | |
| 769-P cells | Renal cell carcinom | Homo sapiens | CVCL_1050 | |
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| Response Description | MITD1 knockdown inhibited clear cell renal cell carcinoma (ccRCC) cell proliferation and migration and induced ferroptosis in ccRCC. Subsequent overexpression experiments demonstrated that MITD1 knockdown induced ferroptosis and suppressed tumor growth and migration through the TAZ/SLC7A11 pathway. | |||
Membrane-associated progesterone receptor component 1 (PGRMC1)
Head neck squamous cell carcinoma [ICD-11: 2D60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [65] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
NH-3 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_8126 | |
| HN4 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_IS30 | ||
| In Vivo Model |
Six-week-old athymic BALB/c male nude mice (nu/nu) were purchased from OrientBio (Seoul, Republic of Korea). HN4 cells with transfection of PGRMC1 overexpression or control vector and HN4PCC with shPGRMC1 or control vector were subcutaneously injected into the bilateral flank of nude mice. When gross nodules were detected in tumor implants, mice were subjected to different treatments: vehicle or sulfasalazine (250 mg/kg daily per intraperitoneal route).
Click to Show/Hide
|
||||
| Response Description | GRMC1-dependent lipophagy promotes ferroptosis in paclitaxel-tolerant persister cancer cells. PGRMC1 expression increased FAO and ferroptosis sensitivity from in vivo mice experiments. And PGRMC1 promotes ferroptosis by xCT (SLC7A11) inhibition in head and neck cancer (HNC) cells. | ||||
MEG8 (IncRNA)
Hemangioma [ICD-11: 2E81]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [60] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Notch signaling pathway | hsa04330 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hHemECs (Human hemangioma endothelial cells) | |||
| Response Description | Knockdown of long non-coding RNA MEG8 inhibited the proliferation and induced the ferroptosis of hemangioma endothelial cells by regulating miR-497-5p/NOTCH2 axis. Importantly, silencing MEG8 significantly decreased the expressions of SLC7A11 and GPX4 both in mRNA and protein level and had no effect on the level of AIFM2. | |||
Lysine-specific demethylase 4A (KDM4A)
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [66] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell metastasis | |||||
In Vitro Model |
143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | ||
| In Vivo Model |
For the xenograft mouse model, 1 x 106 OS 143B cells in 100 uL PBS were subcutaneously injected with 1 x 106 OS cells. For the OS lung metastasis model and in vivo imaging, 1 x 106 143B cells in 10 uL PBS were injected orthotopically into the medullary cavity of the tibia of mice. For bioluminescent imaging, we used 143B cells stably expressing luciferase, and the luciferase substrate D-Luciferin (Selleck, China) was retro-orbitally injected before imaging on days 7 and 21.
Click to Show/Hide
|
||||
| Response Description | KDM4A regulates SLC7A11 transcription and osteosarcoma cell ferroptosis by controlling H3K9me3 demethylation in the promoter region of SLC7A11. The findings suggestes that KDM4A activity may be a potential therapeutic target for future OS treatment. | ||||
Lysine-specific demethylase 3B (KDM3B)
Acute myeloid leukaemia [ICD-11: 2A60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [67] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 |
| HEK293 cells | Normal | Homo sapiens | CVCL_0045 | |
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| Response Description | Since KDM3B has been shown to associate with acute myeloid leukemia (AML). KDM3B protects cells from Erastininduced ferroptosis through transcriptional upregulation of SLC7A11 via interaction with ATF4. | |||
LIM and SH3 domain protein 1 (LASP1)
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [68] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | |
| IHH-4 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_2960 | ||
| BCPAP cells | Thyroid carcinoma | Homo sapiens | CVCL_0153 | ||
| KTC-1 cells | Thyroid carcinoma | Homo sapiens | CVCL_6300 | ||
| TPC-1 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | ||
| In Vivo Model |
Animal experiments were approved by ethics committee of Wuzhou Red Cross Hospital. The TPC-1 cells transfected with sh-CERS6-AS1 or sh-NC were made into cell suspension (2 x 106/ml) with PBS. Then nude mice were randomly divided into sh-CERS6-AS1 group (n = 6) and sh-NC group (n = 6). The mice were subcutaneously inoculated with 200 ul corresponding cell suspension respectively, and then the weight and tumor volume of mice were recorded every other week. After 5 weeks, pentobarbital sodium (120 mg/kg) were intraperitoneally injected to make nude mice euthanasia, and then the tumors were stripped and weighed. Subsequently, immunohistochemistry and RT-PCR were performed.
Click to Show/Hide
|
||||
| Response Description | Silencing CERS6-AS1 suppressed cell viability and increased ferroptosis in papillary thyroid cancer. LASP1 was modulated by CERS6-AS1 through sponging miR-497-5p. The expression of Ki67, PCNA, GPX4, and SLC7A11 was inhibited by si-CERS6-AS1 transfection. | ||||
Kelch domain-containing protein 3
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ubiquitin mediated proteolysis | hsa04120 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell growth | |||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| SJSA-1 cells | Osteosarcoma | Homo sapiens | CVCL_1697 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| HCCLM3 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 | ||
| In Vivo Model |
4-6-week-old female BALB/c nu/nu mice obtained from SLAC Laboratory Animal Co., Ltd. were bred and maintained in our institutional pathogen-free mouse facilities. Ovarian tumors were established by subcutaneously injecting 5 x 106 SKOV3 cells in 100 ul of PBS buffer into the right flank of 6-week-old nude mice (four mice for each group). At the end of 3 weeks, mice were killed and in vivo solid tumors were dissected and weighed.
Click to Show/Hide
|
||||
| Response Description | KLHDC3 expression is elevated in ovarian cancer. KLHDC3 suppresses ferroptosis in vitro and supports tumor growth in vivo by relieving p14ARF-mediated suppression of SLC7A11 transcription. | ||||
Interleukin-6 (IL6)
Head neck squamous cell carcinoma [ICD-11: 2D60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [51] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ferroptosis | hsa04216 | ||||
| JAK-STAT signaling pathway | hsa04630 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HN4 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_IS30 | |
| CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | ||
| In Vivo Model |
Four-week-old male BALB/c-nu mice were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). About 4 x 106 CAL27 cells were stably transfected with lentivirus. After administration of 2 ug/mL puromycin for three days, transfection efficiency was confirmed by western blotting. Approximately 2 x 106 transfected cells were subcutaneously injected into flanks. For the drug-administration study, 20 mg/kg erastin (S7242, Selleck Chemicals) were administrated intraperitoneally twice every other day. Approximately 20 uL IL-6 (10 ug/mL, PeproTech, USA) were given intratumorally twice every other day.
Click to Show/Hide
|
||||
| Response Description | The study demonstrate the critical role of IL-6-induced ferroptosis resistance during head and neck squamous cell carcinoma carcinogenesis. The IL-6/STAT3/xCT (encoded by SLC7A11) axis acts as a novel mechanism driving tumor progression and thus may potentially be utilized as a target for tumor prevention and therapy. | ||||
Interferon gamma (IFNG)
Fibrosarcoma [ICD-11: 2B53]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [70] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 | ||
| B16-F0 cells | Melanoma | Mus musculus | CVCL_0604 | ||
| ID8 cells | Ovarian cancer | Mus musculus | CVCL_IU14 | ||
| In Vivo Model |
Six- to eight-week-old female NSG or C57BL/6 mice were obtained from the Jackson Laboratory. For HT-1080 tumor model, 106 tumor cells were subcutaneously injected on the right flank of NSG mice. For adoptive transfer of OT-I to B16-OVA model, 105 B16-OVA cells were subcutaneously injected on the right flank of C57BL/6 mice. For the B16 tumor model, 105 B16F0 cells were subcutaneously injected on the right flank of C57BL/6 mice. For the ID8 tumor model, 2 x 106 luciferase-expressing ID8 cells were injected into the peritoneal cavity of each female mouse.
Click to Show/Hide
|
||||
| Response Description | Interferon gamma (IFNG) released from CD8T cells downregulates the expression of SLC3A2 and SLC7A11, two subunits of the glutamate-cystine antiporter system xc-, impairs the uptake of cystine by tumour cells, and as a consequence, promotes tumour cell lipid peroxidation and ferroptosis in Fibrosarcoma. | ||||
hsa-miR-770-5p (miRNA)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [44] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mKTs (Mouse knee tissues) | ||||
| HK-2 (Human renal glomerular endothelial cells) | |||||
| In Vivo Model |
All C57BL/6 mice (5-6 weeks, 18-20 g) were obtained from Animal Testing Center of Qinglongshan (Nanjing, Suzhou, China). DN mice were fed with a high-fat diet (HFD) for 12 weeks and then injected with STZ (30 mg/kg of streptozotocin; Sigma-Aldrich, St Louis, MO, USA) i.p. for 7 consecutive days. Blood glucose levels of mice were measured using 16.7 mmol/l after one week of the final injection. Then, mice were killed under anesthesia and their kidneys taken for analysis after induction of STZ at 4 months.
Click to Show/Hide
|
||||
| Response Description | Circular RNA circ ASAP2 decreased inflammation and ferroptosis in diabetic nephropathy through SOX2/ SLC7A11 by miR-770-5p. Importantly, this research indicated that circ ASAP2 might act as a target for improving the role of ferroptosis in diabetes nephropathy. | ||||
hsa-miR-587 (miRNA)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [71] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
ES-2 cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | |
| Caov-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0201 | |
| Response Description | ADAMTS9-AS1 regulated SLC7A11 expression through miR-587, thereby affecting ferroptosis, proliferation and migration of Epithelial ovarian cancer cells. | |||
hsa-miR-545-3p (miRNA)
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [72] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
FTC-133 cells | Thyroid gland follicular carcinoma | Homo sapiens | CVCL_1219 | |
| TPC-1 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | ||
| In Vivo Model |
BALB/c nude mice (18-20 g, 4-week-old, male) (n = 6) were purchased and applied for the tumor growth analysis of FTC133 cellsin vivo. About 1 x 106 FTC133 cells were transfected with control shRNA or circ_0067934 shRNA and were subcutaneously injected into the left fat pad of mice. After 5 days of injection, we measured tumor growth every 5 days. We sacrificed the mice after 30 days of injection. The tumor volume was calculated by (length x width2)/2. And the tumor weight was calculated at day 30.
Click to Show/Hide
|
||||
| Response Description | Circ_0067934 upregulated the expression of the ferroptosis-negative regulator SLC7A11 by sponging and inhibiting miR-545-3p in thyroid cancer cells. The overexpression of SLC7A11 or the inhibitor of miR-545-3p reversed circ_0067934 silencing-regulated thyroid cancer cell proliferation. | ||||
hsa-miR-520d-5p (miRNA)
Oral squamous cell carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [73] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | |
| SCC-15 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1681 | ||
| In Vivo Model |
The tumorigenicity analysis was conducted in BALB/c nude mice (6-weeks-old, male). The mice were maintained at pathogen-free condition. The 5 x 106 CAL27 cells were treated with control shRNA or circFNDC3B shRNA and subcutaneously injected into the nude mice (N = 5). The mice were sacrificed after 30 days and the tumor volume was calculated using the formula of (length x width2)/2.
Click to Show/Hide
|
||||
| Response Description | CircFNDC3B attenuated ferroptosis of Oral squamous cell carcinoma (OSCC) cells and contributed to OSCC progression by regulating the miR-520d-5p/SLC7A11 axis. CircFNDC3B, miR-520d-5p, and SLC7A11 may serve as potential therapeutic targets of OSCC. | ||||
hsa-miR-520a-5p (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [74] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| In Vivo Model |
SCID/nude mice (6 weeks old) were ordered from Laboratory Animal Center of Chinese Academy of Sciences (Beijing, China). HT29 cells were co-transfected with sh-circFOXP1 and pCMV-SLC7A11 or empty vector. Cells were digested, 5 x 106 cells were mixed with Matrigel (Corning) and subcutaneously injected into mice. The width and length of tumor were measured at indicated time, and the tumor size was calculated by the formula: 0.5 x length x width2. Mice were then succumbed to death, the tumors were isolated, weighted, and made into paraffin-embedded slices (5-um). The slices were stained with anti-KI67 antibody (Santa Cruz Biotechnology) and subsequent HRP-labeled secondary antibody and captured in a microscope (Leica Microsystems).
Click to Show/Hide
|
||||
| Response Description | Mechanically, circFOXP1 increased SLC7A11 expression by directly sponging miR-520a-5p in lung cancer cells. The inhibitor of miR-520a-5p or the overexpression of SLC7A11 reversed circFOXP1 shRNA-induced ferroptosis phenotypes in lung cancer cells. | ||||
hsa-miR-515-5p (miRNA)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [75] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Ca Ski cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| hCECs (Human cervical epithelial cells) | |||||
| In Vivo Model |
The 4-week-old female BALB/c nude mice were used for constructing xenograft model. HeLa cells (1 x 107 cells/mL) were injected into the dorsal flanksusing using 1-mL syringes. Then tumor size was measured and mice received intertumoral injection of si-crEPSTI1-1 (40 uL siRNA1 for cicrEPSTI1) and negative control (40 uL negative control) every four days, respectively (5 mice/group). After 28 days, the mice were sacrificed and xenografts were measured.
Click to Show/Hide
|
||||
| Response Description | CircEPSTI1 sponges miR-375, miR-409-3p and miR-515-5p to upregulate SLC7A11 expression. CircEPSTI1-miR-375/409-3P/ 515-5p-SLC7A11 axis affected the proliferation of cervical cancer via the competing endogenous RNAs (ceRNA) mechanism and was relative to ferroptosis. | ||||
Ovarian dysfunction [ICD-11: 5A80]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [76] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
KGN cells | Ovarian granulosa cell tumor | Homo sapiens | CVCL_0375 | |
| SVOG-3e cells | Normal | Homo sapiens | CVCL_IN98 | ||
| In Vivo Model |
We recruited 12 infertility patients (6 PCOS and 6 controls) aged 20 and 38 who underwent in vitro fertilization (IVF) at the Department of Reproductive Medicine Center, the First Affiliated Hospital of Sun Yat-Sen University. All patients were treated the antagonist stimulation protocol. Briefly, on cycle day 2, ovarian stimulation was started by daily injection of recombinant FSH (r-FSH) (Gonal-F; Merck Serono). Gonadotropin-releasing hormone (GnRH) antagonist (Cetrotide, 0.25 mg; Merck Serono) injection was started from the day 6 of stimulation. When at least three follicles had reached 17 mm or two follicles had reached 18 mm in diameter, an intramuscular injection of 5,000 IU-10000 IU of human chorionic gonadotropin (hCG) is used for triggering final oocyte maturation.
Click to Show/Hide
|
||||
| Response Description | circRHBG inhibits ferroptosis in Polycystic ovary syndrome cells through the circRHBG/ miR-515-5p/SLC7A11 axis in PCOS, which may provide new diagnostic molecular markers and therapeutic targets for PCOS. | ||||
hsa-miR-513a-3p (miRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [77] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
hESCCs (Esophageal squamous cancer cells) | |||
| Response Description | Downregulation of BBOX1-AS1 inhibits cell proliferation, and metastasis accelerates cell apoptosis and ferroptosis in esophageal squamous cell cancer by upregulating miR-513a-3p to reduce SLC7A11 expression. These findings may provide novel insights into the diagnosis and treatment of ESCC. | |||
hsa-miR-5096 (miRNA)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [78] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MDA-MB-468 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | |
| MDA-MB-453 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| SK-BR-3 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | ||
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| ZR-75-1 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0588 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| In Vivo Model |
Mating was setup 2 days prior to injection day and zebrafish embryos were collected and incubated in E3 embryo medium (5 mM NaCl, 0.17 mM KCl, 10 mM HEPES, 0.33 mM MgSO4·7H2O, 0.33 mM CaCl2·6H2O, and 0.00001% methylene blue) containing 0.2 mM N-phenyl-thiourea (PTU) (catalog no: P7629, Sigma-Aldrich). Two days post-fertilization, the chorion was removed manually using fine forceps, and the embryos were anesthetized using E3 medium containing 200 mg/L Ethyl 3-aminobenzoate methanesulfonate (Tricaine) (catalog no: A5040, Sigma-Aldrich). Anesthetized embryos were mounted in 0.7% low melting agarose containing 200 ug/ml of Tricaine and were microinjected with 500 cells in the yolk sac using Nanoject III (catalog no: 3-000-207; Drummond Scientific Company, PA, USA). At 1 day post-injection (dpi), embryos with similar graft size were selected and imaged using both bright field and RFP channels and incubated in E3-PTU medium containing 5 ug/ml doxycycline at 34 until further imaging. At 4 dpi, embryos were anesthetized and imaged again using both bright field and RFP channels using Olympus IX-73 microscope. Cells that migrated outside the yolk sac (injection site) were represented by a notable fluorescent dot and considered a metastatic event; these were counted manually for all embryos in each experimental group.
Click to Show/Hide
|
||||
| Response Description | The present study demonstrated that miR-5096 targets and downregulates SLC7A11, thereby providing a mechanistic basis for ferroptosis in human breast cancer cells. In addition, miR-5096 induced cell death via ferroptosis, characterized by mitochondrial shrinkage with partial loss of cristae with simultaneous changes in ACSL4, ROS, lipid ROS, OH-, reactive iron, GSH, and MMP levels. | ||||
hsa-miR-497-5p (miRNA)
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [68] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | |
| IHH-4 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_2960 | ||
| BCPAP cells | Thyroid carcinoma | Homo sapiens | CVCL_0153 | ||
| KTC-1 cells | Thyroid carcinoma | Homo sapiens | CVCL_6300 | ||
| TPC-1 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | ||
| In Vivo Model |
Animal experiments were approved by ethics committee of Wuzhou Red Cross Hospital. The TPC-1 cells transfected with sh-CERS6-AS1 or sh-NC were made into cell suspension (2 x 106/ml) with PBS. Then nude mice were randomly divided into sh-CERS6-AS1 group (n = 6) and sh-NC group (n = 6). The mice were subcutaneously inoculated with 200 ul corresponding cell suspension respectively, and then the weight and tumor volume of mice were recorded every other week. After 5 weeks, pentobarbital sodium (120 mg/kg) were intraperitoneally injected to make nude mice euthanasia, and then the tumors were stripped and weighed. Subsequently, immunohistochemistry and RT-PCR were performed.
Click to Show/Hide
|
||||
| Response Description | Silencing CERS6-AS1 suppressed cell viability and increased ferroptosis in papillary thyroid cancer. LASP1 was modulated by CERS6-AS1 through sponging miR-497-5p. The expression of Ki67, PCNA, GPX4, and SLC7A11 was inhibited by si-CERS6-AS1 transfection. | ||||
Hemangioma [ICD-11: 2E81]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [60] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Notch signaling pathway | hsa04330 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
hHemECs (Human hemangioma endothelial cells) | |||
| Response Description | Knockdown of long non-coding RNA MEG8 inhibited the proliferation and induced the ferroptosis of hemangioma endothelial cells by regulating miR-497-5p/NOTCH2 axis. Importantly, silencing MEG8 significantly decreased the expressions of SLC7A11 and GPX4 both in mRNA and protein level and had no effect on the level of AIFM2. | |||
hsa-miR-409-3p (miRNA)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [75] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Ca Ski cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| hCECs (Human cervical epithelial cells) | |||||
| In Vivo Model |
The 4-week-old female BALB/c nude mice were used for constructing xenograft model. HeLa cells (1 x 107 cells/mL) were injected into the dorsal flanksusing using 1-mL syringes. Then tumor size was measured and mice received intertumoral injection of si-crEPSTI1-1 (40 uL siRNA1 for cicrEPSTI1) and negative control (40 uL negative control) every four days, respectively (5 mice/group). After 28 days, the mice were sacrificed and xenografts were measured.
Click to Show/Hide
|
||||
| Response Description | CircEPSTI1 sponges miR-375, miR-409-3p and miR-515-5p to upregulate SLC7A11 expression. CircEPSTI1-miR-375/ 409-3P/515-5p-SLC7A11 axis affected the proliferation of cervical cancer via the competing endogenous RNAs (ceRNA) mechanism and was relative to ferroptosis. | ||||
hsa-miR-3938 (miRNA)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [56] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
HEB (Human glial cells) | ||||
| SF126 cells | Glioblastoma | Homo sapiens | CVCL_1688 | ||
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
4-week-old female BALB/c nude mice were acquired from the National Laboratory Animal Center (Shanghai, China). Overall, 10 mice (n = 5 each group) were implanted with U251 cells stably knockdown circCDK14 by lentiviruses carrying sh-circCDK14 (Wanleibio, China), or control U251 cells with lentiviruses carrying sh-NC (Wanleibio, China). 5 x 106 cells were resuspended in phosphatebuffered saline (100 ul) and Matrigel substrate (100 ul) and injected into the right flank of nude mice. Tumor volume was documented once 7 days after implantation.
Click to Show/Hide
|
||||
| Response Description | CircCDK14 promotes the migration, invasion and proliferation of glioma cells in vitroas well as tumorigenesisin vivo. An evaluation of the underlying mechanism revealed that circCDK14 sponged miR-3938 to upregulate oncogenic gene PDGFRA expression. After knockdown of circCDK14 in glioma cells, protein levels of SLC7A11 and GPX4 decreased significantly and Fp became more sensitivity. | ||||
hsa-miR-378a-3p (miRNA)
Nerve injury [ICD-11: NA04]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [79] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
All the animals were housed in a temperature- and humidity-controlled room under a 12 h light/dark cycle and maintained in standard cages with free access to food and water. After seven days of breeding, the mice were randomly assigned to four groups of fifteen animals each. The animals in the four groups were treated with Pb acetate indrinking waterat doses of 0, 250 mg/L, 500 mg/L, and 1000 mg/L (control, LPb, MPb, and HPb groups, respectively). The Pb exposure period was 12 weeks.
Click to Show/Hide
|
||||
| Response Description | Inhibition of miR-378a-3p expression reversed the reduction in GSH and the increase in lipid ROS levels induced by lead exposure. MiR-378a-3p exerted an important effect by regulating SLC7A11 expression in nerve injury induced by lead exposure. | ||||
hsa-miR-375-3p (miRNA)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [80] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell stemness | |||||
In Vitro Model |
SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | ||
| In Vivo Model |
Four- to eight-week-old female BALB/c nude mice were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). For the tumor-limiting dilution assay, 1 x 107, 5 x 106, and 2.5 x 106 of LV3-miR-375 cells, LV3-SLC7A11 cells, LV3-shSLC7A11 cells, LV3-miR-375-SLC7A11 cells, and LV3-NC cells (BGC-823 and SGC-7901) were subcutaneously implanted into the underarm of mice. Fifteen days later, all mice were euthanized and tumor tissues were collected and weighed. For metastasis experiment, mouse models were established by intravenous injection of cells. Three mice per group were injected with 2 x 106 cells in 200 uL RPMI-1640 serum-free media. Six weeks after injection, mice were sacrificed for collecting lung tissues.
Click to Show/Hide
|
||||
| Response Description | MiR-375 reduced the stemness of gastric cancer cells in vitro and in vivo. Mechanistically, SLC7A11 was identified as a direct target of miR-375 and miR-375 attenuated the stemness of GC cells mainly through triggering SLC7A11-dependent ferroptosis. | ||||
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [75] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Ca Ski cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| hCECs (Human cervical epithelial cells) | |||||
| In Vivo Model |
The 4-week-old female BALB/c nude mice were used for constructing xenograft model. HeLa cells (1 x 107 cells/mL) were injected into the dorsal flanksusing using 1-mL syringes. Then tumor size was measured and mice received intertumoral injection of si-crEPSTI1-1 (40 uL siRNA1 for cicrEPSTI1) and negative control (40 uL negative control) every four days, respectively (5 mice/group). After 28 days, the mice were sacrificed and xenografts were measured.
