Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10022)
| Target Name | Ferritin heavy chain (FTH1) | ||||
|---|---|---|---|---|---|
| Synonyms |
Cell proliferation-inducing gene 15 protein
Click to Show/Hide
|
||||
| Gene Name | FTH1 | ||||
| Sequence |
MTTASTSQVRQNYHQDSEAAINRQINLELYASYVYLSMSYYFDRDDVALKNFAKYFLHQS
HEEREHAEKLMKLQNQRGGRIFLQDIKKPDCDDWESGLNAMECALHLEKNVNQSLLELHK LATDKNDPHLCDFIETHYLNEQVKAIKELGDHVTNLRKMGAPESGLAEYLFDKHTLGDSD NES Click to Show/Hide
|
||||
| Family | Ferritin family | ||||
| Function |
Stores iron in a soluble, non-toxic, readily available form. Important for iron homeostasis. Has ferroxidase activity. Iron is taken up in the ferrous form and deposited as ferric hydroxides after oxidation. Also plays a role in delivery of iron to cells. Mediates iron uptake in capsule cells of the developing kidney.
Click to Show/Hide
|
||||
| Gene ID | 2495 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
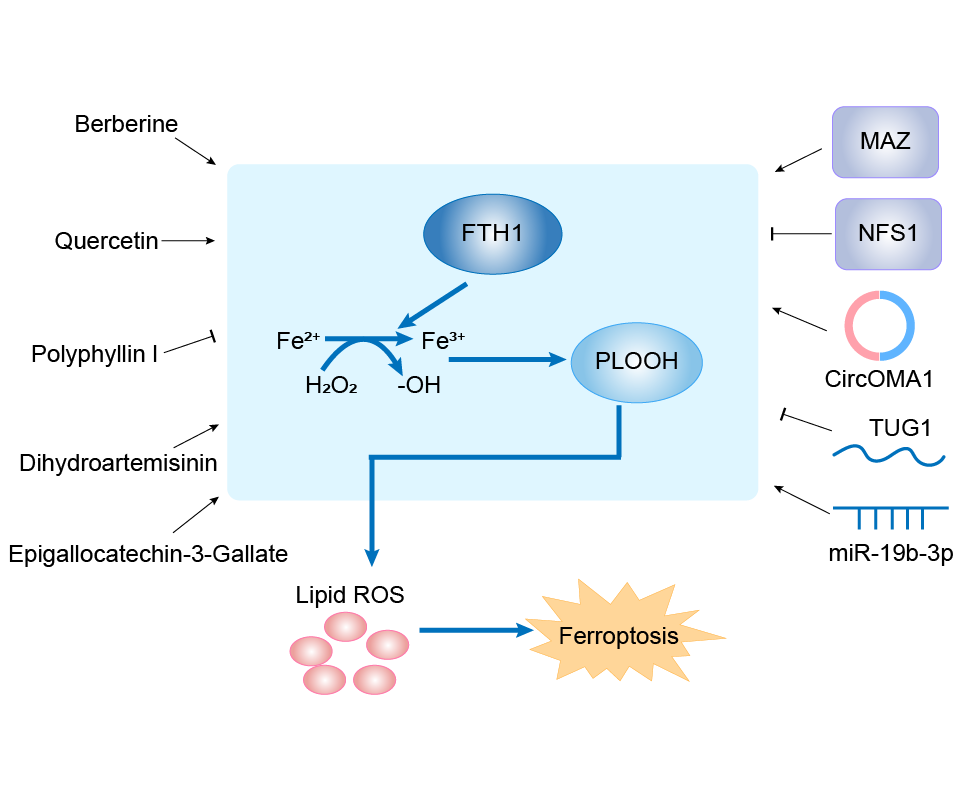
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
FTH1 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Ubiquitin-like modifier-activating enzyme ATG7 (ATG7)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Amentoflavone | Investigative | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response Description | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
TUG1 (IncRNA)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Dihydroartemisinin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
All BALB/C nude mice were purchased from Huafukang Biotechnology (Beijing, China). These mice were 5 weeks old and weighed 14-16 g. We established subcutaneous tumour-forming mouse model by injecting 5 x 106 U87 cells into the lateral abdomen of BALB/C nude mice. Animals were then treated with DHA solvent (50 mg/kg) by intragastric administration once a day for 26 days.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin (DHA) could promote ferroptosis in glioma cells. Low expression of GPX4 and high expression of HMOX1 were identified in DHA treated glioma cells. MAZ was further identified as the direct target of long noncoding RNA (lncRNA) TUG1 through luciferase assay. Downregulated expression of TUG1 and upregulated expression of MAZ were identified in DHA treated glioma cells. TUG1 overexpression or inhibition of FTH1 expression could enhance the antiglioma effect of DHA in vitro and in vivo, providing a promising strategy to enhance the antitumor effect of DHA in glioma. | ||||
Serine/threonine-protein kinase ULK1 (ULK1)
Renal dysfunction [ICD-11: GB6Z]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Bisphenol A | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
TCMK-1 cells | Normal | Mus musculus | CVCL_2772 | |
| In Vivo Model |
Balb/c mice aged 6-8 weeks were purchased from Liaoning Changsheng biotechnology co., Ltd. (Benxi, China). All animal experiments comply with the requirements of the Institutional Animal Care and Use Committee (IACUC) of Jilin University. The mice were housed in separate cages and given enough food and drinking water during the conditions of a 12-h light and dark cycle. The establishment of animal models is as previously described.
Click to Show/Hide
|
||||
| Response Description | Renal dysfunction and renal tubular epithelial damage induced by Bisphenol A (BPA) are linked to ferroptosis, which depends on the activation of ferritinophagy through AMPK-mTOR- ULK1 axis. These findings revealed that after BPA treatment, FTH was significantly reduced and iron was accumulated. | ||||
NAD-dependent protein deacylase sirtuin-6 (SIRT6)
Cataract [ICD-11: 9B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Melatonin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
B-3 cells | Normal | Homo sapiens | CVCL_6367 | |
| In Vivo Model |
Six-week-old albino Sprague Dawley (SD) male rats were provided by the Experimental Animal Centre of the Second Affiliated Hospitalof Harbin Medical University. Fifteen minutes before exposure, the rats were anaesthetized by intraperitoneal injection of a mixture of 90 mg/kg ketamine and 15 mg/kg xylazine. Then, tropicamide phenylephrine was dropped in both eyes; at the same time, the rats that received drug treatment were injected subconjunctivally (5 ul/eye) with 500 mM Fer-1, 200 mM MT or the same dose of DMSO used to dissolve the drug using a 28-gauge needle and a Hamilton microinjector. After another 5 min, a single eye of every experimental group rat was exposed to UVB (312 nm) 5 W/m2 for 30 min. Every time, UVB exposure was synchronized with the drug injection, and the frequency was every other day until it was stopped 9 weeks later.
Click to Show/Hide
|
||||
| Response Description | Melatonin inhibited ferroptosis through the SIRT6/p-Nrf2/GPX4 and SIRT6/COA4/FTH1 pathways to neutralize lipid peroxidation toxicity, which protected cells against ferroptotic stress in vitro and delayed cataract formation caused by UVB exposure in rats. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Cadmium | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/ Sirt1 axis. | |||
Myc-associated zinc finger protein (MAZ)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Dihydroartemisinin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
All BALB/C nude mice were purchased from Huafukang Biotechnology (Beijing, China). These mice were 5 weeks old and weighed 14-16 g. We established subcutaneous tumour-forming mouse model by injecting 5 x 106 U87 cells into the lateral abdomen of BALB/C nude mice. Animals were then treated with DHA solvent (50 mg/kg) by intragastric administration once a day for 26 days.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin (DHA) could promote ferroptosis in glioma cells. Low expression of GPX4 and high expression of HMOX1 were identified in DHA treated glioma cells. MAZ was further identified as the direct target of long noncoding RNA (lncRNA) TUG1 through luciferase assay. Downregulated expression of TUG1 and upregulated expression of MAZ were identified in DHA treated glioma cells. TUG1 overexpression or inhibition of FTH1 expression could enhance the antiglioma effect of DHA in vitro and in vivo, providing a promising strategy to enhance the antitumor effect of DHA in glioma. | ||||
Microtubule-associated proteins 1A/1B light chain 3B {ECO:0000305} (MAP1LC3B)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Amentoflavone | Investigative | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response Description | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
Microtubule-associated proteins 1A/1B light chain 3A (MAP1LC3A)
Pulmonary fibrosis [ICD-11: CB03]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Dihydroquercetin | Preclinical | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hBEs (Human bronchial epithelial cells) | ||||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| In Vivo Model |
Eight-week-old male C57BL/6 mice were purchased from Hubei University of Medicine (Shiyan, China). The SiO2-induced mouse pulmonary fibrosis model was performed. In brief, each group of mice was anesthetized with 1% pentobarbital sodium intraperitoneally at 40 mg/kg body weight and their tracheae had been surgically exposed. In addition, SiO2 suspension (20 mg in 50 ul saline) was instilled in the mice. The vehicle control groups were given an equivalent amount of 0.9% sterile saline. After one week of acclimation, mice were divided randomly into four groups (n = 8 per group). Control group, SiO2 group, SiO2 and low dose of DHQ group (DHQ-L, 10 mg/kg) as well as large dose of DHQ group (DHQ-H, 50 mg/kg).
