Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0076)
| Name |
Sulfasalazine
|
||||
|---|---|---|---|---|---|
| Synonyms |
sulfasalazine; 599-79-1; Salicylazosulfapyridine; Azulfidine; Salazosulfapyridine; Sulphasalazine; Salazopyrin; Asulfidine; Benzosulfa; Salazopyridin; Accucol; Azopyrin; Sulcolon; Colo-Pleon; Salazopiridazin; Azopyrine; Reupirin; Salisulf; Salazosulfapyridin; Sulfasalazinum; w-t Sasp oral; Azulfidine EN; Sulfasalazin; Sulfasalazina; Sulfazalazine; Azulfidine EN-tabs; Salazosulfapiridina; Salazosulfapyridinum; Sas-500; SASP; 5-(p-(2-Pyridylsulfamyl)phenylazo)salicylic acid; NSC 667219; S.A.S.-500; 5-(4-(2-Pyridylsulfamoyl)phenylazo)-2-hydroxybenzoic acid; 4-(Pyridyl-2-amidosulfonyl)-3'-carboxy-4'-hydroxyazobenzene; 5-((p-(2-Pyridylsulfamoyl)phenyl)azo)salicylic acid; 2-Hydroxy-5-((4-((2-pyridinylamino)sulfonyl)phenyl)azo)benzoic acid; 5-[4-(2-Pyridylsulfamoyl)phenylazo]salicylic Acid; CHEBI:9334; NSC 203730; S.A.S. 500; C18H14N4O5S; (E)-2-hydroxy-5-((4-(N-(pyridin-2-yl)sulfamoyl)phenyl)diazenyl)benzoic acid; Sulfasalazopyridine; MFCD00057363; Benzoic acid, 2-Hydroxy-5-[[4-[(2-pyridinylamino)sulfonyl]phenyl]azo]-; 3XC8GUZ6CB; Sulfasalazine (Azulfidine); NSC-203730; NSC-667219; Benzoic acid, 2-hydroxy-5-((4-((2-pyridinylamino)sulfonyl)phenyl)azo)-; S.A.S.; 737754-28-8; Azosulfidin; DTXSID0021256; 2-hydroxy-5-{[4-(pyridin-2-ylsulfamoyl)phenyl]diazenyl}benzoic acid; 5-[p-(2-Pyridylsulfamoyl)phenylazo]salicylic acid; 2-HYDROXY-(5-([4-(2-PYRIDINYLAMINO)SULFONYL]PHENYL)AZO)BENZOIC ACID; 5-(p-(2-Pyridinylsulfamoyl)Phenylazo)Salicylic Acid; NSC203730; NSC667219; Salicylic acid, 5-((p-(2-pyridylsulfamoyl)phenyl)azo)-; SSZ; NCGC00090903-01; Salazo-sulfapyridinum; CAS-599-79-1; 2-hydroxy-5-[[4-(pyridin-2-ylsulfamoyl)phenyl]diazenyl]benzoic acid; 2-Hydroxy-5-[[4-[(2-pyridinylamino)sulfonyl]phenyl]azo]benzoic acid; 2-hydroxy-5-[(E)-{4-[(pyridin-2-ylamino)sulfonyl]phenyl}diazenyl]benzoic acid; 5-[p-(2-Pyridylsulfamyl)phenylazo]salicylic acid; (3Z)-6-Oxo-3-[[4-(pyridin-2-ylsulfamoyl)phenyl]hydrazinylidene]cyclohexa-1,4-diene-1-carboxylic acid; 5-(p-(2-Pyridylsulfamoyl)phenylazo)salicylic acid; Salazopyrin EN-Tabs; DTXCID401256; Sulfasalazinum [INN-Latin]; Sulfasalazina [INN-Spanish]; Salicylic acid, 5-[[p-(2-pyridylsulfamoyl)phenyl]azo]-; 2-Hydroxy-5-[4-(pyridin-2-ylsulfamoyl)-phenylazo]-benzoic acid; Salazosulfapyridinum [INN-Latin]; Salazosulfapiridina [INN-Spanish]; SI-88; 2-Hydroxy-5-((4-(N-(pyridin-2-yl)sulfamoyl)phenyl)diazenyl)benzoic acid; Alti-Sulfasalazine; PMS-Sulfasalazine; 2-hydroxy-5-[(E)-2-{4-[(pyridin-2-yl)sulfamoyl]phenyl}diazen-1-yl]benzoic acid; Azulfidine (TN); SMR000059146; Azlufidine