Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0093)
| Name |
Berberine
|
||||
|---|---|---|---|---|---|
| Synonyms |
berberine; 2086-83-1; Umbellatine; Berberin; Berbericine; Majarine; Thalsine; Umbellatin; Berberone; 0I8Y3P32UF; CHEBI:16118; EINECS 218-229-1; BRN 3570374; UNII-0I8Y3P32UF; Benzo(g)-1,3-benzodioxolo(5,6-a)quinolizinium, 5,6-dihydro-9,10-dimethoxy-; DTXSID9043857; 9,10-Dimethoxy-2,3-(methylenedioxy)-7,8,13,13a-tetrahydroberbinium; Berberal; 9,10-dimethoxy-5,6-dihydro[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolin-7-ium; 16,17-dimethoxy-5,7-dioxa-13-azoniapentacyclo[11.8.0.02,10.04,8.015,20]henicosa-1(13),2,4(8),9,14,16,18,20-octaene; BERBERINE (MART.); BERBERINE [MART.]; Benzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium, 5,6-dihydro-9,10-dimethoxy-; BERBINIUM, 7,8,13,13a-TETRAHYDRO-9,10-DIMETHOXY-2,3-(METHYLENEDIOXY)-; Berberine hydrogen sulfate; CHEMBL12089; 5,6-Dihydro-9,10-dimethoxybenzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium; 9,10-dimethoxy-2,3-(methylenedioxy)-7,8,13,13a-tetradehydroberbinium; 5,6-DIHYDRO-9,10-DIMETHOXY-1,3-BENZODIOXOLO(5,6-A)BENZO(G)QUINOLIZINIUM; 5,6-DIHYDRO-9,10-DIMETHOXYBENZO(G)-1,3-BENZODIOXOLO(5,6-A)QUINOLIZINIUM; 9,10-DIMETHOXY-5,6-DIHYDRO(1,3)DIOXOLO(4,5-G)ISOQUINO(3,2-A)ISOQUINOLIN-7-IUM; GNF-PF-4545; BER; NSC646666; NCGC00016526-02; NCGC00016526-07; CAS-633-65-8; Berbinium; berberine dimer; Coptis rhizome; 7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenebis(oxy))berbinium; 7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-[methylenebis(oxy)]berbinium; C20H18NO4; Umbellatine (6CI); Spectrum_001110; ST055798; Berbinium, 7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenedioxy)-, chloride; BERBERINE [MI]; Prestwick0_000586; Prestwick1_000586; Prestwick2_000586; Prestwick3_000586; Spectrum2_000894; Spectrum3_000618; Spectrum4_000785; Spectrum5_001458; BERBERINE [VANDF]; UPCMLD-DP032; NCIMech_000354; BERBERINE [WHO-DD]; 9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium; SCHEMBL25632; BSPBio_000432; BSPBio_002156; KBioGR_001230; KBioSS_001590; cid_12456; DivK1c_000265; inverted exclamation markY97%; SPBio_000708; SPBio_002651; BPBio1_000476; CHEMBL295124; MEGxp0_001923; DTXCID7023857; UPCMLD-DP032:001; ACon1_001957; BCBcMAP01_000112; GTPL11353; KBio1_000265; KBio2_001590; KBio2_004158; KBio2_006726; KBio3_001656; NINDS_000265; YBHILYKTIRIUTE-UHFFFAOYSA-N; 34MD1011DM; HMS3561D13; HY-N0716; AC-117; BBL029198; BDBM50203126; CCG-35898; s9046; STK870320; 7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenedioxy)berbinium; AKOS002141363; DB04115; SDCCGMLS-0066718.P001; 9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo-[4,5-g]isoquinolino[3,2-a]isoquinolin-7-ium; IDI1_000265; SMP1_000298; NCGC00016526-01; NCGC00016526-03; NCGC00016526-04; NCGC00016526-05; NCGC00016526-06; NCGC00016526-08; NCGC00016526-11; NCGC00091896-03; NCI60_001050; NCI60_001224; NCI60_004319; SBI-0051613.P002; BERBERINE (CONSTITUENT OF GOLDENSEAL); CS-0009734; FT-0603587; C00757; A904183; Q176525; BERBERINE (CONSTITUENT OF GOLDENSEAL) [DSC]; Q-200701; Q-200702; SR-01000711827-5; BRD-K14796088-003-06-0; BRD-K14796088-003-17-7; Benzylpenicillin