Click to Show/Hide
|
||||
| Response Description | Dihydroquercetin suppressed ferritinophagy by down-regulation of microtubule-associated protein 1A/ 1B-light chain 3 (LC3), and up-regulation of ferritin heavy chain 1 (FTH1), nuclear receptor co-activator 4 (NCOA4) in activated HBE cells. Research revealed that inhibition of ferritinophagy-mediated HBE cells ferroptosis was responsible for DHQ to ameliorate SiO2-induced lung fibrosis. | ||||
hsa-miR-34a-5p (miRNA)
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Cadmium | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/Sirt1 axis. | |||
hsa-miR-19b-3p (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Curcumenol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| CCD-19Lu cells | Normal | Homo sapiens | CVCL_2382 | ||
| BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | ||
| In Vivo Model |
A subcutaneous tumor-bearing nude mouse model was established by injecting the flank of BALB/c nude mice with 5 x 106 H460 cells. Ten days later, mice were blindly randomized into four groups and intravenously injected with 200 ul ddH2O containing 0.1% CMC-Na and 1% Tween 80, iron chelators DFO (100 mg/kg/day), curcumenol (200 mg/kg/day), and curcumenol combine DFO. Tumor long diameter, short diameter, and body weight were detected every two days after the first drug treatment. The tumor volume calculation formula: (tumor long diameter x tumor short diameter2)/2. Finally, mice were sacrificed. All tumors were collected for immunohistochemical (IHC) staining.
Click to Show/Hide
|
||||
| Response Description | The natural product curcumenol exerted its antitumor effects on lung cancer by triggering ferroptosis, and the lncRNA H19/miR-19b-3p/FTH1 axis plays an essential role in curcumenol-induced ferroptotic cell death. Mechanistically, we showed that lncRNA H19 functioned as a competing endogenous RNA to bind to miR-19b-3p, thereby enhanced the transcription activity of its endogenous target, ferritin heavy chain 1 (FTH1), a marker of ferroptosis. | ||||
H19 (IncRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Curcumenol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| CCD-19Lu cells | Normal | Homo sapiens | CVCL_2382 | ||
| BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | ||
| In Vivo Model |
A subcutaneous tumor-bearing nude mouse model was established by injecting the flank of BALB/c nude mice with 5 x 106 H460 cells. Ten days later, mice were blindly randomized into four groups and intravenously injected with 200 ul ddH2O containing 0.1% CMC-Na and 1% Tween 80, iron chelators DFO (100 mg/kg/day), curcumenol (200 mg/kg/day), and curcumenol combine DFO. Tumor long diameter, short diameter, and body weight were detected every two days after the first drug treatment. The tumor volume calculation formula: (tumor long diameter x tumor short diameter2)/2. Finally, mice were sacrificed. All tumors were collected for immunohistochemical (IHC) staining.
Click to Show/Hide
|
||||
| Response Description | The natural product curcumenol exerted its antitumor effects on lung cancer by triggering ferroptosis, and the lncRNA H19/miR-19b-3p/FTH1 axis plays an essential role in curcumenol-induced ferroptotic cell death. Mechanistically, we showed that lncRNA H19 functioned as a competing endogenous RNA to bind to miR-19b-3p, thereby enhanced the transcription activity of its endogenous target, ferritin heavy chain 1 (FTH1), a marker of ferroptosis. | ||||
CircOMA1 (circRNA)
Prolactinoma [ICD-11: 2F37]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Cabergoline | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
Carbonyl reductase [NADPH] 1 (CBR1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Chrysin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Capan-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0026 | ||
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| AsPC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0152 | ||
| In Vivo Model |
Male BALB/c nude mice (5 weeks old, weighing 18-20 g) were provided by Jiangsu Jicui Yaokang Biotechnology Co., Ltd. (Nanjing, China). The mice were subcutaneously transplanted with non-targeting shRNA (shcontrol) or CBR1-targeting shRNA (shCBR1)-transfected PANC-1 cells (200 uL, 1 x 107 cells). Tumor volumes and body weights were measured every 4 days (n = 4, each), tumor volume = 0.5 x (a x a x b) (a, smallest diameter; b, largest diameter). The mice were subcutaneously inoculated with PANC-1 cells (200 uL, 1 x 107 cells) in the combination treatment. When the tumor volume reached 80-100 mm3, the mice were treated with chrysin (30 mg/kg/i.P., daily), gemcitabine (20 mg/kg/i.p., once every other day), or in combination for four weeks.
Click to Show/Hide
|
||||
| Response Description | Inhibition of CBR1 by chrysin increased cellular ROS levels and led to ROS-dependent autophagy, which resulted in the degradation of ferritin heavy polypeptide 1 (FTH1) and an increase in the intracellular free iron level that participates in ferroptosis in pancreatic cancer (PC) cells. Finally, chrysin enhanced PC sensitivity to gemcitabine by inducing ferroptotic death in vitro and in vivo.? | ||||
Bromodomain-containing protein 4 (BRD4)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | JQ1 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| In Vivo Model |
Female athymic BALB/c nude mice (4-6-week old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Approximately 1 x 107 cells (A549) in 200 uL of serum-free medium and Matrigel solution were injected directly into the right axilla of each mouse. Tumor growth was measured with calipers every 3 days.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis was induced under (+)-JQ1 treatment and BRD4 knockdown, indicating that (+)-JQ1 induces ferroptosis via BRD4 inhibition in breast adenocarcinoma. In addition, expression of the ferroptosis-associated genes GPX4, SLC7A11, and SLC3A2 was downregulated under (+)-JQ1 treatment. Moreover, JQ1 treatment and BRD4 knockdown led to decreased FTH1 expression. | ||||
Autophagy protein 5 (ATG5)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Amentoflavone | Investigative | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response Description | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
A disintegrin and metalloproteinase with thrombospondin motifs 18 (ADAMTS18)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Curcumin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 |
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | |
| Response Description | Curcumin induces ferroptosis in tumor cells by upregulating the expression of ADAMTS18, thereby enhancing the sensitivity of clear cell renal cell carcinoma (ccRCC) to sunitinib. And Curcumin can significantly inhibit FTH1 and FTL1 gene expression in tumor tissues of nude mice. | |||
ZFAS1 (IncRNA)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
To simulate the animal model of diabetic cardiomyopathy, male db/+ mice and db/db mice (age, 7 weeks, weight, 24 g) were fed a normal diet for 4 weeks and kept at 24 under a 14-h light/8-h dark cycle. The animals were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). Diabetic mice were intracoronarily administered equal volumes (80 ul) of adenoviruses Ad-ZFAS1, Ad-sh-ZFAS1, Ad-CCND2, Ad-sh-CCND2 or Ad-NC.33 miR-150-5p mimics and mimic control (NC) were injected into the tail vein of mice (50 ug/kg) every 15 days for 12 weeks. Db/db mice were treated with or without ferrostatin-1 (Fer-1, ferroptosis inhibitor; Sigma-Aldrich, 5 mg/kg) for an additional 12 weeks.
Click to Show/Hide
|
||||
| Response Description | lncRNA-ZFAS1 acted as a ceRNA to sponge miR-150-5p and downregulate CCND2 to promote cardiomyocyte ferroptosis and Diabetic cardiomyopathy development. Inhibition of ZFAS1 restored the expression of FTH1, reduced the expression of 4HNE, rescued the expression of GPX4 and inhibited the expression of apoptosisrelated genes. | ||||
Transcription regulator protein BACH1 (BACH1)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
In Vitro Model |
mEFs (Mouse embryonic fibroblasts) | ||||
| In Vivo Model |
The generation of Bach1-/-mice on the C57BL/6J background was described previously. Mice 13 weeks of age were analyzed for models of AMI. The mice were subjected to ligation of the proximal LAD to induce AMI. They were randomly assigned to sham or AMI, DMSO, or DFX groups.
