Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10015)
| Target Name | Beclin-1 (BECN1) | ||||
|---|---|---|---|---|---|
| Synonyms |
Coiled-coil myosin-like BCL2-interacting protein; Protein GT197
Click to Show/Hide
|
||||
| Gene Name | BECN1 | ||||
| Sequence |
MEGSKTSNNSTMQVSFVCQRCSQPLKLDTSFKILDRVTIQELTAPLLTTAQAKPGETQEE
ETNSGEEPFIETPRQDGVSRRFIPPARMMSTESANSFTLIGEASDGGTMENLSRRLKVTG DLFDIMSGQTDVDHPLCEECTDTLLDQLDTQLNVTENECQNYKRCLEILEQMNEDDSEQL QMELKELALEEERLIQELEDVEKNRKIVAENLEKVQAEAERLDQEEAQYQREYSEFKRQQ LELDDELKSVENQMRYAQTQLDKLKKTNVFNATFHIWHSGQFGTINNFRLGRLPSVPVEW NEINAAWGQTVLLLHALANKMGLKFQRYRLVPYGNHSYLESLTDKSKELPLYCSGGLRFF WDNKFDHAMVAFLDCVQQFKEEVEKGETRFCLPYRMDVEKGKIEDTGGSGGSYSIKTQFN SEEQWTKALKFMLTNLKWGLAWVSSQFYNK Click to Show/Hide
|
||||
| Family | Beclin family | ||||
| Function |
Plays a central role in autophagy. Acts as core subunit of the PI3K complex that mediates formation of phosphatidylinositol 3-phosphate; different complex forms are believed to play a role in multiple membrane trafficking pathways: PI3KC3-C1 is involved in initiation of autophagosomes and PI3KC3-C2 in maturation of autophagosomes and endocytosis. Involved in regulation of degradative endocytic trafficking and required for the abcission step in cytokinesis, probably in the context of PI3KC3-C2. Essential for the formation of PI3KC3-C2 but not PI3KC3-C1 PI3K complex forms. Involved in endocytosis. Protects against infection by a neurovirulent strain of Sindbis virus. May play a role in antiviral host defense.
Click to Show/Hide
|
||||
| Gene ID | 8678 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
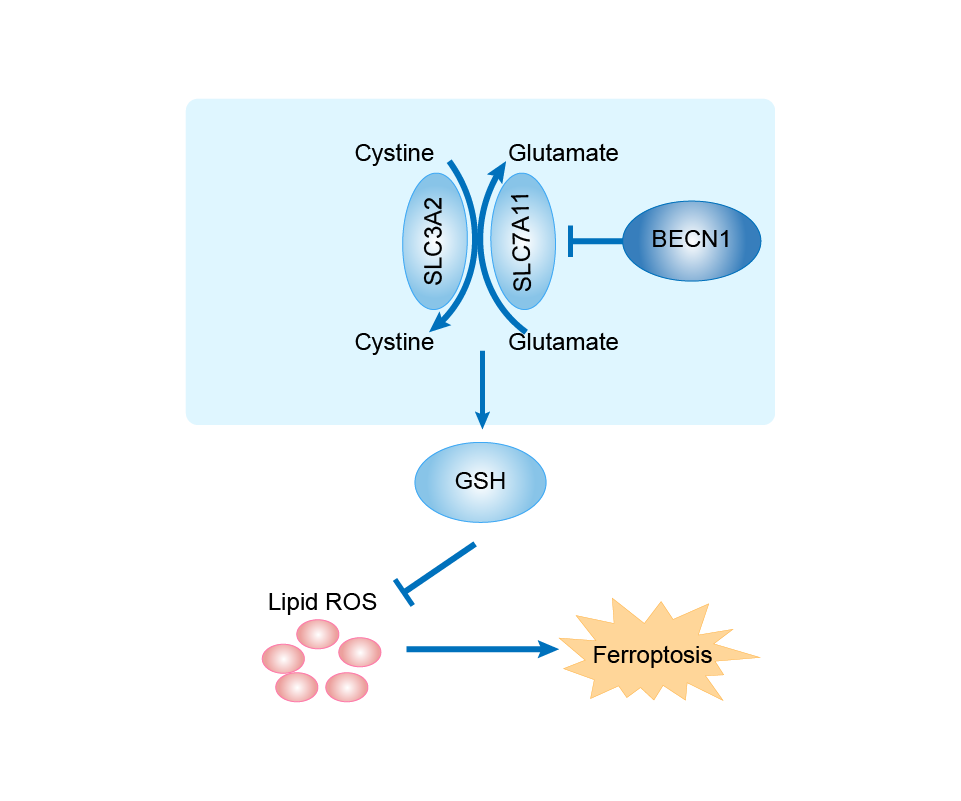
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
BECN1 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
YTH domain-containing family protein 1 (YTHDF1)
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
ICR mice (8-week-old, 18-22 g) were obtained from Yangzhou University (Yangzhou, China). There were 8 mice in each group and they were randomly divided into 6 groups. Mice were treated with Vehicle, CCl4, VA-Lip-control-vector+CCl4+Erastin, VA-Lip-Mettl4-shRNA+CCl4+Erastin, VA-Lip-Fto-plasmid+CCl4+Erastin, VA-Lip- Ythdf1-shRNA+CCl4+Erastin, respectively. A mixture of olive oil and carbon tetrachloride (CCl4) (9:1 (v/v)) was used to trigger liver fibrosis in mouse model by intraperitoneal injection (0.1 ml/20 g body weight), according to our previous reports.