EN-Tabs; Sulphasalazine, N-; CCRIS 4713; HSDB 3395; 13gs; Pms-Sulfasalazine E.C.; SR-05000001721; EINECS 209-974-3; UNII-3XC8GUZ6CB; 5-[[p-(2-Pyridylsulfamoyl)phenyl]azo]salicylic acid; Sulfasalazine (USP/INN); S.A.S. Enteric-500; BRN 0356241; Iwata; Prestwick_848; Sulfasalazine [USAN:USP:INN:BAN]; Spectrum_000998; Prestwick0_000520; Prestwick1_000520; Prestwick2_000520; Prestwick3_000520; Spectrum2_001216; Spectrum3_001364; Spectrum4_000347; Spectrum5_001443; SULFASALAZINE [MI]; CHEMBL421; Epitope ID:122672; SULFASALAZINE [INN]; SCHEMBL4514; SCHEMBL4515; Salazosulfapyridine (JP17); SULFASALAZINE [HSDB]; SULFASALAZINE [IARC]; SULFASALAZINE [USAN]; SCHEMBL18490; BSPBio_000479; BSPBio_002888; KBioGR_000753; KBioGR_002314; KBioSS_001478; KBioSS_002316; SULFASALAZINE [VANDF]; 5-22-08-00433 (Beilstein Handbook Reference); MLS000759399; MLS001424109; MLS006011702; BIDD:GT0161; DivK1c_000860; SPECTRUM1500552; SULFASALAZINE [MART.]; SPBio_001032; SPBio_002400; SULFASALAZINE [USP-RS]; SULFASALAZINE [WHO-DD]; SULFASALAZINE [WHO-IP]; 5-[4-(2-Pyridylsulfamoyl)-phenylazo]-salicylic acid; BPBio1_000527; CHEMBL100848; CHEMBL242373; GTPL4840; SCHEMBL1079598; SCHEMBL1229516; CHEMBL1206016; SCHEMBL10289061; CHEBI:94500; HMS502K22; KBio1_000860; KBio2_001478; KBio2_002314; KBio2_004046; KBio2_004882; KBio2_006614; KBio2_007450; KBio3_002108; KBio3_002794; SALAZOSULFAPYRIDINE [JAN]; cMAP_000018; NCEXYHBECQHGNR-QZQOTICOSA-N; NCEXYHBECQHGNR-UHFFFAOYSA-N; NINDS_000860; OQANPHBRHBJGNZ-BKUYFWCQSA-N; OQANPHBRHBJGNZ-UHFFFAOYSA-N; HMS1569H21; HMS1921C05; HMS2051J21; HMS2090P13; HMS2092K07; HMS2096H21; HMS2232H07; HMS3370D16; HMS3393J21; HMS3655G07; HMS3713H21; HMS3871J13; HMS3884E21; Pharmakon1600-01500552; SULFASALAZINE [ORANGE BOOK]; BCP13311; SULFASALAZINE [EP MONOGRAPH]; Tox21_111037; Tox21_201239; Tox21_300541; BDBM50097125; BDBM50103596; CCG-39145; DL-510; NSC757330; s1576; SULFASALAZINE [USP MONOGRAPH]; SULFASALAZINUM [WHO-IP LATIN]; 2-hydroxy-5-(2-{4-[(pyridin-2-yl)sulfamoyl]phenyl}diazen-1-yl)benzoic acid; AKOS002311709; AKOS025116975; AKOS026749974; AKOS037515748; Tox21_111037_1; WLN: T6NJ BSWMR DNUNR DQ CVQ; CCG-100987; DB00795; HS-0062; NC00237; NSC-757330; IDI1_000860; SMP2_000059; NCGC00016518-01; NCGC00090903-02; NCGC00090903-03; NCGC00090903-04; NCGC00090903-05; NCGC00090903-06; NCGC00090903-07; NCGC00090903-08; NCGC00090903-09; NCGC00090903-11; NCGC00186644-01; NCGC00254313-01; NCGC00258791-01; AC-20497; HY-14655; PD087097; SMR004703430; SY052318; SBI-0051526.P003; FT-0603483; FT-0674746; S0580; SW196979-4; C07316; D00448; EN300-119546; H10652; AB00052101-04; AB00052101-06; AB00052101_07; AB00052101_08; A832559; Q420035; Q-201769; SR-05000001721-1; SR-05000001721-2; SR-05000001721-3; Sulfasalazine, analytical