Click to Show/Hide
|
||||
| Response Description | BACH1 accelerates ferroptosis by suppressing labile iron metabolism. And ferritin genes (Fth1 and Ftl1) and the ferroportin gene (Slc40a1) were dramatically up-regulated in Bach1-/- MEFs. BACH1 controls the threshold of ferroptosis induction and may represent a therapeutic target for alleviating ferroptosis-related diseases, including myocardial infarction. | ||||
Transcription factor AP-2-alpha (TFAP2A)
Gallbladder cancer [ICD-11: 2C13]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
H69 cells | Normal | Homo sapiens | CVCL_8121 |
| GBC-SD cells | Gallbladder carcinoma | Homo sapiens | CVCL_6903 | |
| Response Description | In vitro, gallbladder carcinoma (GBC) exhibited upregulated expression of TFAP2A, whose inhibition reduced GBC cell proliferation, migration, and invasion. Fe2+ and MDA levels were elevated. TFAP2A silencing attenuated the expression of key genes associated with oxidative stress such as heme oxygenase 1 (HO-1), nuclear factor erythroid 2 like 2 (Nrf2), ferritin heavy chain 1 (FTH1) and NAD(P)H quinone dehydrogenase 1 (NQO1). | |||
rno-miR-335 (miRNA)
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In Vivo Model |
A total of 48 male Sprague-Dawley rats (weighing, 180-220 g; 6-8 weeks of age) were purchased from the Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China). The rats were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine, intraperitoneal injection) and placed in a stereotaxic apparatus with the skull flat. An intracerebral injection of 6-OHDA was performed at 2 sites in the right SN pars compacta (SNpc) and ventral tegmental area (VTA): Anteroposterior (A/P)=-4.9 mm; mediolateral (M/L)=-1.9 mm; dorsoventral (D/V)=-7.5 mm; and anteroposterior (A/P)=-4.9 mm; mediolateral (M/L)=-1.1 mm; dorsoventral (D/V)=8.0 mm. During the surgery, body temperature was maintained at ~36.5 using a heating pad. All rats received meticulous post-operative care.
Click to Show/Hide
|
||||
| Response Description | MiR335 promotes ferroptosis by targeting FTH1 inin vitroandin vivomodels of Parkinson's disease, providing a potential therapeutic target for the treatment of PD. Mechanistically, miR335 enhanced ferroptosis through the degradation of FTH1 to increase iron release, lipid peroxidation and reactive oxygen species (ROS) accumulation, and to decrease mitochondrial membrane potential (MMP). | ||||
rno-miR-29a-3p (miRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rBMMSCs (Rat bone marrow mesenchymal stem cells) | ||||
| IAR 20 cells | Normal | Homo sapiens | CVCL_5296 | ||
| In Vivo Model |
Clean-grade male Sprague-Dawley (SD) rats were purchased from China Food and Drug Administration (Beijing, China). SD rats were fed a high-fat diet (Composition: 15% triglyceride, 15% sucrose, 10% egg yolk powder, 1% cholesterol, 0.2% bile salt, 58.8% basic feed) for 20 weeks. Hematoxylin and eosin (HE) and oil red O staining showed that the area of mixed macrovesicular steatosis was more than 60% under the microscope, indicating that a model of severe steatotic liver was established successfully. A 70% liver thermal ischemia model was established, continuously blocked for 80 min, and then, the ischemic liver was obtained 24 h after reperfusion.
Click to Show/Hide
|
||||
| Response Description | miR-29a-3p, which targets IREB2, is abundant in HO-1/BMMSC-exosomes and could decrease the IREB2 protein level. The reduced IREB2 level led to an increase in the level of FTH1 and decreased the level of TFR1 through posttranscriptional regulation, which ultimately reduced the level of intracellular Fe2+ and the production of lipid ROS and inhibited the occurrence of ferroptosis in SHP-HR. In conclusion, ferroptosis plays an important role in HO-1/BMMSC-mediated alleviation of steatotic hepatic ischemia-reperfusion injury. | ||||
Iron-sulfur cluster assembly enzyme ISCU (ISCU)
Acute myeloid leukaemia [ICD-11: 2A60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| Cell cycle | |||||
In Vitro Model |
HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| KG1 cells | Normal | Mus musculus | CVCL_UD72 | ||
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| In Vivo Model |
BALB/c Nude Mice (4 weeks old) were obtained from Shanghai Experimental Animal Center of the Chinese Academy of Sciences (Shanghai, China) and then subcutaneously injection with HL60 cells (1 x 107, suspended in 0.1 mL PBS). After tumors reached 100-200 mm3, the mice were randomly assigned to two groups. DHA was administered intraperitoneal injection once a day at 50 mg/kg body weight and the mice in normal control were received equal amounts of vehicle (10% DMSO in sterile corn oil). On the 28th day, mice were euthanized. The tumor volumes were measured every 4 days with a caliper.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin (DHA) strongly inhibited the viability of acute myeloid leukemia (AML) cell lines and arrest cell cycle at G0/G1 phase. ISCU over-expression robustly alleviated the iron starvation response through regulating the expression of IRPs and downstream FTH. ISCU significantly attenuated DHA induced ferroptosis by regulating iron metabolism, rescuing the mitochondrial function and increasing the level of GSH. | ||||
hsa-miR-224-5p (miRNA)
Congestive heart failure [ICD-11: BD10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mMTs (Mouse myocardial tissues) | ||||
| In Vivo Model |
Six-week-old male C57/BL6J mice (weighting ~18-20 g) were supplied by the Shenyang Military Region General Hospital Experimental Animal Center. The mice were fed a standard diet and had unlimited access to drinking water. After 2 weeks of feeding, the mice were randomly assigned to TAC group (n = 15) and SHAM group (n = 16), and then anesthetized by intraperitoneal injection of 50 mg/kg pentobarbital sodium. To allow direct access to the transverse aorta, the mice a horizontal incision (5 mm in length) was made at the suprasternal notch. TAC operation was then performed by ligating the aorta between the right innominate and left carotid arteries using a 27G needle tied with 7-0 silk suture. The needle was promptly withdrawn, leaving the aortic constriction in place. The surgical procedure of mice in SHAM group was similar to that of mice in TAC group, except that the 7-0 silk suture was only crossed through the aortic arch without ligation.
Click to Show/Hide
|
||||
| Response Description | MiR-224-5p was indeed the downstream target of circSnx12, and miR-224-5p could bind to the 3'-UTR region of FTH1 and regulate its expression level. Therefore, it is speculated that low circSnx12 expression and high miR-224-5p expression can downregulate FTH1 expression, directly regulate iron overload in myocardial cells, and ultimately leads to cardiac cell death. This new approach reveals potential circRNA targets for the treatment of heart failure. | ||||
hsa-miR-150-5p (miRNA)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
To simulate the animal model of diabetic cardiomyopathy, male db/+ mice and db/db mice (age, 7 weeks, weight, 24 g) were fed a normal diet for 4 weeks and kept at 24 under a 14-h light/8-h dark cycle. The animals were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). Diabetic mice were intracoronarily administered equal volumes (80 ul) of adenoviruses Ad-ZFAS1, Ad-sh-ZFAS1, Ad-CCND2, Ad-sh-CCND2 or Ad-NC.33 miR-150-5p mimics and mimic control (NC) were injected into the tail vein of mice (50 ug/kg) every 15 days for 12 weeks. Db/db mice were treated with or without ferrostatin-1 (Fer-1, ferroptosis inhibitor; Sigma-Aldrich, 5 mg/kg) for an additional 12 weeks.
Click to Show/Hide
|
||||
| Response Description | lncRNA-ZFAS1 acted as a ceRNA to sponge miR-150-5p and downregulate CCND2 to promote cardiomyocyte ferroptosis and Diabetic cardiomyopathy development. Inhibition of ZFAS1 restored the expression of FTH1, reduced the expression of 4HNE, rescued the expression of GPX4 and inhibited the expression of apoptosisrelated genes. | ||||
Glutaredoxin-related protein 5, mitochondrial (GLRX5)
Head neck squamous cell carcinoma [ICD-11: 2D60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AMC-HN-3 cells | Laryngeal squamous cell carcinoma | Homo sapiens | CVCL_5961 | |
| HN3R (Human head and neck squamous cell carcinoma cell) | |||||
| HN4 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_IS30 | ||
| HN4R (Human head and neck squamous cell carcinoma cell) | |||||
| In Vivo Model |
Five-week-old athymic BALB/c male nude mice (nu/nu) were purchased from Central Lab Animal Inc. (Seoul, Republic of Korea). HN4R cells with vector control or shGLRX5 were subcutaneously injected into the bilateral flank of nude mice. From the day when gross nodules were detected in tumor implants, mice were subjected to different treatments: vehicle or SAS (250 mg/kg daily per intraperitoneal route). Each group included seven mice.
