Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10006)
| Target Name | Long-chain-fatty-acid--CoA ligase 4 (ACSL4) | ||||
|---|---|---|---|---|---|
| Synonyms |
Arachidonate--CoA ligase; Long-chain acyl-CoA synthetase 4
Click to Show/Hide
|
||||
| Gene Name | ACSL4 | ||||
| Sequence |
MKLKLNVLTIILLPVHLLITIYSALIFIPWYFLTNAKKKNAMAKRIKAKPTSDKPGSPYR
SVTHFDSLAVIDIPGADTLDKLFDHAVSKFGKKDSLGTREILSEENEMQPNGKVFKKLIL GNYKWMNYLEVNRRVNNFGSGLTALGLKPKNTIAIFCETRAEWMIAAQTCFKYNFPLVTL YATLGKEAVVHGLNESEASYLITSVELLESKLKTALLDISCVKHIIYVDNKAINKAEYPE GFEIHSMQSVEELGSNPENLGIPPSRPTPSDMAIVMYTSGSTGRPKGVMMHHSNLIAGMT GQCERIPGLGPKDTYIGYLPLAHVLELTAEISCFTYGCRIGYSSPLTLSDQSSKIKKGSK GDCTVLKPTLMAAVPEIMDRIYKNVMSKVQEMNYIQKTLFKIGYDYKLEQIKKGYDAPLC NLLLFKKVKALLGGNVRMMLSGGAPLSPQTHRFMNVCFCCPIGQGYGLTESCGAGTVTEV TDYTTGRVGAPLICCEIKLKDWQEGGYTINDKPNPRGEIVIGGQNISMGYFKNEEKTAED YSVDENGQRWFCTGDIGEFHPDGCLQIIDRKKDLVKLQAGEYVSLGKVEAALKNCPLIDN ICAFAKSDQSYVISFVVPNQKRLTLLAQQKGVEGTWVDICNNPAMEAEILKEIREAANAM KLERFEIPIKVRLSPEPWTPETGLVTDAFKLKRKELRNHYLKDIERMYGGK Click to Show/Hide
|
||||
| Family | ATP-dependent AMP-binding enzyme family | ||||
| Function |
Catalyzes the conversion of long-chain fatty acids to their active form acyl-CoA for both synthesis of cellular lipids, and degradation via beta-oxidation. Preferentially activates arachidonate and eicosapentaenoate as substrates. Preferentially activates 8,9-EET > 14,15-EET > 5,6-EET > 11,12-EET. Modulates glucose- stimulated insulin secretion by regulating the levels of unesterified EETs. Modulates prostaglandin E2 secretion.
Click to Show/Hide
|
||||
| Gene ID | 2182 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
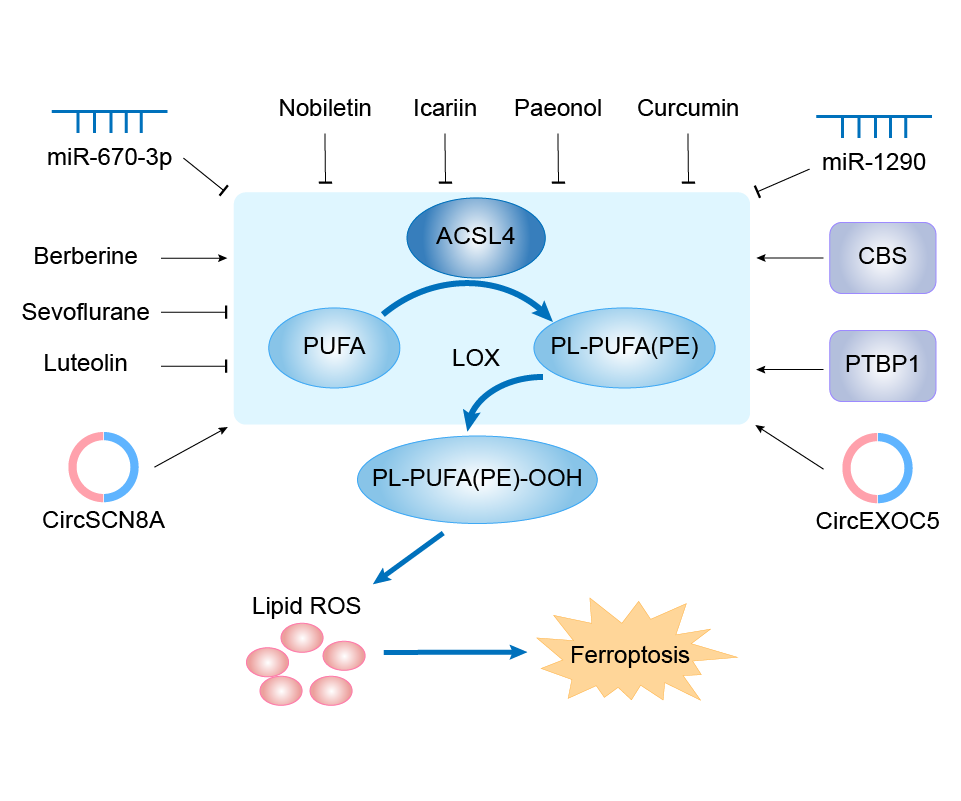
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
ACSL4 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Tumor necrosis factor alpha-induced protein 3 (TNFAIP3)
Lung cancer [ICD-11: 2C25]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Erastin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Cisplatin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | The upregulated expression of individual miRNAs, miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92 were determined by qRT-PCR. This study revealed a novel mechanism through which miR-17-92 protects endothelial cells from erastin-induced ferroptosis by targeting the A20(TNFAIP3)-ACSL4 axis. | |||
Transcription factor Sp1 (SP1)
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Sevoflurane | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Adult male SD rats (250-300 g) were purchased from Charies River (Beijing, China). The animals were placed in laboratory cages, kept on a 12-h light-dark cycle, and had free access to food and water throughout the study. The rats were randomly assigned to the sham (only the left neck was exposed without ligation) group, MACO group, and sevo + MACO (2.5% sevoflurane before refusion) group. The MCAO model was made by a modified nylon suture method. After 1 h of ischemia, the suture was gently pulled to the beginning of the external carotid artery and re-perfused for 24 h. For sevoflurane postconditioning, rats were stabilized in a gas-tight anesthesia chamber with sevoflurane inhalation for 1 h at the onset of blood refusion. Sevoflurane (AbbVie, Japan) was delivered at a concentration of 2.5% through a vaporizer (Vapor 2000, Germany). In the sham or MCAO group, rats were only exposed to the mixed gas (95% O2 and 5% CO2).