benzathine, Antibiotic for Culture Media Use Only; 5,6-dihydro-9,10-dimethoxy-benzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium; Berbinium, 7,8,13,13a-tetradehydro-9,10-dimethoxy-2,3-(methylenedioxy)-; 3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrido[2,1-a]isoquinolin-5-ylium; 9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolin-7-ylium; 9,10-Dimethoxy-5,6-dihydro-7lambda~5~-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinoline; Benzo[g]-1,3-benzodioxolo[5,6-a]quinolizinium, 5,6-dihydro-9,10-dimethoxy- (9CI); 16,17-dimethoxy-5,7-dioxa-13$l^{5}-azapentacyclo[11.8.0.0^{2,10}.0^{4,8}.0^{15,20}]henicosa-1(13),2,4(8),9,14,16,18,20-octaen-13-ylium; 9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolin-7-ylium chloride; 9,10-Dimethoxy-5,6-dihydro-[1,3]dioxolo[4,5-g]isoquino[3,2-a]isoquinolin-7-ylium; chloride; InChI=1/C20H18NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+
Click to Show/Hide
|
||||
| Structure |
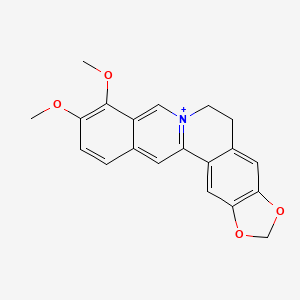 |
||||
| Formula |
C20H18NO4+
|
||||
| IUPAC Name |
16,17-dimethoxy-5,7-dioxa-13-azoniapentacyclo[11.8.0.02,10.04,8.015,20]henicosa-1(13),2,4(8),9,14,16,18,20-octaene
|
||||
| Canonical SMILES |
COC1=C(C2=C[N+]3=C(C=C2C=C1)C4=CC5=C(C=C4CC3)OCO5)OC
|
||||
| InChI |
InChI=1S/C20H18NO4/c1-22-17-4-3-12-7-16-14-9-19-18(24-11-25-19)8-13(14)5-6-21(16)10-15(12)20(17)23-2/h3-4,7-10H,5-6,11H2,1-2H3/q+1
|
||||
| InChIKey |
YBHILYKTIRIUTE-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | Cellular tumor antigen p53 (TP53) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response regulation | Berberine downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against IMA-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [6] | ||||
| Responsed Disease | Liver fibrosis | ICD-11: DB93 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | hHSCs (Human hepatic stellate cells) | ||||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| HSC-T6 cells | Normal | Rattus norvegicus | CVCL_0315 | ||
| BRL-3A cells | Normal | Rattus norvegicus | CVCL_0606 | ||
| In Vivo Model |
6-week-old male mice (20-25 g) were randomly allocated to the indicated groups and housed in cages with ad libitum access to food and water, following habituation to a 12 h light/dark cycle. The experimenter was blinded to the group allocation. The mouse liver fibrosis model group was established via intraperitoneal (i.p.) injection with TAA or carbon tetrachloride (CCl4) for 6 weeks. The control group was injected i.p. with the same volume of olive oil or saline only. For the BBR group, a dose of BBR (200 mg/kg/day) was given by oral gavage. In addition to the i.p. injection. with TAA or CCl4, the mice in the treatment group received BBR intragastrically. In the inhibitor group, besides the injected i.p. with TAA and BBR oral gavage treatment, mice were injected i.p. with a dose of Fer-1 (1 mg/kg/day). Mice were sacrificed under 3% isoflurane anesthesia after 6 weeks of treatment; blood and liver tissue samples were taken and processed for subsequent analyses. At least five mouse hepatic sections were utilized in every group.