Click to Show/Hide
|
||||
| Response Description | Inhibition of GLRX5 predisposes therapy-resistant head and neck cancer (HNC) cells to ferroptosis. Increased IRP and TfR and decreased Fpn and FTH boosted up intracellular free iron, resulting in lipid peroxidation and ferroptosis in vitro and in vivo. | ||||
G1/S-specific cyclin-D2 (CCND2)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
hCMs (Human cardiomyocytes) | ||||
| In Vivo Model |
To simulate the animal model of diabetic cardiomyopathy, male db/+ mice and db/db mice (age, 7 weeks, weight, 24 g) were fed a normal diet for 4 weeks and kept at 24 under a 14-h light/8-h dark cycle. The animals were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). Diabetic mice were intracoronarily administered equal volumes (80 ul) of adenoviruses Ad-ZFAS1, Ad-sh-ZFAS1, Ad-CCND2, Ad-sh-CCND2 or Ad-NC.33 miR-150-5p mimics and mimic control (NC) were injected into the tail vein of mice (50 ug/kg) every 15 days for 12 weeks. Db/db mice were treated with or without ferrostatin-1 (Fer-1, ferroptosis inhibitor; Sigma-Aldrich, 5 mg/kg) for an additional 12 weeks.
Click to Show/Hide
|
||||
| Response Description | lncRNA-ZFAS1 acted as a ceRNA to sponge miR-150-5p and downregulate CCND2 to promote cardiomyocyte ferroptosis and Diabetic cardiomyopathy development. Inhibition of ZFAS1 restored the expression of FTH1, reduced the expression of 4HNE, rescued the expression of GPX4 and inhibited the expression of apoptosisrelated genes. | ||||
Fanconi anemia group D2 protein (FANCD2)
Bone marrow injury [ICD-11: PK81]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
mBMSCs (Mouse bone marrow stromal cells) | |||
| Response Description | Bone marrow injury remains a serious concern in traditional cancer treatment. FANCD2 regulated genes and/or expression of proteins involved in iron metabolism (e.g., FTH1, TF, TFRC, HAMP, HSPB1, SLC40A1, and STEAP3) and lipid peroxidation (e.g., GPX4). | |||
Cysteine desulfurase (NFS1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MCF10DCIS cells | Normal | Homo sapiens | CVCL_5552 | |
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| SW900 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_1731 | ||
| NCI-H196 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1509 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| NCI-H2170 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_1535 | ||
| NCI-H647 cells | Lung adenosquamous carcinoma | Homo sapiens | CVCL_1574 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| NCI-H838 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | ||
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | ||
| NCI-H322 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1556 | ||
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | ||
| In Vivo Model |
Tumours were initiated in 4-8-week-old female NOD. CB17 Scid/J mice. Orthotopically in the mouse mammary gland, by implantation of 500,000 cells in 25 ul 33% Matrigel into the fourth mouse mammary fat pad; subcutaneously, by injection of 500,000 cells in 100 ul 33% Matrigel into the left or right flank of the mouse; via tail vein by injection of 500,000 cells in 150 ul RPMI into the mouse tail vein; and via intratracheal instillation by instilling 200,000 cells in 50 ul 2 mM EDTA as described. Cancer cells were transduced with viral shRNAs, selected for 3 days with puromycin, and allowed to recover for one day before introduction into mice. For experiments comparing subcutaneous and lung tumour formation, shRNA transduced cells were prepared at the same time and injected on the same day. Animals were imaged by IVIS (Perkin Elmer) 15 min following injection subcutaneously into the neck scruff with XenoLight d-Luciferin (165 mg per kg body weight, Perkin Elmer). Average luminescence was quantified per mouse from equal sized bins covering the mouse thorax. For experiments in which tumour growth was measured upon drug treatment, MDA-MB-231 cells, implanted as described above, were allowed to form palpable tumours (~4 mm diameter) and mice were sorted into treatment groups as described below. PEG-Cyst(e)inase was delivered via intraperitoneal injection at 50 mg per kg body weight every 3 days, SSA was delivered by daily intraperitoneal injection at 250 mg per kg body weight, and BSO was delivered in the drinking water at 20 mM with 5 mg ml-1 sucralose.
Click to Show/Hide
|
||||
| Response Description | NFS1 suppression induced TFRC expression and repressed FTH1 and cytoplasmic aconitase activity. Suppression of NFS1 cooperates with inhibition of cysteine transport to trigger ferroptosis in vitro and slow tumour growth. Therefore, lung adenocarcinomas select for expression of a pathway that confers resistance to high oxygen tension and protects cells from undergoing ferroptosis in response to oxidative damage. | ||||
CircSnx12 (circRNA)
Congestive heart failure [ICD-11: BD10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mMTs (Mouse myocardial tissues) | ||||
| In Vivo Model |
Six-week-old male C57/BL6J mice (weighting ~18-20 g) were supplied by the Shenyang Military Region General Hospital Experimental Animal Center. The mice were fed a standard diet and had unlimited access to drinking water. After 2 weeks of feeding, the mice were randomly assigned to TAC group (n = 15) and SHAM group (n = 16), and then anesthetized by intraperitoneal injection of 50 mg/kg pentobarbital sodium. To allow direct access to the transverse aorta, the mice a horizontal incision (5 mm in length) was made at the suprasternal notch. TAC operation was then performed by ligating the aorta between the right innominate and left carotid arteries using a 27G needle tied with 7-0 silk suture. The needle was promptly withdrawn, leaving the aortic constriction in place. The surgical procedure of mice in SHAM group was similar to that of mice in TAC group, except that the 7-0 silk suture was only crossed through the aortic arch without ligation.
Click to Show/Hide
|
||||
| Response Description | MiR-224-5p was indeed the downstream target of circSnx12, and miR-224-5p could bind to the 3'-UTR region of FTH1 and regulate its expression level. Therefore, it is speculated that low circSnx12 expression and high miR-224-5p expression can downregulate FTH1 expression, directly regulate iron overload in myocardial cells, and ultimately leads to cardiac cell death. This new approach reveals potential circRNA targets for the treatment of heart failure. | ||||
Alpha-ketoglutarate-dependent dioxygenase FTO (FTO)
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
ICR mice (8-week-old, 18-22 g) were obtained from Yangzhou University (Yangzhou, China). There were 8 mice in each group and they were randomly divided into 6 groups. Mice were treated with Vehicle, CCl4, VA-Lip-control-vector+CCl4+Erastin, VA-Lip-Mettl4-shRNA+CCl4+Erastin, VA-Lip-Fto-plasmid+CCl4+Erastin, VA-Lip- Ythdf1-shRNA+CCl4+Erastin, respectively. A mixture of olive oil and carbon tetrachloride (CCl4) (9:1 (v/v)) was used to trigger liver fibrosis in mouse model by intraperitoneal injection (0.1 ml/20 g body weight), according to our previous reports.
Click to Show/Hide
|
||||
| Response Description | m6A reader YTHDF1 promoted BECN1 mRNA stability via recognizing the m6A binding site, thus triggering autophagy activation, and eventually leading to HSC ferroptosis. FTO plasmid and METTL4 shRNA markedly impaired erastin-induced upregulation of NCOA4 and downregulation of FTH1 in HSC-LX2 cells. Overall, m6A modification-dependent ferroptosis as a potential target for the treatment of liver fibrosis. | ||||
Unspecific Regulator
Osteosarcoma [ICD-11: 2B51]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Responsed Drug | Ursolic Acid | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
In Vitro Model |
HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| 143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | ||
| In Vivo Model |
NU/NU mice (the Fourth Military Medical University, Shaanxi, China) were injected with 143B cells (100 uL, 5 x 107 cells/mL, i.h.). Seven days after the injection, the mice were divided into 6 different groups (n= 3) and intraperitoneally injected with different drugs twice a week. Then, on day 28, the mice were sacrificed, and the tumours in the different groups were weighed. Body weight and tumour size were measured every 3 days from day 7 to day 28. The tumour tissue was fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 4 um thick sections for haematoxylin-eosin (H&E) and immunofluorescence staining.