Click to Show/Hide
|
||||
| Response Description | CircEPSTI1 sponges miR-375, miR-409-3p and miR-515-5p to upregulate SLC7A11 expression. CircEPSTI1- miR-375/409-3P/515-5p-SLC7A11 axis affected the proliferation of cervical cancer via the competing endogenous RNAs (ceRNA) mechanism and was relative to ferroptosis. | ||||
hsa-miR-372-3p (miRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [82] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 |
| TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| Eca-109 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | |
| HET-1A cells | Normal | Homo sapiens | CVCL_3702 | |
| Response Description | ARHGEF26-AS1 facilitated ferroptosis but restrained cell growth and positively regulated ADAM23 by sponging miR-372-3p in esophageal squamous cell carcinoma (ESCC). Overexpression of ARHGEF26-AS1 upregulated the protein levels of ADAM23 but depleted the protein levels of GPX4, SLC3A2, and SLC7A11. | |||
hsa-miR-367-3p (miRNA)
Multiple sclerosis [ICD-11: 8A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [83] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBMSCs (Bone marrow stromal cells) | ||||
| BV-2 cells | Normal | Mus musculus | CVCL_0182 | ||
| In Vivo Model |
Female C57BL6 mice were randomized into four groups (n = 8/group): the control group (non-EAE mice), the EAE group (EAE mice injected intrathecally with 5 uL PBS when symptoms appeared), the EAE + BMSCs-Exo + mimic negative control (NC) group (EAE mice injected intrathecally with Exos from bone MSCs (BMSCs) transfected with mimic NC [5 uL, 2 ug/L in PBS] when symptoms appeared), and the EAE + BMSCs-Exo + miR-367-3p mimic group (EAE mice injected intrathecally with Exos from BMSCs transfected with miR-367-3p mimic [5 uL, 2 ug/L in PBS] when symptoms appeared).
Click to Show/Hide
|
||||
| Response Description | MiR-367-3p can be delivered by BMSC-Exos into microglia, where miR-367-3p inhibits EZH2 expression and activates the expression of SLC7A11, suppressing microglial ferroptosis and relieving the symptoms of EAE. Experimental autoimmune encephalomyelitis (EAE) is a typical animal model of multiple sclerosis. | ||||
hsa-miR-34c-3p (miRNA)
Oral squamous cell carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [84] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
SCC-25 cells | Squamous carcinoma | Homo sapiens | CVCL_1682 |
| CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | |
| HOK cells | Normal | Hexagrammos otakii | CVCL_YE19 | |
| Response Description | Low expression of miR-34c-3p in oral squamous cell carcinoma, negatively regulating SLC7A11 expression, promoting ferroptosis, and suppressing cell proliferation. | |||
hsa-miR-339-3p (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [35] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| 16HBE14o- cells | Normal | Homo sapiens | CVCL_0112 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| In Vivo Model |
6-8 weeks mice were divided into 4 groups randomly. Mice were injected with 2.5 x 105 wild type LLC cells, Uc.339 OE-LLC cells, with or without miR-339 inhibitors, respectively, through the lateral tail vain. The mice were killed after 4 weeks by carbon dioxide asphyxiation followed by cervical dislocation to ensure death. The lungs were removed, rinsed with PBS, and the number of metastatic foci on the lung surface was counted. The pulmonary lobes were subsequently kept in 4% paraformaldehyde for later paraffin embedding and hematoxylin and eosin staining.
Click to Show/Hide
|
||||
| Response Description | LncRNA Uc.339 competitively binds to pri-miR-339 and inhibits the production of mature miR-339. At the same time, it is the first to clarify the reason for the negative correlation between miR-339 and SLC7A11 expression in lung cancer, and for the first verification that the inhibition of miR-339 led to increased expression of SLC7A11 and weakens ferroptosis, which constituted an important carcinogenesis mechanism for lung adenocarcinoma metastasis. | ||||
hsa-miR-30b-5p (miRNA)
Preeclampsia [ICD-11: JA24]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [85] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HTR-8/SVneo cells | Normal | Homo sapiens | CVCL_7162 | |
| hETCs (Human first-trimester extravillous trophoblast cells) | |||||
| In Vivo Model |
Pregnant SD rats were randomly dived into four groups: sham group (n = 8), PE group (n = 8), PE + ferrostatin-1 group ( n= 8), and PE + miR-30b-5p inhibition group (n = 8). On day 14 of pregnancy, PE rat model was established through reduced uterine perfusion pressure (RUPP) surgery, wherein constrictive silver clips were placed on the aorta (0.203-mm clips) superior to the iliac bifurcation and on the ovarian vessels (0.100-mm clips), according to a previous description. Sham rats were operated on in a fashion similar to that of RUPP rats, but without clipping. Mini-pumps were also intraperitoneally inserted into rats on day 14 of pregnancy. The mini-pump in each rat delivered the ferrostatin-1 at a dose of 10 umol/kg/day for 5 days. An miR-30b-5p antagonist (GenePharma) was injected from the tail veins on day 13 of gestation, at a rate of 100 uL/day for 6 days. The blood pressure was measured on days 14, 16, and 19 of gestation using catheters inserted into the carotid artery and jugular vein.
Click to Show/Hide
|
||||
| Response Description | Abnormally up-regulated miR-30b-5p triggered the ferroptosis in trophoblasts under hypoxic conditions by down-regulating SLC7A11, Pax3, and Pax3-downstream target, FPN1. Blockage of miR-30b-5p up-regulation or direct inhibition of ferroptosis attenuated preeclampsia (PE) symptoms in the rat model, making miR-30b-5p a potential therapeutic target for preeclampsia. | ||||
hsa-miR-30a-5p (miRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [86] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 | |
| KYSE-70 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1356 | ||
| hEECs (Human esophageal epithelial cells) | |||||
| In Vivo Model |
BALB/c nude male mice of 4 weeks old were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). After one week of adaptive feeding, EC9706 cells (3 x 106) stably expressing sh-NC and sh-circPVT1, sh-NC + 5-FU and sh-circPVT1 + 5-FU were subcutaneously were injected into the right flank of the nude mice in a serum-free DMEM medium.
Click to Show/Hide
|
||||
| Response Description | CircPVT1 regulated the chemosensitivity of esophageal squamous cell carcinoma cells through ROS and Wnt/-catenin pathways via miR-30a-5p/FZD3. Knockdown of circPVT1 promoted chemosensitivity in ESCC by increasing ferroptosis via downregulating GPX4 and SLC7A11. | ||||
hsa-miR-27a-3p (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [87] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| Response Description | MiR-27a-3p, was an essential modulator of ferroptosisviadirectly targeting SLC7A11 in non-small cell lung cancer cells. Overexpressing miR-27a-3p led to SLC7A11 suppressionviadirectly binding to its 3'-UTR, followed by the reduction of erastin-caused ferroptosis. | |||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [88] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
The rats were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg/kg). Before the experiment, the rats were fasted for 8-10 h and ensured sufficient water. The left common carotid artery (CCA) and left external carotid artery (ECA) were ligated after skin incision in the middle of the neck. In the common carotid artery cut a small incision. In order to induce ischemia, a cord plug was inserted through the incision into the internal carotid artery (ICA) and then to the origin of the middle cerebral artery (MCA). After ischemia for 2 h, the rats were anesthetized and gently pulled out the cord plug. At the end of the reperfusion time, the rats were euthanized by injection of a minimal lethal dose of anesthesia.
Click to Show/Hide
|
||||
| Response Description | The results of dual luciferase reporter gene technique indicated SLC7A11 as the target gene of miR-27a. In the current study, miR-27a upregulates ferroptosis to aggravate cerebral ischemia-reperfusion injury by SLC7A11. | ||||
hsa-mir-27a (Precursor RNA)
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [89] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
The male Sprague Dawley rats were randomly divided into control group, sham group, middle cerebral artery occlusion/reperfusion model group (MCAO/R group) (according to different ischemia reperfusion time, the model group was divided into the 3, 6, 12 and 24 hours reperfusion groups after 2 hour-ischemia), and vehicle group, agomir-27a group, antagomir-27a group. The Zea-Longa method was used to establish rat MCAO model.
Click to Show/Hide
|
||||
| Response Description | In the process of cerebral ischemia and reperfusion, the up-regulated miR-27a may induce ferroptosis through inhibiting SLC7A11, thus causing brain tissue damage. | ||||
hsa-miR-25-3p (miRNA)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [45] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | ||
| In Vivo Model |
PC3 and PC3/DR cells (5 x 106 cells) were subcutaneously injected into each flank of six-week-old male BALB/c nude mice (HFK Biotech, China). When the tumor volume reached 100 mm3, the mice were treated with Dimethyl Sulfoxide (DMSO) alone, DTX (5 mg/kg body weight, every two days) with DMSO or erastin (20 mg/kg body weight in 20 ul DMSO plus 130 ul corn oil, daily) by intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | Docetaxel (DTX)-resistant prostate cancer cells develop tolerance toward ferroptosis and that lncRNAPCAT1 promotes chemoresistance by blocking DTX-induced ferroptosis. Mechanistic studies indicated that PCAT1 activates the expression of SLC7A11 by interacting with c-Myc and sponging with miR-25-3p. In addition, TFAP2C activates PCAT1 expression to reduce ferroptosis susceptibility and enhance chemoresistance. | ||||
hsa-miR-206 (miRNA)
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [48] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SJSA-1 cells | Osteosarcoma | Homo sapiens | CVCL_1697 |
| Response Description | LncRNA SNHG14 targeted and down-regulated the expression of miR-206, further affecting the common ferroptosis inhibitor SLC7A11, and preventing NR-SJSA1 cells from undergoing ferroptosis. In conclusion, our findings highlight the involvement of lncRNA SNHG14 in ferroptosis and chemotherapy resistance of nutlin3a-resistant NR-SJSA1 cells, thus shedding new insight on how to overcome drug resistance in osteosarcoma cells and improve treatment efficacy. | |||
hsa-miR-194-5p (miRNA)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [90] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | |
| Response Description | circSnx12 can be a molecular sponge of miR-194-5p, which targets SLC7A11. According to our findings, circSnx12 ameliorates cisplatin resistance by blocking ferroptosis via a miR-194-5p/SLC7A11 pathway. CircARNT2 may thus serve as an effective therapeutic target for overcoming cisplatin resistance in ovarian cancer. | |||
hsa-miR-143-3p (miRNA)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [49] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 |
| Response Description | SLC16A1-AS1 served as a sponge of miR-143-3p, and knockdown SLC16A1-AS1 significantly increased the enrichment of miR-143-3p. And then, SLC7A11 was identified as the target protein of miR-143-3p, and overexpression miR-143-3p remarkably inhibited the expression of SLC7A11. And silencing lncRNA SLC16A1-AS1 can induce ferroptosis through miR-143-3p/SLC7A11 signaling in renal cell carcinoma. | |||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [52] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
AC16 [Human hybrid cardiomyocyte] cells | Normal | Homo sapiens | CVCL_4U18 |
| Response Description | SEMA5A-IT1 overexpression upregulated the expression of BCL2 and SLC7A11 through sponging miR-143-3p, thereby protecting cardiomyocytes against apoptotic and ferroptosis cell death. In conclusion, we propose that SEMA5A-IT1, which is transported to cardiomyocytes through circulating sEVs, is an important regulatory molecule that protects cardiomyocytes from ischemia-reperfusion injury. | |||
hsa-miR-140-5p (miRNA)
Myocardial injury [ICD-11: NB31]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [91] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Six-week-old C57BL/6 male mice were randomly divided into two groups; the first group was fed a 45% high fat diet for 20 weeks while the control group were fed a normal chow diet. ATM-exosomes isolated from obese and lean mice were transferred into normal mice via tailvein injection(30 ug per week), as previously described. To investigate glutathione synthesis, 20 mM l-buthionine-(S,R)-sulfoximine (BSO) was added to the drinking water and administered to mice for 2 weeks. To inhibit ferroptosis, mice were given an intraperitoneal injection with Fer-1 (1 mg/kg).
Click to Show/Hide
|
||||
| Response Description | Solute carrier family 7 member 11 (SLC7A11) is a downstream target of miR-140-5p, which induces ferroptosis via inhibition of GSH synthesis by targeting SLC7A11. Attenuating exosomal-miR-140-5p expression alleviates ferroptosis and obesity-induced cardiac injury by alleviating GSH inhibition. | ||||
hsa-miR-128-3p (miRNA)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [58] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| DU145 cells | Prostate carcinoma | Homo sapiens | CVCL_0105 | ||
| In Vivo Model |
A total of 2 x 106 PC3 and PC3/Cd cells were subcutaneously injected into the right flanks of 4-week-old male Balb/c nude mice. Tumor burdens were closely monitored by tumor volumes. When the largest tumors reached a size of 1.0 cm3, all mice were sacrificed due to ethical considerations. Moreover, the final tumor weight was also recorded.
Click to Show/Hide
|
||||
| Response Description | OIP5-AS1 served as an endogenous sponge of miR-128-3p to regulate the expression of SLC7A11, a surrogate marker of ferroptosis. Moreover, miR-128-3p decreased cell viability by enhancing ferroptosis. Taken together, lncRNA OIP5-AS1 promotes prostate cancer progression and ferroptosis resistance through miR-128-3p/SLC7A11 signaling. | ||||
hsa-miR-1261 (miRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [92] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
| Cell metastasis | |||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | ||
| MHCC97-H cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_4972 | ||
| In Vivo Model |
Four-week-old female BALB/c nude mice were subcutaneously inoculated with 2 x 106 cells (5 mice per group). Intratumoral injection (40 uL of si-circ0097009 or negative control siRNA) was administered every 4 days. Mice were sacrificed, and tumor weights were measured after 4 weeks. To establish lung metastases, HepG2 cells were treated with 20 umol/L si-circ0097009. After 48 hours, cells (1 x 105) were intravenously injected into the tail veins of mice (6 mice per group).
Click to Show/Hide
|
||||
| Response Description | Circ0097009 acts as a competing endogenous RNA to regulate the expression of SLC7A11, a key regulator of cancer cell ferroptosis, by sponging miR-1261 in hepatocellular cancer. Circ0097009 may be used as a diagnostic biomarker for HCC and as a potential target for HCC therapy. | ||||
hsa-miR-125b-5p (miRNA)
Oral squamous cell carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [93] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
Tca8113 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6851 |
| TSCCA cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_VL15 | |
| CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | |
| SCC-9 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1685 | |
| hTECs (Human tongue epithelial cells) | ||||
| Response Description | EZH2 inhibits the ferroptosis of tongue squamous cell carcinoma cells by inhibiting miR-125b-5p and enhancing SLC7A11. MiR-125b-5p regulates ferroptosis in TSCC cells by targeting SLC7A11. | |||
Histone-lysine N-methyltransferase EZH2 (EZH2)
Oral squamous cell carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [93] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
Tca8113 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6851 |
| TSCCA cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_VL15 | |
| CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | |
| SCC-9 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1685 | |
| hTECs (Human tongue epithelial cells) | ||||
| Response Description | EZH2 inhibits the ferroptosis of tongue squamous cell carcinoma cells by inhibiting miR-125b-5p and enhancing SLC7A11. MiR-125b-5p regulates ferroptosis in TSCC cells by targeting SLC7A11. | |||
Multiple sclerosis [ICD-11: 8A40]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [83] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBMSCs (Bone marrow stromal cells) | ||||
| BV-2 cells | Normal | Mus musculus | CVCL_0182 | ||
| In Vivo Model |
Female C57BL6 mice were randomized into four groups (n = 8/group): the control group (non-EAE mice), the EAE group (EAE mice injected intrathecally with 5 uL PBS when symptoms appeared), the EAE + BMSCs-Exo + mimic negative control (NC) group (EAE mice injected intrathecally with Exos from bone MSCs (BMSCs) transfected with mimic NC [5 uL, 2 ug/L in PBS] when symptoms appeared), and the EAE + BMSCs-Exo + miR-367-3p mimic group (EAE mice injected intrathecally with Exos from BMSCs transfected with miR-367-3p mimic [5 uL, 2 ug/L in PBS] when symptoms appeared).
Click to Show/Hide
|
||||
| Response Description | MiR-367-3p can be delivered by BMSC-Exos into microglia, where miR-367-3p inhibits EZH2 expression and activates the expression of SLC7A11, suppressing microglial ferroptosis and relieving the symptoms of EAE. Experimental autoimmune encephalomyelitis (EAE) is a typical animal model of multiple sclerosis. | ||||
Hepatoblastoma [ICD-11: DB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [94] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| mPHs (Mouse primary hepatocytes) | |||||
| In Vivo Model |
Male C57BL/6 mice (n = 20, 6-8-week-old, b.w. 18-23 g) were purchased from SJA Laboratory Animal Co Ltd., Changsha, Hunan, China. Mice were given 600 mg/kg D-GalN (Sigma-Aldrich) and 30 ug/kg LPS (Sigma-Aldrich) by intraperitoneal injection. Mice in Sham group were received saline injection. For Fer-1 treatment, mice were received 10 mg/kg Fer-1 at 1 h prior to D-GalN and LPS injection. The wild-type (WT) or HBx-Tg mice were then subjected to D-GalN and LPS administration. For GSK126 treatment, wild-type (WT) or HBx-Tg mice were received GSK126 (150 mg/kg) prior to D-GalN and LPS administration.
Click to Show/Hide
|
||||
| Response Description | HBx facilitates ferroptosis in acute liver failure (ALF) via EZH2/H3K27me3-mediated SLC7A11 suppression. | ||||
HEPFAL (IncRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [95] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | |
| Response Description | LncRNA HEPFAL promotes the ubiquitination of SLC7A11, resulting in a decrease in GSH production, which in turn affects the activity of GPX4 and ultimately leads to the occurrence of ferroptosis. And LncRNA HEPFAL has the potential as a target for the diagnosis and treatment of hepatocellular carcinoma. | |||
Heat shock protein 105 kDa (HSPH1)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [96] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | |
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
Four-week-old female BALB/c nude mice were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). For subsequent studies, the nude mice were randomly divided into four groups as follows: sh-Ctrl, sh-ATF2, sh-Ctrl + sorafenib and sh-ATF2 + sorafenib. Approximately 5 x 106 ATF2 knockdown or control MGC803 cells were subcutaneously injected into the axilla of nude mice. Beginning on Day 8, mice in the sorafenib treatment group received 10 mg/kg sorafenib by intraperitoneal injection every 2 days for 3 weeks.
Click to Show/Hide
|
||||
| Response Description | Using ChIP-Seq and RNA-Seq, HSPH1 as a target of ATF2 and further validated it by ChIPqPCR analysis. HSPH1 can interact with SLC7A11 (cystine/glutamate transporter) and increase its protein stability. Importantly, knockdown of HSPH1 partly reversed the effects caused by ATF2 overexpression on sorafenib-induced ferroptosis in gastric cancer cells. | ||||
Growth/differentiation factor 15 (GDF15)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [97] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 |
| Response Description | GDF15 knockdown promotes erastin-induced ferroptosis in gastric cancer cell MGC803 by attenuating the expression of SLC7A11 and the function of system Xc-. | |||
gga-miR-129-3p (miRNA)
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [98] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
LMH cells | Hepatoma | Gallus gallus | CVCL_2580 |
| LMH cells | Hepatoma | Gallus gallus | CVCL_2580 | |
| Response Description | miR-129-3p affected ferroptosis under Se deficiency conditions through the SLC7A11 pathway. Our research provides a new perspective for the mechanism of Se deficiency on the liver damage. | |||
Frizzled-3 (FZD3)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [86] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 | |
| KYSE-70 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1356 | ||
| hEECs (Human esophageal epithelial cells) | |||||
| In Vivo Model |
BALB/c nude male mice of 4 weeks old were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). After one week of adaptive feeding, EC9706 cells (3 x 106) stably expressing sh-NC and sh-circPVT1, sh-NC + 5-FU and sh-circPVT1 + 5-FU were subcutaneously were injected into the right flank of the nude mice in a serum-free DMEM medium.
Click to Show/Hide
|
||||
| Response Description | CircPVT1 regulated the chemosensitivity of esophageal squamous cell carcinoma cells through ROS and Wnt/-catenin pathwaysviamiR-30a-5p/FZD3. Knockdown of circPVT1 promoted chemosensitivity in ESCC by increasing ferroptosis via downregulating GPX4 and SLC7A11. | ||||
ETS translocation variant 4 (ETV4)
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [99] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell cycle | |||||
In Vitro Model |
TPC-1 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | |
| IHH-4 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_2960 | ||
| Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | ||
| In Vivo Model |
Six-week-old BALB/c female nude mice (HFK Bioscience, Beijing, China) were used to perform experimentsin vivo. One hundred forty-four mice were randomly divided into four groups (36 mice in each group). TPC-1 cells were stably transfected with NC shRNA or ETV4-shRNA, and GLAG-66 cells were stably transfected with ETV4-OE or Vector. 5 x 106 cells were injected subcutaneously into the right armpit in mice. After 7 days, the diameter of tumors was measured every 3 days to calculate the tumor volume.
Click to Show/Hide
|
||||
| Response Description | The downregulation of ETV4 repressed the tumor development through the low expression of SLC7A11, and the ETV4 overexpression obtained the contrary effects. Overall, knockdown of ETV4 suppressed the papillary thyroid cancer progression by promoting ferroptosis upon SLC7A11 downregulation. | ||||
Estrogen receptor (ESR1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [100] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 |
| ZR-75-1 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0588 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Response Description | SLC7A11 expression was regulated by both ESR1 and NEDD4L, in opposite ways. For the first time, the study elucidated that ESR1 and NEDD4L functioned together after radiation treatment and finally induced ferroptosis in breast cancer cells, which provides novel insight into the guidance of clinical treatment of breast cancer. | |||
ELAV-like protein 1 (ELAVL1)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [55] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| In Vivo Model |
For animal models of gastric subserosal injection, we collected MGC-803 cell lines (5 x 10 cells) that were infected by lentivirus with or without PMAN-OE, and suspended in 40 ul serum-free medium (50% Matrigel). After that, nude mice (six mice per group) were anesthetized by intraperitoneal injection of 100 ul of pentobarbital (1%). After disinfection, the abdominal cavity was opened to expose the greater curvature of the stomach. The tumor suspension (40 ul) was implanted under the serosa of the greater curvature of the stomach of the nude mice through an insulin needle.
Click to Show/Hide
|
||||
| Response Description | HIF-1 could act as a protective factor against ferroptosis in gastric cancer (GC) cells. HIF-1 activates PMAN at the transcriptional level, which greatly improves the output of ELAVL1 in the cytoplasm. ELAVL1 directly combines with the AREs of SLC7A11 mRNA 3-UTR and improves the stability of SLC7A11mRNA, thereby increasing the expression of SLC7A11 and reducing the accumulation of ROS and iron in ferroptosis, ultimately promoting the proliferation and development of tumor cells. | ||||
E3 ubiquitin-protein ligase NEDD4-like (NEDD4L)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [100] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 |
| ZR-75-1 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0588 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Response Description | SLC7A11 expression was regulated by both ESR1 and NEDD4L, in opposite ways. For the first time, the study elucidated that ESR1 and NEDD4L functioned together after radiation treatment and finally induced ferroptosis in breast cancer cells, which provides novel insight into the guidance of clinical treatment of breast cancer. | |||
Disintegrin and metalloproteinase domain-containing protein 23 (ADAM23)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [82] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 |
| TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| Eca-109 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | |
| HET-1A cells | Normal | Homo sapiens | CVCL_3702 | |
| Response Description | ARHGEF26-AS1 facilitated ferroptosis but restrained cell growth and positively regulated ADAM23 by sponging miR-372-3p in esophageal squamous cell carcinoma (ESCC). Overexpression of ARHGEF26-AS1 upregulated the protein levels of ADAM23 but depleted the protein levels of GPX4, 3SLC3A2, and SLC7A11. | |||
DAZ-associated protein 1 (DAZAP1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [101] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| SMMC-7721 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| In Vivo Model |
Male BALB/c nude mice (4-5 weeks, 14-18 g) were purchased from Vital River Laboratories (Beijing, China). We randomly (random number grouping method) divided the mice into five groups: the blank group, the DMSO group, the SF group, the SF + sh-NC group and the SF + sh-DAZAP1 group. For SF-intervention mice, we dissolved 10 mg/kg of SF into 0.2 ml DMSO and injected the mixture intraperitoneally every other day for two weeks. 1 x 107 cells (with or without lentivirus) suspended in 500 ul of ice-cold PBS were subcutaneously injected into the left flank of mice.