standard, >=98% (HPLC); 5-[[4-(2-Pyridylsulfamoyl)phenyl]azo]salicylic acid; BRD-K10670311-001-06-4; BRD-K10670311-001-08-0; Q27166356; Q63398427; Sulphasalazine, Antibiotic for Culture Media Use Only; F2173-1125; Z1521554012; 4-(Pyridyl-2-amidosulfonyl)-3''-carboxy-4''-hydroxyazobenzene; Sulfasalazine, European Pharmacopoeia (EP) Reference Standard; 2-hydroxy-5-[(E)-[4-(2-pyridylsulfamoyl)phenyl]azo]benzoic acid; 5-{4-[(2-pyridylideneamino)sulfonyl]phenyldiazenyl}salicylic acid; Sulfasalazine, United States Pharmacopeia (USP) Reference Standard; (E)-2-hydroxy-5-((4-(N-pyridin-2-ylsulfamoyl)phenyl)diazenyl)benzoic acid; 2-hydroxy-5-((4-(N-pyridin-2-ylsulfamoyl)phenyl)diazenyl)benzoic acid; 2-Hydroxy-5-((4-[(2-pyridinylamino)sulfonyl]phenyl)diazenyl)benzoic acid #; 2-hydroxy-5-{(E)-[4-(pyridin-2-ylsulfamoyl)phenyl]diazenyl}benzoic acid; 2-HYDROXY-5-((4-((2-PYRIDINYLAMINO)SULFONYL)PHENYL)AZO)BENZOIC ACID [WHO-IP]; 6-oxo-3-(2-[4-(n-pyridin-2-ylsulfamoyl)phenyl]hydrazono)cyclohexa-1,4-dienecarboxylic acid; BENZOIC ACID, 2-HYDROXY-5-(2-(4-((2-PYRIDINYLAMINO)SULFONYL)PHENYL)DIAZENYL)-; 6-oxo-3-((4-(pyridin-2-ylsulfamoyl)phenyl) hydrazinylidene]cyclohexa-1,4-diene-1-carboxylic acid; 6-oxo-3-[[4-(2-pyridinylsulfamoyl)phenyl]hydrazinylidene]-1-cyclohexa-1,4-dienecarboxylic acid
Click to Show/Hide
|
||||
| Structure |
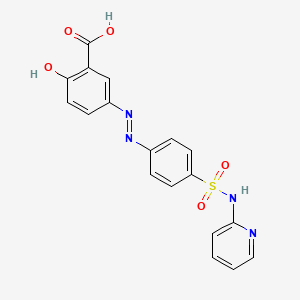 |
||||
| Formula |
C18H14N4O5S
|
||||
| IUPAC Name |
2-hydroxy-5-[[4-(pyridin-2-ylsulfamoyl)phenyl]diazenyl]benzoic acid
|
||||
| Canonical SMILES |
C1=CC=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)N=NC3=CC(=C(C=C3)O)C(=O)O
|
||||
| InChI |
InChI=1S/C18H14N4O5S/c23-16-9-6-13(11-15(16)18(24)25)21-20-12-4-7-14(8-5-12)28(26,27)22-17-3-1-2-10-19-17/h1-11,23H,(H,19,22)(H,24,25)
|
||||
| InChIKey |
NCEXYHBECQHGNR-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Cystine/glutamate transporter (SLC7A11)
| In total 5 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colon cancer | ICD-11: 2B90 | |||
| Responsed Regulator | 5'-AMP-activated protein kinase catalytic subunit alpha-2 (PRKAA2) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 HCT116, CX-1, or HT1080 cells in 100 ul phosphate buffered saline (PBS; Thermo Fisher Scientific, AM9625) were injected subcutaneously right of the dorsal midline in athymic nude immunodeficient mice (six- to eight-week-old, female). To generate orthotopic tumors, 1 x 106 KPC cells in 10 ul PBS were surgically implanted into the pancreases of immunocompetent C57BL/6J mice (six- to eight-week-old, female).