Click to Show/Hide
|
||||
| Response Description | Ursolic acid inhibited tumour cell proliferation and promoted the apoptosis of a variety of osteosarcoma cells. Mechanistic studies showed that ursolic acid degraded ferritin by activating autophagy and induced intracellular overload of ferrous ions, leading to ferroptosis. Ferritin, which includes ferritin light peptide 1 (FTL1) and ferritin heavy peptide 1 (FTH1). | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | ||||
| Responsed Drug | Bavachin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | ||
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | ||||
Gastric cancer [ICD-11: 2B72]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [25] | ||||
| Responsed Drug | Polyphyllin I | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| In Vivo Model |
A subcutaneous gastric tumor model was established by subcutaneously injecting 1 x 106 AGS cells or 2 x 106 MKN-45 cells near the right axilla of mice. Seven days after tumor cell inoculation, mice received daily i. p. Injection of PPI (3 mg/kg, dissolved in 1% DMSO + 5% PEG300 + 5% Tween 80 + 89% deionized water), as described previously, or the control solution with the same solvent. Mice were weighed at day 15, while tumor volumes were measured every 3 days and calculated using a formula: length x width2/2.
Click to Show/Hide
|
||||
| Response Description | For the first time, our results have demonstrated that Polyphyllin I exerts its antitumor activity on the gastric cancer by, at least partially, inducing cancer cell ferroptosisviaregulating NRF2/FTH1 pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | ||||
| Responsed Drug | Polyphyllin B | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
NUGC-3 cells | Gastric carcinoma | Homo sapiens | CVCL_1612 | |
| MKN-1 cells | Gastric carcinoma | Homo sapiens | CVCL_1415 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| In Vivo Model |
The nude mice were raised in our laboratory for a week before the experiment. Then, 5 x 106 MKN-1 cells were subcutaneously injected to establish the subcutaneous xenograft tumour model in nude mice. When the maximum diameter of the xenograft tumours grew steadily to 1 cm, they were dissected completely and cut into 1 mm3 tissue fragments. Then, the tissue fragment was inserted into the surface of the serosa on the greater curvature of the stomach. Different doses of PB (2.5 mg/kg or 5.0 mg/kg) were given by intraperitoneal injection once a day for 3 weeks. The control group was given the same volume of vehicle. The positive control group was given 5-Fu at the dose of 10 mg/kg. The body weight and tumour size of nude mice were recorded. Mice were administered fluorescein substrate (150 mg/kg) intraperitoneally for in vivo imaging twice a week on a Xenogen IVIS 200 imaging system (Caliper Life Sciences, USA). The tumour inhibition rate was analysed using LT Living Image 4.3 Software.
Click to Show/Hide
|
||||
| Response Description | We identified a novel GPx4 inhibitor, polyphyllin B (PB), which can induce ferroptosis by down-regulating GPx4 expression in gastric cancer cells. It has also been shown to inhibit cell proliferation, suppress invasion and migration, induce apoptosis, and block the cell cycle progression in GC cellsin vitro. Then, immunofluorescence and western blotting assay confirmed that PB can regulate the expression of LC3B, TFR1, NOCA4 and FTH1 in vitro, which suggested that suggest that PB may increase the level of Fe2+by transporting Fe3+into the cell by TFR1 and promoting NCOA4-dependent iron autophagy. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [27] | |||
| Responsed Drug | Formosanin C | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response Description | The saponin formosanin C (FC) is a novel natural ferroptosis inducer, which triggered a stronger ferroptosis in human hepatocellular carcinoma HepG2 cells containing a higher level of NCOA4 and a lower level of FTH1 compared to Hep3B cells. | |||
Lung cancer [ICD-11: 2C25]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [28] | ||||
| Responsed Drug | Gefitinib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRAS mutant non-small-cell lung cancer (NSCLC) cells. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [28] | ||||
| Responsed Drug | Betulin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRAS mutant non-small-cell lung cancer (NSCLC) cells. | ||||
Bladder cancer [ICD-11: 2C94]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [29] | ||||
| Responsed Drug | Baicalin | Terminated | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
5637 cells | Bladder carcinoma | Homo sapiens | CVCL_0126 | |
| BLSC-KU-19 cells | Leukemia | Bos taurus | CVCL_VN09 | ||
| In Vivo Model |
All mouse experiments were approved by the Use and Care of Animals Committee at Hangzhou Normal University. About 6 x 106 KU-19-19cells were injected into the about 3-5 weeks old female BALB/c nude mice (about 18 g,n = 5).Once palpable tumors appeared, the mice were randomized in four groups: the control (deionized water containing 7% Tween 80 and 0.1% CMC-Na) group, the DFO (100 mg/kg/day) group, the baicalin (200 mg/kg/day) group, and DFO + baicalin group. After 10 days of drug administration (intraperitoneal injection, once daily), mice were sacrificed, and tumor specimens resected were collected for immunohistochemical staining and Perl's staining (Solarbio Life Sciences, G1420).
Click to Show/Hide
|
||||
| Response Description | Baicalin triggered ferroptosis in vitro and in vivo, as evidenced by ROS accumulation and intracellular chelate iron enrichment. Baicalin exerts its anticancer activity in bladder cancer by inducing FTH1-dependent ferroptosis, which will hopefully provide great therapeutic potential for bladder cancer treatment. | ||||
Nervous system disease [ICD-11: 8E7Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [30] | |||
| Responsed Drug | N2L | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | N2L recovered glutathione peroxidase 4 (GPX4) expression and blocked the increase of Cyclooxygenase-2 (cox-2) and acyl-CoA synthetase long-chain family member 4 (ACSL4) protein expressions. Moreover, N2L also significantly prevented Ferritin Heavy Chain 1 (FTH1) from downregulation and maintained iron homeostasis. And N2L could be a ferroptosis inhibitor for the therapy of ferroptosis-related neurodegenerative diseases, such as Alzheimer's disease. | |||
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [31] | ||||
| Responsed Drug | Berberine | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response Description | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
Cardiovascular diseases [ICD-11: BE2Z]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [32] | ||||
| Responsed Drug | Sesamin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
Forty specific pathogen-free normal Sprague Dawley (SD) rats (7 weeks old and 251-275 g in weight) were supplied by Charles River Laboratories. The SD rats were randomly allocated into five groups (n = 8). In the PM2.5 exposure group, the rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times. In the saline control group, the SD rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and intratracheally instilled with 0.9% saline (1 mL per kg b.w.) every other day for a total of three times. In the Ses pretreatment groups, the SD rats were gavaged with low (L-Ses, 40 mg per kg b.w), medium (M-Ses, 80 mg per kg b.w.), and high (H-Ses, 160 mg per kg b.w.) doses of Ses. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times.
Click to Show/Hide
|
||||
| Response Description | Sesamin pretreatment upregulated the expression levels of GPX4, SLC7A11, TFRC, and FPN1 and inhibited the expression levels of FTH1 and FTL. Ses pretreatment could ameliorate PM2.5-induced cardiovascular injuries perhaps by inhibiting ferroptosis. | ||||
Drug-induced or toxic liver disease [ICD-11: DB95]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [33] | ||||
| Responsed Drug | Nickel Chloride | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| In Vivo Model |
Totally 128 7-week-old ICR male mice (22-25 g) were provided by Dashuo Biological Technology (Chengdu, China). The animals were divided into four groups (32 mice per group) randomly. The mice in the three experimental groups were gavage administered with Ni (NiCl2·6H2O) at doses of 7.5, 15, and 30 mg/kg body weight respectively, while those in the control group were given distilled water. The Ni dose adopted here was determined according to the value of median lethal dose (LD50, 306.11 mg/kg) attained in the research on acute oral toxicity of male mice. We selected 1/40, 1/20 and 1/10 LD50 (306.11 mg/kg) of NiCl2 in this study.
Click to Show/Hide
|
||||
| Response Description | Nickel chloride caused hepatic ferroptosis accompanied by increased iron content in the liver and up-regulation of cyclooxygenase 2 (COX-2) protein and mRNA expression levels, down-regulation of glutathione eroxidase 4 (GPX4), ferritin heavy chain 1 (FTH1) and nuclear receptor coactivator 4 (NCOA4) protein and mRNA expression levels. Altogether, Mitochondria damage and ferroptosis involved in Ni-induced hepatotoxicity in mice. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [34] | ||||
| Responsed Drug | Epigallocatechin Gallate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [35] | ||||
| Responsed Drug | Quercetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| In Vivo Model |
Six-week-old male C57BL/6J mice (18-20 g) were obtained from Zhejiang Vital River Laboratory (Zhejiang, China). 32 mice were divided randomly into 4 groups: Saline group (CONT), 25 mg/kg/day ACR group (ACR), 25 mg/kg/day ACR with a low dose of 25 mg/kg/day QCT group (ACR + QCT (L)), and 25 mg/kg/day ACR with a high dose of 50 mg/kg/day QCT group (ACR + QCT (H)), 8 animals in each group.