Click to Show/Hide
|
||||
| Response Description | m6A reader YTHDF1 promoted BECN1 mRNA stability via recognizing the m6A binding site, thus triggering autophagy activation, and eventually leading to HSC ferroptosis. FTO plasmid and METTL4 shRNA markedly impaired erastin-induced upregulation of NCOA4 and downregulation of FTH1 in HSC-LX2 cells. Overall, m6A modification-dependent ferroptosis as a potential target for the treatment of liver fibrosis. | ||||
Ubiquitin carboxyl-terminal hydrolase 11 (USP11)
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| In Vivo Model |
The mouse SCIRI model was established in 6- to 8-week-old C57BL/6 male mice. Mice were anesthetized by isoflurane inhalation and fixed in a supine position, and a midline abdominal incision method was used. After positioning of the left kidney through the peritoneum, we carefully searched for and separated the abdominal aorta along the left renal artery. The abdominal aorta was clamped under the exit of the left renal artery (which was not clamped in sham-operated mice). After 60 min, the aneurysm clip was removed and blood perfusion was restored. After the operation, the mice were placed in a cage alone and kept warm, and the bladder was massaged once every 8 h until the bladder reflex was restored.
Click to Show/Hide
|
||||
| Response Description | USP11 plays a key role in regulating ferroptosis and additionally identifies USP11-mediated autophagy-dependent ferroptosis as a promising target for the treatment of spinal cord ischemia-reperfusion injury (SCIRI). USP11 promotes autophagy activation by stabilizing Beclin 1, thereby leading to ferroptosis. | ||||
Unspecific Regulator
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Responsed Drug | Amentoflavone | Investigative | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response Description | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Responsed Drug | Sulfasalazine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 HCT116, CX-1, or HT1080 cells in 100 ul phosphate buffered saline (PBS; Thermo Fisher Scientific, AM9625) were injected subcutaneously right of the dorsal midline in athymic nude immunodeficient mice (six- to eight-week-old, female). To generate orthotopic tumors, 1 x 106 KPC cells in 10 ul PBS were surgically implanted into the pancreases of immunocompetent C57BL/6J mice (six- to eight-week-old, female).