Click to Show/Hide
|
||||
| Response Description | Sevoflurane treatment inhibits ferroptosis and increases apoptosis events by inhibiting the SP1/ASCL4 axis, thereby reducing cerebral ischemia-reperfusion injury damage. | ||||
RAF proto-oncogene serine/threonine-protein kinase (RAF1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Tetraarsenic tetrasulfide | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
In Vitro Model |
NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| Response Description | On H23 cells treated with realgar, the expression of GPX4, SCL7A11 decreased while ACSL4 expression increased; this effect could also be amplified by Sorafenib. In conclusion, the present study indicated that realgar may induce ferroptosis by regulating the Raf, and hence plays a role in antiKRAS mutant lung cancer. | |||
Protein C-ets-1 (ETS1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MHCC97-L cells | Hepatocellular carcinoma | Homo sapiens | CVCL_4973 | |
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| HEK293 FT cells | Normal | Homo sapiens | CVCL_6911 | ||
| In Vivo Model |
Parental MHCC97L cells (2 x 106 cells/mouse) were subcutaneously injected into the 4-to-5-week-old NOD-SCID mice. When the tumours reached a volume of around 50-100 mm3 (calculated by the formula 4/3(D/2)(d/2)2, where D and d represent the minor and major axis of the tumour, respectively), the maximum tolerated dose of sorafenib (50 mg/kg) was given to the mice by oral gavage daily until the drug resistance occurred, denoted as the drug resistant group. For the control, the wild type group was treated with the vehicle (0.5% CMC-Na). The tumour size and body weight were measured every 3 days.
Click to Show/Hide
|
||||
| Response Description | Sorafenib treatment triggered ferroptosis via lipid ROS production and chelatable iron accumulation. The ETS1 upregulated by sorafenib was a key transcription factor of miR-23a-3p that directly enhanced miR-23a-3p expression. MiR-23a-3p recognized and bound to ACSL4 3UTR to limit lipid ROS production, thus attenuating sorafenib-induced ferroptotic cell death in hepatocellular carcinoma. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Supraventricular tachycardia [ICD-11: BC81]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Icariin | Phase 3 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response Description | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Cadmium | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/ Sirt1 axis. | |||
mmu-miR-7a-5p (miRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Bromelain | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
Mitofusin-2 (MFN2)
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Arsenic | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (300 g-350 g, specific pathogen free) were obtained from Institute of Genome Engineered Animal Models for Human Disease of Dalian Medical University (Dalian, China). To explore the influence of NaAsO2 (CAS No.7784-46-5, Sigma-Aldrich, USA) on the liver, the rats were subjected to NaAsO2 at the dosage of 0, 2.5, and 5 mg/kg by gavage for 9 months. The control group was gavaged with distilled water as vehicle.
Click to Show/Hide
|
||||
| Response Description | Arsenic induces rat liver nonalcoholic steatohepatitis (NASH) and Ferroptosis via interacting between Mitofusin-2 with IRE1. NaAsO2 increases IRE1 and Mfn2 expression, subsequently led to upregulated ACSL4 expression and 5-HETE via the directly combination Mfn2 with IRE1, ultimately induced ferroptotic cell death. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Sodium arsenite | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (300 g-350 g, specific pathogen free) were obtained from Institute of Genome Engineered Animal Models for Human Disease of Dalian Medical University (Dalian, China). To explore the influence of NaAsO2 (CAS No.7784-46-5, Sigma-Aldrich, USA) on the liver, the rats were subjected to NaAsO2 at the dosage of 0, 2.5, and 5 mg/kg by gavage for 9 months. The control group was gavaged with distilled water as vehicle.
Click to Show/Hide
|
||||
| Response Description | Arsenic induces rat liver nonalcoholic steatohepatitis (NASH) and Ferroptosis via interacting between Mitofusin-2 with IRE1. NaAsO2 increases IRE1 and Mfn2 expression, subsequently led to upregulated ACSL4 expression and 5-HETE via the directly combination Mfn2 with IRE1, ultimately induced ferroptotic cell death. | ||||
Hydroxymethylglutaryl-CoA synthase, cytoplasmic (HMGCS1)
Squamous cell carcinoma of skin [ICD-11: 2C31]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Itraconazole | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-431 cells | Skin squamous cell carcinoma | Homo sapiens | CVCL_0037 | |
| COLO 16 cells | Skin squamous cell carcinoma | Homo sapiens | CVCL_D607 | ||
| In Vivo Model |
Female BALB/c nude mice (6weeks old and 18-22 g weight) were purchased from the Model Animal Research Center of Nanjing University. A431 cells (5 x 106) in cold DMEM (50 ul) were mixed with Matrigel (50 ul) and injected into mice subcutaneously. After 6 days, the tumor volume was measured and the mice were assigned to three groups. Mice were treated with either normal saline or itraconazole (40 mg/kg oral twice daily; 80 mg/kg oral twice daily).
Click to Show/Hide
|
||||
| Response Description | Itraconazole inhibited the cell proliferation, induced apoptosis and blocked cell cycle of Cutaneous squamous cell carcinoma cells. And 3-hydroxy-3-methylglutaryl-CoA synthase 1 (HMGCS1) and acyl-CoA synthetase long-chain family member 4 (ACSL4) were significantly upregulated in A431 cells treated with itraconazole. Itraconazole may induce ferroptosis via HMGCS1/ACSL4 axis in A431 cells. | ||||
Hydroxycarboxylic acid receptor 1 (HCAR1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Lactate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
CAF cells | Normal | Carassius auratus | CVCL_R883 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
Female mice aged around 6-7 weeks were used for this study, which were purchased through Laboratory Animal Center of Chongqing Medical University from Vital River Co. Ltd (Beijing, China).After one week, each mouse was injected subcutaneously with 100 uL of Huh-7 cell suspension (5 x 106 units) to establish the tumor model. The mice were grouped randomly, and then subjected to different treatments after subcutaneous tumors became visually detectable.
Click to Show/Hide
|
||||
| Response Description | Lactate regulates the ferroptosis of hepatocellular carcinoma cells. And blocking the lactate uptake via hydroxycarboxylic acid receptor 1 (HCAR1)/MCT1 inhibition promotes ferroptosis by activating the AMPK to downregulate SCD1, which may synergize with its acyl-coenzyme A synthetase 4 (ACSL4)-promoting effect to amplify the ferroptotic susceptibility. | ||||
hsa-miR-34a-5p (miRNA)
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Cadmium | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/Sirt1 axis. | |||
hsa-miR-23a-3p (miRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MHCC97-L cells | Hepatocellular carcinoma | Homo sapiens | CVCL_4973 | |
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| HEK293 FT cells | Normal | Homo sapiens | CVCL_6911 | ||
| In Vivo Model |
Parental MHCC97L cells (2 x 106 cells/mouse) were subcutaneously injected into the 4-to-5-week-old NOD-SCID mice. When the tumours reached a volume of around 50-100 mm3 (calculated by the formula 4/3(D/2)(d/2)2, where D and d represent the minor and major axis of the tumour, respectively), the maximum tolerated dose of sorafenib (50 mg/kg) was given to the mice by oral gavage daily until the drug resistance occurred, denoted as the drug resistant group. For the control, the wild type group was treated with the vehicle (0.5% CMC-Na). The tumour size and body weight were measured every 3 days.
