Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0166)
| Name |
Amentoflavone
|
||||
|---|---|---|---|---|---|
| Synonyms |
Amentoflavone; 1617-53-4; Didemethyl-ginkgetin; 3',8''-Biapigenin; Amenthoflavone; Tridemethylsciadopitysin; MLS000574827; CHEBI:2631; 9I1VC79L77; MFCD00017470; NSC-295677; 8-[5-(5,7-dihydroxy-4-oxo-chromen-2-yl)-2-hydroxy-phenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one; 8-[5-(5,7-dihydroxy-4-oxochromen-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one; SMR000156235; 8-(5-(5,7-Dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydroxyphenyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one; 4H-1-Benzopyran-4-one, 8-(5-(5,7-dihydroxy-4-oxo-4H-1-benzopyran-2-yl)-2-hydroxyphenyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-; 8-(5-(5,7-dihydroxy-4-oxo-4H-1-benzopyran-2-yl)-2-hydroxyphenyl)-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 8-[5-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one; SR-01000721725; UNII-9I1VC79L77; 4H-1-Benzopyran-4-one, 8-[5-(5,7-dihydroxy-4-oxo-4H-1-benzopyran-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)-; NSC 295677; AMENTOFLAVONE [INCI]; BIDD:PXR0028; I3',II8-BIAPIGENIN; GINKGETIN, DIDEMETHYL-; SCHEMBL312563; MEGxp0_000924; med.21724, Compound 138; DTXSID20167225; Amentoflavone, analytical standard; YUSWMAULDXZHPY-UHFFFAOYSA-N; BDBM429466; HMS2228B12; HMS3343J17; HMS3885A08; -hydroxyphenyl)-5,7-dihydroxy-2-; BCP13255; BEA14006; HY-N0662; Amentoflavone, >=99.0% (HPLC); LMPK12040009; NSC295677; s3833; (4-hydroxyphenyl)-4H-chromen-4-one; AKOS015896819; CCG-269950; CS-4945; 3',8-Bi[4',5,7-trihydroxyflavone]; NCGC00247542-01; NCGC00247542-02; AC-34718; BS-15502; FT-0622262; A11476; A810291; 8-(5-(5,7-dihydroxy-4-oxo-4H-chromen-2-yl)-2; Q-100192; Q4742425; SR-01000721725-2; SR-01000721725-3; 4',5,7-Trihydroxyflavone(3'->8)-4',5,7-trihydroxyflavone; 3''',8-BIFLAVONE, 4',4''',5,5'',7,7''-HEXAHYDROXY; 4',4''',5,5'',7,7''-Hexahydroxy-3''',8-biflavone, 8CI; 4H-1-Benzopyran-4-one, 8-[5-(5,7-dihydroxy-4-oxo-4H-1-benzopyran-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl); 8-[5-(5,7-dihydroxy-4-oxo-chromen-2-yl)-2-hydroxy-phenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one;Amentoflavone; 8-[5-(5,7-dihydroxy-4-oxochromen-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one.
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
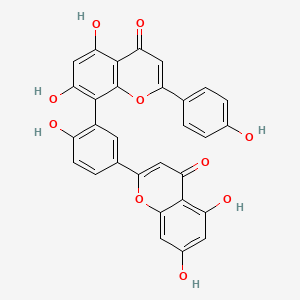 |
||||
| Formula |
C30H18O10
|
||||
| IUPAC Name |
8-[5-(5,7-dihydroxy-4-oxochromen-2-yl)-2-hydroxyphenyl]-5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one
|
||||
| Canonical SMILES |
C1=CC(=CC=C1C2=CC(=O)C3=C(O2)C(=C(C=C3O)O)C4=C(C=CC(=C4)C5=CC(=O)C6=C(C=C(C=C6O5)O)O)O)O
|
||||
| InChI |
InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-36H
|
||||
| InChIKey |
YUSWMAULDXZHPY-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Ferritin heavy chain (FTH1)
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Microtubule-associated proteins 1A/1B light chain 3B {ECO:0000305} (MAP1LC3B) | Driver | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response regulation | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Autophagy protein 5 (ATG5) | Driver | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response regulation | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Ubiquitin-like modifier-activating enzyme ATG7 (ATG7) | Driver | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response regulation | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
Unspecific Target
| In total 4 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Responsed Regulator | Cyclic AMP-dependent transcription factor ATF-2 (ATF2) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | GSE-1 (Human gastric mucosal epithelial cells) | ||||
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| In Vivo Model |
The BALB/c nude mice (n = 15, 4-6 weeks old) were purchased from Charles River Labs and kept under controlled conditions. Then 1 x 106 AGS cells were inoculated subcutaneously into nude mice. When the tumor reached to 100 mm3, mice were randomly divided into three groups, the control group was intraperitoneally injected with saline, the AF group was intraperitoneally injected with AF at dosages of 80 mg/kg/day, and AF + anti-miR-496 group received intraperitoneal injection, followed by the administration of miR-496 antagonist via intra-tumor injection once a week for 4 weeks.
Click to Show/Hide
|
||||
| Response regulation | Amentoflavone suppressed the proliferation and induced ferroptotic cell death in gastric cancer cells via miR-496/ATF2 axis, indicating a novel therapeutic approach for GC patients. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Responsed Regulator | hsa-miR-496 (miRNA) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | GSE-1 (Human gastric mucosal epithelial cells) | ||||
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| In Vivo Model |
The BALB/c nude mice (n = 15, 4-6 weeks old) were purchased from Charles River Labs and kept under controlled conditions. Then 1 x 106 AGS cells were inoculated subcutaneously into nude mice. When the tumor reached to 100 mm3, mice were randomly divided into three groups, the control group was intraperitoneally injected with saline, the AF group was intraperitoneally injected with AF at dosages of 80 mg/kg/day, and AF + anti-miR-496 group received intraperitoneal injection, followed by the administration of miR-496 antagonist via intra-tumor injection once a week for 4 weeks.
Click to Show/Hide
|
||||
| Response regulation | Amentoflavone suppressed the proliferation and induced ferroptotic cell death in gastric cancer cells via miR-496/ATF2 axis, indicating a novel therapeutic approach for GC patients. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Responsed Disease | Corpus uteri cancer | ICD-11: 2C76 | |||
| Responsed Regulator | Serine/threonine-protein kinase mTOR (MTOR) | Suppressor | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | hESCs (Human endometrial stromal cells) | ||||
| KLE cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 | ||
| Response regulation | Amentoflavone inhibited the viability and proliferation of endometrial carcinoma cells (KLE) cells but promoted apoptosis and ferroptosis. The expressions of ROS and AMPK were increased, while mTOR expression was decreased in AF-treated KLE cells. NAC reversed the effects of AF on biological behaviors of KLE cells by inactivating ROS/AMPK/mTOR signaling. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Responsed Disease | Corpus uteri cancer | ICD-11: 2C76 | |||
| Responsed Regulator | 5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1) | Driver | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | hESCs (Human endometrial stromal cells) | ||||
| KLE cells | Endometrial adenocarcinoma | Homo sapiens | CVCL_1329 | ||
| Response regulation | Amentoflavone inhibited the viability and proliferation of endometrial carcinoma cells (KLE) cells but promoted apoptosis and ferroptosis. The expressions of ROS and AMPK were increased, while mTOR expression was decreased in AF-treated KLE cells. NAC reversed the effects of AF on biological behaviors of KLE cells by inactivating ROS/AMPK/mTOR signaling. | ||||
Beclin-1 (BECN1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response regulation | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
References