Click to Show/Hide
|
||||
| Response Description | BECN1 plays a novel role in lipid peroxidation that could be exploited to improve anticancer therapy by the induction of ferroptosis in Colon cancer. Mechanistically, phosphorylation of BECN1 at Ser90/93/96 by PRKAA/AMPK contributes to the formation of a BECN1-SLC7A11 complex and system Xc-inhibition. Knockdown of BECN1 by shRNA inhibits ferroptosis induced by system X-c- inhibitors (e.g., erastin, sulfasalazine, and sorafenib). | ||||
Traumatic brain injury [ICD-11: NA07]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Responsed Drug | Baicalin | Terminated | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
In Vitro Model |
rPNs (Rat primary neurons) | ||||
| In Vivo Model |
Rats were injected with 4 mL/kg of chloral hydrate for anesthesia and then put on a stereotactic apparatus. Subsequently, the needle was tilted at 55 in the sagittal plane and fixed anterior to the bregma (7.5 mm). The needle tip was toward the right and lowered the anterior to the chiasma (2 mm). Finally, the nonheparinized autologous femoral arterial blood (0.3 mL) was injected into a prechiasmatic cistern using a syringe pump. Rat temperature was maintained at 37 ± 0.5 during the surgery. The rats in the sham group were injected with the same dose of saline into a prechiasmatic cistern. At last, rats were monitored for recovery and then returned to cages.
Click to Show/Hide
|
||||
| Response Description | Baicalin was confirmed to suppress the beclin1, LC3-II, and LC3-I protein levels in rat brain tissues. Moreover, we found that baicalin inhibited neuronal apoptosis. Overall, baicalin suppressed autophagy-dependent ferroptosis in early brain injury after subarachnoid hemorrhage. | ||||
Unspecific Regulator
Amentoflavone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response Description | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
Sulfasalazine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Responsed Disease | Colon cancer [ICD-11: 2B90] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | ||
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 HCT116, CX-1, or HT1080 cells in 100 ul phosphate buffered saline (PBS; Thermo Fisher Scientific, AM9625) were injected subcutaneously right of the dorsal midline in athymic nude immunodeficient mice (six- to eight-week-old, female). To generate orthotopic tumors, 1 x 106 KPC cells in 10 ul PBS were surgically implanted into the pancreases of immunocompetent C57BL/6J mice (six- to eight-week-old, female).
Click to Show/Hide
|
||||
| Response Description | BECN1 plays a novel role in lipid peroxidation that could be exploited to improve anticancer therapy by the induction of ferroptosis in Colon cancer. Mechanistically, phosphorylation of BECN1 at Ser90/93/96 by PRKAA/AMPK contributes to the formation of a BECN1-SLC7A11 complex and system Xc-inhibition. Knockdown of BECN1 by shRNA inhibits ferroptosis induced by system X-c- inhibitors (e.g., erastin, sulfasalazine, and sorafenib). | ||||
Baicalin
[Terminated]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | ||||
| Responsed Disease | Traumatic brain injury [ICD-11: NA07] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
| In Vitro Model | rPNs (Rat primary neurons) | ||||
| In Vivo Model |
Rats were injected with 4 mL/kg of chloral hydrate for anesthesia and then put on a stereotactic apparatus. Subsequently, the needle was tilted at 55 in the sagittal plane and fixed anterior to the bregma (7.5 mm). The needle tip was toward the right and lowered the anterior to the chiasma (2 mm). Finally, the nonheparinized autologous femoral arterial blood (0.3 mL) was injected into a prechiasmatic cistern using a syringe pump. Rat temperature was maintained at 37 ± 0.5 during the surgery. The rats in the sham group were injected with the same dose of saline into a prechiasmatic cistern. At last, rats were monitored for recovery and then returned to cages.
Click to Show/Hide
|
||||
| Response Description | Baicalin was confirmed to suppress the beclin1, LC3-II, and LC3-I protein levels in rat brain tissues. Moreover, we found that baicalin inhibited neuronal apoptosis. Overall, baicalin suppressed autophagy-dependent ferroptosis in early brain injury after subarachnoid hemorrhage. | ||||
References