Click to Show/Hide
|
||||
| Response Description | DAZAP1 knockdown by small interfering RNA markedly inhibited hepatocellular carcinoma (HCC) cell proliferation, migration and invasion. At the mechanistic level, DAZAP1 was identified as a potent inhibitor of ferroptosis and an efficient binding partner of SLC7A11 mRNA. | ||||
Cyclic AMP-dependent transcription factor ATF-4 (ATF4)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [40] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| SNU-398 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0077 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| HLE cells | Hepatocellular carcinoma | Homo sapiens | CVCL_1281 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| In Vivo Model |
SNU398-parental cells, SNU398-shLuc, or SNU398-shYAP/TAZ cells (106 in 100 ul PBS) were implanted into the left flanks of immunodeficient NOD/SCID; common receptor-/-(NSG) mice. When tumors were palpable, Sorafenib (LC Laboratories, S-8502) was applied at 20 mg/kg daily via gavage, SSA (Sulfasalazine, Sigma, S0883) was given at 120 mg/kg daily by intraperitoneal injection, 20 mM BSO (Lbuthionine-sulfoximine, Sigma, B2515) was given in the drinking water for 3 weeks.
Click to Show/Hide
|
||||
| Response Description | In a TEAD-dependent manner, YAP/TAZ induce the expression of SLC7A11, a key transporter maintaining intracellular glutathione homeostasis, thus enabling hepatocellular carcinoma cells to overcome Sorafenib-induced ferroptosis. At the same time, YAP/TAZ sustain the protein stability, nuclear localization, and transcriptional activity of ATF4 which in turn cooperates to induce SLC7A11 expression. | ||||
Cyclic AMP-dependent transcription factor ATF-2 (ATF2)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [96] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | |
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
Four-week-old female BALB/c nude mice were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). For subsequent studies, the nude mice were randomly divided into four groups as follows: sh-Ctrl, sh-ATF2, sh-Ctrl + sorafenib and sh-ATF2 + sorafenib. Approximately 5 x 106 ATF2 knockdown or control MGC803 cells were subcutaneously injected into the axilla of nude mice. Beginning on Day 8, mice in the sorafenib treatment group received 10 mg/kg sorafenib by intraperitoneal injection every 2 days for 3 weeks.
Click to Show/Hide
|
||||
| Response Description | Using ChIP-Seq and RNA-Seq, HSPH1 as a target of ATF2 and further validated it by ChIPqPCR analysis. HSPH1 can interact with SLC7A11 (cystine/glutamate transporter) and increase its protein stability. Importantly, knockdown of HSPH1 partly reversed the effects caused by ATF2 overexpression on sorafenib-induced ferroptosis in gastric cancer cells. | ||||
Coiled-coil domain-containing protein 6 (CCDC6)
Testicular cancer [ICD-11: 2C80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [102] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
NTERA-2 cells | Embryonal carcinoma | Homo sapiens | CVCL_0034 |
| TM4 cells | Normal | Mus musculus | CVCL_4327 | |
| MZ-GC-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4U28 | |
| MZ-GC-2 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4U29 | |
| Response Description | Testicular cancers are among the most common malignancies in young males. The loss of CCDC6 was associated with an enhancement of the xCT/SLC7A11 cystine antiporter expression which, by promoting the accumulation of ROS, interfered with the activation of ferroptosis pathway. | |||
Circ_0067934 (circRNA)
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [72] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
FTC-133 cells | Thyroid gland follicular carcinoma | Homo sapiens | CVCL_1219 | |
| TPC-1 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | ||
| In Vivo Model |
BALB/c nude mice (18-20 g, 4-week-old, male) (n = 6) were purchased and applied for the tumor growth analysis of FTC133 cellsin vivo. About 1 x 106 FTC133 cells were transfected with control shRNA or circ_0067934 shRNA and were subcutaneously injected into the left fat pad of mice. After 5 days of injection, we measured tumor growth every 5 days. We sacrificed the mice after 30 days of injection. The tumor volume was calculated by (length x width2)/2. And the tumor weight was calculated at day 30.
Click to Show/Hide
|
||||
| Response Description | Circ_0067934 upregulated the expression of the ferroptosis-negative regulator SLC7A11 by sponging and inhibiting miR-545-3p in thyroid cancer cells. The overexpression of SLC7A11 or the inhibitor of miR-545-3p reversed circ_0067934 silencing-regulated thyroid cancer cell proliferation. | ||||
CircSnx12 (circRNA)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [90] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 |
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | |
| Response Description | circSnx12 can be a molecular sponge of miR-194-5p, which targets SLC7A11. According to our findings, circSnx12 ameliorates cisplatin resistance by blocking ferroptosis via a miR-194-5p/SLC7A11 pathway. CircARNT2 may thus serve as an effective therapeutic target for overcoming cisplatin resistance in ovarian cancer. | |||
CircRPPH1 (circRNA)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [81] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| Hs746T cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0333 | |
| NCI-N87 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1603 | |
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| Response Description | circRPPH1 promoted the stemness of gastric cancer cells dependent on the miR-375/SLC7A11. This study provides a potential target for gastric cancer progression based on the circRPPH1/miR-375/SLC7A11 regulatory axis. | |||
CircRHBG (circRNA)
Ovarian dysfunction [ICD-11: 5A80]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [76] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
KGN cells | Ovarian granulosa cell tumor | Homo sapiens | CVCL_0375 | |
| SVOG-3e cells | Normal | Homo sapiens | CVCL_IN98 | ||
| In Vivo Model |
We recruited 12 infertility patients (6 PCOS and 6 controls) aged 20 and 38 who underwent in vitro fertilization (IVF) at the Department of Reproductive Medicine Center, the First Affiliated Hospital of Sun Yat-Sen University. All patients were treated the antagonist stimulation protocol. Briefly, on cycle day 2, ovarian stimulation was started by daily injection of recombinant FSH (r-FSH) (Gonal-F; Merck Serono). Gonadotropin-releasing hormone (GnRH) antagonist (Cetrotide, 0.25 mg; Merck Serono) injection was started from the day 6 of stimulation. When at least three follicles had reached 17 mm or two follicles had reached 18 mm in diameter, an intramuscular injection of 5,000 IU-10000 IU of human chorionic gonadotropin (hCG) is used for triggering final oocyte maturation.
Click to Show/Hide
|
||||
| Response Description | circRHBG inhibits ferroptosis in Polycystic ovary syndrome cells through the circRHBG/miR-515-5p/SLC7A11 axis in PCOS, which may provide new diagnostic molecular markers and therapeutic targets for PCOS. | ||||
CircPVT1 (circRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [86] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Wnt signaling pathway | hsa04310 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 | |
| KYSE-70 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1356 | ||
| hEECs (Human esophageal epithelial cells) | |||||
| In Vivo Model |
BALB/c nude male mice of 4 weeks old were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). After one week of adaptive feeding, EC9706 cells (3 x 106) stably expressing sh-NC and sh-circPVT1, sh-NC + 5-FU and sh-circPVT1 + 5-FU were subcutaneously were injected into the right flank of the nude mice in a serum-free DMEM medium.
Click to Show/Hide
|
||||
| Response Description | CircPVT1 regulated the chemosensitivity of esophageal squamous cell carcinoma cells through ROS and Wnt/-catenin pathwaysviamiR-30a-5p/FZD3. Knockdown of circPVT1 promoted chemosensitivity in ESCC by increasing ferroptosis via downregulating GPX4 and SLC7A11. | ||||
CircFOXP1 (circRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [74] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| In Vivo Model |
SCID/nude mice (6 weeks old) were ordered from Laboratory Animal Center of Chinese Academy of Sciences (Beijing, China). HT29 cells were co-transfected with sh-circFOXP1 and pCMV-SLC7A11 or empty vector. Cells were digested, 5 x 106 cells were mixed with Matrigel (Corning) and subcutaneously injected into mice. The width and length of tumor were measured at indicated time, and the tumor size was calculated by the formula: 0.5 x length x width2. Mice were then succumbed to death, the tumors were isolated, weighted, and made into paraffin-embedded slices (5-um). The slices were stained with anti-KI67 antibody (Santa Cruz Biotechnology) and subsequent HRP-labeled secondary antibody and captured in a microscope (Leica Microsystems).
Click to Show/Hide
|
||||
| Response Description | Mechanically, circFOXP1 increased SLC7A11 expression by directly sponging miR-520a-5p in lung cancer cells. The inhibitor of miR-520a-5p or the overexpression of SLC7A11 reversed circFOXP1 shRNA-induced ferroptosis phenotypes in lung cancer cells. | ||||
CircFNDC3B (circRNA)
Oral squamous cell carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [73] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
CAL-27 cells | Tongue adenosquamous carcinom | Homo sapiens | CVCL_1107 | |
| SCC-15 cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1681 | ||
| In Vivo Model |
The tumorigenicity analysis was conducted in BALB/c nude mice (6-weeks-old, male). The mice were maintained at pathogen-free condition. The 5 x 106 CAL27 cells were treated with control shRNA or circFNDC3B shRNA and subcutaneously injected into the nude mice (N = 5). The mice were sacrificed after 30 days and the tumor volume was calculated using the formula of (length x width2)/2.
Click to Show/Hide
|
||||
| Response Description | CircFNDC3B attenuated ferroptosis of Oral squamous cell carcinoma (OSCC) cells and contributed to OSCC progression by regulating the miR-520d-5p/SLC7A11 axis. CircFNDC3B, miR-520d-5p, and SLC7A11 may serve as potential therapeutic targets of OSCC. | ||||
CircEPSTI1 (circRNA)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [75] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Ca Ski cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| hCECs (Human cervical epithelial cells) | |||||
| In Vivo Model |
The 4-week-old female BALB/c nude mice were used for constructing xenograft model. HeLa cells (1 x 107 cells/mL) were injected into the dorsal flanksusing using 1-mL syringes. Then tumor size was measured and mice received intertumoral injection of si-crEPSTI1-1 (40 uL siRNA1 for cicrEPSTI1) and negative control (40 uL negative control) every four days, respectively (5 mice/group). After 28 days, the mice were sacrificed and xenografts were measured.
Click to Show/Hide
|
||||
| Response Description | CircEPSTI1 sponges miR-375, miR-409-3p and miR-515-5p to upregulate SLC7A11 expression. CircEPSTI1-miR-375/409-3P/515-5p-SLC7A11 axis affected the proliferation of cervical cancer via the competing endogenous RNAs (ceRNA) mechanism and was relative to ferroptosis. | ||||
CircCDK14 (circRNA)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [56] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
HEB (Human glial cells) | ||||
| SF126 cells | Glioblastoma | Homo sapiens | CVCL_1688 | ||
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
4-week-old female BALB/c nude mice were acquired from the National Laboratory Animal Center (Shanghai, China). Overall, 10 mice (n = 5 each group) were implanted with U251 cells stably knockdown circCDK14 by lentiviruses carrying sh-circCDK14 (Wanleibio, China), or control U251 cells with lentiviruses carrying sh-NC (Wanleibio, China). 5 x 106 cells were resuspended in phosphatebuffered saline (100 ul) and Matrigel substrate (100 ul) and injected into the right flank of nude mice. Tumor volume was documented once 7 days after implantation.
Click to Show/Hide
|
||||
| Response Description | CircCDK14 promotes the migration, invasion and proliferation of glioma cells in vitroas well as tumorigenesisin vivo. An evaluation of the underlying mechanism revealed that circCDK14 sponged miR-3938 to upregulate oncogenic gene PDGFRA expression. After knockdown of circCDK14 in glioma cells, protein levels of SLC7A11 and GPX4 decreased significantly and Fp became more sensitivity. | ||||
CircASAP2 (circRNA)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [44] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mKTs (Mouse knee tissues) | ||||
| HK-2 (Human renal glomerular endothelial cells) | |||||
| In Vivo Model |
All C57BL/6 mice (5-6 weeks, 18-20 g) were obtained from Animal Testing Center of Qinglongshan (Nanjing, Suzhou, China). DN mice were fed with a high-fat diet (HFD) for 12 weeks and then injected with STZ (30 mg/kg of streptozotocin; Sigma-Aldrich, St Louis, MO, USA) i.p. for 7 consecutive days. Blood glucose levels of mice were measured using 16.7 mmol/l after one week of the final injection. Then, mice were killed under anesthesia and their kidneys taken for analysis after induction of STZ at 4 months.
Click to Show/Hide
|
||||
| Response Description | Circular RNA circ ASAP2 decreased inflammation and ferroptosis in diabetic nephropathy through SOX2/ SLC7A11 by miR-770-5p. Importantly, this research indicated that circ ASAP2 might act as a target for improving the role of ferroptosis in diabetes nephropathy. | ||||
Circ0097009 (circRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [92] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
| Cell metastasis | |||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| BEL-7402 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_5492 | ||
| MHCC97-H cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_4972 | ||
| In Vivo Model |
Four-week-old female BALB/c nude mice were subcutaneously inoculated with 2 x 106 cells (5 mice per group). Intratumoral injection (40 uL of si-circ0097009 or negative control siRNA) was administered every 4 days. Mice were sacrificed, and tumor weights were measured after 4 weeks. To establish lung metastases, HepG2 cells were treated with 20 umol/L si-circ0097009. After 48 hours, cells (1 x 105) were intravenously injected into the tail veins of mice (6 mice per group).
Click to Show/Hide
|
||||
| Response Description | Circ0097009 acts as a competing endogenous RNA to regulate the expression of SLC7A11, a key regulator of cancer cell ferroptosis, by sponging miR-1261 in hepatocellular cancer. Circ0097009 may be used as a diagnostic biomarker for Hepatocellular carcinoma and as a potential target for HCC therapy. | ||||
Circ-BGN (circRNA)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [36] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BT-474 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0179 | |
| SK-BR-3 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | ||
| In Vivo Model |
A total of 24 NOD/SCID mice were purchased from Model Animal Research Center and grown under specific-pathogen-free condition. Mice were randomly divided into three groups (n = 8 per group), BT474-Tr cells were inoculated orthotopically onto the abdominal mammary fat pad. After 1 week, mice were treated with erastin (15 mg/kg intraperitoneal, twice every other day). Erastin was dissolved in 5% DMSO + corn oil (C8267, Sigma). To better dissolve erastin, we warmed the tube at 37 water bath and shook it gently. At the end of the sixth week, all mice were sacrificed, tumor tissues were collected and weighed.
Click to Show/Hide
|
||||
| Response Description | Knockdown of circ-BGN inhibited breast cancer cell viability and notably restored its sensitivity to trastuzumab. Further, we found that circ-BGN could directly bind to OTUB1 and SLC7A11, enhancing OTUB1-mediated SLC7A11 deubiquitination and thereby inhibiting ferroptosis. | ||||
CERS6-AS1 (IncRNA)
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [68] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | |
| IHH-4 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_2960 | ||
| BCPAP cells | Thyroid carcinoma | Homo sapiens | CVCL_0153 | ||
| KTC-1 cells | Thyroid carcinoma | Homo sapiens | CVCL_6300 | ||
| TPC-1 cells | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | ||
| In Vivo Model |
Animal experiments were approved by ethics committee of Wuzhou Red Cross Hospital. The TPC-1 cells transfected with sh-CERS6-AS1 or sh-NC were made into cell suspension (2 x 106/ml) with PBS. Then nude mice were randomly divided into sh-CERS6-AS1 group (n = 6) and sh-NC group (n = 6). The mice were subcutaneously inoculated with 200 ul corresponding cell suspension respectively, and then the weight and tumor volume of mice were recorded every other week. After 5 weeks, pentobarbital sodium (120 mg/kg) were intraperitoneally injected to make nude mice euthanasia, and then the tumors were stripped and weighed. Subsequently, immunohistochemistry and RT-PCR were performed.
Click to Show/Hide
|
||||
| Response Description | Silencing CERS6-AS1 suppressed cell viability and increased ferroptosis in papillary thyroid cancer. LASP1 was modulated by CERS6-AS1 through sponging miR-497-5p. The expression of Ki67, PCNA, GPX4, and SLC7A11 was inhibited by si- CERS6-AS1 transfection. | ||||
BBOX1-AS1 (IncRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [77] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
hESCCs (Esophageal squamous cancer cells) | |||
| Response Description | Downregulation of BBOX1-AS1 inhibits cell proliferation, and metastasis accelerates cell apoptosis and ferroptosis in esophageal squamous cell cancer by upregulating miR-513a-3p to reduce SLC7A11 expression. These findings may provide novel insights into the diagnosis and treatment of ESCC. | |||
ATP-binding cassette sub-family C member 5 (ABCC5)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [103] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| In Vivo Model |
All animal experiments were carried out with the approval of the Southern Medical University Animal Care and Use Committee in accordance with the guidelines for the ethical treatment of animals. Nude nu/nu mice were maintained in a barrier facility in racks filtered with a high-efficiency particulate air filter. The animals were fed an autoclaved laboratory rodent diet. The mice in this study were purchased from the Experimental Animal Centre of Southern Medical University, which is certified by the Guangdong Provincial Bureau of Science.
Click to Show/Hide
|
||||
| Response Description | ABCC5 increased intracellular glutathione (GSH) and attenuated lipid peroxidation accumulation by stabilizing SLC7A11 protein, which inhibited ferroptosis. Additionally, the inhibition of ABCC5 enhanced the anti-cancer activity of sorafenib in hepatocellular carcinoma. | ||||
ARHGEF26-AS1 (IncRNA)
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [82] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
EC9706 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_E307 |
| TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| Eca-109 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | |
| HET-1A cells | Normal | Homo sapiens | CVCL_3702 | |
| Response Description | ARHGEF26-AS1 facilitated ferroptosis but restrained cell growth and positively regulated ADAM23 by sponging miR-372-3p in esophageal squamous cell carcinoma (ESCC). Overexpression of ARHGEF26-AS1 upregulated the protein levels of ADAM23 but depleted the protein levels of GPX4, SLC3A2, and SLC7A11. | |||
Aldo-keto reductase family 1 member C3 (AKR1C3)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [41] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
In Vitro Model |
Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| MHCC97-H cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_4972 | ||
| HCC-LY10 (Human hepatoma cells) | |||||
| In Vivo Model |
To generate murine subcutaneous tumors, 2 x 106 HCC cells were injected subcutaneously to the right of the dorsal midline in nude mice. Once the tumors reached approximately 100 mm3 at day 15, mice were randomly allocated into groups and treated with erastin or sorafenib for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Overexpression of AKR1C3 protected against ferroptosis in hepatocellular carcinoma (HCC) cells. Mechanistically, AKR1C3 regulated ferroptosis through YAP/SLC7A11 signaling in HCC. | ||||
ADAMTS9-AS1 (IncRNA)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [71] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
ES-2 cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | |
| Caov-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0201 | |
| Response Description | ADAMTS9-AS1 regulated SLC7A11 expression through miR-587, thereby affecting ferroptosis, proliferation and migration of Epithelial ovarian cancer cells. | |||
3'-5' RNA helicase YTHDC2
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [104] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| NCI-H1975 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | ||
| NCI-H441 cells | Lung papillary adenocarcinoma | Homo sapiens | CVCL_1561 | ||
| NCI-H1650 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1483 | ||
| HCC827 cells | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| NCI-H292 cells | Lung mucoepidermoid carcinoma | Homo sapiens | CVCL_0455 | ||
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | ||
| In Vivo Model |
For xenograft experiments, 1.5 x 107 Doxocycline (Dox)-inducible YTHDC2-expressing H1299 cells were subcutaneously injected into 4-6-week-oldathymic nude mice. At day 14 post inoculation, mice were randomly divided into 2 groups for further administrating with or without Dox (30 mg/kg) every other day. Tumors were assessed after sacrificing the mice at day 28 after implantation.
Click to Show/Hide
|
||||
| Response Description | The m6A reader YT521-B homology containing 2 (YTHDC2) has been identified to inhibit lung adenocarcinoma (LUAD) tumorigenesis by suppressing solute carrier 7A11 (SLC7A11)-dependent antioxidant function. YTHDC2 also suppresses SLC3A2 subunit via inhibiting HOXA13-mediated SLC3A2 transcription. | ||||
Unspecific Regulator
Acute myeloid leukaemia [ICD-11: 2A60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [105] | |||
| Responsed Drug | Indole-3-pyruvic acid | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 |
| MLL/AF4+ RS4 cells | Adult B acute lymphoblastic leukemia | Homo sapiens | CVCL_0093 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| NIH3T3 cells | Normal | Mus musculus | CVCL_0594 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| Response Description | Indole-3-pyruvate (I3P) suppresses ferroptosis by direct free radical scavenging and through the activation of an anti-oxidative gene expression program in Childhood acute monocytic leukemia. And I3P elevated the activation of compensatory gene expression as indicated by increased protein amounts of SLC7A11 and HO-1, two important target genes of anti-oxidative stress pathways. | |||
Diffuse large B-cell lymphoma [ICD-11: 2A81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [106] | ||||
| Responsed Drug | Imidazole ketone erastin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SU-DHL-1 cells | Anaplastic large cell lymphoma | Homo sapiens | CVCL_0538 | |
| SU-DHL-2 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_9550 | ||
| SU-DHL-6 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_2206 | ||
| SU-DHL-8 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_2207 | ||
| SU-DHL-10 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1889 | ||
| SU-DHL-16 cells | B-cell non-Hodgkin lymphoma | Homo sapiens | CVCL_1890 | ||
| A3/Kawakami cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1062 | ||
| OCI-LY8 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_8803 | ||
| U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | ||
| DoHH2 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1179 | ||
| HBL-1 cells | Non-Hodgkin lymphoma | Homo sapiens | CVCL_M572 | ||
| U-2932 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1896 | ||
| SU-DHL-7 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_4380 | ||
| SU-DHL-9 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_4379 | ||
| A4/Fukuda cells | B acute lymphoblastic leukemia | Homo sapiens | CVCL_1064 | ||
| WSU-NHL cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1793 | ||
| SU-DHL-5 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1735 | ||
| Karpas-422 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1325 | ||
| In Vivo Model |
NOD/SCID mice (12-weeks of age and ~28 g weight) were weighed before injection and divided into groups of 3 mice per cage. Mice were dosed using three different routes, IP and PO with 50 mg/kg IKE, and IV with 17 mg/kg IKE. Samples were collected at 0, 1, 3, 4, and 8 h from three mice per time point. Additionally, three mice per group were used as controls by administration with equivalent amount of vehicle 1 by IP, PO, and IV, and samples were collected at 8 h. At the appropriate time, mice were sacrificed by CO2 asphyxiation for 3 min and ~0.5 mL of blood was collected via cardiac puncture.
Click to Show/Hide
|
||||
| Response Description | Imidazole ketone erastin (IKE) is a potent, selective, and metabolically stable system xc- (SLC7A11)inhibitor. In addition, biodegradable polyethylene glycol-poly(lactic-co-glycolic acid) nanoparticles were employed to aid in IKE delivery and exhibited reduced toxicity compared with free IKE in a diffuse large B cell lymphoma (DLBCL) xenograft model. | ||||
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [107] | |||
| Responsed Drug | Tirapazamine | Phase 3 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 |
| MNNG/HOS Cl #5 cells | Osteosarcoma | Homo sapiens | CVCL_0439 | |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| Response Description | SLC7A11 overexpression could restored the proliferation and migration abilities inhibited by Tirazamine. Thus, TPZ could inhibit the proliferation and migration of osteosarcoma cells, and induce ferroptosis in part through inhibiting SLC7A11. | |||
Gastrointestinal cancer [ICD-11: 2B5B]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [108] | ||||
| Responsed Drug | Berberine | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| TMK-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4384 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
Five-week-old male BALB/c mice were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained in a pathogen-free animal facility under a 12 h light/dark cycle in a temperature (22 )- and humidity-controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following the current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval no. 12924, 5 November 2020). Animals were acclimated to their housing for seven days before the start of the experiment. For the peritoneal dissemination tumor model, CT26 cancer cells (1 x 107 in 0.2 mL per mouse) were injected into the mouse peritoneal cavity. To measure tumor weight, mice were euthanized on Day 12 and the tumors were excised, while the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, with gross removal of non-tumor tissues. The largest tumor was formed on the diaphragm, and paraffin-embedded sections of the excised diaphragmatic tumor were prepared and stained with hematoxylin-eosin. BBR was diluted with distilled water to produce a final concentration of 48 mg/mL. The solutions were ultrasonically treated for 1 h, and fully vortexed for 30 min. BBR solution was administered by free drinking. The intake calculated from the amount of water consumed was 15.2 mg/kg body weight/day.