Click to Show/Hide
|
||||
| Response Description | Quercetin (QCT) specifically reacted with autophagic cargo receptor NCOA4, blocked the degradation of iron storage protein FTH1, and eventually downregulated the intracellular iron levels and the consequent ferroptosis. Collectively, our results presented a unique approach to alleviate ACR-induced liver injury by targeting ferroptosis with QCT. | ||||
Ubiquitin-like modifier-activating enzyme ATG7 (ATG7)
Amentoflavone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response Description | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
TUG1 (IncRNA)
Dihydroartemisinin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
All BALB/C nude mice were purchased from Huafukang Biotechnology (Beijing, China). These mice were 5 weeks old and weighed 14-16 g. We established subcutaneous tumour-forming mouse model by injecting 5 x 106 U87 cells into the lateral abdomen of BALB/C nude mice. Animals were then treated with DHA solvent (50 mg/kg) by intragastric administration once a day for 26 days.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin (DHA) could promote ferroptosis in glioma cells. Low expression of GPX4 and high expression of HMOX1 were identified in DHA treated glioma cells. MAZ was further identified as the direct target of long noncoding RNA (lncRNA) TUG1 through luciferase assay. Downregulated expression of TUG1 and upregulated expression of MAZ were identified in DHA treated glioma cells. TUG1 overexpression or inhibition of FTH1 expression could enhance the antiglioma effect of DHA in vitro and in vivo, providing a promising strategy to enhance the antitumor effect of DHA in glioma. | ||||
Serine/threonine-protein kinase ULK1 (ULK1)
Bisphenol A
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Renal dysfunction [ICD-11: GB6Z] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | TCMK-1 cells | Normal | Mus musculus | CVCL_2772 | |
| In Vivo Model |
Balb/c mice aged 6-8 weeks were purchased from Liaoning Changsheng biotechnology co., Ltd. (Benxi, China). All animal experiments comply with the requirements of the Institutional Animal Care and Use Committee (IACUC) of Jilin University. The mice were housed in separate cages and given enough food and drinking water during the conditions of a 12-h light and dark cycle. The establishment of animal models is as previously described.
Click to Show/Hide
|
||||
| Response Description | Renal dysfunction and renal tubular epithelial damage induced by Bisphenol A (BPA) are linked to ferroptosis, which depends on the activation of ferritinophagy through AMPK-mTOR- ULK1 axis. These findings revealed that after BPA treatment, FTH was significantly reduced and iron was accumulated. | ||||
NAD-dependent protein deacylase sirtuin-6 (SIRT6)
Melatonin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cataract [ICD-11: 9B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | B-3 cells | Normal | Homo sapiens | CVCL_6367 | |
| In Vivo Model |
Six-week-old albino Sprague Dawley (SD) male rats were provided by the Experimental Animal Centre of the Second Affiliated Hospitalof Harbin Medical University. Fifteen minutes before exposure, the rats were anaesthetized by intraperitoneal injection of a mixture of 90 mg/kg ketamine and 15 mg/kg xylazine. Then, tropicamide phenylephrine was dropped in both eyes; at the same time, the rats that received drug treatment were injected subconjunctivally (5 ul/eye) with 500 mM Fer-1, 200 mM MT or the same dose of DMSO used to dissolve the drug using a 28-gauge needle and a Hamilton microinjector. After another 5 min, a single eye of every experimental group rat was exposed to UVB (312 nm) 5 W/m2 for 30 min. Every time, UVB exposure was synchronized with the drug injection, and the frequency was every other day until it was stopped 9 weeks later.
Click to Show/Hide
|
||||
| Response Description | Melatonin inhibited ferroptosis through the SIRT6/p-Nrf2/GPX4 and SIRT6/COA4/FTH1 pathways to neutralize lipid peroxidation toxicity, which protected cells against ferroptotic stress in vitro and delayed cataract formation caused by UVB exposure in rats. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Cadmium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Kidney injury [ICD-11: NB92] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/ Sirt1 axis. | |||
Myc-associated zinc finger protein (MAZ)
Dihydroartemisinin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| In Vivo Model |
All BALB/C nude mice were purchased from Huafukang Biotechnology (Beijing, China). These mice were 5 weeks old and weighed 14-16 g. We established subcutaneous tumour-forming mouse model by injecting 5 x 106 U87 cells into the lateral abdomen of BALB/C nude mice. Animals were then treated with DHA solvent (50 mg/kg) by intragastric administration once a day for 26 days.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin (DHA) could promote ferroptosis in glioma cells. Low expression of GPX4 and high expression of HMOX1 were identified in DHA treated glioma cells. MAZ was further identified as the direct target of long noncoding RNA (lncRNA) TUG1 through luciferase assay. Downregulated expression of TUG1 and upregulated expression of MAZ were identified in DHA treated glioma cells. TUG1 overexpression or inhibition of FTH1 expression could enhance the antiglioma effect of DHA in vitro and in vivo, providing a promising strategy to enhance the antitumor effect of DHA in glioma. | ||||
Microtubule-associated proteins 1A/1B light chain 3B {ECO:0000305} (MAP1LC3B)
Amentoflavone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response Description | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
Microtubule-associated proteins 1A/1B light chain 3A (MAP1LC3A)
Dihydroquercetin
[Preclinical]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Pulmonary fibrosis [ICD-11: CB03] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hBEs (Human bronchial epithelial cells) | ||||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| In Vivo Model |
Eight-week-old male C57BL/6 mice were purchased from Hubei University of Medicine (Shiyan, China). The SiO2-induced mouse pulmonary fibrosis model was performed. In brief, each group of mice was anesthetized with 1% pentobarbital sodium intraperitoneally at 40 mg/kg body weight and their tracheae had been surgically exposed. In addition, SiO2 suspension (20 mg in 50 ul saline) was instilled in the mice. The vehicle control groups were given an equivalent amount of 0.9% sterile saline. After one week of acclimation, mice were divided randomly into four groups (n = 8 per group). Control group, SiO2 group, SiO2 and low dose of DHQ group (DHQ-L, 10 mg/kg) as well as large dose of DHQ group (DHQ-H, 50 mg/kg).
Click to Show/Hide
|
||||
| Response Description | Dihydroquercetin suppressed ferritinophagy by down-regulation of microtubule-associated protein 1A/ 1B-light chain 3 (LC3), and up-regulation of ferritin heavy chain 1 (FTH1), nuclear receptor co-activator 4 (NCOA4) in activated HBE cells. Research revealed that inhibition of ferritinophagy-mediated HBE cells ferroptosis was responsible for DHQ to ameliorate SiO2-induced lung fibrosis. | ||||
hsa-miR-34a-5p (miRNA)
Cadmium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Kidney injury [ICD-11: NB92] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/Sirt1 axis. | |||
hsa-miR-19b-3p (miRNA)
Curcumenol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| CCD-19Lu cells | Normal | Homo sapiens | CVCL_2382 | ||
| BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | ||
| In Vivo Model |
A subcutaneous tumor-bearing nude mouse model was established by injecting the flank of BALB/c nude mice with 5 x 106 H460 cells. Ten days later, mice were blindly randomized into four groups and intravenously injected with 200 ul ddH2O containing 0.1% CMC-Na and 1% Tween 80, iron chelators DFO (100 mg/kg/day), curcumenol (200 mg/kg/day), and curcumenol combine DFO. Tumor long diameter, short diameter, and body weight were detected every two days after the first drug treatment. The tumor volume calculation formula: (tumor long diameter x tumor short diameter2)/2. Finally, mice were sacrificed. All tumors were collected for immunohistochemical (IHC) staining.
Click to Show/Hide
|
||||
| Response Description | The natural product curcumenol exerted its antitumor effects on lung cancer by triggering ferroptosis, and the lncRNA H19/miR-19b-3p/FTH1 axis plays an essential role in curcumenol-induced ferroptotic cell death. Mechanistically, we showed that lncRNA H19 functioned as a competing endogenous RNA to bind to miR-19b-3p, thereby enhanced the transcription activity of its endogenous target, ferritin heavy chain 1 (FTH1), a marker of ferroptosis. | ||||
H19 (IncRNA)
Curcumenol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| CCD-19Lu cells | Normal | Homo sapiens | CVCL_2382 | ||
| BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | ||
| In Vivo Model |
A subcutaneous tumor-bearing nude mouse model was established by injecting the flank of BALB/c nude mice with 5 x 106 H460 cells. Ten days later, mice were blindly randomized into four groups and intravenously injected with 200 ul ddH2O containing 0.1% CMC-Na and 1% Tween 80, iron chelators DFO (100 mg/kg/day), curcumenol (200 mg/kg/day), and curcumenol combine DFO. Tumor long diameter, short diameter, and body weight were detected every two days after the first drug treatment. The tumor volume calculation formula: (tumor long diameter x tumor short diameter2)/2. Finally, mice were sacrificed. All tumors were collected for immunohistochemical (IHC) staining.