Click to Show/Hide
|
||||
| Response regulation | Berberine induced iron disruption in HSCs by modulating ferritin degradation in both the autophagy/ROS and UPS pathways, driving HSC ferroptosis to attenuate liver fibrosis. | ||||
Prostaglandin G/H synthase 2 (PTGS2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | |||
| Target for Ferroptosis | Marker | |||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response regulation | Berberine (BBR) inhibited ferroptosis via reducing ROS generation and reducing lipid peroxidation in erastin and RSL3-treated cardiac cells.Furthermore, quantitative polymerase chain reaction results showed that Ptgs2 mRNA was reduced in BBR-treated cells. BBR has the potential to treat ferroptosis-induced cardiomyopathy. | |||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Gastrointestinal cancer | ICD-11: 2B5B | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| TMK-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4384 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
Five-week-old male BALB/c mice were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained in a pathogen-free animal facility under a 12 h light/dark cycle in a temperature (22 )- and humidity-controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following the current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval no. 12924, 5 November 2020). Animals were acclimated to their housing for seven days before the start of the experiment. For the peritoneal dissemination tumor model, CT26 cancer cells (1 x 107 in 0.2 mL per mouse) were injected into the mouse peritoneal cavity. To measure tumor weight, mice were euthanized on Day 12 and the tumors were excised, while the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, with gross removal of non-tumor tissues. The largest tumor was formed on the diaphragm, and paraffin-embedded sections of the excised diaphragmatic tumor were prepared and stained with hematoxylin-eosin. BBR was diluted with distilled water to produce a final concentration of 48 mg/mL. The solutions were ultrasonically treated for 1 h, and fully vortexed for 30 min. BBR solution was administered by free drinking. The intake calculated from the amount of water consumed was 15.2 mg/kg body weight/day.
Click to Show/Hide
|
||||
| Response regulation | Berberine induces apoptosis and ferroptosis by inhibiting mitochondrial complex I and promoting autophagy, leading to combined cell death in the GIC and suppressing stemness. BBR induces cell death in gastrointestinal cancer cells accompanied by increased mitochondrial superoxide and ACSL4 levels, decreased SLC7A11, and impaired antioxidant mechanisms, indicated by decreased GPX4 expression and decreased GSH. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Diabetes mellitus | ICD-11: 5A10 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Response regulation | Berberine (BBR) stimulated GPX4 expression to reduce the content of Fe2+ and ROS, thereby repressing the ferroptosis of islet cells in diabetes mellitus, which functioned similarly as ferroptosis inhibitor Fer-1. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response regulation | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response regulation | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [7] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Health | ICD-11: N.A. | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| Response regulation | Berberine can inhibit erastin-induced ferroptosis in HT22 cells possibly by activating the Nrf2-HO-1/ GPX4 pathway. | ||||
NAD(P)H dehydrogenase [quinone] 1 (NQO1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response regulation | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Gastrointestinal cancer | ICD-11: 2B5B | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| TMK-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4384 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
Five-week-old male BALB/c mice were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained in a pathogen-free animal facility under a 12 h light/dark cycle in a temperature (22 )- and humidity-controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following the current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval no. 12924, 5 November 2020). Animals were acclimated to their housing for seven days before the start of the experiment. For the peritoneal dissemination tumor model, CT26 cancer cells (1 x 107 in 0.2 mL per mouse) were injected into the mouse peritoneal cavity. To measure tumor weight, mice were euthanized on Day 12 and the tumors were excised, while the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, with gross removal of non-tumor tissues. The largest tumor was formed on the diaphragm, and paraffin-embedded sections of the excised diaphragmatic tumor were prepared and stained with hematoxylin-eosin. BBR was diluted with distilled water to produce a final concentration of 48 mg/mL. The solutions were ultrasonically treated for 1 h, and fully vortexed for 30 min. BBR solution was administered by free drinking. The intake calculated from the amount of water consumed was 15.2 mg/kg body weight/day.