Click to Show/Hide
|
||||
| Response Description | Berberine induces apoptosis and ferroptosis by inhibiting mitochondrial complex I and promoting autophagy, leading to combined cell death in the gastrointestinal cancer and suppressing stemness. BBR induces cell death in GIC cells accompanied by increased mitochondrial superoxide and ACSL4 levels, decreased SLC7A11, and impaired antioxidant mechanisms, indicated by decreased GPX4 expression and decreased GSH. | ||||
Gastric cancer [ICD-11: 2B72]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [109] | ||||
| Responsed Drug | Atranorin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hGCCs (Gastric cancer cells) | ||||
| In Vivo Model |
NOD-scid mice (NOD.CB17-Prkdcscid/NcrCrl) aged 6-7 weeks and weighing 20-22 g were used in the experiment. The animal study was performed at the Shanghai University of Traditional Chinese Medicine with approval from the Institutional Animal Care and Use Committee in accordance with the institutional guidelines. All mice were randomly divided into two groups, and each group consisted of four mice. In experimental group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul Atranorin@SPION (10 mg/kg) every 2 days. In control group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul SPION (10 mg/kg) alone every 2 days. After 2 months, the mice were sacrificed, and their tumors were excised.
Click to Show/Hide
|
||||
| Response Description | Atranorin@SPIONs significantly reduced the 5-hydroxymethylcytidine modification level of GPX4 and SLC7A11 mRNA 3' untranslated region in gastric cancer cells. This study revealed the molecular biological mechanism by which Atranorin@SPION inhibit the in vitro and in vivo activity of GCSCs, that is, Atranorin@SPION reduced the expression of members of the Xc-/GPX4 axis and reduced their mRNA 5-hydroxymethylcytidine modification, finally induced ferroptosis of GCSCs. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [110] | ||||
| Responsed Drug | Actinidia chinensis Planch | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
In Vitro Model |
HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| In Vivo Model |
Wild type AB strain of zebrafish (Danio rerio) was obtained from Southern Medical University. The HGC-27 cells labeled with EGFP were resuspended in PBS in the concentration of 5*107/ml. 10 nl cell suspension containing approximately 300 cells were loaded into capillary needles and injected into the abdominal perivitelline space of zebrafish embryos by a nanoliter injector (Narishige, Tokyo, Japan). After injection, the tumor-bearing embryos were transferred into a 24-well plate and acclimated in embryo water at 35 for 24 h and then incubated at 0, 90, 180 mg/ml ACP decoction for 48 h.
Click to Show/Hide
|
||||
| Response Description | Actinidia chinensis Planch (ACP) increased the accumulation of ROS via inhibited the glutathione peroxidase 4 (GPx4) and xCT (SLC7A11) proteins, while were inhibited by Ferrostatin-1 (Fer-1) significantly. In conclusion, ACP was a promising antineoplastic agent for the treatment of gastric cancer by regulating apoptosis, ferroptosis and mesenchymal phenotype. | ||||
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [111] | ||||
| Responsed Drug | Pt3R5G | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| NCM460 cells | Normal | Homo sapiens | CVCL_0460 | ||
| In Vivo Model |
Five-week-old male BALB/c-nude mice from Central Laboratory of Animal, Xi'an Jiaotong University Health Science Center were housed 4 per cage under controlled temperature (23 ± 2 ), a 12 h/12 h light/dark cycle with ad libitum access to food and water and specific pathogen-free conditions. Twelve BALB/c-nude mice were randomly divided into three groups (control, 25 mg/kg, 50 mg/kg). 1 x 106 RKO cells were subcutaneously injected into either side of the same mice dorsal flanks. After 14 days, animals then received Pt3R5G (25 mg/kg, 50 mg/kg) byintraperitoneal injectionfor 15 days. The weight of mouse and tumor nodules sizer were measured every 3 days for 29 days.
Click to Show/Hide
|
||||
| Response Description | Pt3R5G significantly down-regulated SLC7A11 expression and up-regulated TFR1 in RKO cells. Pt3R5G inhibits cell proliferation through inducing ferroptosis by down-regulating SLC7A11 in colon cancer. | ||||
Colorectal cancer [ICD-11: 2B91]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [112] | ||||
| Responsed Drug | Propofol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response Description | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [113] | ||||
| Responsed Drug | 2-Imino-6-methoxy-2H-chromene-3-carbothioamide | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| mTOR signaling pathway | hsa04150 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| In Vivo Model |
Five-week-old female BALB/c nude mice were purchased from Beijing Weitong Lihua Experimental Animal Technical Co., Ltd. (Beijing, China). The mice were randomly assigned to the treatment and control groups until the tumor size reached approximately 100 mm3. The mice in the treatment group were injected with 0.174 mg/mL IMCA (100 uL), and those in the control group were injected with an equal volume of normal saline. The nude mice were euthanized, and samples were obtained from their tumor, heart, hepar, kidney, and blood after 33 days of IMCA treatment.
Click to Show/Hide
|
||||
| Response Description | 2-imino-6-methoxy-2H-chromene-3-carbothioamide (IMCA) significantly induced the ferroptosis of colorectal cancer cells. Mechanistically, IMCA downregulated the expression of SLC7A11 and decreased the contents of cysteine and glutathione, which resulted in reactive oxygen species accumulation and ferroptosis. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [114] | ||||
| Responsed Drug | Talaroconvolutin A | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| In Vivo Model |
5 x 106 HCT116 cells were inoculated subcutaneously in the underarm of Balb/c nude female mice (5-week old). The inoculated mice were randomly divided into two groups (6 mice each group). When the tumor reached 300 mm3, the drug group was given TalaA intraperitoneally at a dose of 6.0 mg/kg, and the control group was given the same dose of cosolvent-corn oil. The drug (or cosolvent) was injected every 2 days. Body weight and tumor volume were measured every 2 days.
Click to Show/Hide
|
||||
| Response Description | Talaroconvolutin A (TalaA) downregulated the expression of the channel protein solute carrier family 7 member 11 (SLC7A11) but upregulated arachidonate lipoxygenase 3 (ALOXE3), promoting ferroptosis. TalaA causes upregulation of HMOX1 which lead to the degradation of heme and the release of free iron, accumulating in mitochondria and giving rise to lipid peroxidation. TalaA could be a new potential powerful drug candidate for colorectal cancer therapy. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [115] | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| SNU-182 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0090 | ||
| SNU-449 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | ||
| In Vivo Model |
A total of 20 male Balb/c nude mice aged 6-8 weeks were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). Five million Huh7 cells were inoculated into the right flanks of the mice. When the tumor size reached 80-100 mm3, the mice were randomly divided into four groups and administered artesunate (30 mg/kg mouse weight) alone, sorafenib (20 mg/kg mouse weight) alone, a combination of artesunate and sorafenib, or the same volume of PBS by gavage every other day.
Click to Show/Hide
|
||||
| Response Description | Sorafenib at low dose mainly caused oxidative stress through mitochondrial impairments and SLC7A11-invovled glutathione depletion. Artesunate-induced lysosome activation synergized with sorafenib-mediated pro-oxidative effects by promoting sequential reactions including lysosomal cathepsin B/L activation, ferritin degradation, lipid peroxidation, and consequent ferroptosis. Taken together, artesunate could be repurposed to sensitize sorafenib in hepatocellular carcinoma treatment. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [116] | ||||
| Responsed Drug | Necrostatin-1 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| Response Description | Necrostatin-1 potentiated sulfasalazine-induced expression of SLC7A11, a catalytic subunit of system xc- in these cells. And necrostatin-1 Prevents Ferroptosis in a RIPK1- and IDO-Independent Manner in Hepatocellular Carcinoma. | ||||
Lung cancer [ICD-11: 2C25]
| In total 5 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [117] | ||||
| Responsed Drug | Ginkgetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SPC-A1 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | ||
| In Vivo Model |
Briefly, when tumours on transplanted nude mice reached around 100 mm3, the mice were randomized divided into eight groups: control, ginkgetin, DDP, ginkgetin + DDP, UAMC 3203, ginkgetin + UAMC 3203, DDP + UAMC 3203, ginkgetin + DDP + UAMC 3203. Both DDP (3 mg/kg) and ginkgetin (30 mg/kg) were administered by intraperitoneal injection, with 2 - 3 times per week and once per day, respectively. UAMC 3203 (10 mg/kg) was administered 5 days/week by intraperitoneally injection. Tumour size and body weight were measured 3 times per week. After dosing 31 days, the nude mice were sacrificed, and tumours were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | The induction of ferroptosis mediated by ginkgetin was further confirmed by the decreased expression of SLC7A11 and GPX4, and a decreased GSH/GSSG ratio. Simultaneously, ginkgetin disrupted redox hemostasis in DDP-treated cells, as demonstrated by the enhanced ROS formation and inactivation of the Nrf2/HO-1 axis. Ginkgetin also enhanced DDP-induced mitochondrial membrane potential (MMP) loss and apoptosis in cultured non-small cell lung cancer (NSCLC) cells. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [118] | ||||
| Responsed Drug | Sulforaphane | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
H69 cells | Normal | Homo sapiens | CVCL_8121 | |
| NCI-H82 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1591 | ||
| H69AR cells | Lung small cell carcinoma | Homo sapiens | CVCL_3513 | ||
| Response Description | Sulforaphane (SFN)-induced cell death was mediated via ferroptosis and inhibition of the mRNA and protein expression levels of SLC7A11 in small-cell lung cancer (SCLC) cells. The anticancer effects of SFN may provide novel options for SCLC treatment. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [119] | ||||
| Responsed Drug | Capsaicin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 | ||
| Response Description | Capsaicin inhibited the proliferation of A549 and NCI-H23 cells and induced ferroptosis by inactivating SLC7A11/GPX4 signaling. Capsaicin could be used as a potential anticancer agent in the treatment of non-small cell lung cancer. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [120] | ||||
| Responsed Drug | Manoalide | Phase 2 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| H157 cells | Oral cavity Squamous cell carcinoma | Homo sapiens | CVCL_2458 | ||
| HCC827 cells | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| In Vivo Model |
The LSL-KrasG12D mouse model was obtained from the Jackson Laboratory (Sacramento, CA). Adeno-Cre (Genechem, Shanghai, China) was introduced into the trachea of mice at a dose of 1.25 x 1011 PFU in a total volume of 50 uL. Tumor tissues from 12-week post-infection mice were washed with cold PBS, cut into small pieces, and washed with DMEM/F12 (containing 1 x Glutamine, 10 mM HEPES, and antibiotics), digested with collagenase I and IV for 0.5-1 h at 37. After washing twice with DMEM/F12 and centrifugation (500 g, 5 min), the dissociated cells were seeded into growth factor-reduced matrigel (Corning, #356237) at 37 for 30 min.
Click to Show/Hide
|
||||
| Response Description | Manoalide (MA) induces ferroptosis by suppressing the NRF2-SLC7A11 axis and mitochondrial Ca2+overload induced-FTH1 pathways to promote the sensitivity of osimertinib-resistant lung cancer cells to osimertinib. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [121] | ||||
| Responsed Drug | XAV939 | Preclinical | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
In Vitro Model |
NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| Response Description | The downregulation of the lncRNA MIR503HG induced by XAV939 may serve an important role in suppressing the progression of non-small cell lung cancer via sponging miR1273c, to downregulate its target SOX4. Furthermore, the downregulation of SLC7A11 induced by XAV939 may inhibit NSCLC development via participation in the ferroptosis pathway. | ||||
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [122] | |||
| Responsed Drug | Sulfasalazine | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| Response Description | The combined effects of vorinostat with salazosulfapyridine (SASP) depend on the accumulation of ROS caused by a decrease in intracellular GSH levels, possibly due to SASP-mediated inhibition of xCT. xCT (coded by the SLC7A11 gene), a light chain subunit of the glutamate-cystine antiporter system Xc(-) in Breast adenocarcinoma. | |||
Uterine serous carcinoma [ICD-11: 2C72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [123] | ||||
| Responsed Drug | Sulfasalazine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hUPSCs (Human uterine serous papillary carcinoma-1 cells) | ||||
| Abcam HeLa ERGIC2 KO cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_B1RG | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| In Vivo Model |
The mean weight of the mice at the start was 19.4 ± 0.87 g. To generate the subcutaneous xenograft model, USPC1 (5 x 106 cells) or PTX1 (5 x 106 cells) were suspended in 200 ul of PBS following determination of cellular viability and injected into the subcutaneous tissue of 6-week-old female Crj:SHO-PrkdcscidHrhr hairless SCID mice (n = 2) (Charles River Laboratories Inc.). Tumor formation was visually confirmed in mice inoculated with USPC1 cells, but not in those inoculated with PTX1 cells, thus the animal study was performed using USPC1 cells. The recipient mice were monitored for general health status and presence of subcutaneous tumors once a week.
Click to Show/Hide
|
||||
| Response Description | The effect of the xCT (SLC7A11) inhibitor, sulfasalazine on cytotoxicity was stronger in paclitaxel-resistant uterine serous carcinoma (USC) cells compared with that in paclitaxel-sensitive USC cells. Furthermore, the synthetic lethal interaction between the accumulation of ROS and the activation of the Ras effector, JNK, induced cell-proliferation inhibition and ferroptotic cell death in paclitaxel-resistant USC cells. | ||||
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [124] | ||||
| Responsed Drug | Norcantharidin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Athymic nu/nu female mice aged 6-8 weeks (n = 9; mean weight, 20.21 ± 1.54 g) were purchased from the specific pathogen SPF (Beijing) Lab Animals Technology Co. Ltd. Mice were housed in a temperature- and humidity-controlled environment (20-24 , 45-55% humidity), with free access to food and water and in groups of three. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC ID: 17-3256) at Nantong University and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Nuclear factor erythroid 2-related factor 2 (NRF2), heme oxygenase 1 (HO-1), glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (xCT) expression levels were significantly decreased following norcantharidin (NCTD) treatment. Collectively, NCTD may represent a potent anticancer agent in ovarian cancer cells, and NCTD-induced ferroptotic cell death may be achieved by inhibiting the NRF2/HO-1/GPX4/xCT axis. | ||||
Diabetes mellitus [ICD-11: 5A10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [125] | ||||
| Responsed Drug | Cryptochlorogenic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
INS-1 cells | Insulinoma | Rattus norvegicus | CVCL_0352 | |
| In Vivo Model |
Sixty Sprague-Dawley (SD) rats with weights ranging from 250-270 g were obtained from experimental animal center of Xiamen university. For diabetes model group, fasting was performed for 12 h before experiment. The rats (ten rats per group) were assigned into Control group, Model (DM) treated with 50 mg/kg streptozotocin (STZ) via abdominal injection, positive control group and experimental groups. The blood glucose level, which is served as the indicator for the diabetes, was monitored herein. The glucose level after modeling is above 16.7 mmol/l, supporting that the modeling is successful.
Click to Show/Hide
|
||||
| Response Description | Cryptochlorogenic acid (CCA) functions via inhibition of ferroptosis by activation of cystine/glutamate transporter system (XC)/glutathione peroxidase 4(GPX4)/Nrf2 and inhibition of nuclear receptor coactivator 4 (NCOA4) in diabetes. System xc- which is composed of SLC7A11 and SLC3A2, served as the provider of GSH synthesis. | ||||
Ovarian dysfunction [ICD-11: 5A80]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [126] | ||||
| Responsed Drug | N-acetylcysteine | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell differentiation | |||||
In Vitro Model |
hPTs (Human placental tissues) | ||||
| In Vivo Model |
Adult Sprague-Dawley rats (70 days old) of both sexes were purchased from the Laboratory Animal Centre of Harbin Medical University, Harbin, China. Before the experiment, female rats were allowed to acclimatize for a minimum of 1 week and then were monitored daily by vaginal lavage to determine the stage of the estrous cycle as previously described (Zhanget al., 2016). Pregnancy was achieved by housing female rats on the night of proestrus with fertile males of the same strain at a 2:1 ratio. Confirmation of mating was performed the morning after by the presence of a vaginal plug, and this was considered as GD 0.5. The rats were sacrificed between 8:00 a.m. and 9:00 a.m. hours on GD 14.5.
Click to Show/Hide
|
||||
| Response Description | Previous studies demonstrate that increased uterine and placental ferroptosis is associated with oxidative stress-induced fetal loss in a pre-clinical polycystic ovary syndrome (PCOS)-like rat model. N-acetylcysteine treatment results in increased mRNA expression of Aifm2, a negative regulator of GPX4-independent ferroptosis in the placenta. Moreover, NAC reverses HAIR-induced uterine and placental ferroptosis through activation of the Slc7a11/GSH/GPX4 axis. | ||||
Cognitive disorder [ICD-11: 6D71]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [127] | ||||
| Responsed Drug | Liraglutide | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
Male diabetic db/db mice and nondiabetic littermate db/m mice that were 4 weeks of age were purchased from Changzhou Cavens Experimental Animal Co., Ltd. After 1week of adaptive feeding, 20 db/db mice were randomly divided into two groups: a model group (db/db, n = 10) and a treatment group (LIRA, n = 10). Another 10 db/m mice were used as the control group (db/m, n = 10). After feeding to 10weeks old, the LIRA group was given liraglutide (CSN11311, CSNpharm, China) diluent (200 ug/kg/d) by intraperitoneal injection for 5 weeks, with equivoluminal 0.9% saline intraperitoneally administered to the other two groups.
Click to Show/Hide
|
||||
| Response Description | Liraglutide was shown to prevent ferroptosis in the hippocampus by elevating the expression of GPX4 and SLC7A11 and suppressing the excessive amount of ACSL4. LIRA can reduce oxidative stress, lipid peroxidation and iron overload in diabetes-induced cognitive dysfunction and further inhibit ferroptosis, thereby weakening the damage to hippocampal neurons and synaptic plasticity and ultimately restoring cognitive function. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [128] | ||||
| Responsed Drug | Paraquat | Investigative | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| Ferroptosis | hsa04216 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Twenty male C57BL/6 mice at 12 weeks old were purchased from Hebei Medical University Experimental Animal Center. 10 mice of the experimental group were intraperitoneally injected with PQ (10 mg PQ (salt)/kg/dose) three times a week for 3 weeks according to the previous report. Ten mice of the control group were intraperitoneally injected with the same dose of normal saline. Once the experimental schedule was completed, firstly, the animals were used for behavioral tests. Then, the mice were anesthetized with 0.4% pentobarbital sodium (1 mL/100 g) solution and perfused. The substantia nigra tissue was exfoliated for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Paraquat (PQ) significantly caused the iron accumulation in cytoplasm and mitochondria through ferritinophagy pathway induced by NCOA4. Iron overload initiated lipid peroxidation through 12Lox, further inducing ferroptosis by producing lipid ROS. PQ downregulated SLC7A11 and GPX4 expression and upregulated Cox2 expression. Bcl2/Bax and P-p38/p38 pathways mediated the cross-talk between ferroptosis and apoptosis induced by PQ. These data further demonstrated the complexity of Parkinson's disease occurrence. | ||||
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [129] | ||||
| Responsed Drug | Baicalin | Terminated | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In Vivo Model |
A total of 60 male C57BL/6 mice (10weeks old, 25-28g) were purchased from Guangzhou University of Chinese Medicine Experimental Animal Center (Guangzhou, China). The mice were maintained with enough food and water at 24, 60% relative humidity and 12/12h light/dark cycle. The mice were randomly divided into three groups: sham operation group (Sham), ICH model group (Mod) and baicalin group (Bai) (n = 20/group). Baicalin was suspended in 0.5% carboxymethylcellulose sodium solution. Given the extremely low solubility of baicalin, the concentration of baicalin solution was 0.5 mg/ml. To achieve 20 mg/kg/day dosage, the baicalin solution was administered to the mice in the Bai group by oral route twice at an interval of 1 h within 2 h after ICH injury onset. The remaining two groups received an equal volumes of saline through oral gavage. Since the second day after ICH, mice in the Bai group received 20 mg/kg of baicalin solution while those in the remaining two groups received equal volumes of saline once a day for three consecutive days.
Click to Show/Hide
|
||||
| Response Description | Baicalin significantly increased the mRNA expression of GPX4 and SLC7A11 in the perihematoma brain tissues of intracerebral hemorrhage (ICH) model mice. Baicalin can inhibit the development of ferroptosis in ICH. Baicalin is a potential therapeutic drug for ICH treatment. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [130] | ||||
| Responsed Drug | Galangin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
Male gerbils weighing 70-90 g (12 weeks) were selected for this study. Gerbils were anesthetized with 7% chloral hydrate (350 mg/kg) and the bilateral common carotid arteries were occluded using artery clips. After 5 min, the clips were removed to restore cerebral blood flow. After the operation, place the gerbil on an electric blanket to keep the gerbil's body temperature. The sham group underwent the same surgical procedure without ligation of carotid arteries and was given an equal volume of physiological saline as in the treated groups. The model + galangin (Jiangsu Yongjian Pharmaceutical Technology, 548-83-4, Purity: >=98% (HPLC)) groups underwent the same procedure as the model group and then were received galangin at 25, 50, or 100 mg/kg/day for two continuous weeks.
Click to Show/Hide
|
||||
| Response Description | Gerbils treated with galangin after ischemia-reperfusion (I/R) injury showed significant improvements in learning and memory. In addition, galangin treatment reduced the levels of lipid peroxide in the brains of gerbils that underwent I/R as well as reduced the amount of cell death and increased the expression of SLC7A11 and glutathione peroxidase 4 (GPX4). | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [131] | ||||
| Responsed Drug | Chrysin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [132] | |||
| Responsed Drug | Kaempferol | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
mPCNs (Mouse primary cortical neurons) | |||
| Response Description | Kaempferol provides protection from OGD/R-induced ferroptosis, at least in part, by activating Nrf2/SLC7A11/GPX4 signaling pathway. Therefore, pharmacological inhibition of ferroptosis may be an attractive therapeutic target for the treatment of ischemic stroke. | |||
Cardiomyopathy [ICD-11: BC43]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [133] | ||||
| Responsed Drug | Canagliflozin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57BL/6J mice aged 6-8 weeks with weights of 18-20 g were obtained from the Slack Laboratory Animal Co., Ltd. (Shanghai, China). Mice were allowed to acclimatize in the laboratory environment for 1 week before the beginning of the experiment. DCM model establishment: The mice were given a single intraperitoneal injection of 150 mg/kg 1% streptozotocin (STZ, V900890, Sigma, USA, dissolved in 0.1 mol/L sodium citrate buffer, pH = 4.4 - 4.6). Mouse blood from the tail vein was collected in each group of the model mice and tested by glucose meter (Accu-Chek Performa test strips, Roche, Accu-Chek Performa Combo, Roche, USA) on day 3, 5 and 7 after injection.