Click to Show/Hide
|
||||
| Response Description | Sorafenib treatment triggered ferroptosis via lipid ROS production and chelatable iron accumulation. The ETS1 upregulated by sorafenib was a key transcription factor of miR-23a-3p that directly enhanced miR-23a-3p expression. MiR-23a-3p recognized and bound to ACSL4 3UTR to limit lipid ROS production, thus attenuating sorafenib-induced ferroptotic cell death in hepatocellular carcinoma. | ||||
hsa-miR-19b-3p (miRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Bromelain | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | The upregulated expression of individual miRNAs, miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92 were determined by qRT-PCR. This study revealed a novel mechanism through which miR-17-92 protects endothelial cells from erastin-induced ferroptosis by targeting the A20-ACSL4 axis. | |||
hsa-miR-17-5p (miRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Bromelain | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
hsa-miR-16-5p (miRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Bromelain | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
hsa-miR-150-5p (miRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Bromelain | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
hsa-miR-144-3p (miRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Bromelain | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | |
| SW1353 cells | Bone chondrosarcoma | Homo sapiens | CVCL_0543 | ||
| MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | ||
| SAOS-2 cells | Osteosarcoma | Homo sapiens | CVCL_0548 | ||
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | ||
| HOB (Human normal osteoblastic cells) | |||||
| In Vivo Model |
The OS model of nude mice was constructed using the CDTX model. After transfection, the h143B cells were prepared into a single-cell suspension and subcutaneously injected into the left proximal tibia of 36 (3 mice per group) 4-weeks-old nude mice (1 x 107 cells per mouse).
Click to Show/Hide
|
||||
| Response Description | MiR-144-3p can induce the occurrence of ferroptosis by negatively regulating the expression of ZEB1, thereby inhibiting the proliferation, migration, and invasion of osteosarcoma (OS) cells. The overexpression of ZEB1 caused the lower expression level of ACSL4 and higher expression level of xCT and GPX4. | ||||
hsa-miR-130a-3p (miRNA)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Bromelain | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
HOTAIR (IncRNA)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Paeonol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
C57BL/6 mice (aged 8-10 weeks, Vital River, Beijing, China) were housed in SPF conditions. The animal study was performed according to the National Institutes of Health Guide and approved by the Ethics Committees of Affiliated Jiangmen Traditional Chinese Medicine Hospital of Jinan University.
Click to Show/Hide
|
||||
| Response Description | Paeonol (PAN) inhibits the progression of intracerebral haemorrhage via the HOTAIR/UPF1/ACSL4 signalling pathway. Thus, PAN could act as a new agent for the treatment of ferroptosis in intracerebral haemorrhage. | ||||
Elongation of very long chain fatty acids protein 6 (ELOVL6)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Apatinib | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| HIEC-6 cells | Normal | Homo sapiens | CVCL_6C21 | |
| Response Description | ACSL4, a vital regulator of ferroptosis, could interact with ELOVL6 directly. Apatinib promoted ferroptosis in colorectal cancer (CRC) cells by targeting ELOVL6/ACSL4, providing a new mechanism support for apatinib application in the clinical treatment of CRC. | |||
Aquaporin-11 (AQP11)
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Penicillamine | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were provided by at the Centre for Animals of Central South University (Changsha, China). To prepare the seizure mouse model, the mice underwent the intrahippocampal injection of KA as described in our previous investigation. For short, mice were anesthetized with sodium phenobarbital (50 mg/kg, i.p.) and carefully placed on a stereotaxic apparatus. Then, KA (1 uL, 250 ng/uL dissolved in saline) was stereotactically injected into the hippocampus according to the following coordinates: anteroposterior -2.0 mm; lateral -1.3 mm; dorsoventral -1.2 mm. After injection, the infusion needle was kept in place for 5-10 min to avoid liquid reflux. Mice in the control group underwent the same surgical procedure but received injection with an equal volume of phosphate buffered saline (PBS) instead of KA.
Click to Show/Hide
|
||||
| Response Description | D-penicillamine can be repurposed to cure seizure disorders such as epilepsy. D-penicillamine reveals the amelioration of seizure-induced neuronal injury via inhibiting Aqp11-dependent ferroptosis. Furthermore, ferroptosis-associated indices including acyl-coA synthetase long chain family member 4 (ACSL4), prostaglandin-endoperoxide synthase 2 (Ptgs2) gene and lipid peroxide (LPO) level were significantly decreased in KA mouse model after DPA treatment. | ||||
Zinc finger protein basonuclin-1 (BNC1)
Primary ovarian insufficiency [ICD-11: GA30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
ES-2 cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Ovaries collected from 12-week-old mice were fixed in 2.5% glutaraldehyde at room temperature for 2 h and then at 4 overnight. The ovaries were washed with PBS three times for 10 min. Then, the ovaries were fixed with 1% osmic acid for 1 h and washed with PBS three times for 10 min each. The ovaries were fixed with 2% uranyl acetate for 30 min; dehydrated with 50%, 70%, 90% and 100% ethanol for 10 min each; and washed with 100% acetone twice for 15 min each.
Click to Show/Hide
|
||||
| Response Description | Pharmacologic inhibition of YAP signaling or ferroptosis significantly rescues Bnc mutation-induced primary ovarian insufficiency (POI). BNC1 directly regulates Nf2 expression. BNC1 deficiency downregulates NF2 expression, which reduces YAP phosphorylation and promote YAP nuclear accumulation. YAP activation upregulates Tfrc and Acsl4 expression. | ||||
Zinc finger E-box-binding homeobox 1 (ZEB1)
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | |
| SW1353 cells | Bone chondrosarcoma | Homo sapiens | CVCL_0543 | ||
| MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | ||
| SAOS-2 cells | Osteosarcoma | Homo sapiens | CVCL_0548 | ||
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | ||
| HOB (Human normal osteoblastic cells) | |||||
| In Vivo Model |
The OS model of nude mice was constructed using the CDTX model. After transfection, the h143B cells were prepared into a single-cell suspension and subcutaneously injected into the left proximal tibia of 36 (3 mice per group) 4-weeks-old nude mice (1 x 107 cells per mouse).
Click to Show/Hide
|
||||
| Response Description | MiR-144-3p can induce the occurrence of ferroptosis by negatively regulating the expression of ZEB1, thereby inhibiting the proliferation, migration, and invasion of osteosarcoma (OS) cells. The overexpression of ZEB1 caused the lower expression level of ACSL4 and higher expression level of xCT and GPX4. | ||||
ZFAS1 (IncRNA)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hRECs (Human retinal endothelial cells) | ||||
| In Vivo Model |
For in vivo experiments, the eyes in each group (n = 6) were enucleated carefully and processed for indirect immunofluorescence in whole-mount or cross-section as previously described. For cryosections, the eyes (n = 3 retinae from 3 mice) were fixed in 4% PFA at room temperature for 15 min. The frozen samples were then sliced transversely (6 um) at -20. For retinal flat-mounts, the eyes (n = 3 eyes from 3 mice) were fixed in 4% PFA at room temperature for 15 min, and the retinae were dissected out as cups. Both cryosections and retinal cups were blocked with PBS containing 0.5% Triton-X100 and 5% BSA at 4 overnight and included with the anti-CD31 and anti-GPX4 (1:100, ab125066, Abcam) primary antibodies.