Click to Show/Hide
|
||||
| Response regulation | BECN1 plays a novel role in lipid peroxidation that could be exploited to improve anticancer therapy by the induction of ferroptosis. Mechanistically, phosphorylation of BECN1 at Ser90/93/96 by PRKAA/ AMPK contributes to the formation of a BECN1-SLC7A11 complex and system Xc-inhibition. Knockdown of BECN1 by shRNA inhibits ferroptosis induced by system X-c- inhibitors (e.g., erastin, sulfasalazine, and sorafenib) in Colon carcinoma. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colon cancer | ICD-11: 2B90 | |||
| Responsed Regulator | 5'-AMP-activated protein kinase catalytic subunit alpha-2 (PRKAA2) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 HCT116, CX-1, or HT1080 cells in 100 ul phosphate buffered saline (PBS; Thermo Fisher Scientific, AM9625) were injected subcutaneously right of the dorsal midline in athymic nude immunodeficient mice (six- to eight-week-old, female). To generate orthotopic tumors, 1 x 106 KPC cells in 10 ul PBS were surgically implanted into the pancreases of immunocompetent C57BL/6J mice (six- to eight-week-old, female).
Click to Show/Hide
|
||||
| Response regulation | BECN1 plays a novel role in lipid peroxidation that could be exploited to improve anticancer therapy by the induction of ferroptosis. Mechanistically, phosphorylation of BECN1 at Ser90/93/96 by PRKAA/AMPK contributes to the formation of a BECN1-SLC7A11 complex and system Xc-inhibition. Knockdown of BECN1 by shRNA inhibits ferroptosis induced by system X-c- inhibitors (e.g., erastin, sulfasalazine, and sorafenib) in Colon carcinoma. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| Response regulation | The combined effects of vorinostat with salazosulfapyridine (SASP) depend on the accumulation of ROS caused by a decrease in intracellular GSH levels, possibly due to SASP-mediated inhibition of xCT. xCT (coded by the SLC7A11 gene), a light chain subunit of the glutamate-cystine antiporter system Xc(-) in Breast adenocarcinoma. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| HCC1937 cells | Breast ductal carcinoma | Homo sapiens | CVCL_0290 | ||
| Bcap37 cells | Breast carcinoma | Homo sapiens | CVCL_0164 | ||
| HBL-100 cells | Normal | Homo sapiens | CVCL_4362 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| In Vivo Model |
T47D xenografts were established in 5-week-old nude mice (Shanghai SLAC Laboratory Animal Corporation) by inoculating 1 x 107 cells mixed with Matrigel (BD Biosciences) at 1:1 ratio (volume) into the abdominal mammary fat pad. When the tumor reached 50-100 mm3, the mice were assigned randomly into different treatment groups (DMSO, Metformin, SAS, and Metformin + SAS groups). Metformin (200 mg/kg/day) was provided in drinking water. Sulfasalazine was dissolved in dimethyl sulfoxide (DMSO), diluted in PBS, and then intraperitoneally injected into mice at a dose of 250 mg/kg once a day.