Click to Show/Hide
|
||||
| Response Description | Canagliflozin (Cana) promotes upregulation of SLC7A11 and downregulation of TfR1 and FTN-H, which protect the cardiomyocytes from ferroptosis. These finding suggests that Cana inhibit ferroptosis by balancing cardiac iron homeostasis and promoting the system Xc/GSH/GPX4 axis in diabetic cardiomyopathy. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [134] | ||||
| Responsed Drug | Liquiritin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
The set of animal experiments was designed to evaluate the effectiveness of liquiritin on doxorubicin-induced cardiotoxicity as were as ferroptosis were explored. Mice were randomly divided into 5 groups: (1) the control group; (2) doxorubicin group; (3) the doxorubicin plus liquiritin group (20 mg/kg); (4) the doxorubicin plus liquiritin group (40 mg/kg); (5) the doxorubicin plus liquiritin group (80 mg/kg) (Han et al.2022; Mou et al.2021). The control group and doxorubicin group were given equal volume of 0.5% sodium carboxymethylcellulose; the doxorubicin plus liquiritin groups were given different doses of liquiritin (0.5%) sodium carboxymethylcellulose co-suspension) by intragastric administration 7 days in advance once a daily. On day 8, groups (2), (3), (4), and (5) were given a single intraperitoneal injection of 15 mg/kg of DOX to establish a model of doxorubicin-induced cardiotoxicity; and group (1) was given an equal volume of saline intraperitoneally.
Click to Show/Hide
|
||||
| Response Description | Liquiritin can protect the doxorubicin-induce mice's cardiotoxicity, and its beneficial effect is related to the reduction of ferroptosis through a mechanism involving the regulation of the SLC7A11/GPX4 pathway. | ||||
Congestive heart failure [ICD-11: BD10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [135] | ||||
| Responsed Drug | Atorvastatin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
8-week C57BL/6J male mice purchased from Comparative Medicine Center of Yangzhou University were retained with unrestricted access to sterilized diet and water at standard bio-clean laboratory settings (Experimental Animal Center of College of Veterinary Medicine of Yangzhou University). Animals were randomly divided into four groups(n = 6-8 mice per group): control group or ISO group: injected with saline or ISO (5 mg/kg) subcutaneously for 14 days and, meanwhile, received vehicle saline via gavage for 14 days respectively; ATV (Pfizer,USA) group or ISO + ATV group: injected with saline or 5 mg/kg ISO (Sigma, USA) subcutaneously for 14 days and, meanwhile, received 20 mg/kg ATV via gavage for 14 days respectively.
Click to Show/Hide
|
||||
| Response Description | Atorvastatin showed significantly protective effects through suppressing the activation of ferroptosis related signaling, as evidenced by decreasing the mRNA levels of PTGS2 (a marker of ferroptosis), contents of malonaldehyde and protein levels of NOX4 and increasing the contents of glutathione (GSH), the ratio of GSH/GSSG and protein levels of GPX4 and SLC7A11. ATV reduced cardiac hypertrophy and fibrosis and accumulation of iron in heart failure. | ||||
Cardiovascular diseases [ICD-11: BE2Z]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [136] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | ||||
| Response Description | Astragaloside IV partially upregulated the levels of SLC7A11 and GPX4 expression which were reduced by LPC. The LPC-suppressed proliferation and LPC-induced apoptosis and senescence of endothelial cells were greatly attenuated by AS-IV treatment. In conclusion, AS-IV could serve as a novel drug for treating ferroptosis-related cardiovascular diseases. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [137] | ||||
| Responsed Drug | Sesamin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
Forty specific pathogen-free normal Sprague Dawley (SD) rats (7 weeks old and 251-275 g in weight) were supplied by Charles River Laboratories. The SD rats were randomly allocated into five groups (n = 8). In the PM2.5 exposure group, the rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times. In the saline control group, the SD rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and intratracheally instilled with 0.9% saline (1 mL per kg b.w.) every other day for a total of three times. In the Ses pretreatment groups, the SD rats were gavaged with low (L-Ses, 40 mg per kg b.w), medium (M-Ses, 80 mg per kg b.w.), and high (H-Ses, 160 mg per kg b.w.) doses of Ses. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times.
Click to Show/Hide
|
||||
| Response Description | Sesamin pretreatment upregulated the expression levels of GPX4, SLC7A11, TFRC, and FPN1 and inhibited the expression levels of FTH1 and FTL. Ses pretreatment could ameliorate PM2.5-induced cardiovascular injuries perhaps by inhibiting ferroptosis. | ||||
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [138] | |||
| Responsed Drug | Chrysophanol | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HSC-T6 cells | Normal | Rattus norvegicus | CVCL_0315 |
| Response Description | Chrysophanol significantly induced HBx-transfected HSC-T6 death by inducing ferroptosis, as demonstrated by lipid ROS accumulation and upregulation of expression of ER markers, such as Bip, CHOP, and p-IRE1, and ferroptotic markers, such as GPX4 and SLC7A11. Therefore, chrysophanol may exert ferroptotic effects on activated HSCs to prevent liver fibrosis. | |||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [139] | ||||
| Responsed Drug | Salidroside | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | ||
| In Vivo Model |
Following endotracheal intubation, mice were ventilated with room air at a rate of 120 cycles/min and atidal volumeof 7 mL/kg (MiniVent, Harvard Apparatus, USA). To induce ischemia, mice underwent left thoracotomy, and the left pulmonary hilum was blocked for 60 min with a microvascular clamp. After ischemia, the coronary artery was reperfused for 120 min by removing the clamp. The mice were euthanized at the end of the experiment through CO2 asphyxiation and cervical dislocation. Next, bronchoalveolar lavage fluid (BALF), blood, and lung samples were collected for testing.
Click to Show/Hide
|
||||
| Response Description | Salidroside postconditioning attenuates ferroptosis-mediated lung ischemia-reperfusion injury by activating the Nrf2/SLC7A11 signaling axis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [140] | ||||
| Responsed Drug | Dimethyl fumarate | Approved | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
The mice were randomly divided into four groups of six: sham + vehicle, sham + DMF, IR + vehicle, and IR + DMF. The mice were supplemented with DMF at a concentration of 100 mg/kg or DMSO by daily oral gavage for a week before surgery, as previously reported. As stated in a prior study, the partial warm liver IRI model was developed. Briefly, the sham group only had free hepatic portal blood vessels after laparotomy, and the blood flow was not obstructed. As for the hepatic IR group, the blood supply to the left and mid-hepatic lobes was blocked, resulting in 70% mouse liver IRI for 90 min. The mice were put on a heated blanket after surgery in order to maintain body temperature and monitor vital signs. Blood supply was restored for 6 h. Died mice were eliminated for testing prior to sample collection. The mice were euthanized after the sample were obtained. The same experimenter carried out all surgeries.
Click to Show/Hide
|
||||
| Response Description | NRF2 knockdown notably decreased the expression of SLC7A11 and HO-1 and blocked the anti-ferroptosis effects of dimethyl fumarate (DMF). DMF inhibits ferroptosis by activating the NRF2/SLC7A11/HO-1 axis and exerts a protective effect against hepatic ischemia-reperfusion injury. | ||||
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [141] | ||||
| Responsed Drug | Aspirin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
These mice were on eight weeks old male DBA/2J background (n = 36, HFK Bioscience, Beijing, China). They were randomized one of the six groups: control normal mice group (NC); diabetic mice group (DM); diabetic mice group (Fer-1), who intraperitoneal injected Fer-1 (Selleck, Houston, TX, USA); diabetic mice group (vehicle-P), who intraperitoneal injected 1% dimethyl sulfoxide (DMSO); diabetic mice group (As), who intragastric administrated Aspirin (Solarbio, Beijing, China); diabetic mice group (vehicle-G), who intragastric administrated 0.5% sodium carboxymethyl cellulose (Na-CMC; Solarbio, Beijing, China). Diabetes models were induced with 5 consecutive days of a single intraperitoneal injection of streptozotocin 40 mg/kg (dissolved in 0.1 M citrate buffer, pH 4.5; SigmaAldrich, St Louis, MO, USA). Control mice only was injected the same volume of citrate buffer. In the Fer-1 or vehicle-P groups, the diabetic mice were treated respectively with Fer-1 (2.5 umol/kg, dissolved in 1% DMSO) or 1% DMSO during the duration of treatment for 12-week every day. And in the AS and vehicle-G groups, the diabetic mice were treated respectively with aspirin (50 mg/kg, dissolved in 0.5% Na-CMC) or 0.5% Na-CMC for 12-week every day.
Click to Show/Hide
|
||||
| Response Description | Aspirin can upregulate SLC7A11 and GPX4 expression by suppressing COX2. Our results demonstrated that ferroptosis in renal tubular cells contributes to Diabetic kidney disease (DKD) development and that diabetes-related ferroptosis was inhibited through the downregulation of COX2 by aspirin, thus retarding the progression of DKD. | ||||
Lung injury [ICD-11: NB32]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [142] | ||||
| Responsed Drug | Astragaloside IV | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hT2AECs (Type II alveolar epithelial cells) | ||||
| In Vivo Model |
The animals were randomly assigned to six groups (7 mice in each) as follows: (I) Normal saline (NS) group, (II) Ast-IV 100 mg/kg (Ast) group, (III) PM2.5 group, (IV) Ast-IV 50 mg/kg + PM2.5 (Ast-L) group, (V) Ast 100 mg/kg + PM2.5 (Ast-H) group, and (VI) Ast-IV 100 mg/kg + erastin 20 mg/kg + PM2.5 (Era) group. Based on our previous results, this study adopted anintraperitoneal injection(i.p.) of Ast-IV (dissolved in normal saline containing 0.1% DMSO for preventive treatment. After all the mice were adaptively fed for 5 days, in the NS and PM2.5 groups, mice received the normal saline containing 0.1% DMSO viai.p.once a day for the next three consecutive days. Similar to the NS group, in the Ast, Ast-H, and Era groups, mice received Ast-IV (100 mg/kg) viai.p. Ast-L group received Ast-IV (50 mg/kg) viai.p. To evaluate the effect of Ast-IV on ferroptosis in PM2.5-induced lung injury, we used the ferroptosis agonist erastin to activate ferroptosisin vivo. In the Era group, mice received erastin (20 mg/kg, 10% DMSO + 40% PEG300 + 5%Tween80 + 45% normal saline) 30 min before each preventive treatment of Ast-IV.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (Ast-IV) reduced the lung wet-dry ratio and the levels of interleukin 6 (IL-6), tumor necrosis factor- (TNF-) and interleukin 1 (IL-1) in serum. Ast-IV could also improve the oxidative stress level in BALF, restore the GSH level in the lung tissue, and reduce the iron content in the lung tissue. Western blot outcomes revealed that Ast-IV regulated the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to protect PM2.5-mediated lung injury. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [143] | ||||
| Responsed Drug | Lipopolysaccharide | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
The male C57BL/6 mice were divided randomly into 4 groups (n = 4 per group, 8-10 weeks old, weight = 23-25 g): the control group receiving 0.9% NaCl (containing 0.1% DMSO), the LPS group receiving LPS plus 0.9% NaCl (containing 0.1% DMSO), the Fer-1 group receiving Fer-1 only, and the LPS + Fer-1 group receiving both Fer-1 and LPS. The LPS-induced ALI model was induced by instilling intratracheally 50 ul of LPS solution (0.2 g/L), then Fer-1 (0.8 mg/kg) was administered after LPS challenge via tail vein injection. The Fer-1 was dissolved in DMSO first, and diluted with 0.9% NaCl. The final concentration of Fer-1 and DMSO was 0.2 mg/ml and 0.1% respectively.
Click to Show/Hide
|
||||
| Response Description | The cell viability of BEAS-2B was down-regulated by lipopolysaccharide (LPS) treatment, together with the ferroptosis markers SLC7A11 and GPX4, while the levels of MDA, 4-HNE and total iron were increased by LPS treatment in a dose-dependent manner, which could be rescued by ferrostatin-1. Fer-1 exerted therapeutic action against LPS-induced acute lung injury, and down-regulated the ferroptosis level in lung tissues. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [143] | ||||
| Responsed Drug | Ferrostatin-1 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
The male C57BL/6 mice were divided randomly into 4 groups (n = 4 per group, 8-10 weeks old, weight = 23-25 g): the control group receiving 0.9% NaCl (containing 0.1% DMSO), the LPS group receiving LPS plus 0.9% NaCl (containing 0.1% DMSO), the Fer-1 group receiving Fer-1 only, and the LPS + Fer-1 group receiving both Fer-1 and LPS. The LPS-induced ALI model was induced by instilling intratracheally 50 ul of LPS solution (0.2 g/L), then Fer-1 (0.8 mg/kg) was administered after LPS challenge via tail vein injection. The Fer-1 was dissolved in DMSO first, and diluted with 0.9% NaCl. The final concentration of Fer-1 and DMSO was 0.2 mg/ml and 0.1% respectively.
Click to Show/Hide
|
||||
| Response Description | The cell viability of BEAS-2B was down-regulated by lipopolysaccharide (LPS) treatment, together with the ferroptosis markers SLC7A11 and GPX4, while the levels of MDA, 4-HNE and total iron were increased by LPS treatment in a dose-dependent manner, which could be rescued by ferrostatin-1. Fer-1 exerted therapeutic action against LPS-induced acute lung injury, and down-regulated the ferroptosis level in lung tissues. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [144] | ||||
| Responsed Drug | Lycopene | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mSCs (Mouse splenocytes) | ||||
| In Vivo Model |
Three-week-old specific pathogen-free ICR (Institute of Cancer Research) male mice (weights of 18-22 g) were provided by Liaoning Changsheng Biotech Co. Ltd. The mice were housed under conditions at 22 ± 2 with 35-65% humidity and a light/dark cycle of 12 h/12 h in the cage. The animals were quarantined for a week before formal experiments, then randomly divided into seven groups: vehicle control group (Vcon), control group (Con), 5 mg/kg BW/d Lyc group (Lyc), 500 and 1000 mg/kg BW/d DEHP group (D5 and D10, respectively), DEHP + Lyc group (DL5 and DL10, respectively) (n = 20). The animals were exposed to DEHP via oral gavage, which lasted for 28 d, and then sacrificed after being anesthetized.
Click to Show/Hide
|
||||
| Response Description | DEHP disrupted the GSH metabolism via the xc/ GPX4 antioxidant system and, subsequently, caused the ferroptotic cell death, but Lycopene (Lyc) could effectively mitigate DEHP-induced damage to the antioxidant system. These findings indicated that Lyc may be an effective strategy for the prevention of DEHP-induced splenic toxicity via the regulation of ferroptosis. | ||||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [146] | |||
| Responsed Drug | T-2 Toxin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| Response Description | SLC7A11 overexpression significantly rescued the enhanced ferroptosis caused by T-2 toxin. T-2 toxin induces ferroptosis by downregulating SLC7A11 expression. Ferroptosis mediates T-2 toxin-induced cytotoxicity by increasing ROS and downregulating SLC7A11 expression. | |||
Ubiquitin-fold modifier 1 (UFM1)
Metformin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| HCC1937 cells | Breast ductal carcinoma | Homo sapiens | CVCL_0290 | ||
| Bcap37 cells | Breast carcinoma | Homo sapiens | CVCL_0164 | ||
| HBL-100 cells | Normal | Homo sapiens | CVCL_4362 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| In Vivo Model |
T47D xenografts were established in 5-week-old nude mice (Shanghai SLAC Laboratory Animal Corporation) by inoculating 1 x 107 cells mixed with Matrigel (BD Biosciences) at 1:1 ratio (volume) into the abdominal mammary fat pad. When the tumor reached 50-100 mm3, the mice were assigned randomly into different treatment groups (DMSO, Metformin, SAS, and Metformin + SAS groups). Metformin (200 mg/kg/day) was provided in drinking water. Sulfasalazine was dissolved in dimethyl sulfoxide (DMSO), diluted in PBS, and then intraperitoneally injected into mice at a dose of 250 mg/kg once a day.
Click to Show/Hide
|
||||
| Response Description | Metformin reduces the protein stability of SLC7A11, which is a critical ferroptosis regulator, by inhibiting its UFMylation process. Furthermore, metformin combined with sulfasalazine, the system xc-inhibitor, can work in a synergistic manner to induce ferroptosis and inhibit the proliferation of breast cancer cells. | ||||
Ubiquitin thioesterase OTUB1 (OTUB1)
Solasonine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| CFPAC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_1119 | ||
| In Vivo Model |
For xenograft assays, we subcutaneously injected 1 x 106 PANC-1 and CFPAC-1 into the right side of each male nude mouse (n = 6). Tumor volumes (length x width2 x 0.5) were measured at specified time points. For one treatment cycle in a week (starting from week 1 to week 5), solasonine (40 or 80 mg/kg, oral administration, 2 times) were given. A total of five treatment cycles were conducted in this experiment.
Click to Show/Hide
|
||||
| Response Description | Solasonine is involved in ferroptosis by suppressing TFAP2A-mediated transcriptional upregulation of OTUB1, thereby activating ubiquitinated degradation of SLC7A11 and promoting pancreatic cancer cell ferroptosis. | ||||
Tumor necrosis factor alpha-induced protein 3 (TNFAIP3)
Erastin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
Cisplatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
Transcription factor AP-2-alpha (TFAP2A)
Solasonine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| CFPAC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_1119 | ||
| In Vivo Model |
For xenograft assays, we subcutaneously injected 1 x 106 PANC-1 and CFPAC-1 into the right side of each male nude mouse (n = 6). Tumor volumes (length x width2 x 0.5) were measured at specified time points. For one treatment cycle in a week (starting from week 1 to week 5), solasonine (40 or 80 mg/kg, oral administration, 2 times) were given. A total of five treatment cycles were conducted in this experiment.
Click to Show/Hide
|
||||
| Response Description | Solasonine is involved in ferroptosis by suppressing TFAP2A-mediated transcriptional upregulation of OTUB1, thereby activating ubiquitinated degradation of SLC7A11 and promoting pancreatic cancer cell ferroptosis. | ||||
Signal transducer and activator of transcription 3 (STAT3)
Bavachin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | |||
rno-miR-23a-3p (miRNA)
GW4869
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Supraventricular tachycardia [ICD-11: BC81] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| rCFs (Rat cardiac fibroblasts) | |||||
| In Vivo Model |
Eighteen beagles, randomly divided into three groups, both sexes and an average age of 1 year, weighing 7.5 ± 1.5 kg, were used for the study as follows: Sham group (n = 6), Pacing group (n = 6), and GW4869 + Pacing group (n = 6). Each beagle canine was given an intramuscular injection of 25 mg/kg ketamine sulfate before being premedicated with pentobarbital sodium (30 mg/kg, intravenous injection) and ventilated with room air by a respirator (MAO01746, Harvard Apparatus Holliston, United States). Venous access was established to supply saline (50-100 mL/h) or pentobarbital sodium (2.5 mg/kg/h).
Click to Show/Hide
|
||||
| Response Description | The exosome inhibitor GW4869 reduced ferroptosis, fibrosis, and inflammation and improved histological and electrophysiological remodeling. Pacing-CF-exos highly expressed miR-23a-3p by informatics analysis and experimental verification. Inhibitor- miR-23a-3p protected h9c2 cells from ferroptosis accompanying with upregulation of SLC7A11. The development of atrial fibrillation (AF) in a persistent direction could be prevented by intervening with exosomal miRNAs to reduce oxidative stress injury and ferroptosis. | ||||
RNA-binding motif, single-stranded-interacting protein 1 (RBMS1)
Nortriptyline hydrochloride
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Doxorubicin (Dox)- inducible RBMS1 knockdown stable cells (3 x 106 ) were injected subcutaneously into the abdomen side of 6-week-old BALB/c nude mice (Vital River). Mice were fed either with sucrose water or sucrose water containing 0.1% doxycycline hyclate. H1299 vector, 4 H1299 pLKO.1 RBMS1 and H1299 pLKO.1 RBMS1/SLC7A11 cells (2.5 x 106 ) were injected subcutaneously into the abdomen side of 6-week-old BALB/c nude mice(Vital River). The xenograft tumour formation was monitored using callipers every 3 days.
Click to Show/Hide
|
||||
| Response Description | RBMS1 ablation inhibited the translation of SLC7A11, reduced SLC7A11-mediated cystine uptake, and promoted ferroptosis. Nortriptyline hydrochloride decreased the level of RBMS1, thereby promoting ferroptosis. Importantly, RBMS1 depletion or inhibition by nortriptyline hydrochloride sensitized radioresistant lung cancer cells to radiotherapy. | ||||
RAF proto-oncogene serine/threonine-protein kinase (RAF1)
Tetraarsenic tetrasulfide
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| In Vitro Model | NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| Response Description | On H23 cells treated with realgar, the expression of GPX4, SCL7A11 decreased while ACSL4 expression increased; this effect could also be amplified by Sorafenib. In conclusion, the present study indicated that realgar may induce ferroptosis by regulating the Raf, and hence plays a role in antiKRAS mutant lung cancer. | |||
Protein FAM98A (FAM98A)
Metformin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| LoVo cells | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| Caco-2 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| HCT 15 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0292 | ||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| FHC cells | Normal | Homo sapiens | CVCL_3688 | ||
| In Vivo Model |
A total 1 x 106 SW620-vector or SW620-FAM98A cells were suspended in 100 ul PBS and injected s.c. into the back of 4- to 6-week-old male BALB/cnude mice (Laboratory Animal Unit, Southern Medical University, China). The sizes of the resulting tumors were measured weekly. Tumor volumes were calculated as follows: total tumor volume (mm3) = (Length x Width2)/2, where Length is the longest length. When the tumor sizes reached about 200 mm3, nude mice in the four groups were given PBS or 5-FU treatment (30 mg/kg, intraperitoneal injection, twice a week), respectively. Nude mice were maintained in a barrier facility in racks filtered with a high-efficiency particulate air filter.
Click to Show/Hide
|
||||
| Response Description | The expressions of FAM98A and SLC7A11 were also downregulated after metformin treatment. And FAM98A is predominantly expressed in the colorectal cancer tissues and high FAM98A expression is usually accompanied by the high expression of SLC7A11, which usually means ferroptosis resistance. | ||||
NF-kappa-B inhibitor alpha (NFKBIA)
RSL3
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
Female B-NDG mice (4-6 weeks old, 16-20 g) were purchased from Biocytogen (Biocytogen Jiangsu Co., Ltd., Jiangsu, China) and housed under specific pathogen-free conditions. 5 x 106 U87 cells were resuspended in 200 uL PBS buffer and then inoculated into the left hind limb of each mouse. Once tumor volumes reached >=50 mm3, the mice were randomly divided into four groups (n = 5): the control, RSL3-only, BAY-only, and RSL3 plus BAY groups. Chemicals were administered through intratumor injection (100 mg/kg for RSL3 and 1 mg/kg for BAY 11-7082) biweekly for two weeks.
Click to Show/Hide
|
||||
| Response Description | NF-kB pathway activation is vital for RSL3-induced ferroptosis in glioblastoma cells both in vitro and in vivo. Furthermore, RNAi-mediated GPX4 silencing cannot trigger ferroptosis in glioblastoma cells unless the NF-kB pathway is activated simultaneously. Finally, NF-kB pathway activation promotes ferroptosis by downregulating the expression of ATF4 and SLC7A11. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Quercetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from Gempharmatech Co., Ltd (Changzhou, China). All mice were housed in cages with standard laboratory conditions: a consistent temperature of 24 , a 12 h light/dark cycle, and free access to water and food. The mice were randomized into four groups: 1) the KA group (n = 6), injected intraperitoneally with 20 mg/kg KA, as described in a previous study; while 2) the control group (n = 6), injected intraperitoneally with an equal volume of PBS; 3) the KA + QCT group (n = 6): this group was givenintragastric administrationof 50 mg/kg of QCT once daily for 21 days before KA injection based on the literature; and 4) the KA+ferrostatin1 (Fer-1) group (n = 6), injected intraperitoneally with a well-known ferroptosis inhibitor (3 mg/kg Fer-1) for 21 days before KA administration, as described in a previous study.