Click to Show/Hide
|
||||
| Response regulation | Berberine induces apoptosis and ferroptosis by inhibiting mitochondrial complex I and promoting autophagy, leading to combined cell death in the GIC and suppressing stemness. BBR induces cell death in gastrointestinal cancer cells accompanied by increased mitochondrial superoxide and ACSL4 levels, decreased SLC7A11, and impaired antioxidant mechanisms, indicated by decreased GPX4 expression and decreased GSH. | ||||
Glutathione peroxidase 1 (GPX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cerebral ischemia | ICD-11: 8B10 | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
All animal experiments described in this study were carried out in accordance with the U.K. Animals (Scientific Procedures) Act and were approved by the Experimental Animal Center of Wenzhou Medical University (No. wydw2022-0032). Six-to-eight-weeks old male ICR mice were obtained from Beijing Weitonglihua Experimental Animal Technology Co. Ltd. (Beijing, China). Mice were group-housed in the breeding environment under a 12/12h light/dark cycle, controlled temperature of 20-22 and 50-60% humidity with ad libitum chow and water. All animals were randomized for the research and procedures.
Click to Show/Hide
|
||||
| Response regulation | This study revealed the therapeutic potential of berberine on cerebral ischemia-reperfusion injury via inhibiting neuronal ferroptosis, in which upregulated glutathione peroxidase 1 (GPX1) was possibly involved. | ||||
Ferritin heavy chain (FTH1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
All animal experiment protocols were implemented in accordance with the National Institutes of Health (NIH) guidelines, and the procedures were approved by the Animal Ethics Committee of Southwest University. C57BL/6J male mice, 8-10 weeks old, weighing 20 ± 2 g, were used in this study. Mice were housed under standard conditions at 22-24 with a 12 h light/12 h darkness cycle and free access to food and tap water. Thirty-six mice were randomly divided into six groups: control (N = 8), IMA group (50 mg/kg) (N = 8), Low-Ber (20 mg/kg) + IMA group (N = 8), Medium-Ber (40 mg kg1) + IMA group (N = 8), High-Ber (80 mg/kg) + IMA group (N = 8), and Fer-1 (1 mg/kg) + IMA group (N = 8). IMA was given intraperitoneally for 14 days. Ber was given orally 2 h before IMA treatment and Fer-1 was given intraperitoneally 2 h before IMA treatment.
Click to Show/Hide
|
||||
| Response regulation | Berberine (Ber) downregulated the expression of transferrin receptor (TfR) and P53 and upregulated the expression of nuclear factor erythroid 2-related factor 2 (Nrf2), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO1), ferritin heavy chain-1 (FTH1), and glutathione peroxidase 4 (GPX4) in H9c2 cells and mice. The present data indicated that Ber has the potential to protect against imatinib mesylate-induced cardiotoxicity, partlyviainhibiting Nrf2-dependent ferroptosis. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Gastrointestinal cancer | ICD-11: 2B5B | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| TMK-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4384 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
Five-week-old male BALB/c mice were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained in a pathogen-free animal facility under a 12 h light/dark cycle in a temperature (22 )- and humidity-controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following the current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval no. 12924, 5 November 2020). Animals were acclimated to their housing for seven days before the start of the experiment. For the peritoneal dissemination tumor model, CT26 cancer cells (1 x 107 in 0.2 mL per mouse) were injected into the mouse peritoneal cavity. To measure tumor weight, mice were euthanized on Day 12 and the tumors were excised, while the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, with gross removal of non-tumor tissues. The largest tumor was formed on the diaphragm, and paraffin-embedded sections of the excised diaphragmatic tumor were prepared and stained with hematoxylin-eosin. BBR was diluted with distilled water to produce a final concentration of 48 mg/mL. The solutions were ultrasonically treated for 1 h, and fully vortexed for 30 min. BBR solution was administered by free drinking. The intake calculated from the amount of water consumed was 15.2 mg/kg body weight/day.
Click to Show/Hide
|
||||
| Response regulation | Berberine induces apoptosis and ferroptosis by inhibiting mitochondrial complex I and promoting autophagy, leading to combined cell death in the gastrointestinal cancer and suppressing stemness. BBR induces cell death in GIC cells accompanied by increased mitochondrial superoxide and ACSL4 levels, decreased SLC7A11, and impaired antioxidant mechanisms, indicated by decreased GPX4 expression and decreased GSH. | ||||
References