Click to Show/Hide
|
||||
| Response regulation | Metformin reduces the protein stability of SLC7A11, which is a critical ferroptosis regulator, by inhibiting its UFMylation process. Furthermore, metformin combined with sulfasalazine, the system xc-inhibitor, can work in a synergistic manner to induce ferroptosis and inhibit the proliferation of breast cancer cells. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Uterine serous carcinoma | ICD-11: 2C72 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | hUPSCs (Human uterine serous papillary carcinoma-1 cells) | ||||
| Abcam HeLa ERGIC2 KO cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_B1RG | ||
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | ||
| In Vivo Model |
The mean weight of the mice at the start was 19.4 ± 0.87 g. To generate the subcutaneous xenograft model, USPC1 (5 x 106 cells) or PTX1 (5 x 106 cells) were suspended in 200 ul of PBS following determination of cellular viability and injected into the subcutaneous tissue of 6-week-old female Crj:SHO-PrkdcscidHrhr hairless SCID mice (n = 2) (Charles River Laboratories Inc.). Tumor formation was visually confirmed in mice inoculated with USPC1 cells, but not in those inoculated with PTX1 cells, thus the animal study was performed using USPC1 cells. The recipient mice were monitored for general health status and presence of subcutaneous tumors once a week.
Click to Show/Hide
|
||||
| Response regulation | The effect of the xCT (SLC7A11) inhibitor, sulfasalazine on cytotoxicity was stronger in paclitaxel-resistant uterine serous carcinoma (USC) cells compared with that in paclitaxel-sensitive USC cells. Furthermore, the synthetic lethal interaction between the accumulation of ROS and the activation of the Ras effector, JNK, induced cell-proliferation inhibition and ferroptotic cell death in paclitaxel-resistant USC cells. | ||||
Transferrin receptor protein 1 (TFRC)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | |||
| Target for Ferroptosis | Marker/Suppressor/Driver | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | |
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| Response regulation | Sulfasalazine (SAS) upregulated TFRC and DMT1. Knockdown of the ER increased TFRC expression in breast cancer cells. In conclusion SAS could trigger ferroptosis in breast cancer cells, especially in cells with low ER expression. | |||
Natural resistance-associated macrophage protein 2 (SLC11A2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | |||
| Target for Ferroptosis | Driver | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 |
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | |
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | |
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| Response regulation | Sulfasalazine (SAS) upregulated TFRC and DMT1. Knockdown of the ER increased TFRC expression in breast cancer cells. In conclusion SAS could trigger ferroptosis in breast cancer cells, especially in cells with low ER expression. | |||
Beclin-1 (BECN1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Colon cancer | ICD-11: 2B90 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 HCT116, CX-1, or HT1080 cells in 100 ul phosphate buffered saline (PBS; Thermo Fisher Scientific, AM9625) were injected subcutaneously right of the dorsal midline in athymic nude immunodeficient mice (six- to eight-week-old, female). To generate orthotopic tumors, 1 x 106 KPC cells in 10 ul PBS were surgically implanted into the pancreases of immunocompetent C57BL/6J mice (six- to eight-week-old, female).
Click to Show/Hide
|
||||
| Response regulation | BECN1 plays a novel role in lipid peroxidation that could be exploited to improve anticancer therapy by the induction of ferroptosis in Colon cancer. Mechanistically, phosphorylation of BECN1 at Ser90/93/96 by PRKAA/AMPK contributes to the formation of a BECN1-SLC7A11 complex and system Xc-inhibition. Knockdown of BECN1 by shRNA inhibits ferroptosis induced by system X-c- inhibitors (e.g., erastin, sulfasalazine, and sorafenib). | ||||
References