Click to Show/Hide
|
||||
| Response Description | The association between the Nrf2-mediated ferroptosis pathway and seizures in a clinical setting. Quercetin effectively protects against seizure-induced neuron death in vivo and in vitro and alleviates cognitive function impairment via the SIRT1/Nrf2/SLC7A11/GPX4 pathway. | ||||
Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1)
Micafungin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
The surgical procedure for establishing the myocardial I/R injury rat model was carried out as we did before. Briefly, a left thoracotomy was performed in the fourth intercostal space and the heart was exposed via opening thepericardium. The left coronary artery was surrounded with a 4-0 silk suture and a snare was formed by passing both ends of the suture via a short polyethylene tubing. Blockage of the coronary artery was conducted via clamping the snare against the heart surface. Reperfusion was performed by release of the snare. The sham group conducted the same procedure but without ischemia (the snare was not tightened). To establish the I/R injury model, the rat hearts were subjected to 1 h-ischemia plus 3 h-reperfusion. At the end, the blood and hearts were collected for assay of the creatine kinase(CK) activity and infarct size to determine the success of I/R injury model. To explore the role of MALT1 in myocardial I/R injury the underlying mechanisms, three sets of experiment were performed.
Click to Show/Hide
|
||||
| Response Description | The inhibition of MALT1 can reduce ischemia/reperfusion-induced myocardial ferroptosis through enhancing the Nrf2/SLC7A11 pathway; and MALT1 may be used as a potential target to seek novel or existing drugs (such as micafungin) for treating myocardial infarction. | ||||
Mothers against decapentaplegic homolog 3 (SMAD3)
Formononetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPRTECs (Mouse primary renal tubular epithelial cells) | ||||
| In Vivo Model |
For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium(30 mg/kg). Then, UUO surgery orsham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10% DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10% DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO. For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg). Then, UUO surgery or sham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10 % DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10 % DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO.
Click to Show/Hide
|
||||
| Response Description | Formononetin (FN) alleviates chronic kidney disease (CKD) by impeding ferroptosis-associated fibrosis by suppressing the Smad3/ATF3/SLC7A11 signaling and could serve as a candidate therapeutic drug for CKD. In addition, FN also promoted the separation of the Nrf2/Keap1 complex and enhanced Nrf2 nuclear accumulation. | ||||
Mitogen-activated protein kinase 8 (MAPK8)
Seratrodast
[Discontinued in Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Drugs were dissolved in vehicle (0.1% DMSO + 20% PEG 300 + 0.5% CMC-Na + ddH2O). Mice in Control and PTZ groups were administered for five days with an equivalent volume of vehicle. PTZ-induced seizure model was done for the subsequent 1 h after the last administration of drugs. We performed a preliminary doseresponse trial, the dose of 60 mg/kg was established as being sufficient to trigger seizures with lower mortality and chosen as the optimal dose. One mouse in PTZ group was dead due to a severe seizure. At the end of the experiment, the mice were anesthetized or euthanized. For histopathological studies, the mice were anesthetized and intracardially perfused with 0.9% saline, followed by 0.4% paraformaldehyde for fixation of the brain. For immunoblot analysis, the hippocampus was rapidly isolated.
Click to Show/Hide
|
||||
| Response Description | Seratrodast could reduce lipid ROS production, regulate the system xc-/glutathione (GSH)/glutathione peroxidase 4 (GPX4) axis, and inhibit JNK (MAPK8) phosphorylation and p53 expression. JNK can directly or indirectly modulate the expression and activation of p53, which could regulate ferroptosis through inhibition of SLC7A11 transcription. Seratrodast increased the latency of seizures and reduced seizure duration in pentylenetetrazole-induced seizures. | ||||
Mitogen-activated protein kinase 1 (MAPK1)
Simvastatin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [14] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Corpus uteri cancer [ICD-11: 2C76] | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | Ishikawa cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_2529 |
| Response Description | Simvastatin has the potential to be a targeted drug for endometrial cancer (EC) treatment. Besides, the inhibition to the RAS/MAPK signaling pathway allows simvastatin to induce ferroptosis through up-regulating the level of ROS, MDA, Fe2+, and TRF1 (TF) and reducing the level of GSH, SLC7A11, and FPN in cells. | |||
LINC00618 (IncRNA)
Vincristine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [15] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Myeloid leukaemia [ICD-11: 2B33] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 |
| K-562 cells | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | |
| Response Description | LINC00618, is reduced in human leukemia and strongly increased by vincristine (VCR) treatment. Furthermore, LINC00618 promotes apoptosis by increasing the levels of BCL2-Associated X (BAX) and cleavage of caspase-3. LINC00618 also accelerates ferroptosis by increasing the levels of lipid reactive oxygen species (ROS) and iron, two surrogate markers of ferroptosis, and decreasing the expression of solute carrier family 7 member 11 (SLC7A11). | |||
Krueppel-like factor 4 (KLF4)
Polyphyllin III
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| HBL-100 cells | Normal | Homo sapiens | CVCL_4362 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| MDA-MB-453 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | ||
| In Vivo Model |
MDA-MB-231 xenografts were established in 5 week-old BALB/C nude mice (Shanghai SLAC Laboratory Animal Corporation) by inoculating 1 x 106 cells mixed with Matrigel (BD Biosciences) at a 1:1 ratio into the abdominal mammary fat pad. When the tumor reached 50-100 mm3, the mice were assigned randomly into different treatment groups (DMSO, PPIII, SAS, and PPIII + SAS groups), and each group consisted of 5 mice. PPIII (5 mg/kg/day) and SAS (200 mg/kg/day) were dissolved in dimethyl sulfoxide (DMSO), diluted in PBS, and then intraperitoneally injected into mice at a dose of 10 ml/kg/d once a day.
Click to Show/Hide
|
||||
| Response Description | Polyphyllin III, which is a major saponin extracted fromParis polyphyllarhizomes, exerted its proliferation-inhibitory effect on MDA-MB-231 triple-negative breast cancer cells mainly through ACSL4-mediated lipid peroxidation elevation and ferroptosis induction. Polyphyllin III treatment also induced KLF4-mediated protective upregulation of xCT(SLC7A11), which is the negative regulator of ferroptosis. | ||||
Krueppel-like factor 15 (KLF15)
Elabela
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [17] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | rAFs (Rat adventitial fibroblasts) | |||
| Response Description | KLF15 siRNA impeded the beneficial roles of elabela (ELA) in DOX-pretreated rat aortic AFs by suppressing the Nrf2/SLC7A11/GPX4 signaling. In conclusion, ELA prevents DOX-triggered promotion of cytotoxicity, and exerts anti-oxidative and anti-ferroptotic effects in rat aortic AFs via activation of the KLF15/GPX4 signaling, indicating a promising therapeutic value of ELA in antagonizing DOX-mediated cardiovascular abnormality and disorders. | |||
hsa-miR-489-3p (miRNA)
Levobupivacaine
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [18] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | |
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| In Vivo Model |
Ten SCID nude mice aged 6-8 weeks were purchased from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), and subcutaneously injected with SGC7901 cells (5 x 106 cells per mouse) in left back. One week after feeding, the mice were randomly divided into two groups, the control and treatment group. For the next 25 days, the mice in treatment group were injected with erastin (15 mg/kg intraperitoneally) or co-treated with 40 mol/kg body weight of levobupivacaine once a day. The body weight and tumor size were measured every 3 days.
Click to Show/Hide
|
||||
| Response Description | Levobupivacaine-upregulated miR-489-3p enhanced ferroptosis of gastric cancer cells by targeting SLC7A11. MiR-489-3p was involved in levobupivacaine-induced ferroptosis of gastric cancer cells. Levobupivacaine/miR-489-3p/SLC7A11 axis attenuates gastric cancer cell proliferationin vitro. | ||||
hsa-miR-382-5p (miRNA)
Lidocaine
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| In Vivo Model |
SPF-level male nude mice aged 56weeks and weighted around 20 g were purchased from Vitalriver (China). All mice were maintained in a 12-hour circadian rhythm, and had free access to water and food. Cancer cells were subcutaneously injected into the right flank of mice. Lidocaine was administrated to mice at a dose of 1.5 mg per kg injected through the vail tails. For control group, the mice were treated with saline. Tumor volume and mice body weight were monitored every 5 days.
Click to Show/Hide
|
||||
| Response Description | The ovarian and breast cancer cell proliferation was suppressed while cell apoptosis was induced by lidocaine in vitro. Lidocaine attenuated invasion and migration of ovarian and breast cancer cells as well. Regarding the mechanism, lidocaine downregulated solute carrier family 7 member 11 (SLC7A11) expression by enhancing microRNA-382-5p (miR-382-5p) in the cells. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| In Vivo Model |
SPF-level male nude mice aged 56weeks and weighted around 20 g were purchased from Vitalriver (China). All mice were maintained in a 12-hour circadian rhythm, and had free access to water and food. Cancer cells were subcutaneously injected into the right flank of mice. Lidocaine was administrated to mice at a dose of 1.5 mg per kg injected through the vail tails. For control group, the mice were treated with saline. Tumor volume and mice body weight were monitored every 5 days.
Click to Show/Hide
|
||||
| Response Description | The ovarian and breast cancer cell proliferation was suppressed while cell apoptosis was induced by lidocaine in vitro. Lidocaine attenuated invasion and migration of ovarian and breast cancer cells as well. Regarding the mechanism, lidocaine downregulated solute carrier family 7 member 11 (SLC7A11) expression by enhancing microRNA-382-5p (miR-382-5p) in the cells. | ||||
hsa-miR-122-5p (miRNA)
Isorhynchophylline
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| p53 signaling pathway | hsa04115 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (SD rats, weighing 250-300 g) aged 11-12 weeks were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All 96 rats were randomly divided into four groups of 24 rats each: Sham group, Sham + IRN (30 mg/Kg) group, ICH group, and ICH + IRN (30 mg/Kg) group. The rats in sham group were injected with PBS solution, and the Sham + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection) after the sham operation. After ICH, the rats in ICH group were injected with PBS solution, and the ICH + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection).
Click to Show/Hide
|
||||
| Response Description | Isorhynchophylline (IRN) decreased ferroptosis and lipid ROS level, upregulated the expression of miR-122-5p and SLC7A11 mRNA, and inhibited TP53 expression. In conclusion, IRN protects neurocyte from intracerebral hemorrhage (ICH)-induced ferroptosis via miR-122-5p/TP53/SLC7A11 pathway, which may provide a potential therapeutic mechanism for ICH. | ||||
Gap junction alpha-1 protein (GJA1)
GAP 27
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [21] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Glutathione metabolism | hsa00480 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Thirty-two male C57BL/6 mice (20 ± 2) g (Beijing Weitonglihua Experimental Animal Technology Co., Ltd.) were bred in individually ventilated cages (IVC) at SPF conditions, kept on a 12 h light/dark cycle, relative humidity conditions (40-70%) and controlled temperature (24 ± 2 ). After one-week acclimation mice were divided randomly into four groups: control group, cisplatin group (20 mg/kg cisplatin dissolved in saline), cisplatin + Fer-1 group (5 mg/kg Fer-1 dissolved in DMSO), and cisplatin + gap27 group (35 ug/kg gap27 dissolved in DMSO). There were eight animals in each group and 20 mg/kg cisplatin was given to each animal once by intraperitoneal injection except mice in the control group. Fer-1 and gap27 was administered 1 h before the injection of cisplatin.
Click to Show/Hide
|
||||
| Response Description | Downregulation of Cx43 expression by gap27 reduced acute kidney injury in the animal model by inhibiting cisplatin-induced ferroptosis. Therefore, our results indicated that downregulation of Cx43 can inhibit ferroptosis by restoring the level of SLC7A11 in the system xctransporter and alleviate cisplatin-induced acute kidney injury. | ||||
Endothelial PAS domain-containing protein 1 (EPAS1)
D-Mannose
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [22] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Degenerative arthritis [ICD-11: FA05] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hCDs (Chondrocytes) | ||||
| In Vivo Model |
C57BL/6 J mice (8 weeks old, female) were purchased from Dossy Experimental Animal Limited Company (Chengdu, China). For surgery, mice were anaesthetized with pentobarbital sodium (100 mg/kg, injected intraperitoneally) and subjected to unilateral ACLT procedures. 28 The sham group received a skin incision and suturing without patellar dislocation or ligament transection. For virus injection, mice were intraarticularly injected with 1 x 109 pfu (8 ul) of mock or AdEpas1 virus after one week of surgery. For Fer1 (MCE, Monmouth Junction, HY100579) injection, mice were intraarticularly injected with 1 mg/kg Fer1 or with vehicle two weeks after surgery, the injection was repeated once a week.
Click to Show/Hide
|
||||
| Response Description | D-mannose alleviates osteoarthritis (OA) progression by suppressing HIF-2a-mediated chondrocyte sensitivity to ferroptosis. Overexpression of HIF-2a in chondrocytes by Ad- Epas1 intra-articular injection abolished the chondroprotective effect of D-mannose during OA progression and eliminated the role of D-mannose as a ferroptosis suppressor. Also, the RNA and protein levels of the two key ferroptosis suppressors, Gpx4 and Slc7a11, were increased in Dmannosetreated chondrocytes. | ||||
Cyclic GMP-AMP synthase (CGAS)
Niraparib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cytosolic DNA-sensing pathway | hsa04623 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| MC-38 cells | Colon adenocarcinoma | Homo sapiens | CVCL_B288 | ||
| In Vivo Model |
Six-week-old male BALB/c athymic nude mice were purchased from the Experimental Animal Center of Peking (Beijing, China). Stable cells (5 x 106) were seeded into the right flanks of the mice. After the xenografts had grown to 200 mm3, saline as a vehicle or sorafenib (30 mg/kg) was administered by gavage every day, and the mice were euthanized by the cervical dislocation method five weeks later. Before sacrifice, the tumor sizes and body weights were measured twice per week. The tumor volume (V) was calculated as follows: (L x W2)/2 (length, L, and width, W). The xenografts were excised and further assessed.
Click to Show/Hide
|
||||
| Response Description | Niraparib, a widely used PARPi, augmented cGAS-mediated ferroptosis and immune activation. In colorectal cancer models, cGAS signaling exerts tumor control via ATF3SLC7A11GPX4-mediated ferroptosis and IFNCD8 T cell-mediated antitumor immune response. | ||||
Cyclic AMP-dependent transcription factor ATF-3 (ATF3)
Brucine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [24] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U118 cells | Astrocytoma | Homo sapiens | CVCL_0633 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| In Vivo Model |
The athymic BALB/c nude mice (4 weeks; 20-22 g; Beijing Vital River Laboratory Animal Technology Company, China) were housed in a specific pathogen-free environment under a 12-h lightdark cycle with free access to food and water. The animals were allowed to acclimatize to their surroundings for 3 days. U87 cells (1 x 106) in the logarithmic growth phase in 100 uL PBS were subcutaneously injected into the right flank. Therapeutic experiments were started when the tumor reached around 150 mm3 after about 10 days. Mice were allocated to receive intraperitoneal injections of vehicle (control group, n = 6) or 40 mg/kg bodyweight (n = 6) in the same volume (50 uL) once a day for 13 times.
Click to Show/Hide
|
||||
| Response Description | Brucine inhibited glioma cell growth in vitro and in vivo, and brucine induced ATF3 upregulation and translocation into nuclei via activation of ER stress. ATF3 promoted brucine-induced H2O2 accumulation via upregulating NOX4 and SOD1 to generate H2O2 on one hand, and downregulating catalase and xCT (SLC7A11) to prevent H2O2 degradation on the other hand. | ||||
Formononetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mPRTECs (Mouse primary renal tubular epithelial cells) | ||||
| In Vivo Model |
For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium(30 mg/kg). Then, UUO surgery orsham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10% DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10% DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO. For UUO-induced CKD, the mice were randomly assigned into four groups (n = 6 per group): UUO, UUO + FN, UUO + VST, and Sham. The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (30 mg/kg). Then, UUO surgery or sham operation was performed as previously described. Mice in the UUO + FN group were orally administrated with 40 mg/kg/day FN (dissolved in 10 % DMSO). For positive control, mice in UUO + VST group were orally treated with 20 mg/kg/day VST (dissolved in 10 % DMSO). Mice in the UUO and Sham groups were given equivalent solvent by oral. All mice were sacrificed 7 days post-UUO.
Click to Show/Hide
|
||||
| Response Description | Formononetin (FN) alleviates chronic kidney disease (CKD) by impeding ferroptosis-associated fibrosis by suppressing the Smad3/ATF3/SLC7A11 signaling and could serve as a candidate therapeutic drug for CKD. In addition, FN also promoted the separation of the Nrf2/Keap1 complex and enhanced Nrf2 nuclear accumulation. | ||||
Cellular tumor antigen p53 (TP53)
Pseudolaric acid B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [25] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-87MG cells | Glioblastoma | Homo sapiens | CVCL_0022 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| SHG-44 cells | Astrocytoma | Homo sapiens | CVCL_6728 | ||
| In Vivo Model |
Twenty athymic BALB/c nude mice (aged 4 weeks, weight 20-22 g, from Shanghai laboratory animal Center, Shanghai, China) were housed in a specific pathogen-free environment. A total of 1 x 106 logarithmically growing C6 cells in 100 uL of PBS were subcutaneously injected into the right flank of each mouse. Therapeutic experiments were started when the tumor reached about 150 mm3 after about 7 days. The mice were allocated to receive intraperitoneal injections of vehicle (control group, n = 5/group), PAB at the dosage of 10 mg/kg body weight (n = 10/group) and 20 mg/kg body weight (n = 10/group) in the same volume 50 uL once a days for 8 times.
Click to Show/Hide
|
||||
| Response Description | Pseudolaric acid B (PAB) improved intracellular iron by upregulation of transferrin receptor. The increased iron activated Nox4, which resulted in overproduction of H2O2and lipid peroxides. Moreover, PAB depleted intracellular GSH via p53-mediated xCT (SLC7A11) pathway, which further exacerbated accumulation of H2O2and lipid peroxides. Thus, PAB triggers ferroptosis in glioma cells and is a potential medicine for glioma treatment. | ||||
Kayadiol
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [26] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | T-cell lymphoma [ICD-11: 2B01] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | YT cells | Natural killer cell lymphoblastic leukemia | Homo sapiens | CVCL_1797 |
| hPBLs (Human peripheral blood lymphocytes) | ||||
| Response Description | Kayadiol decreased the expression of SLC7A11 and GPX4, the negative regulatory proteins for ferroptosis. And p53 was the key mediator of kayadiol-induced ferroptosis by SLC7A11/GPX4 axis through p53 knockout experiments. Kayadiol can serve as an effective alternative in the treatment of NK/T cell lymphoma. | |||
Bavachin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | |||
Tanshinone IIA
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [27] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| NCI-N87 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1603 | ||
| In Vivo Model |
All mice were housed under a setting of 12-h light/dark cycle at 22 ± 1, 55% humidity and fed with water and food provided at regular time. During the entire maintenance period, all mice were permitted free cage activity without joint immobilization. The initial body weights of the mice were between 20 and 23 grams. After subcutaneous injection of 2 x 106 BGC-823 gastric cancer cells into the back of NOD-SCID mice, the mice were treated with or without Tan IIA (50 mg/kg) or Tan IIA in combination with Fer-1 (50 mg/kg). Tan IIA was diluted in DMSO:Methanol:Hydroxypropyl-b-cydodextrin (HP-b-CD) = 1:1:1. Fer-1 was also dissolved in DMSO:Methanol:HP-b-CD. Seven days after BGC-823 gastric cancer cells injection, intraperitoneal injection with Tan IIA was carried out every other day followed by killing at day 22 of tumor cell inoculation. All mice were killed by dislocation of the cervical vertebrae. Before killing, the tumor volume was measured every 3 days. All experiments were carried out using six mice each group in three independent experiments of a time-dependent manner with three time points.
Click to Show/Hide
|
||||
| Response Description | Tanshinone IIA increased lipid peroxidation and up-regulated Ptgs2 and Chac1 expression, two markers of ferroptosis. In addition, Tan IIA also up-regulated p53 expression and down-regulated xCT (SLC7A11) expression. Therefore, Tan IIA could suppress the proliferation of gastric cancer via inducing p53 upregulation-mediated ferroptosis. | ||||
Gambogenic Acid
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [28] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Melanoma [ICD-11: 2C30] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell adhesion molecules | hsa04514 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
| In Vitro Model | A-375 cells | Amelanotic melanoma | Homo sapiens | CVCL_0132 |
| A2058 cells | Amelanotic melanoma | Homo sapiens | CVCL_1059 | |
| Response Description | Gambogenic acid (GNA) significantly inhibited the invasion, migration and EMT in melanoma cells, and these cells exhibited small mitochondrial wrinkling (an important feature of ferroptosis). GNA upregulated the expression of p53, solute carrier family 7 member 11 (SLC7A11) and glutathione peroxidase 4 (GPX4) in the model cells, contributing to the mechanisms underlying GNA-induced ferroptosis. | |||
Cyperquat
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [29] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | As a classic drug employed inin vitromodels of Parkinson's disease, 1-methyl-4-phenylpyridinium (MPP) can induce senescence in PC12 cells. The expression of the ferroptosis-related proteins ASCL4 was upregulated and FTH1 was downregulated, which promoted accumulation of lipid peroxides and eventually led to ferroptosis. By rescuing MPP-induced ferroptosis, cell senescence could be inhibited, and its molecular mechanism was related to a p53/SLC7A11/GPX4 signaling pathway. | |||
Seratrodast
[Discontinued in Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Drugs were dissolved in vehicle (0.1% DMSO + 20% PEG 300 + 0.5% CMC-Na + ddH2O). Mice in Control and PTZ groups were administered for five days with an equivalent volume of vehicle. PTZ-induced seizure model was done for the subsequent 1 h after the last administration of drugs. We performed a preliminary doseresponse trial, the dose of 60 mg/kg was established as being sufficient to trigger seizures with lower mortality and chosen as the optimal dose. One mouse in PTZ group was dead due to a severe seizure. At the end of the experiment, the mice were anesthetized or euthanized. For histopathological studies, the mice were anesthetized and intracardially perfused with 0.9% saline, followed by 0.4% paraformaldehyde for fixation of the brain. For immunoblot analysis, the hippocampus was rapidly isolated.
Click to Show/Hide
|
||||
| Response Description | Seratrodast could reduce lipid ROS production, regulate the system xc-/glutathione (GSH)/glutathione peroxidase 4 (GPX4) axis, and inhibit JNK (MAPK8) phosphorylation and p53 expression. JNK can directly or indirectly modulate the expression and activation of p53, which could regulate ferroptosis through inhibition of SLC7A11 transcription. Seratrodast increased the latency of seizures and reduced seizure duration in pentylenetetrazole-induced seizures in Epilepsy. | ||||
Isorhynchophylline
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| p53 signaling pathway | hsa04115 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (SD rats, weighing 250-300 g) aged 11-12 weeks were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). All 96 rats were randomly divided into four groups of 24 rats each: Sham group, Sham + IRN (30 mg/Kg) group, ICH group, and ICH + IRN (30 mg/Kg) group. The rats in sham group were injected with PBS solution, and the Sham + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection) after the sham operation. After ICH, the rats in ICH group were injected with PBS solution, and the ICH + IRN (30 mg/Kg) group was received an equal amount of 30 mg/Kg IRN solution (intra-peritoneal injection).
Click to Show/Hide
|
||||
| Response Description | Isorhynchophylline (IRN) decreased ferroptosis and lipid ROS level, upregulated the expression of miR-122-5p and SLC7A11 mRNA, and inhibited TP53 expression. In conclusion, IRN protects neurocyte from intracerebral hemorrhage (ICH)-induced ferroptosis via miR-122-5p/TP53/SLC7A11 pathway, which may provide a potential therapeutic mechanism for ICH. | ||||
Ferric citrate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [30] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Sarcopenia [ICD-11: FB32] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Glutathione metabolism | hsa00480 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| In Vivo Model |
The 8-week- and 40-week-old male SAMP8 mice were purchased from the model animal research center of Zhishan Institute of Healthcare Research Co., Ltd. (Beijing, China). All the mice were kept in an SPF grade animal facility at 24 with a relative humidity of 50%-60%, and in a light/dark cycle of 12 h/12 h.