Click to Show/Hide
|
||||
| Response Description | The natural product curcumenol exerted its antitumor effects on lung cancer by triggering ferroptosis, and the lncRNA H19/miR-19b-3p/FTH1 axis plays an essential role in curcumenol-induced ferroptotic cell death. Mechanistically, we showed that lncRNA H19 functioned as a competing endogenous RNA to bind to miR-19b-3p, thereby enhanced the transcription activity of its endogenous target, ferritin heavy chain 1 (FTH1), a marker of ferroptosis. | ||||
CircOMA1 (circRNA)
Cabergoline
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Prolactinoma [ICD-11: 2F37] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MMQ cells | Pituitary gland neoplasm | Rattus norvegicus | CVCL_2117 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
All animal studies were performed in the Laboratory Animal Center of Sun Yat-sen University and conducted in accordance with the institutional policies for animal care. Approximately 5 x 106 MMQ_vector cells or MMQ_circOMA1 cells in 150 uL were injected into the right flank of BALB/c nude mice (total of 12 female mice, 4-6 weeks, SCXK2021-0029). After tumor formation (10 days), mice were randomly divided into four groups (n = 3 mice/group) as follows: vector (saline solution, intraperitoneally injected), circOMA1 (saline solution, intraperitoneally injected), vector + CAB (0.5 mg/kg, intraperitoneally injected), and circOMA1 + CAB (0.5 mg/kg, intraperitoneally injected) in accordance with previous studies. CAB was injected intraperitoneally every 2 days for 14 days. The size of the tumor was measured every 3 days. On Day 15, mice were anesthetized with 0.3% pentobarbital sodium solution and then sacrificed by cervical dislocation, and the xenograft tumors were removed and weighed.
Click to Show/Hide
|
||||
| Response Description | GCLM was directly targeted by miR-145-5p and indirectly regulated by circOMA1. Importantly, circOMA1 induced ferroptosis resistance through the increased expression of Nrf2, GPX4, and FTH1, and circOMA1 attenuated cabergoline (CAB)-induced ferroptosis in MMQ cells in vivo and in vitro. circOMA1 may be a new therapeutic target for the individualized treatment of DA-resistant prolactinoma patients. | ||||
Carbonyl reductase [NADPH] 1 (CBR1)
Chrysin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Capan-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0026 | ||
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| AsPC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0152 | ||
| In Vivo Model |
Male BALB/c nude mice (5 weeks old, weighing 18-20 g) were provided by Jiangsu Jicui Yaokang Biotechnology Co., Ltd. (Nanjing, China). The mice were subcutaneously transplanted with non-targeting shRNA (shcontrol) or CBR1-targeting shRNA (shCBR1)-transfected PANC-1 cells (200 uL, 1 x 107 cells). Tumor volumes and body weights were measured every 4 days (n = 4, each), tumor volume = 0.5 x (a x a x b) (a, smallest diameter; b, largest diameter). The mice were subcutaneously inoculated with PANC-1 cells (200 uL, 1 x 107 cells) in the combination treatment. When the tumor volume reached 80-100 mm3, the mice were treated with chrysin (30 mg/kg/i.P., daily), gemcitabine (20 mg/kg/i.p., once every other day), or in combination for four weeks.
Click to Show/Hide
|
||||
| Response Description | Inhibition of CBR1 by chrysin increased cellular ROS levels and led to ROS-dependent autophagy, which resulted in the degradation of ferritin heavy polypeptide 1 (FTH1) and an increase in the intracellular free iron level that participates in ferroptosis in pancreatic cancer (PC) cells. Finally, chrysin enhanced PC sensitivity to gemcitabine by inducing ferroptotic death in vitro and in vivo.? | ||||
Bromodomain-containing protein 4 (BRD4)
JQ1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| In Vivo Model |
Female athymic BALB/c nude mice (4-6-week old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Approximately 1 x 107 cells (A549) in 200 uL of serum-free medium and Matrigel solution were injected directly into the right axilla of each mouse. Tumor growth was measured with calipers every 3 days.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis was induced under (+)-JQ1 treatment and BRD4 knockdown, indicating that (+)-JQ1 induces ferroptosis via BRD4 inhibition in breast adenocarcinoma. In addition, expression of the ferroptosis-associated genes GPX4, SLC7A11, and SLC3A2 was downregulated under (+)-JQ1 treatment. Moreover, JQ1 treatment and BRD4 knockdown led to decreased FTH1 expression. | ||||
Autophagy protein 5 (ATG5)
Amentoflavone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| U-373MG cells | Astrocytoma | Homo sapiens | CVCL_2219 | ||
| In Vivo Model |
1 x 107 U251 cells were subcutaneously injected into the right back of the four-week-old BALB/c nude mice. After the tumor grown to 100 mm3, mice were randomly divided into 3 groups: mice in control group receivedintraperitoneal injectionof saline, while mice in AF treatment group received intraperitoneal injection at dosages of 40 mg/kg/day or 80 mg/kg/day, respectively.
Click to Show/Hide
|
||||
| Response Description | Amentoflavone (AF) triggered ferroptosis in autophagy-dependent manner. Furthermore, the expression of LC3B, Beclin1, ATG5, ATG7 were increased, and the expression of FTH were decreased by AF in a dose-dependent manner in vivo. AF has the potential to be considered as a novel treatment agent in glioma. | ||||
A disintegrin and metalloproteinase with thrombospondin motifs 18 (ADAMTS18)
Curcumin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Hereditary Leiomyomatosis [ICD-11: 2C90] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 |
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | |
| Response Description | Curcumin induces ferroptosis in tumor cells by upregulating the expression of ADAMTS18, thereby enhancing the sensitivity of clear cell renal cell carcinoma (ccRCC) to sunitinib. And Curcumin can significantly inhibit FTH1 and FTL1 gene expression in tumor tissues of nude mice. | |||
Unspecific Regulator
Ursolic Acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | ||||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| In Vitro Model | HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| 143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | ||
| In Vivo Model |
NU/NU mice (the Fourth Military Medical University, Shaanxi, China) were injected with 143B cells (100 uL, 5 x 107 cells/mL, i.h.). Seven days after the injection, the mice were divided into 6 different groups (n= 3) and intraperitoneally injected with different drugs twice a week. Then, on day 28, the mice were sacrificed, and the tumours in the different groups were weighed. Body weight and tumour size were measured every 3 days from day 7 to day 28. The tumour tissue was fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 4 um thick sections for haematoxylin-eosin (H&E) and immunofluorescence staining.
Click to Show/Hide
|
||||
| Response Description | Ursolic acid inhibited tumour cell proliferation and promoted the apoptosis of a variety of osteosarcoma cells. Mechanistic studies showed that ursolic acid degraded ferritin by activating autophagy and induced intracellular overload of ferrous ions, leading to ferroptosis. Ferritin, which includes ferritin light peptide 1 (FTL1) and ferritin heavy peptide 1 (FTH1). | ||||
Bavachin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [24] | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | |||
Polyphyllin I
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [25] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| In Vivo Model |
A subcutaneous gastric tumor model was established by subcutaneously injecting 1 x 106 AGS cells or 2 x 106 MKN-45 cells near the right axilla of mice. Seven days after tumor cell inoculation, mice received daily i. p. Injection of PPI (3 mg/kg, dissolved in 1% DMSO + 5% PEG300 + 5% Tween 80 + 89% deionized water), as described previously, or the control solution with the same solvent. Mice were weighed at day 15, while tumor volumes were measured every 3 days and calculated using a formula: length x width2/2.
Click to Show/Hide
|
||||
| Response Description | For the first time, our results have demonstrated that Polyphyllin I exerts its antitumor activity on the gastric cancer by, at least partially, inducing cancer cell ferroptosisviaregulating NRF2/FTH1 pathway. | ||||
Polyphyllin B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [26] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | NUGC-3 cells | Gastric carcinoma | Homo sapiens | CVCL_1612 | |
| MKN-1 cells | Gastric carcinoma | Homo sapiens | CVCL_1415 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| In Vivo Model |
The nude mice were raised in our laboratory for a week before the experiment. Then, 5 x 106 MKN-1 cells were subcutaneously injected to establish the subcutaneous xenograft tumour model in nude mice. When the maximum diameter of the xenograft tumours grew steadily to 1 cm, they were dissected completely and cut into 1 mm3 tissue fragments. Then, the tissue fragment was inserted into the surface of the serosa on the greater curvature of the stomach. Different doses of PB (2.5 mg/kg or 5.0 mg/kg) were given by intraperitoneal injection once a day for 3 weeks. The control group was given the same volume of vehicle. The positive control group was given 5-Fu at the dose of 10 mg/kg. The body weight and tumour size of nude mice were recorded. Mice were administered fluorescein substrate (150 mg/kg) intraperitoneally for in vivo imaging twice a week on a Xenogen IVIS 200 imaging system (Caliper Life Sciences, USA). The tumour inhibition rate was analysed using LT Living Image 4.3 Software.