Click to Show/Hide
|
||||
| Response Description | ZFAS1 may act as a competing endogenous RNA by competitively binding with microRNA-7-5p (miR-7-5p) and modulating the expression of its downstream molecule acyl-CoA synthetase long-chain family member 4 (ACSL4), which is now identified as a classic driver gene of ferroptosis process. In conclusion, our results demonstrate that HG-induced ZFAS1 elevation activates ferroptosis in hRECs and the ZFAS1/miR-7-5p/ACSL4 axis may serve as a therapeutic target for endothelial dysfunction in diabetic retinopathy. | ||||
YY1-associated protein 1 (YY1AP1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
mEFs (Mouse embryonic fibroblasts) | ||||
| NF639 (Mouse breast epithelial cells) | |||||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| BT-474 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0179 | ||
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | ||
| In Vivo Model |
Six- to eight-week-old female athymic nu/nu mice were purchased from Envigo (East Millstone, NJ, USA). For s.c. tumour models, mice were injected in the right flank with 1 x 107 shNT-GPX4 iKO MSTO-211H cells or shMerlin-GPX4 iKO MSTO-211H cells suspended in 150 uL Matrigel. Tumours were measured with callipers every 3 days. When tumours reached a mean volume of 100 mm3, mice with similarly sized tumours were grouped into four treatment groups. For control or knockout cohorts, mice were given intraperitoneal (i.p.) injections of 0.9% sterile saline or Doxycycline (100 mg/kg body weight) for two days. At the same time, mice were provided with either a normal diet or Doxycycline diet for control or knockout cohorts, respectively.
Click to Show/Hide
|
||||
| Response Description | In epithelial cells, such interactions mediated by E-cadherin suppress ferroptosis by activating the intracellular NF2 (also known as merlin) and Hippo signalling pathway in breast adenocarcinoma. Antagonizing this signalling axis allows the proto-oncogenic transcriptional co-activator YAP to promote ferroptosis by upregulating several ferroptosis modulators, including ACSL4 and TFRC. | ||||
WD repeat domain phosphoinositide-interacting protein 2 (WIPI2)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| Response Description | The expression level of ACSL4 decreased and that of GPX4 increased when WIPI2 was knocked down, suggesting that WIPI2 can potentially positively regulate colorectal cancer ferroptosis. | |||
TUG1 (IncRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Mouse renal I/R model was performed in male C57BL/6 mice (8-12 weeks old). Briefly, the mice were anesthetized with pentobarbital sodium by intraperitoneal injection and lay on the right side. Dorsal incisions of both left and right sides were made to expose kidneys. The right kidney artery was gently separated with cotton swabs and occluded with a microvascular clamp to induce renal ischemia for 45 min. The left renal pedicle clamping and ischemia were the same as right. After ischemia, the micro-aneurysm clips were removed to start the reperfusion. The wounds were sutured and resuscitated with warm sterile saline intraperitoneally. All operations were the same in the sham group except for clamping and ischemia.
Click to Show/Hide
|
||||
| Response Description | Human urine-derived stem cells (USCs)-derived exosomes (USC-Exo) could improve kidney ischemia/reperfusion injury (IRI). Mechanistically, LncRNA TUG1 was carried by USC-Exo downregulation of ACSL4 expression in kidney cells by interacting with SRSF1, then inhibited ACSL4-mediated cell ferroptosis, and thus improved kidney injury in IRI-induced AKI. | ||||
Transcriptional coactivator YAP1 (YAP1)
Primary ovarian insufficiency [ICD-11: GA30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
ES-2 cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Ovaries collected from 12-week-old mice were fixed in 2.5% glutaraldehyde at room temperature for 2 h and then at 4 overnight. The ovaries were washed with PBS three times for 10 min. Then, the ovaries were fixed with 1% osmic acid for 1 h and washed with PBS three times for 10 min each. The ovaries were fixed with 2% uranyl acetate for 30 min; dehydrated with 50%, 70%, 90% and 100% ethanol for 10 min each; and washed with 100% acetone twice for 15 min each.
Click to Show/Hide
|
||||
| Response Description | Pharmacologic inhibition of YAP signaling or ferroptosis significantly rescues Bnc1 mutation-induced primary ovarian insufficiency (POI). BNC1 directly regulates Nf2 expression. BNC1 deficiency downregulates NF2 expression, which reduces YAP phosphorylation and promote YAP nuclear accumulation. YAP activation upregulates Tfrc and Acsl4 expression. | ||||
Serine/arginine-rich splicing factor 1 (SRSF1)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Mouse renal I/R model was performed in male C57BL/6 mice (8-12 weeks old). Briefly, the mice were anesthetized with pentobarbital sodium by intraperitoneal injection and lay on the right side. Dorsal incisions of both left and right sides were made to expose kidneys. The right kidney artery was gently separated with cotton swabs and occluded with a microvascular clamp to induce renal ischemia for 45 min. The left renal pedicle clamping and ischemia were the same as right. After ischemia, the micro-aneurysm clips were removed to start the reperfusion. The wounds were sutured and resuscitated with warm sterile saline intraperitoneally. All operations were the same in the sham group except for clamping and ischemia.
Click to Show/Hide
|
||||
| Response Description | Human urine-derived stem cells (USCs)-derived exosomes (USC-Exo) could improve kidney ischemia/reperfusion injury (IRI). Mechanistically, LncRNA TUG1 was carried by USC-Exo downregulation of ACSL4 expression in kidney cells by interacting with SRSF1, then inhibited ACSL4-mediated cell ferroptosis, and thus improved kidney injury in IRI-induced AKI. | ||||
Sentrin-specific protease 1 (SENP1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
Retinoblastoma-associated protein (RB1)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
In Vitro Model |
LNCaP cells | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| PC-3 cells | Prostate carcinoma | Homo sapiens | CVCL_0035 | ||
| 22Rv1 cells | Prostate carcinoma | Homo sapiens | CVCL_1045 | ||
| DU145 cells | Prostate carcinoma | Homo sapiens | CVCL_0105 | ||
| LNCaP C4-2 cells | Prostate carcinoma | Homo sapiens | CVCL_4782 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| RWPE-1 cells | Normal | Homo sapiens | CVCL_3791 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
1 x 106 shCT or shRB PC-3 cells were mixed with 100 uL Matrigel (Corning) and implanted subcutaneously into the right flanks of 6- to 8-week-old male nude mice. When tumor volumes were approximately 80-100 mm3 in PC3 xenografts or circulating RFP tumor cells had begun to emerge in peripheral blood of PPR-RFP mice (around 7.5 months), vehicle or JKE-1674 (25 mg/kg, dissolved in 10% ethanol and 90% PEG-400, Sigma-Aldrich) were administered orally to mice every other day.
Click to Show/Hide
|
||||
| Response Description | The regulation of ferroptosis by the RB1/E2F/ACSL4 axis and highlight the therapeutic potential of ferroptosis induction in the treatment of RB1 loss driven prostate cancer growth and metastasis and perhaps other RB1-deficient malignancies. | ||||
Pumilio homolog 2 (PUM2)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| hBMSCs (Bone marrow stromal cells) | |||||
| In Vivo Model |
A total of 96 C57BL/6 male mice (20-25 g) aged 11-12 weeks were purchased from experimental animal center of experimental animal center of Guangdong Medical University. 96 mice were randomly divided into four groups (24 mice per group): Sham group (200 ul of PBS), Sham + BMSCs-Exo group (200 ul of BMSCs-Exo), I/R group (200 ul of PBS) and I/R + BMSCs-Exo group (200 ul of BMSCs-Exo). After 10 days of adaptive feeding, all mice were injected intraperitoneally with 0.4-0.5 mL/100 g 1%Pentobarbital Sodium. I/R and I/R + BMSCs-Exo group mice were subjected to cardiac I/R injury induced by ligation of the left anterior descending artery (LAD) for 30 min followed by 24 h reperfusion. Sham and Sham + BMSCs-Exo mice were sham treated and subjected to the same surgical procedures as I/R mice except that they did not receive ligation of the left anterior descending coronary artery.