Click to Show/Hide
|
||||
| Response Description | Ferric citrate induced ferroptosis in C2C12 cells, as well as impaired their differentiation from myoblasts to myotubes. Iron overload upregulated the expression of P53, which subsequently repressed the protein level of Slc7a11 (solute carrier family 7, member 11), a known ferroptosis-related gene. Targeting iron accumulation and ferroptosis might be a therapeutic strategy for treating sarcopenia. | ||||
CD44 antigen (CD44)
Sodium butyrate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [31] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | FHC cells | Normal | Homo sapiens | CVCL_3688 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| In Vivo Model |
Six-week-old male C57BL/6J mice were purchased from the Medical Laboratory Animal Center of Guangdong Province (Foshan, China). Forty-five C57BL/6L mice were randomized into 4 groups after 1 week of adaptive feeding: (1) Control group (n = 9); (2) AOM/DSS group (n = 12); (3) AOM/DSS + NaB (orally) group (n = 12); 4) AOM/DSS + NaB (intraperitoneal injection) group (n = 12). The Control group received an intraperitoneal injection of saline solution beginning on day 1, and received sterile drinking water throughout the study. Other three groups received an intraperitoneal injection of 10 mg/kg AOM (Sigma Aldrich) beginning on day 1, and received drinking water containing 2.5% DSS at the second and eighth weeks (2% DSS in the fifth week). Besides, 0.1 M NaB (Sigma Aldrich) was given in drinking water during the whole experiment process in AOM/DSS + NaB (p.o.) group, while AOM/DSS + NaB (i.p.) group was injected intraperitoneally (IP) with 1 g/kg NaB per day.
Click to Show/Hide
|
||||
| Response Description | Sodium butyrate (NaB) induces ferroptosis in colorectal cancer cells through the CD44/SLC7A11 signaling pathway and has synergistic effects with Erastin. | ||||
Bromodomain-containing protein 4 (BRD4)
JQ1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [32] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| In Vivo Model |
Female athymic BALB/c nude mice (4-6-week old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Approximately 1 x 107 cells (A549) in 200 uL of serum-free medium and Matrigel solution were injected directly into the right axilla of each mouse. Tumor growth was measured with calipers every 3 days.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis was induced under (+)-JQ1 treatment and BRD4 knockdown, indicating that (+)-JQ1 induces ferroptosis via BRD4 inhibition in breast adenocarcinoma. In addition, expression of the ferroptosis-associated genes GPX4, SLC7A11, and SLC3A2 was downregulated under (+)-JQ1 treatment. Moreover, JQ1 treatment and BRD4 knockdown led to decreased FTH1 expression. | ||||
5'-AMP-activated protein kinase catalytic subunit alpha-2 (PRKAA2)
Sulfasalazine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Colon cancer [ICD-11: 2B90] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 HCT116, CX-1, or HT1080 cells in 100 ul phosphate buffered saline (PBS; Thermo Fisher Scientific, AM9625) were injected subcutaneously right of the dorsal midline in athymic nude immunodeficient mice (six- to eight-week-old, female). To generate orthotopic tumors, 1 x 106 KPC cells in 10 ul PBS were surgically implanted into the pancreases of immunocompetent C57BL/6J mice (six- to eight-week-old, female).
Click to Show/Hide
|
||||
| Response Description | BECN1 plays a novel role in lipid peroxidation that could be exploited to improve anticancer therapy by the induction of ferroptosis. Mechanistically, phosphorylation of BECN1 at Ser90/93/96 by PRKAA/ AMPK contributes to the formation of a BECN1-SLC7A11 complex and system Xc-inhibition. Knockdown of BECN1 by shRNA inhibits ferroptosis induced by system X-c- inhibitors (e.g., erastin, sulfasalazine, and sorafenib) in Colon carcinoma. | ||||
5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1)
Fingolimod
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [34] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Multiple myeloma [ICD-11: 2A83] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| AMPK signaling pathway | hsa04152 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | U266B1 cells | Plasma cell myeloma | Homo sapiens | CVCL_0566 |
| RPMI-8226 cells | Plasma cell myeloma | Homo sapiens | CVCL_0014 | |
| Response Description | Multiple myeloma (MM) is the second hematological plasma cell malignany and sensitive to fingolimod (FTY720), a novel immunosuppressant. Fingolimod (FTY720) can activate PP2A subunit C and dephosphorylate AMPKa, and then inhibit the expression of SLC7A11 and GPX4. In conclusion, FTY720 induces ferroptosis and autophagy through the PP2A/AMPK pathway. | |||
Unspecific Regulator
Indole-3-pyruvic acid
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [105] | |||
| Responsed Disease | Acute myeloid leukaemia [ICD-11: 2A60] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 |
| MLL/AF4+ RS4 cells | Adult B acute lymphoblastic leukemia | Homo sapiens | CVCL_0093 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| NIH3T3 cells | Normal | Mus musculus | CVCL_0594 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| Response Description | Indole-3-pyruvate (I3P) suppresses ferroptosis by direct free radical scavenging and through the activation of an anti-oxidative gene expression program in Childhood acute monocytic leukemia. And I3P elevated the activation of compensatory gene expression as indicated by increased protein amounts of SLC7A11 and HO-1, two important target genes of anti-oxidative stress pathways. | |||
Imidazole ketone erastin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [106] | ||||
| Responsed Disease | Diffuse large B-cell lymphoma [ICD-11: 2A81] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SU-DHL-1 cells | Anaplastic large cell lymphoma | Homo sapiens | CVCL_0538 | |
| SU-DHL-2 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_9550 | ||
| SU-DHL-6 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_2206 | ||
| SU-DHL-8 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_2207 | ||
| SU-DHL-10 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1889 | ||
| SU-DHL-16 cells | B-cell non-Hodgkin lymphoma | Homo sapiens | CVCL_1890 | ||
| A3/Kawakami cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1062 | ||
| OCI-LY8 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_8803 | ||
| U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | ||
| DoHH2 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1179 | ||
| HBL-1 cells | Non-Hodgkin lymphoma | Homo sapiens | CVCL_M572 | ||
| U-2932 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1896 | ||
| SU-DHL-7 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_4380 | ||
| SU-DHL-9 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_4379 | ||
| A4/Fukuda cells | B acute lymphoblastic leukemia | Homo sapiens | CVCL_1064 | ||
| WSU-NHL cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1793 | ||
| SU-DHL-5 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1735 | ||
| Karpas-422 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1325 | ||
| In Vivo Model |
NOD/SCID mice (12-weeks of age and ~28 g weight) were weighed before injection and divided into groups of 3 mice per cage. Mice were dosed using three different routes, IP and PO with 50 mg/kg IKE, and IV with 17 mg/kg IKE. Samples were collected at 0, 1, 3, 4, and 8 h from three mice per time point. Additionally, three mice per group were used as controls by administration with equivalent amount of vehicle 1 by IP, PO, and IV, and samples were collected at 8 h. At the appropriate time, mice were sacrificed by CO2 asphyxiation for 3 min and ~0.5 mL of blood was collected via cardiac puncture.
Click to Show/Hide
|
||||
| Response Description | Imidazole ketone erastin (IKE) is a potent, selective, and metabolically stable system xc- (SLC7A11)inhibitor. In addition, biodegradable polyethylene glycol-poly(lactic-co-glycolic acid) nanoparticles were employed to aid in IKE delivery and exhibited reduced toxicity compared with free IKE in a diffuse large B cell lymphoma (DLBCL) xenograft model. | ||||
Tirapazamine
[Phase 3]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [107] | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| In Vitro Model | 143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 |
| MNNG/HOS Cl #5 cells | Osteosarcoma | Homo sapiens | CVCL_0439 | |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| Response Description | SLC7A11 overexpression could restored the proliferation and migration abilities inhibited by Tirazamine. Thus, TPZ could inhibit the proliferation and migration of osteosarcoma cells, and induce ferroptosis in part through inhibiting SLC7A11. | |||
Berberine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [108] | ||||
| Responsed Disease | Gastrointestinal cancer [ICD-11: 2B5B] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| TMK-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4384 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
Five-week-old male BALB/c mice were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained in a pathogen-free animal facility under a 12 h light/dark cycle in a temperature (22 )- and humidity-controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following the current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval no. 12924, 5 November 2020). Animals were acclimated to their housing for seven days before the start of the experiment. For the peritoneal dissemination tumor model, CT26 cancer cells (1 x 107 in 0.2 mL per mouse) were injected into the mouse peritoneal cavity. To measure tumor weight, mice were euthanized on Day 12 and the tumors were excised, while the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, with gross removal of non-tumor tissues. The largest tumor was formed on the diaphragm, and paraffin-embedded sections of the excised diaphragmatic tumor were prepared and stained with hematoxylin-eosin. BBR was diluted with distilled water to produce a final concentration of 48 mg/mL. The solutions were ultrasonically treated for 1 h, and fully vortexed for 30 min. BBR solution was administered by free drinking. The intake calculated from the amount of water consumed was 15.2 mg/kg body weight/day.
Click to Show/Hide
|
||||
| Response Description | Berberine induces apoptosis and ferroptosis by inhibiting mitochondrial complex I and promoting autophagy, leading to combined cell death in the gastrointestinal cancer and suppressing stemness. BBR induces cell death in GIC cells accompanied by increased mitochondrial superoxide and ACSL4 levels, decreased SLC7A11, and impaired antioxidant mechanisms, indicated by decreased GPX4 expression and decreased GSH. | ||||
Atranorin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [109] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hGCCs (Gastric cancer cells) | ||||
| In Vivo Model |
NOD-scid mice (NOD.CB17-Prkdcscid/NcrCrl) aged 6-7 weeks and weighing 20-22 g were used in the experiment. The animal study was performed at the Shanghai University of Traditional Chinese Medicine with approval from the Institutional Animal Care and Use Committee in accordance with the institutional guidelines. All mice were randomly divided into two groups, and each group consisted of four mice. In experimental group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul Atranorin@SPION (10 mg/kg) every 2 days. In control group, approximately 1 x 105 GCSCs in logarithmic growth phase were harvested and inoculated subcutaneously into NOD-scid mice, and intraperitoneal injection of 100 ul SPION (10 mg/kg) alone every 2 days. After 2 months, the mice were sacrificed, and their tumors were excised.
Click to Show/Hide
|
||||
| Response Description | Atranorin@SPIONs significantly reduced the 5-hydroxymethylcytidine modification level of GPX4 and SLC7A11 mRNA 3' untranslated region in gastric cancer cells. This study revealed the molecular biological mechanism by which Atranorin@SPION inhibit the in vitro and in vivo activity of GCSCs, that is, Atranorin@SPION reduced the expression of members of the Xc-/GPX4 axis and reduced their mRNA 5-hydroxymethylcytidine modification, finally induced ferroptosis of GCSCs. | ||||
Actinidia chinensis Planch
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [110] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| In Vitro Model | HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| In Vivo Model |
Wild type AB strain of zebrafish (Danio rerio) was obtained from Southern Medical University. The HGC-27 cells labeled with EGFP were resuspended in PBS in the concentration of 5*107/ml. 10 nl cell suspension containing approximately 300 cells were loaded into capillary needles and injected into the abdominal perivitelline space of zebrafish embryos by a nanoliter injector (Narishige, Tokyo, Japan). After injection, the tumor-bearing embryos were transferred into a 24-well plate and acclimated in embryo water at 35 for 24 h and then incubated at 0, 90, 180 mg/ml ACP decoction for 48 h.
Click to Show/Hide
|
||||
| Response Description | Actinidia chinensis Planch (ACP) increased the accumulation of ROS via inhibited the glutathione peroxidase 4 (GPx4) and xCT (SLC7A11) proteins, while were inhibited by Ferrostatin-1 (Fer-1) significantly. In conclusion, ACP was a promising antineoplastic agent for the treatment of gastric cancer by regulating apoptosis, ferroptosis and mesenchymal phenotype. | ||||
Pt3R5G
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [111] | ||||
| Responsed Disease | Colon cancer [ICD-11: 2B90] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | RKO cells | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| NCM460 cells | Normal | Homo sapiens | CVCL_0460 | ||
| In Vivo Model |
Five-week-old male BALB/c-nude mice from Central Laboratory of Animal, Xi'an Jiaotong University Health Science Center were housed 4 per cage under controlled temperature (23 ± 2 ), a 12 h/12 h light/dark cycle with ad libitum access to food and water and specific pathogen-free conditions. Twelve BALB/c-nude mice were randomly divided into three groups (control, 25 mg/kg, 50 mg/kg). 1 x 106 RKO cells were subcutaneously injected into either side of the same mice dorsal flanks. After 14 days, animals then received Pt3R5G (25 mg/kg, 50 mg/kg) byintraperitoneal injectionfor 15 days. The weight of mouse and tumor nodules sizer were measured every 3 days for 29 days.
Click to Show/Hide
|
||||
| Response Description | Pt3R5G significantly down-regulated SLC7A11 expression and up-regulated TFR1 in RKO cells. Pt3R5G inhibits cell proliferation through inducing ferroptosis by down-regulating SLC7A11 in colon cancer. | ||||
Propofol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [112] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
CT26 (1 x 105 cells/100 uL) were injected into thetail veinof male BALB/c mice. Then the mice were randomly divided into saline, vehicle, propofol, and sevoflurane groups (n = 5 per group). Saline, fat emulsion (as vehicle control of propofol), and propofol (200 mg/kg) were intraperitoneally injected, while sevoflurane (1.8-2.0%) was administered by inhalation for 2 h. In another set of experiments, coloncancer cells (CT26 and HT29) were pretreated with two doses of propofol (5 ug/mL, 10 ug/mL) or fat emulsion (as vehicle control of propofol) in a cell culture medium for 2 h. After washing with phosphate-buffered saline (PBS), the cells were harvested,counted on a hemacytometer and prepared. Cells (CT26: 1 x 105 cells/100 uL, HT29: 1 x 106 cells/100 uL) were finnally injected into mice through the tail vein.Lung metastasiswas detected via hematoxylin and eosin staining (HE) or ex vivo bioluminescence imaging.
Click to Show/Hide
|
||||
| Response Description | Further studies showed that propofol treatment upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2) and its downstream target genes, including HO-1, NQO1, and SLC7A11. Collectively, we demonstrated the risk of a specific type of anesthetic, propofol, in promoting colorectal cancer cell metastasis through Nrf2-mediated ferroptosis inhibition. | ||||
2-Imino-6-methoxy-2H-chromene-3-carbothioamide
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [113] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| mTOR signaling pathway | hsa04150 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| In Vivo Model |
Five-week-old female BALB/c nude mice were purchased from Beijing Weitong Lihua Experimental Animal Technical Co., Ltd. (Beijing, China). The mice were randomly assigned to the treatment and control groups until the tumor size reached approximately 100 mm3. The mice in the treatment group were injected with 0.174 mg/mL IMCA (100 uL), and those in the control group were injected with an equal volume of normal saline. The nude mice were euthanized, and samples were obtained from their tumor, heart, hepar, kidney, and blood after 33 days of IMCA treatment.
Click to Show/Hide
|
||||
| Response Description | 2-imino-6-methoxy-2H-chromene-3-carbothioamide (IMCA) significantly induced the ferroptosis of colorectal cancer cells. Mechanistically, IMCA downregulated the expression of SLC7A11 and decreased the contents of cysteine and glutathione, which resulted in reactive oxygen species accumulation and ferroptosis. | ||||
Talaroconvolutin A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [114] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| In Vivo Model |
5 x 106 HCT116 cells were inoculated subcutaneously in the underarm of Balb/c nude female mice (5-week old). The inoculated mice were randomly divided into two groups (6 mice each group). When the tumor reached 300 mm3, the drug group was given TalaA intraperitoneally at a dose of 6.0 mg/kg, and the control group was given the same dose of cosolvent-corn oil. The drug (or cosolvent) was injected every 2 days. Body weight and tumor volume were measured every 2 days.
Click to Show/Hide
|
||||
| Response Description | Talaroconvolutin A (TalaA) downregulated the expression of the channel protein solute carrier family 7 member 11 (SLC7A11) but upregulated arachidonate lipoxygenase 3 (ALOXE3), promoting ferroptosis. TalaA causes upregulation of HMOX1 which lead to the degradation of heme and the release of free iron, accumulating in mitochondria and giving rise to lipid peroxidation. TalaA could be a new potential powerful drug candidate for colorectal cancer therapy. | ||||
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [115] | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| SNU-182 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0090 | ||
| SNU-449 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | ||
| In Vivo Model |
A total of 20 male Balb/c nude mice aged 6-8 weeks were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). Five million Huh7 cells were inoculated into the right flanks of the mice. When the tumor size reached 80-100 mm3, the mice were randomly divided into four groups and administered artesunate (30 mg/kg mouse weight) alone, sorafenib (20 mg/kg mouse weight) alone, a combination of artesunate and sorafenib, or the same volume of PBS by gavage every other day.
Click to Show/Hide
|
||||
| Response Description | Sorafenib at low dose mainly caused oxidative stress through mitochondrial impairments and SLC7A11-invovled glutathione depletion. Artesunate-induced lysosome activation synergized with sorafenib-mediated pro-oxidative effects by promoting sequential reactions including lysosomal cathepsin B/L activation, ferritin degradation, lipid peroxidation, and consequent ferroptosis. Taken together, artesunate could be repurposed to sensitize sorafenib in hepatocellular carcinoma treatment. | ||||
Necrostatin-1
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [116] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| Response Description | Necrostatin-1 potentiated sulfasalazine-induced expression of SLC7A11, a catalytic subunit of system xc- in these cells. And necrostatin-1 Prevents Ferroptosis in a RIPK1- and IDO-Independent Manner in Hepatocellular Carcinoma. | |||
Ginkgetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [117] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SPC-A1 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | ||
| In Vivo Model |
Briefly, when tumours on transplanted nude mice reached around 100 mm3, the mice were randomized divided into eight groups: control, ginkgetin, DDP, ginkgetin + DDP, UAMC 3203, ginkgetin + UAMC 3203, DDP + UAMC 3203, ginkgetin + DDP + UAMC 3203. Both DDP (3 mg/kg) and ginkgetin (30 mg/kg) were administered by intraperitoneal injection, with 2 - 3 times per week and once per day, respectively. UAMC 3203 (10 mg/kg) was administered 5 days/week by intraperitoneally injection. Tumour size and body weight were measured 3 times per week. After dosing 31 days, the nude mice were sacrificed, and tumours were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | The induction of ferroptosis mediated by ginkgetin was further confirmed by the decreased expression of SLC7A11 and GPX4, and a decreased GSH/GSSG ratio. Simultaneously, ginkgetin disrupted redox hemostasis in DDP-treated cells, as demonstrated by the enhanced ROS formation and inactivation of the Nrf2/HO-1 axis. Ginkgetin also enhanced DDP-induced mitochondrial membrane potential (MMP) loss and apoptosis in cultured non-small cell lung cancer (NSCLC) cells. | ||||
Sulforaphane
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [118] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Glutathione metabolism | hsa00480 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | H69 cells | Normal | Homo sapiens | CVCL_8121 |
| NCI-H82 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1591 | |
| H69AR cells | Lung small cell carcinoma | Homo sapiens | CVCL_3513 | |
| Response Description | Sulforaphane (SFN)-induced cell death was mediated via ferroptosis and inhibition of the mRNA and protein expression levels of SLC7A11 in small-cell lung cancer (SCLC) cells. The anticancer effects of SFN may provide novel options for SCLC treatment. | |||
Capsaicin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [119] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 | |
| Response Description | Capsaicin inhibited the proliferation of A549 and NCI-H23 cells and induced ferroptosis by inactivating SLC7A11/GPX4 signaling. Capsaicin could be used as a potential anticancer agent in the treatment of non-small cell lung cancer. | |||
Manoalide
[Phase 2]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [120] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| H157 cells | Oral cavity Squamous cell carcinoma | Homo sapiens | CVCL_2458 | ||
| HCC827 cells | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| In Vivo Model |
The LSL-KrasG12D mouse model was obtained from the Jackson Laboratory (Sacramento, CA). Adeno-Cre (Genechem, Shanghai, China) was introduced into the trachea of mice at a dose of 1.25 x 1011 PFU in a total volume of 50 uL. Tumor tissues from 12-week post-infection mice were washed with cold PBS, cut into small pieces, and washed with DMEM/F12 (containing 1 x Glutamine, 10 mM HEPES, and antibiotics), digested with collagenase I and IV for 0.5-1 h at 37. After washing twice with DMEM/F12 and centrifugation (500 g, 5 min), the dissociated cells were seeded into growth factor-reduced matrigel (Corning, #356237) at 37 for 30 min.
Click to Show/Hide
|
||||
| Response Description | Manoalide (MA) induces ferroptosis by suppressing the NRF2-SLC7A11 axis and mitochondrial Ca2+overload induced-FTH1 pathways to promote the sensitivity of osimertinib-resistant lung cancer cells to osimertinib. | ||||
XAV939
[Preclinical]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [121] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell apoptosis | ||||
| In Vitro Model | NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 |
| Response Description | The downregulation of the lncRNA MIR503HG induced by XAV939 may serve an important role in suppressing the progression of non-small cell lung cancer via sponging miR1273c, to downregulate its target SOX4. Furthermore, the downregulation of SLC7A11 induced by XAV939 may inhibit NSCLC development via participation in the ferroptosis pathway. | |||
Sulfasalazine
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [122] | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| Response Description | The combined effects of vorinostat with salazosulfapyridine (SASP) depend on the accumulation of ROS caused by a decrease in intracellular GSH levels, possibly due to SASP-mediated inhibition of xCT. xCT (coded by the SLC7A11 gene), a light chain subunit of the glutamate-cystine antiporter system Xc(-) in Breast adenocarcinoma. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [123] | ||||
| Responsed Disease | Uterine serous carcinoma [ICD-11: 2C72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | hUPSCs (Human uterine serous papillary carcinoma-1 cells) | ||||
| Abcam HeLa ERGIC2 KO cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_B1RG | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| In Vivo Model |
The mean weight of the mice at the start was 19.4 ± 0.87 g. To generate the subcutaneous xenograft model, USPC1 (5 x 106 cells) or PTX1 (5 x 106 cells) were suspended in 200 ul of PBS following determination of cellular viability and injected into the subcutaneous tissue of 6-week-old female Crj:SHO-PrkdcscidHrhr hairless SCID mice (n = 2) (Charles River Laboratories Inc.). Tumor formation was visually confirmed in mice inoculated with USPC1 cells, but not in those inoculated with PTX1 cells, thus the animal study was performed using USPC1 cells. The recipient mice were monitored for general health status and presence of subcutaneous tumors once a week.
Click to Show/Hide
|
||||
| Response Description | The effect of the xCT (SLC7A11) inhibitor, sulfasalazine on cytotoxicity was stronger in paclitaxel-resistant uterine serous carcinoma (USC) cells compared with that in paclitaxel-sensitive USC cells. Furthermore, the synthetic lethal interaction between the accumulation of ROS and the activation of the Ras effector, JNK, induced cell-proliferation inhibition and ferroptotic cell death in paclitaxel-resistant USC cells. | ||||
Norcantharidin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [124] | ||||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 cells | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| In Vivo Model |
Athymic nu/nu female mice aged 6-8 weeks (n = 9; mean weight, 20.21 ± 1.54 g) were purchased from the specific pathogen SPF (Beijing) Lab Animals Technology Co. Ltd. Mice were housed in a temperature- and humidity-controlled environment (20-24 , 45-55% humidity), with free access to food and water and in groups of three. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC ID: 17-3256) at Nantong University and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Click to Show/Hide
|
||||
| Response Description | Nuclear factor erythroid 2-related factor 2 (NRF2), heme oxygenase 1 (HO-1), glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (xCT) expression levels were significantly decreased following norcantharidin (NCTD) treatment. Collectively, NCTD may represent a potent anticancer agent in ovarian cancer cells, and NCTD-induced ferroptotic cell death may be achieved by inhibiting the NRF2/HO-1/GPX4/xCT axis. | ||||
Cryptochlorogenic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [125] | ||||
| Responsed Disease | Diabetes mellitus [ICD-11: 5A10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | INS-1 cells | Insulinoma | Rattus norvegicus | CVCL_0352 | |
| In Vivo Model |
Sixty Sprague-Dawley (SD) rats with weights ranging from 250-270 g were obtained from experimental animal center of Xiamen university. For diabetes model group, fasting was performed for 12 h before experiment. The rats (ten rats per group) were assigned into Control group, Model (DM) treated with 50 mg/kg streptozotocin (STZ) via abdominal injection, positive control group and experimental groups. The blood glucose level, which is served as the indicator for the diabetes, was monitored herein. The glucose level after modeling is above 16.7 mmol/l, supporting that the modeling is successful.