Click to Show/Hide
|
||||
| Response Description | We identified a novel GPx4 inhibitor, polyphyllin B (PB), which can induce ferroptosis by down-regulating GPx4 expression in gastric cancer cells. It has also been shown to inhibit cell proliferation, suppress invasion and migration, induce apoptosis, and block the cell cycle progression in GC cellsin vitro. Then, immunofluorescence and western blotting assay confirmed that PB can regulate the expression of LC3B, TFR1, NOCA4 and FTH1 in vitro, which suggested that suggest that PB may increase the level of Fe2+by transporting Fe3+into the cell by TFR1 and promoting NCOA4-dependent iron autophagy. | ||||
Formosanin C
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [27] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response Description | The saponin formosanin C (FC) is a novel natural ferroptosis inducer, which triggered a stronger ferroptosis in human hepatocellular carcinoma HepG2 cells containing a higher level of NCOA4 and a lower level of FTH1 compared to Hep3B cells. | |||
Gefitinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [28] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRAS mutant non-small-cell lung cancer (NSCLC) cells. | ||||
Betulin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [28] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| In Vivo Model |
Nude mice (5 weeks) were purchased from SLAC Int. (Shanghai, China). A549 cells (6 x 107 /ml) were collected and mixed with Matrigel (Corning, USA) at a 1:1 ratio by volume. Then, 100 ul cells were injected subcutaneously into the back region of nude mice to generate tumors with a size of 100 mm3 . Mice were randomly divided into four groups (n = 5/group): the control group, betulin group (10 mg/kg), gefitinib group (30 mg/kg), and the combined group. The control group was orally administered vehicle, while the betulin group, gefitinib group, and the combined group were orally administered betulin, gefitinib, and betulin plus gefitinib every other day. The tumor size and mice body weight were measured every other day too, and the volume was calculated according to the formula: tumor size (mm3 ) = (length x width2 ) x 0.5.
Click to Show/Hide
|
||||
| Response Description | The expression of SCL7A11, GPX4, and FTH1, which are negative regulators of ferroptosis, was significantly decreased under the combinative treatment of betulin and gefitinib. Moreover, the positive regulatory protein HO-1 was increased. These findings reiterated that the combination of betulin with gefitinib could trigger ferroptosis in KRAS mutant non-small-cell lung cancer (NSCLC) cells. | ||||
Baicalin
[Terminated]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [29] | ||||
| Responsed Disease | Bladder cancer [ICD-11: 2C94] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | 5637 cells | Bladder carcinoma | Homo sapiens | CVCL_0126 | |
| BLSC-KU-19 cells | Leukemia | Bos taurus | CVCL_VN09 | ||
| In Vivo Model |
All mouse experiments were approved by the Use and Care of Animals Committee at Hangzhou Normal University. About 6 x 106 KU-19-19cells were injected into the about 3-5 weeks old female BALB/c nude mice (about 18 g,n = 5).Once palpable tumors appeared, the mice were randomized in four groups: the control (deionized water containing 7% Tween 80 and 0.1% CMC-Na) group, the DFO (100 mg/kg/day) group, the baicalin (200 mg/kg/day) group, and DFO + baicalin group. After 10 days of drug administration (intraperitoneal injection, once daily), mice were sacrificed, and tumor specimens resected were collected for immunohistochemical staining and Perl's staining (Solarbio Life Sciences, G1420).
Click to Show/Hide
|
||||
| Response Description | Baicalin triggered ferroptosis in vitro and in vivo, as evidenced by ROS accumulation and intracellular chelate iron enrichment. Baicalin exerts its anticancer activity in bladder cancer by inducing FTH1-dependent ferroptosis, which will hopefully provide great therapeutic potential for bladder cancer treatment. | ||||
N2L
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [30] | |||
| Responsed Disease | Nervous system disease [ICD-11: 8E7Z] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | N2L recovered glutathione peroxidase 4 (GPX4) expression and blocked the increase of Cyclooxygenase-2 (cox-2) and acyl-CoA synthetase long-chain family member 4 (ACSL4) protein expressions. Moreover, N2L also significantly prevented Ferritin Heavy Chain 1 (FTH1) from downregulation and maintained iron homeostasis. And N2L could be a ferroptosis inhibitor for the therapy of ferroptosis-related neurodegenerative diseases, such as Alzheimer's disease. | |||
Berberine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [31] | ||||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response Description | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
Sesamin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [32] | ||||
| Responsed Disease | Cardiovascular diseases [ICD-11: BE2Z] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
Forty specific pathogen-free normal Sprague Dawley (SD) rats (7 weeks old and 251-275 g in weight) were supplied by Charles River Laboratories. The SD rats were randomly allocated into five groups (n = 8). In the PM2.5 exposure group, the rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times. In the saline control group, the SD rats were treated with 0.5% CMC (10 mL per kg b.w.) for 21 days. The SD rats were anesthetized with isoflurane and intratracheally instilled with 0.9% saline (1 mL per kg b.w.) every other day for a total of three times. In the Ses pretreatment groups, the SD rats were gavaged with low (L-Ses, 40 mg per kg b.w), medium (M-Ses, 80 mg per kg b.w.), and high (H-Ses, 160 mg per kg b.w.) doses of Ses. The SD rats were anesthetized with isoflurane and administered with PM2.5 suspension by intratracheal instillation (10 mg per kg b.w.) every other day for a total of three times.
Click to Show/Hide
|
||||
| Response Description | Sesamin pretreatment upregulated the expression levels of GPX4, SLC7A11, TFRC, and FPN1 and inhibited the expression levels of FTH1 and FTL. Ses pretreatment could ameliorate PM2.5-induced cardiovascular injuries perhaps by inhibiting ferroptosis. | ||||
Nickel Chloride
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [33] | ||||
| Responsed Disease | Drug-induced or toxic liver disease [ICD-11: DB95] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
Totally 128 7-week-old ICR male mice (22-25 g) were provided by Dashuo Biological Technology (Chengdu, China). The animals were divided into four groups (32 mice per group) randomly. The mice in the three experimental groups were gavage administered with Ni (NiCl2·6H2O) at doses of 7.5, 15, and 30 mg/kg body weight respectively, while those in the control group were given distilled water. The Ni dose adopted here was determined according to the value of median lethal dose (LD50, 306.11 mg/kg) attained in the research on acute oral toxicity of male mice. We selected 1/40, 1/20 and 1/10 LD50 (306.11 mg/kg) of NiCl2 in this study.
Click to Show/Hide
|
||||
| Response Description | Nickel chloride caused hepatic ferroptosis accompanied by increased iron content in the liver and up-regulation of cyclooxygenase 2 (COX-2) protein and mRNA expression levels, down-regulation of glutathione eroxidase 4 (GPX4), ferritin heavy chain 1 (FTH1) and nuclear receptor coactivator 4 (NCOA4) protein and mRNA expression levels. Altogether, Mitochondria damage and ferroptosis involved in Ni-induced hepatotoxicity in mice. | ||||
Epigallocatechin Gallate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [34] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
Quercetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [35] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| In Vivo Model |
Six-week-old male C57BL/6J mice (18-20 g) were obtained from Zhejiang Vital River Laboratory (Zhejiang, China). 32 mice were divided randomly into 4 groups: Saline group (CONT), 25 mg/kg/day ACR group (ACR), 25 mg/kg/day ACR with a low dose of 25 mg/kg/day QCT group (ACR + QCT (L)), and 25 mg/kg/day ACR with a high dose of 50 mg/kg/day QCT group (ACR + QCT (H)), 8 animals in each group.
Click to Show/Hide
|
||||
| Response Description | Quercetin (QCT) specifically reacted with autophagic cargo receptor NCOA4, blocked the degradation of iron storage protein FTH1, and eventually downregulated the intracellular iron levels and the consequent ferroptosis. Collectively, our results presented a unique approach to alleviate ACR-induced liver injury by targeting ferroptosis with QCT. | ||||
References