Click to Show/Hide
|
||||
| Response Description | Cellular ferroptosis is involved in the pathogenesis of Ischemia-Reperfusion Injury. BMSCs-Exo lncRNA Mir9-3hg can inhibit ferroptosis by modulating the Pum2/PRDX6 axis to exhibit cardioprotective effectsinvivoandinvitro. Silence of PRDX6 markedly decreased cell proliferation, GSH content and Gpx4 protein level, as well as prominently increased iron ion concentration and levels of ROS content and ACSL4 protein in H/R-treated HL-1 cells. | ||||
Prokineticin-2 (PROK2)
Traumatic brain injury [ICD-11: NA07]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ubiquitin mediated proteolysis | hsa04120 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rPCNs (Rat primary cortical neurons) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
Eight-week-old male mice were subjected to severe CCI. Anesthesia was induced with 3% isoflurane in nitrous oxide: oxygen (7:3) and maintained with 1.5% isoflurane via nose cone. Temperature was maintained at 37 ± 0.5 using a heating blanket. After anesthesia, mice were placed in a stereotaxic frame (R.W.D. Shenzhen, China). A 4-mm-diameter craniotomy was performed over the left parietal bone and the bone flap was removed for trauma. A vertically directed CCI was applied (6.0 ± 0.2 m/s, 50 ms dwell time, 1.4 mm depth) using an impactor (R.W.D., Shenzhen, China). After the injury, the skin incision was closed. Mice were monitored with supplemental oxygen (100%) for 1h before returning to their cages. Fer-1 (1 mg/kg per day) was given i.p. at 7 days before CCI and once daily until euthanasia or the MWM test. Before the brain tissues were obtained, mice were perfused intracardially with 4 phosphate-buffer saline (PBS) solution.
Click to Show/Hide
|
||||
| Response Description | Prok2 mediates neuronal cell deaths in traumatic brain injury via ferroptosis. Prok2 prevents neuronal cell death by suppressing the biosynthesis of lipid peroxidation substrates, arachidonic acid-phospholipids, via accelerated F-box only protein 10 (Fbxo10)-driven ubiquitination, degradation of long-chain-fatty-acid-CoA ligase 4 (Acsl4), and inhibition of lipid peroxidation. | ||||
Polypyrimidine tract-binding protein 1 (PTBP1)
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mLVECs (Mouse lung microvascular endothelial cells) | ||||
| In Vivo Model |
The sepsis mouse model was established by cecal ligation and puncture (CLP) of male C57BL/6 mouse (6-8 weeks, Guangzhou Animal Medical Center). Firstly, the mice were anesthetized by intraperitoneal injection of 4% chloral hydrate (0.1 ml/10g). The cecum was found by cutting a 1 cm longitudinally in the abdomen of the mice, and ligating the cecum from the end of the cecum to half the length of the ileocecal valve with 5-0 silk suture. Then a 21 G needle was used to puncture the ligature and the midpoint of the cecum once, and finally the wound was sutured. In the sham operation group, only laparotomy was performed, without ligation and perforation of the cecum. All animal-related experiments in this study were approved by hospital ethics committee according to following the Nation Institutes of Health Guide for the Laboratory Animals Care and Use (approval No. Med-Eth-Re [2022] 168).
Click to Show/Hide
|
||||
| Response Description | CircEXOC5 can enhance the stability of the target gene ACSL4 by binding to the RNA binding protein PTBP1 and up-regulate its expression, thereby promoting ferroptosis and exacerbating sepsis-induced acute lung injury. | ||||
Pirin (PIR)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 cells in 100 ul PBS were injected subcutaneously to the right of the dorsal midline in 6- to 8-week-oldathymic nude mice(n = 5 mice/group). After the tumor reached 60-80 mm3 on day 7, the mice were randomly grouped and treated with IKE (imidazole ketone erastin; 40 mg/kg; i.p., once every other day) for 3 weeks, and then samples were collected and assayed.
Click to Show/Hide
|
||||
| Response Description | Pirin (PIR), an iron-binding nuclear protein, plays a previously unrecognized role in mediating ferroptosis resistance in human pancreatic cancer cells. The depletion of PIR initiates HMGB1-dependent autophagy by binding to BECN1, and subsequently promotes ferroptosis by activating ACSL4. | ||||
Phosphatidylglycerophosphatase and protein-tyrosine phosphatase 1 (PTPMT1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [25] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 |
| Response Description | PTPMT1 is upregulated in PDAC and PTPMT1 inhibits ferroptosis by suppressing the expression of ACSL4 and upregulating SLC7A11 in Panc-1 cells, suggesting PTPMT1 might be a potential prognosis biomarker and therapeutic target in pancreatic cancer. | |||
Peroxisome proliferator-activated receptor alpha (PPARA)
Immunoglobulin A nephropathy [ICD-11: MF8Y]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hMCs (Human mesangial cells) | |||
| Response Description | In PPAR lentivirus-transfected HMCs stimulated by Gd-IgA1, ROS, MDA, and ACSL4 were decreased; glutathione and GPX4, and immunofluorescence colocalization of PPAR and GPX4, increased; and damaged mitochondria reduced. Hence, PPAR pathway downregulation can reduce FABP1 expression, affecting GPX4 and ACSL4 levels, causing HMC ferroptosis, and contributing to immunoglobulin A nephropathy (IgAN) pathogenesis. | |||
Peroxiredoxin-6 (PRDX6)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| hBMSCs (Bone marrow stromal cells) | |||||
| In Vivo Model |
A total of 96 C57BL/6 male mice (20-25 g) aged 11-12 weeks were purchased from experimental animal center of experimental animal center of Guangdong Medical University. 96 mice were randomly divided into four groups (24 mice per group): Sham group (200 ul of PBS), Sham + BMSCs-Exo group (200 ul of BMSCs-Exo), I/R group (200 ul of PBS) and I/R + BMSCs-Exo group (200 ul of BMSCs-Exo). After 10 days of adaptive feeding, all mice were injected intraperitoneally with 0.4-0.5 mL/100 g 1%Pentobarbital Sodium. I/R and I/R + BMSCs-Exo group mice were subjected to cardiac I/R injury induced by ligation of the left anterior descending artery (LAD) for 30 min followed by 24 h reperfusion. Sham and Sham + BMSCs-Exo mice were sham treated and subjected to the same surgical procedures as I/R mice except that they did not receive ligation of the left anterior descending coronary artery.