Click to Show/Hide
|
||||
| Response Description | Cryptochlorogenic acid (CCA) functions via inhibition of ferroptosis by activation of cystine/glutamate transporter system (XC)/glutathione peroxidase 4(GPX4)/Nrf2 and inhibition of nuclear receptor coactivator 4 (NCOA4) in diabetes. System xc- which is composed of SLC7A11 and SLC3A2, served as the provider of GSH synthesis. | ||||
N-acetylcysteine
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [126] | ||||
| Responsed Disease | Ovarian dysfunction [ICD-11: 5A80] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell differentiation | |||||
| In Vitro Model | hPTs (Human placental tissues) | ||||
| In Vivo Model |
Adult Sprague-Dawley rats (70 days old) of both sexes were purchased from the Laboratory Animal Centre of Harbin Medical University, Harbin, China. Before the experiment, female rats were allowed to acclimatize for a minimum of 1 week and then were monitored daily by vaginal lavage to determine the stage of the estrous cycle as previously described (Zhanget al., 2016). Pregnancy was achieved by housing female rats on the night of proestrus with fertile males of the same strain at a 2:1 ratio. Confirmation of mating was performed the morning after by the presence of a vaginal plug, and this was considered as GD 0.5. The rats were sacrificed between 8:00 a.m. and 9:00 a.m. hours on GD 14.5.
Click to Show/Hide
|
||||
| Response Description | Previous studies demonstrate that increased uterine and placental ferroptosis is associated with oxidative stress-induced fetal loss in a pre-clinical polycystic ovary syndrome (PCOS)-like rat model. N-acetylcysteine treatment results in increased mRNA expression of Aifm2, a negative regulator of GPX4-independent ferroptosis in the placenta. Moreover, NAC reverses HAIR-induced uterine and placental ferroptosis through activation of the Slc7a11/GSH/GPX4 axis. | ||||
Liraglutide
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [127] | ||||
| Responsed Disease | Cognitive disorder [ICD-11: 6D71] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
Male diabetic db/db mice and nondiabetic littermate db/m mice that were 4 weeks of age were purchased from Changzhou Cavens Experimental Animal Co., Ltd. After 1week of adaptive feeding, 20 db/db mice were randomly divided into two groups: a model group (db/db, n = 10) and a treatment group (LIRA, n = 10). Another 10 db/m mice were used as the control group (db/m, n = 10). After feeding to 10weeks old, the LIRA group was given liraglutide (CSN11311, CSNpharm, China) diluent (200 ug/kg/d) by intraperitoneal injection for 5 weeks, with equivoluminal 0.9% saline intraperitoneally administered to the other two groups.
Click to Show/Hide
|
||||
| Response Description | Liraglutide was shown to prevent ferroptosis in the hippocampus by elevating the expression of GPX4 and SLC7A11 and suppressing the excessive amount of ACSL4. LIRA can reduce oxidative stress, lipid peroxidation and iron overload in diabetes-induced cognitive dysfunction and further inhibit ferroptosis, thereby weakening the damage to hippocampal neurons and synaptic plasticity and ultimately restoring cognitive function. | ||||
Paraquat
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [128] | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Apoptosis | hsa04210 | |||
| Ferroptosis | hsa04216 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Twenty male C57BL/6 mice at 12 weeks old were purchased from Hebei Medical University Experimental Animal Center. 10 mice of the experimental group were intraperitoneally injected with PQ (10 mg PQ (salt)/kg/dose) three times a week for 3 weeks according to the previous report. Ten mice of the control group were intraperitoneally injected with the same dose of normal saline. Once the experimental schedule was completed, firstly, the animals were used for behavioral tests. Then, the mice were anesthetized with 0.4% pentobarbital sodium (1 mL/100 g) solution and perfused. The substantia nigra tissue was exfoliated for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Paraquat (PQ) significantly caused the iron accumulation in cytoplasm and mitochondria through ferritinophagy pathway induced by NCOA4. Iron overload initiated lipid peroxidation through 12Lox, further inducing ferroptosis by producing lipid ROS. PQ downregulated SLC7A11 and GPX4 expression and upregulated Cox2 expression. Bcl2/Bax and P-p38/p38 pathways mediated the cross-talk between ferroptosis and apoptosis induced by PQ. These data further demonstrated the complexity of Parkinson's disease occurrence. | ||||
Baicalin
[Terminated]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [129] | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In Vivo Model |
A total of 60 male C57BL/6 mice (10weeks old, 25-28g) were purchased from Guangzhou University of Chinese Medicine Experimental Animal Center (Guangzhou, China). The mice were maintained with enough food and water at 24, 60% relative humidity and 12/12h light/dark cycle. The mice were randomly divided into three groups: sham operation group (Sham), ICH model group (Mod) and baicalin group (Bai) (n = 20/group). Baicalin was suspended in 0.5% carboxymethylcellulose sodium solution. Given the extremely low solubility of baicalin, the concentration of baicalin solution was 0.5 mg/ml. To achieve 20 mg/kg/day dosage, the baicalin solution was administered to the mice in the Bai group by oral route twice at an interval of 1 h within 2 h after ICH injury onset. The remaining two groups received an equal volumes of saline through oral gavage. Since the second day after ICH, mice in the Bai group received 20 mg/kg of baicalin solution while those in the remaining two groups received equal volumes of saline once a day for three consecutive days.
Click to Show/Hide
|
||||
| Response Description | Baicalin significantly increased the mRNA expression of GPX4 and SLC7A11 in the perihematoma brain tissues of intracerebral hemorrhage (ICH) model mice. Baicalin can inhibit the development of ferroptosis in ICH. Baicalin is a potential therapeutic drug for ICH treatment. | ||||
Galangin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [130] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
Male gerbils weighing 70-90 g (12 weeks) were selected for this study. Gerbils were anesthetized with 7% chloral hydrate (350 mg/kg) and the bilateral common carotid arteries were occluded using artery clips. After 5 min, the clips were removed to restore cerebral blood flow. After the operation, place the gerbil on an electric blanket to keep the gerbil's body temperature. The sham group underwent the same surgical procedure without ligation of carotid arteries and was given an equal volume of physiological saline as in the treated groups. The model + galangin (Jiangsu Yongjian Pharmaceutical Technology, 548-83-4, Purity: >=98% (HPLC)) groups underwent the same procedure as the model group and then were received galangin at 25, 50, or 100 mg/kg/day for two continuous weeks.
Click to Show/Hide
|
||||
| Response Description | Gerbils treated with galangin after ischemia-reperfusion (I/R) injury showed significant improvements in learning and memory. In addition, galangin treatment reduced the levels of lipid peroxide in the brains of gerbils that underwent I/R as well as reduced the amount of cell death and increased the expression of SLC7A11 and glutathione peroxidase 4 (GPX4). | ||||
Chrysin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [131] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
Kaempferol
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [132] | |||
| Responsed Disease | Cerebral ischaemic stroke [ICD-11: 8B11] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | mPCNs (Mouse primary cortical neurons) | |||
| Response Description | Kaempferol provides protection from OGD/R-induced ferroptosis, at least in part, by activating Nrf2/SLC7A11/GPX4 signaling pathway. Therefore, pharmacological inhibition of ferroptosis may be an attractive therapeutic target for the treatment of ischemic stroke. | |||
Canagliflozin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [133] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male C57BL/6J mice aged 6-8 weeks with weights of 18-20 g were obtained from the Slack Laboratory Animal Co., Ltd. (Shanghai, China). Mice were allowed to acclimatize in the laboratory environment for 1 week before the beginning of the experiment. DCM model establishment: The mice were given a single intraperitoneal injection of 150 mg/kg 1% streptozotocin (STZ, V900890, Sigma, USA, dissolved in 0.1 mol/L sodium citrate buffer, pH = 4.4 - 4.6). Mouse blood from the tail vein was collected in each group of the model mice and tested by glucose meter (Accu-Chek Performa test strips, Roche, Accu-Chek Performa Combo, Roche, USA) on day 3, 5 and 7 after injection.
Click to Show/Hide
|
||||
| Response Description | Canagliflozin (Cana) promotes upregulation of SLC7A11 and downregulation of TfR1 and FTN-H, which protect the cardiomyocytes from ferroptosis. These finding suggests that Cana inhibit ferroptosis by balancing cardiac iron homeostasis and promoting the system Xc/GSH/GPX4 axis in diabetic cardiomyopathy. | ||||
Liquiritin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [134] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
The set of animal experiments was designed to evaluate the effectiveness of liquiritin on doxorubicin-induced cardiotoxicity as were as ferroptosis were explored. Mice were randomly divided into 5 groups: (1) the control group; (2) doxorubicin group; (3) the doxorubicin plus liquiritin group (20 mg/kg); (4) the doxorubicin plus liquiritin group (40 mg/kg); (5) the doxorubicin plus liquiritin group (80 mg/kg) (Han et al.2022; Mou et al.2021). The control group and doxorubicin group were given equal volume of 0.5% sodium carboxymethylcellulose; the doxorubicin plus liquiritin groups were given different doses of liquiritin (0.5%) sodium carboxymethylcellulose co-suspension) by intragastric administration 7 days in advance once a daily. On day 8, groups (2), (3), (4), and (5) were given a single intraperitoneal injection of 15 mg/kg of DOX to establish a model of doxorubicin-induced cardiotoxicity; and group (1) was given an equal volume of saline intraperitoneally.
Click to Show/Hide
|
||||
| Response Description | Liquiritin can protect the doxorubicin-induce mice's cardiotoxicity, and its beneficial effect is related to the reduction of ferroptosis through a mechanism involving the regulation of the SLC7A11/GPX4 pathway. | ||||
Atorvastatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [135] | ||||
| Responsed Disease | Congestive heart failure [ICD-11: BD10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
8-week C57BL/6J male mice purchased from Comparative Medicine Center of Yangzhou University were retained with unrestricted access to sterilized diet and water at standard bio-clean laboratory settings (Experimental Animal Center of College of Veterinary Medicine of Yangzhou University). Animals were randomly divided into four groups(n = 6-8 mice per group): control group or ISO group: injected with saline or ISO (5 mg/kg) subcutaneously for 14 days and, meanwhile, received vehicle saline via gavage for 14 days respectively; ATV (Pfizer,USA) group or ISO + ATV group: injected with saline or 5 mg/kg ISO (Sigma, USA) subcutaneously for 14 days and, meanwhile, received 20 mg/kg ATV via gavage for 14 days respectively.
Click to Show/Hide
|
||||
| Response Description | Atorvastatin showed significantly protective effects through suppressing the activation of ferroptosis related signaling, as evidenced by decreasing the mRNA levels of PTGS2 (a marker of ferroptosis), contents of malonaldehyde and protein levels of NOX4 and increasing the contents of glutathione (GSH), the ratio of GSH/GSSG and protein levels of GPX4 and SLC7A11. ATV reduced cardiac hypertrophy and fibrosis and accumulation of iron in heart failure. | ||||
Astragaloside IV
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [136] | ||||
| Responsed Disease | Cardiovascular diseases [ICD-11: BE2Z] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HUVECs (Human umbilical vein endothelial cells) | ||||
| Response Description | Astragaloside IV partially upregulated the levels of SLC7A11 and GPX4 expression which were reduced by LPC. The LPC-suppressed proliferation and LPC-induced apoptosis and senescence of endothelial cells were greatly attenuated by AS-IV treatment. In conclusion, AS-IV could serve as a novel drug for treating ferroptosis-related cardiovascular diseases. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [142] | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hT2AECs (Type II alveolar epithelial cells) | ||||
| In Vivo Model |
The animals were randomly assigned to six groups (7 mice in each) as follows: (I) Normal saline (NS) group, (II) Ast-IV 100 mg/kg (Ast) group, (III) PM2.5 group, (IV) Ast-IV 50 mg/kg + PM2.5 (Ast-L) group, (V) Ast 100 mg/kg + PM2.5 (Ast-H) group, and (VI) Ast-IV 100 mg/kg + erastin 20 mg/kg + PM2.5 (Era) group. Based on our previous results, this study adopted anintraperitoneal injection(i.p.) of Ast-IV (dissolved in normal saline containing 0.1% DMSO for preventive treatment. After all the mice were adaptively fed for 5 days, in the NS and PM2.5 groups, mice received the normal saline containing 0.1% DMSO viai.p.once a day for the next three consecutive days. Similar to the NS group, in the Ast, Ast-H, and Era groups, mice received Ast-IV (100 mg/kg) viai.p. Ast-L group received Ast-IV (50 mg/kg) viai.p. To evaluate the effect of Ast-IV on ferroptosis in PM2.5-induced lung injury, we used the ferroptosis agonist erastin to activate ferroptosisin vivo. In the Era group, mice received erastin (20 mg/kg, 10% DMSO + 40% PEG300 + 5%Tween80 + 45% normal saline) 30 min before each preventive treatment of Ast-IV.
Click to Show/Hide
|
||||
| Response Description | Astragaloside IV (Ast-IV) reduced the lung wet-dry ratio and the levels of interleukin 6 (IL-6), tumor necrosis factor- (TNF-) and interleukin 1 (IL-1) in serum. Ast-IV could also improve the oxidative stress level in BALF, restore the GSH level in the lung tissue, and reduce the iron content in the lung tissue. Western blot outcomes revealed that Ast-IV regulated the ferroptosis signaling pathway via the Nrf2/SLC7A11/GPX4 axis to protect PM2.5-mediated lung injury. | ||||
Sesamin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [137] | ||||
| Responsed Disease | Cardiovascular diseases [ICD-11: BE2Z] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
Forty specific pathogen-free normal Sprague Dawley (SD) rats (7 weeks old and 251-275 g in weight) were supplied by Charles River Laboratories. The SD rats were randomly allocated into five groups (n = 8). In the PM2.5 exposure group, the rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times. In the saline control group, the SD rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and intratracheally instilled with 0.9% saline (1 mL per kg b.w.) every other day for a total of three times. In the Ses pretreatment groups, the SD rats were gavaged with low (L-Ses, 40 mg per kg b.w), medium (M-Ses, 80 mg per kg b.w.), and high (H-Ses, 160 mg per kg b.w.) doses of Ses. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times.
Click to Show/Hide
|
||||
| Response Description | Sesamin pretreatment upregulated the expression levels of GPX4, SLC7A11, TFRC, and FPN1 and inhibited the expression levels of FTH1 and FTL. Ses pretreatment could ameliorate PM2.5-induced cardiovascular injuries perhaps by inhibiting ferroptosis. | ||||
Chrysophanol
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [138] | |||
| Responsed Disease | Liver fibrosis [ICD-11: DB93] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HSC-T6 cells | Normal | Rattus norvegicus | CVCL_0315 |
| Response Description | Chrysophanol significantly induced HBx-transfected HSC-T6 death by inducing ferroptosis, as demonstrated by lipid ROS accumulation and upregulation of expression of ER markers, such as Bip, CHOP, and p-IRE1, and ferroptotic markers, such as GPX4 and SLC7A11. Therefore, chrysophanol may exert ferroptotic effects on activated HSCs to prevent liver fibrosis. | |||
Salidroside
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [139] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | ||
| In Vivo Model |
Following endotracheal intubation, mice were ventilated with room air at a rate of 120 cycles/min and atidal volumeof 7 mL/kg (MiniVent, Harvard Apparatus, USA). To induce ischemia, mice underwent left thoracotomy, and the left pulmonary hilum was blocked for 60 min with a microvascular clamp. After ischemia, the coronary artery was reperfused for 120 min by removing the clamp. The mice were euthanized at the end of the experiment through CO2 asphyxiation and cervical dislocation. Next, bronchoalveolar lavage fluid (BALF), blood, and lung samples were collected for testing.
Click to Show/Hide
|
||||
| Response Description | Salidroside postconditioning attenuates ferroptosis-mediated lung ischemia-reperfusion injury by activating the Nrf2/SLC7A11 signaling axis. | ||||
Dimethyl fumarate
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [140] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | AML12 cells | Normal | Mus musculus | CVCL_0140 | |
| In Vivo Model |
The mice were randomly divided into four groups of six: sham + vehicle, sham + DMF, IR + vehicle, and IR + DMF. The mice were supplemented with DMF at a concentration of 100 mg/kg or DMSO by daily oral gavage for a week before surgery, as previously reported. As stated in a prior study, the partial warm liver IRI model was developed. Briefly, the sham group only had free hepatic portal blood vessels after laparotomy, and the blood flow was not obstructed. As for the hepatic IR group, the blood supply to the left and mid-hepatic lobes was blocked, resulting in 70% mouse liver IRI for 90 min. The mice were put on a heated blanket after surgery in order to maintain body temperature and monitor vital signs. Blood supply was restored for 6 h. Died mice were eliminated for testing prior to sample collection. The mice were euthanized after the sample were obtained. The same experimenter carried out all surgeries.
Click to Show/Hide
|
||||
| Response Description | NRF2 knockdown notably decreased the expression of SLC7A11 and HO-1 and blocked the anti-ferroptosis effects of dimethyl fumarate (DMF). DMF inhibits ferroptosis by activating the NRF2/SLC7A11/HO-1 axis and exerts a protective effect against hepatic ischemia-reperfusion injury. | ||||
Aspirin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [141] | ||||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
These mice were on eight weeks old male DBA/2J background (n = 36, HFK Bioscience, Beijing, China). They were randomized one of the six groups: control normal mice group (NC); diabetic mice group (DM); diabetic mice group (Fer-1), who intraperitoneal injected Fer-1 (Selleck, Houston, TX, USA); diabetic mice group (vehicle-P), who intraperitoneal injected 1% dimethyl sulfoxide (DMSO); diabetic mice group (As), who intragastric administrated Aspirin (Solarbio, Beijing, China); diabetic mice group (vehicle-G), who intragastric administrated 0.5% sodium carboxymethyl cellulose (Na-CMC; Solarbio, Beijing, China). Diabetes models were induced with 5 consecutive days of a single intraperitoneal injection of streptozotocin 40 mg/kg (dissolved in 0.1 M citrate buffer, pH 4.5; SigmaAldrich, St Louis, MO, USA). Control mice only was injected the same volume of citrate buffer. In the Fer-1 or vehicle-P groups, the diabetic mice were treated respectively with Fer-1 (2.5 umol/kg, dissolved in 1% DMSO) or 1% DMSO during the duration of treatment for 12-week every day. And in the AS and vehicle-G groups, the diabetic mice were treated respectively with aspirin (50 mg/kg, dissolved in 0.5% Na-CMC) or 0.5% Na-CMC for 12-week every day.
Click to Show/Hide
|
||||
| Response Description | Aspirin can upregulate SLC7A11 and GPX4 expression by suppressing COX2. Our results demonstrated that ferroptosis in renal tubular cells contributes to Diabetic kidney disease (DKD) development and that diabetes-related ferroptosis was inhibited through the downregulation of COX2 by aspirin, thus retarding the progression of DKD. | ||||
Lipopolysaccharide
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [143] | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
The male C57BL/6 mice were divided randomly into 4 groups (n = 4 per group, 8-10 weeks old, weight = 23-25 g): the control group receiving 0.9% NaCl (containing 0.1% DMSO), the LPS group receiving LPS plus 0.9% NaCl (containing 0.1% DMSO), the Fer-1 group receiving Fer-1 only, and the LPS + Fer-1 group receiving both Fer-1 and LPS. The LPS-induced ALI model was induced by instilling intratracheally 50 ul of LPS solution (0.2 g/L), then Fer-1 (0.8 mg/kg) was administered after LPS challenge via tail vein injection. The Fer-1 was dissolved in DMSO first, and diluted with 0.9% NaCl. The final concentration of Fer-1 and DMSO was 0.2 mg/ml and 0.1% respectively.
Click to Show/Hide
|
||||
| Response Description | The cell viability of BEAS-2B was down-regulated by lipopolysaccharide (LPS) treatment, together with the ferroptosis markers SLC7A11 and GPX4, while the levels of MDA, 4-HNE and total iron were increased by LPS treatment in a dose-dependent manner, which could be rescued by ferrostatin-1. Fer-1 exerted therapeutic action against LPS-induced acute lung injury, and down-regulated the ferroptosis level in lung tissues. | ||||
Ferrostatin-1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [143] | ||||
| Responsed Disease | Lung injury [ICD-11: NB32] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
The male C57BL/6 mice were divided randomly into 4 groups (n = 4 per group, 8-10 weeks old, weight = 23-25 g): the control group receiving 0.9% NaCl (containing 0.1% DMSO), the LPS group receiving LPS plus 0.9% NaCl (containing 0.1% DMSO), the Fer-1 group receiving Fer-1 only, and the LPS + Fer-1 group receiving both Fer-1 and LPS. The LPS-induced ALI model was induced by instilling intratracheally 50 ul of LPS solution (0.2 g/L), then Fer-1 (0.8 mg/kg) was administered after LPS challenge via tail vein injection. The Fer-1 was dissolved in DMSO first, and diluted with 0.9% NaCl. The final concentration of Fer-1 and DMSO was 0.2 mg/ml and 0.1% respectively.
Click to Show/Hide
|
||||
| Response Description | The cell viability of BEAS-2B was down-regulated by lipopolysaccharide (LPS) treatment, together with the ferroptosis markers SLC7A11 and GPX4, while the levels of MDA, 4-HNE and total iron were increased by LPS treatment in a dose-dependent manner, which could be rescued by ferrostatin-1. Fer-1 exerted therapeutic action against LPS-induced acute lung injury, and down-regulated the ferroptosis level in lung tissues. | ||||
Lycopene
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [144] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mSCs (Mouse splenocytes) | ||||
| In Vivo Model |
Three-week-old specific pathogen-free ICR (Institute of Cancer Research) male mice (weights of 18-22 g) were provided by Liaoning Changsheng Biotech Co. Ltd. The mice were housed under conditions at 22 ± 2 with 35-65% humidity and a light/dark cycle of 12 h/12 h in the cage. The animals were quarantined for a week before formal experiments, then randomly divided into seven groups: vehicle control group (Vcon), control group (Con), 5 mg/kg BW/d Lyc group (Lyc), 500 and 1000 mg/kg BW/d DEHP group (D5 and D10, respectively), DEHP + Lyc group (DL5 and DL10, respectively) (n = 20). The animals were exposed to DEHP via oral gavage, which lasted for 28 d, and then sacrificed after being anesthetized.
Click to Show/Hide
|
||||
| Response Description | DEHP disrupted the GSH metabolism via the xc/ GPX4 antioxidant system and, subsequently, caused the ferroptotic cell death, but Lycopene (Lyc) could effectively mitigate DEHP-induced damage to the antioxidant system. These findings indicated that Lyc may be an effective strategy for the prevention of DEHP-induced splenic toxicity via the regulation of ferroptosis. | ||||
T-2 Toxin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [146] | |||
| Responsed Disease | Health [ICD-11: N.A.] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| Response Description | SLC7A11 overexpression significantly rescued the enhanced ferroptosis caused by T-2 toxin. T-2 toxin induces ferroptosis by downregulating SLC7A11 expression. Ferroptosis mediates T-2 toxin-induced cytotoxicity by increasing ROS and downregulating SLC7A11 expression. | |||
References
