Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0353)
| Name |
Imidazole ketone erastin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Imidazole ketone erastin; 1801530-11-9; IKE; PUN30119; Imidazole ketone erastinIKE; CHEMBL3629671; 3-(5-(2-(1H-imidazol-1-yl)acetyl)-2-isopropoxyphenyl)-2-((4-(2-(4-chlorophenoxy)acetyl)piperazin-1-yl)methyl)quinazolin-4(3H)-one; SCHEMBL16924899; BCP31858; BXC53011; EX-A3112; BDBM50126162; NSC819610; s8877; ZB1594; AKOS037648788; Ferroptosis inducer IKE; PUN-30119; NSC-819610; AC-35771; BS-15620; HY-114481; CS-0086985; D70076; IKE; Ferroptosis inducer IKE; PUN30119; PUN-30119; PUN 30119; 2-[[4-[2-(4-chlorophenoxy)acetyl]piperazin-1-yl]methyl]-3-[5-(2-imidazol-1-ylacetyl)-2-propan-2-yloxyphenyl]quinazolin-4-one
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
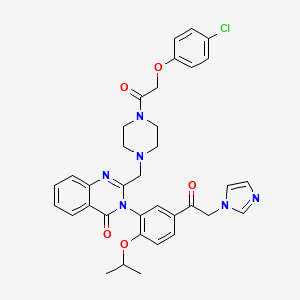 |
||||
| Formula |
C35H35ClN6O5
|
||||
| IUPAC Name |
2-[[4-[2-(4-chlorophenoxy)acetyl]piperazin-1-yl]methyl]-3-[5-(2-imidazol-1-ylacetyl)-2-propan-2-yloxyphenyl]quinazolin-4-one
|
||||
| Canonical SMILES |
CC(C)OC1=C(C=C(C=C1)C(=O)CN2C=CN=C2)N3C(=NC4=CC=CC=C4C3=O)CN5CCN(CC5)C(=O)COC6=CC=C(C=C6)Cl
|
||||
| InChI |
InChI=1S/C35H35ClN6O5/c1-24(2)47-32-12-7-25(31(43)20-40-14-13-37-23-40)19-30(32)42-33(38-29-6-4-3-5-28(29)35(42)45)21-39-15-17-41(18-16-39)34(44)22-46-27-10-8-26(36)9-11-27/h3-14,19,23-24H,15-18,20-22H2,1-2H3
|
||||
| InChIKey |
PSPXJPWGVFNGQI-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Diffuse large B-cell lymphoma | ICD-11: 2A81 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SU-DHL-1 cells | Anaplastic large cell lymphoma | Homo sapiens | CVCL_0538 | |
| SU-DHL-2 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_9550 | ||
| SU-DHL-6 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_2206 | ||
| SU-DHL-8 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_2207 | ||
| SU-DHL-10 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1889 | ||
| SU-DHL-16 cells | B-cell non-Hodgkin lymphoma | Homo sapiens | CVCL_1890 | ||
| A3/Kawakami cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1062 | ||
| OCI-LY8 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_8803 | ||
| U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | ||
| DoHH2 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1179 | ||
| HBL-1 cells | Non-Hodgkin lymphoma | Homo sapiens | CVCL_M572 | ||
| U-2932 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1896 | ||
| SU-DHL-7 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_4380 | ||
| SU-DHL-9 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_4379 | ||
| A4/Fukuda cells | B acute lymphoblastic leukemia | Homo sapiens | CVCL_1064 | ||
| WSU-NHL cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1793 | ||
| SU-DHL-5 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1735 | ||
| Karpas-422 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1325 | ||
| In Vivo Model |
NOD/SCID mice (12-weeks of age and ~28 g weight) were weighed before injection and divided into groups of 3 mice per cage. Mice were dosed using three different routes, IP and PO with 50 mg/kg IKE, and IV with 17 mg/kg IKE. Samples were collected at 0, 1, 3, 4, and 8 h from three mice per time point. Additionally, three mice per group were used as controls by administration with equivalent amount of vehicle 1 by IP, PO, and IV, and samples were collected at 8 h. At the appropriate time, mice were sacrificed by CO2 asphyxiation for 3 min and ~0.5 mL of blood was collected via cardiac puncture.
Click to Show/Hide
|
||||
| Response regulation | Imidazole ketone erastin (IKE) is a potent, selective, and metabolically stable system xc- (SLC7A11)inhibitor. In addition, biodegradable polyethylene glycol-poly(lactic-co-glycolic acid) nanoparticles were employed to aid in IKE delivery and exhibited reduced toxicity compared with free IKE in a diffuse large B cell lymphoma (DLBCL) xenograft model. | ||||