Click to Show/Hide
|
||||
| Response Description | Cellular ferroptosis is involved in the pathogenesis of Ischemia-Reperfusion Injury. BMSCs-Exo lncRNA Mir9-3hg can inhibit ferroptosis by modulating the Pum2/ PRDX6 axis to exhibit cardioprotective effectsinvivoandinvitro. Silence of PRDX6 markedly decreased cell proliferation, GSH content and Gpx4 protein level, as well as prominently increased iron ion concentration and levels of ROS content and ACSL4 protein in H/R-treated HL-1 cells. | ||||
NEAT1 (IncRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [27] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HBE1 cells | Normal | Homo sapiens | CVCL_0287 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1975 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| 95D cells | Lung giant cell carcinoma | Homo sapiens | CVCL_7110 | |
| Response Description | NEAT1 regulated levels of ACSL4 and proteins related to the ferroptosis and classical apoptosis pathways. And NEAT1 regulates ferroptosis and ferroptosis sensitivity, with the latter depending on ACSL4, suggesting that targeting NEAT1 or ACSL4 may be a viable therapeutic approach to the treatment of non-small-cell lung cancer (NSCLC). | |||
NAD-dependent protein deacetylase sirtuin-3, mitochondrial (SIRT3)
Gallbladder cancer [ICD-11: 2C13]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [28] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell adhesion molecules | hsa04514 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
GBC-SD cells | Gallbladder carcinoma | Homo sapiens | CVCL_6903 | |
| EH-GB1 cells | Gallbladder carcinoma | Homo sapiens | CVCL_IU73 | ||
| OCUG-1 cells | Gallbladder carcinoma | Homo sapiens | CVCL_3083 | ||
| NOZ cells | Gallbladder carcinoma | Homo sapiens | CVCL_3079 | ||
| In Vivo Model |
Forty male BALB/cnude mice (4 weeks old, 15-16 g) were purchased from the Shanghai Laboratory Animal Center (Shanghai, China) and divided into eight groups. Cells (1.0 x 106) were subcutaneously injected into the leftaxillaof nude mice.
Click to Show/Hide
|
||||
| Response Description | Expression levels of SIRT3 in patients with gallbladder cancer were lower than those in the adjacent normal tissue. Silence of SIRT3 gene also suppressed AKT-dependent ferroptosis, an iron-dependent and lipid peroxide-mediated cell death. Blockade of AKT activity in sh-SIRT3 cells induced ACSL4 expression that drives ferroptosis, and inhibited epithelial-mesenchymal (EMT) markers and invasive activity. | ||||
mmu-miR-3098-3p (miRNA)
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [29] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Circ-Carm1 was evidently abundant in acute cerebral infarction model cells, and knockdown of circ-Carm1 notably restored cell viability and inhibited ferroptosis in ACI model cells. Mechanistically, circ-Carm1 sponged miR-3098-3p to upregulate ACSL4 expression in ACI model cells to participate in ACI progressionin vitro. | |||
MIR9-3HG (IncRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| hBMSCs (Bone marrow stromal cells) | |||||
| In Vivo Model |
A total of 96 C57BL/6 male mice (20-25 g) aged 11-12 weeks were purchased from experimental animal center of experimental animal center of Guangdong Medical University. 96 mice were randomly divided into four groups (24 mice per group): Sham group (200 ul of PBS), Sham + BMSCs-Exo group (200 ul of BMSCs-Exo), I/R group (200 ul of PBS) and I/R + BMSCs-Exo group (200 ul of BMSCs-Exo). After 10 days of adaptive feeding, all mice were injected intraperitoneally with 0.4-0.5 mL/100 g 1%Pentobarbital Sodium. I/R and I/R + BMSCs-Exo group mice were subjected to cardiac I/R injury induced by ligation of the left anterior descending artery (LAD) for 30 min followed by 24 h reperfusion. Sham and Sham + BMSCs-Exo mice were sham treated and subjected to the same surgical procedures as I/R mice except that they did not receive ligation of the left anterior descending coronary artery.
Click to Show/Hide
|
||||
| Response Description | Cellular ferroptosis is involved in the pathogenesis of Ischemia-Reperfusion Injury. BMSCs-Exo lncRNA Mir9-3hg can inhibit ferroptosis by modulating the Pum2/PRDX6 axis to exhibit cardioprotective effectsinvivoandinvitro. Silence of PRDX6 markedly decreased cell proliferation, GSH content and Gpx4 protein level, as well as prominently increased iron ion concentration and levels of ROS content and ACSL4 protein in H/R-treated HL-1 cells. | ||||
Merlin (NF2)
Primary ovarian insufficiency [ICD-11: GA30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
ES-2 cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Ovaries collected from 12-week-old mice were fixed in 2.5% glutaraldehyde at room temperature for 2 h and then at 4 overnight. The ovaries were washed with PBS three times for 10 min. Then, the ovaries were fixed with 1% osmic acid for 1 h and washed with PBS three times for 10 min each. The ovaries were fixed with 2% uranyl acetate for 30 min; dehydrated with 50%, 70%, 90% and 100% ethanol for 10 min each; and washed with 100% acetone twice for 15 min each.
Click to Show/Hide
|
||||
| Response Description | Pharmacologic inhibition of YAP signaling or ferroptosis significantly rescues Bnc1 mutation-induced primary ovarian insufficiency (POI). BNC1 directly regulates Nf2 expression. BNC1 deficiency downregulates NF2 expression, which reduces YAP phosphorylation and promote YAP nuclear accumulation. YAP activation upregulates Tfrc and Acsl4 expression. | ||||
LncAABR07025387.1 (IncRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [30] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Adult male Sprague Dawley rats (3 months old, 200 g) purchased from the Animal Center of Nanjing University were housed at a controlled temperature of 18-22 and humidity of 50-70% under a 12-h light/dark cycle with free access to food and water. Before the operation, the rats were fasted overnight with free access to water. At the beginning of the operation, the rats were intraperitoneally injected with chloral hydrate (0.5 ml/100 g) for sedation) and 5% additional first dose for unsatisfactory sedation and made to inhale isoflurane for anesthesia. After incising the skin, the muscle was carefully separated layer by layer, and the trachea was exposed and fixed locally with a self-made pull hook. The trachea was then cut with a 10 ml syringe needle. The anesthesia mask was then replaced with the tracheal intubation. The ventilator was connected to the anesthesia machine for isopentane inhalation (2%). The proximal left anterior descending artery (LAD) was ligated using a 6.0 Prolene suture to induce myocardial ischemia. A small semi-cylindrical plastic hose was inserted to the LAD to facilitate the opening of the knot during reperfusion and to reduce the mechanical damage to the heart tissue and blood vessels. About 5 min later, the chest cavity was temporarily closed with a non-damaging hemostatic clip. After 30 min of ischemia, the noninjury hemostatic clip was loosened, the ligation and the protective tube were removed for reperfusion, and the chest was closed. The rats in the sham group underwent the same operation without being subjected to MI/R.
Click to Show/Hide
|
||||
| Response Description | LncAABR07025387.1 acts as a competing endogenous RNA during myocardial ischemia/reperfusion injury. Mechanistically, lncAABR07025387.1 negatively regulates miR-205 expression and subsequently upregulates ACSL4-mediated ferroptosis. | ||||
hsa-miR-7-5p (miRNA)
Retinopathy [ICD-11: 9B71]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hRECs (Human retinal endothelial cells) | ||||
| In Vivo Model |
For in vivo experiments, the eyes in each group (n = 6) were enucleated carefully and processed for indirect immunofluorescence in whole-mount or cross-section as previously described. For cryosections, the eyes (n = 3 retinae from 3 mice) were fixed in 4% PFA at room temperature for 15 min. The frozen samples were then sliced transversely (6 um) at -20. For retinal flat-mounts, the eyes (n = 3 eyes from 3 mice) were fixed in 4% PFA at room temperature for 15 min, and the retinae were dissected out as cups. Both cryosections and retinal cups were blocked with PBS containing 0.5% Triton-X100 and 5% BSA at 4 overnight and included with the anti-CD31 and anti-GPX4 (1:100, ab125066, Abcam) primary antibodies.
Click to Show/Hide
|
||||
| Response Description | ZFAS1 may act as a competing endogenous RNA by competitively binding with microRNA-7-5p (miR-7-5p) and modulating the expression of its downstream molecule acyl-CoA synthetase long-chain family member 4 (ACSL4), which is now identified as a classic driver gene of ferroptosis process. In conclusion, our results demonstrate that HG-induced ZFAS1 elevation activates ferroptosis in hRECs and the ZFAS1/ miR-7-5p/ACSL4 axis may serve as a therapeutic target for endothelial dysfunction in diabetic retinopathy. | ||||
hsa-miR-670-3p (miRNA)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [31] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 |
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | |
| SVG p12 cells | Normal | Homo sapiens | CVCL_3797 | |
| hMGCs (Human normal brain astroglia cells) | ||||
| Response Description | miR-670-3p level was elevated in human glioblastoma, but decreased upon ferroptotic stimulation. Mechanistically, ACSL4 was required for the regulation on ferroptosis and growth of glioblastoma cells by miR-670-3p. Moreover, U87MG and A172 cells treated with miR-670-3p inhibitor showed an increased chemosensitivity to TMZ. | |||
hsa-miR-5096 (miRNA)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [32] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MDA-MB-468 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | |
| MDA-MB-453 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| SK-BR-3 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | ||
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| ZR-75-1 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0588 | ||
| MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | ||
| In Vivo Model |
Mating was setup 2 days prior to injection day and zebrafish embryos were collected and incubated in E3 embryo medium (5 mM NaCl, 0.17 mM KCl, 10 mM HEPES, 0.33 mM MgSO4·7H2O, 0.33 mM CaCl2·6H2O, and 0.00001% methylene blue) containing 0.2 mM N-phenyl-thiourea (PTU) (catalog no: P7629, Sigma-Aldrich). Two days post-fertilization, the chorion was removed manually using fine forceps, and the embryos were anesthetized using E3 medium containing 200 mg/L Ethyl 3-aminobenzoate methanesulfonate (Tricaine) (catalog no: A5040, Sigma-Aldrich). Anesthetized embryos were mounted in 0.7% low melting agarose containing 200 ug/ml of Tricaine and were microinjected with 500 cells in the yolk sac using Nanoject III (catalog no: 3-000-207; Drummond Scientific Company, PA, USA). At 1 day post-injection (dpi), embryos with similar graft size were selected and imaged using both bright field and RFP channels and incubated in E3-PTU medium containing 5 ug/ml doxycycline at 34 until further imaging. At 4 dpi, embryos were anesthetized and imaged again using both bright field and RFP channels using Olympus IX-73 microscope. Cells that migrated outside the yolk sac (injection site) were represented by a notable fluorescent dot and considered a metastatic event; these were counted manually for all embryos in each experimental group.
Click to Show/Hide
|
||||
| Response Description | The present study demonstrated that miR-5096 targets and downregulates SLC7A11, thereby providing a mechanistic basis for ferroptosis in human breast cancer cells. In addition, miR-5096 induced cell death via ferroptosis, characterized by mitochondrial shrinkage with partial loss of cristae with simultaneous changes in ACSL4, ROS, lipid ROS, OH-, reactive iron, GSH, and MMP levels. | ||||
hsa-miR-4291 (miRNA)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
SiHa cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| Ca Ski cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | ||
| C33A cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1094 | ||
| In Vivo Model |
Male BALB/c nude mice (6 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The mice were kept in a constant temperature (25) and pathogen-free room with free access to food and water ad libitum. The animal experiments were approved by the Ethics Committee for Animal Experimentation of The Second Affiliated Hospital and Yuying Childrens Hospital. Mice were euthanised with isoflurane inhalation. CaSki cells overexpressing circLMO1 (7 x 106 cells/100 uL PBS) were injected subcutaneously into the flank of mice. Tumor growth was measured with a caliper 3 times a week and tumor-bearing mice were euthanised at 5 weeks after inoculation.
Click to Show/Hide
|
||||
| Response Description | CircLMO1 acted as a competing endogenous RNA (ceRNA) by sponging miR-4192 to repress target gene ACSL4. CircLMO1 promoted cervical cancer cell ferroptosis through up-regulating ACSL4 expression. Overexpression of miR-4291 or knockdown of ACSL4 reversed the effect of circLMO1 on facilitating ferroptosis and repressing cervical cancer cell proliferation and invasion. | ||||
hsa-miR-424-5p (miRNA)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [34] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HO8910 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6868 |
| SK-OV-3 cells | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| Response Description | MiR-424-5p negatively regulates ferroptosis by directly targeting ACSL4 in ovarian cancer cells. Upregulation of miR-424-5p suppressed ACSL4 by directly binding to its 3'-UTR, which subsequently reduced erastin- and RSL3-induced ferroptosis. | |||
hsa-miR-205-3p (miRNA)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [30] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Adult male Sprague Dawley rats (3 months old, 200 g) purchased from the Animal Center of Nanjing University were housed at a controlled temperature of 18-22 and humidity of 50-70% under a 12-h light/dark cycle with free access to food and water. Before the operation, the rats were fasted overnight with free access to water. At the beginning of the operation, the rats were intraperitoneally injected with chloral hydrate (0.5 ml/100 g) for sedation) and 5% additional first dose for unsatisfactory sedation and made to inhale isoflurane for anesthesia. After incising the skin, the muscle was carefully separated layer by layer, and the trachea was exposed and fixed locally with a self-made pull hook. The trachea was then cut with a 10 ml syringe needle. The anesthesia mask was then replaced with the tracheal intubation. The ventilator was connected to the anesthesia machine for isopentane inhalation (2%). The proximal left anterior descending artery (LAD) was ligated using a 6.0 Prolene suture to induce myocardial ischemia. A small semi-cylindrical plastic hose was inserted to the LAD to facilitate the opening of the knot during reperfusion and to reduce the mechanical damage to the heart tissue and blood vessels. About 5 min later, the chest cavity was temporarily closed with a non-damaging hemostatic clip. After 30 min of ischemia, the noninjury hemostatic clip was loosened, the ligation and the protective tube were removed for reperfusion, and the chest was closed. The rats in the sham group underwent the same operation without being subjected to MI/R.
Click to Show/Hide
|
||||
| Response Description | LncAABR07025387.1 acts as a competing endogenous RNA during myocardial ischemia/reperfusion injury. Mechanistically, lncAABR07025387.1 negatively regulates miR-205 expression and subsequently upregulates ACSL4-mediated ferroptosis. | ||||
hsa-miR-1290 (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [35] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| NCI-H520 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | ||
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | ||
| BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | ||
| In Vivo Model |
Male BALB/c nude mice (3-4 weeks, 16-20 g) were purchased from Vital River Laboratory (Beijing, China) and housed under standard conditions. After acclimatization for one week, mice were randomly divided into two groups (n = 6/group). A549 cells infected with lentivirus circSCN8A (Lv-circSNC8A) or control (Lv-vector) were selected in the presence of puromycin (1 ug/ml). A549 cells (7 x 106) with Lv-circSNC8A or Lv-vector were suspended in 100 uL PBS and respectively injected into each mouse at the right flank.
Click to Show/Hide
|
||||
| Response Description | CircSCN8A represses cell proliferation and metastasis in NSCLC by regulating the miR-1290/ACSL4 axis to induce ferroptosis. Thus, circSCN8A may represent a promising therapeutic target against NSCLC. | ||||
hsa-miR-106b-5p (miRNA)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [36] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
mBMVECs (Mouse brain microvascular endothelial cells) | |||
| Response Description | LncRNA H19 was overexpressed in Intracerebral hemorrhage (ICH). Knockdown of H19 promoted cell proliferation and suppressed BMVECs ferroptosis by regulating the miR-106b-5p/ACSL4 axis. Therefore, H19 knockdown may be a promising therapeutic strategy for ICH. | |||
High mobility group protein B1 (HMGB1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 cells in 100 ul PBS were injected subcutaneously to the right of the dorsal midline in 6- to 8-week-oldathymic nude mice(n = 5 mice/group). After the tumor reached 60-80 mm3 on day 7, the mice were randomly grouped and treated with IKE (imidazole ketone erastin; 40 mg/kg; i.p., once every other day) for 3 weeks, and then samples were collected and assayed.
Click to Show/Hide
|
||||
| Response Description | Pirin (PIR), an iron-binding nuclear protein, plays a previously unrecognized role in mediating ferroptosis resistance in human pancreatic cancer cells. The depletion of PIR initiates HMGB1-dependent autophagy by binding to BECN1, and subsequently promotes ferroptosis by activating ACSL4. | ||||
H19 (IncRNA)
Intracerebral hemorrhage [ICD-11: 8B00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [36] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Glutathione metabolism | hsa00480 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
mBMVECs (Mouse brain microvascular endothelial cells) | |||
| Response Description | LncRNA H19 was overexpressed in Intracerebral hemorrhage (ICH). Knockdown of H19 promoted cell proliferation and suppressed BMVECs ferroptosis by regulating the miR-106b-5p/ACSL4 axis. Therefore, H19 knockdown may be a promising therapeutic strategy for ICH. | |||
Fatty acid-binding protein, liver (FABP1)
Immunoglobulin A nephropathy [ICD-11: MF8Y]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hMCs (Human mesangial cells) | |||
| Response Description | In PPAR lentivirus-transfected HMCs stimulated by Gd-IgA1, ROS, MDA, and ACSL4 were decreased; glutathione and GPX4, and immunofluorescence colocalization of PPAR and GPX4, increased; and damaged mitochondria reduced. Hence, PPAR pathway downregulation can reduce FABP1 expression, affecting GPX4 and ACSL4 levels, causing HMC ferroptosis, and contributing to immunoglobulin A nephropathy (IgAN) pathogenesis. | |||
Fatty acid-binding protein, heart (FABP3)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [37] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| NCI-H1975 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | ||
| NCI-H358 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1559 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| In Vivo Model |
All athymic nude mice (6-week-old) were purchased from Jiesijie (Shanghai, China). To generate routine cell-derived xenograft (CDX) mouse models, established LUAD cells (initial 5 x 106) were subcutaneously injected into the bilateral dorsal flank of athymic nude mice. To generate H1975/A549 cell-implanted intrapulmonary LUAD mice, athymic nude mice were intrapulmonarily injected with cells (5 x 106) under anesthesia and then intranasally administered adeno-associated virus 5 (AAV5) particles (2 x 1012 viral particles/mL, Genomeditech, Shanghai, China) 3 weeks later. To generate patient-derived xenograft (PDX) mouse models, fresh LUAD tissues with a size of 2-3 mm3 were subcutaneously implanted into athymic nude mice. After successful passage, the PDX mice were used for further studies.
Click to Show/Hide
|
||||
| Response Description | Intracellular cir93FABP3 interactions are critical to upregulate FABP3 to reduce global AA via reactions with taurine. The product of AA and taurine (i.e., NAT) prevents AA incorporation into the plasma membrane, thus further reducing the opportunity for PUFA peroxidation in the membrane. NAT reduces ACSL4, LPCAT3 and PLTP. Exosome and cir93 are critical to desensitize lung adenocarcinoma to ferroptosis. | ||||
Cystathionine beta-synthase (CBS)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [38] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| MKN-28 cells | Gastric epithelial carcinoma | Homo sapiens | CVCL_1416 | ||
| In Vivo Model |
Female non-obese diabetic severe combined immune-deficient mice at 5 weeks of age were divided into indicated groups and injected subcutaneously at either side of flank area with indicated cell lines (1 x 106 cells) suspended in 0.1 ml phosphate-buffered saline (PBS). Tumor sizes in all groups were measured every 3 days using Vernier calipers and calculated using the following formula: (length x width2)/2. For the xenograft Cisplatin treatment assay, day 0 was designed when tumors reached around 50 mm3 in volume. DDP 7 mg/kg or carrier (PBS, 100 uL)) was injected intraperitoneally 1 time per week. 21 days after treatment, all mice were sacrificed and tumors were harvested and weighed. Representative images were presented, and all experiments were repeated at least 3 times.
Click to Show/Hide
|
||||
| Response Description | Schematic diagram showing that HIF-1 induces lncRNA-CBSLR to recruit YTHDF2 protein and CBS mRNA to form CBSLR/ YTHDF2/CBS complex, which in turn decreases CBS mRNA stability in an m6A dependent manner. The decreased CBS expression reduced methylation of ACSL4 protein, thus, the protein is degraded via the ubiquitination-proteasome pathway. Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. | ||||
CircSCN8A (circRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [35] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| NCI-H520 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | ||
| SK-MES-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | ||
| BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | ||
| In Vivo Model |
Male BALB/c nude mice (3-4 weeks, 16-20 g) were purchased from Vital River Laboratory (Beijing, China) and housed under standard conditions. After acclimatization for one week, mice were randomly divided into two groups (n = 6/group). A549 cells infected with lentivirus circSCN8A (Lv-circSNC8A) or control (Lv-vector) were selected in the presence of puromycin (1 ug/ml). A549 cells (7 x 106) with Lv-circSNC8A or Lv-vector were suspended in 100 uL PBS and respectively injected into each mouse at the right flank.
Click to Show/Hide
|
||||
| Response Description | CircSCN8A represses cell proliferation and metastasis in NSCLC by regulating the miR-1290/ACSL4 axis to induce ferroptosis. Thus, circSCN8A may represent a promising therapeutic target against NSCLC. | ||||
CircLMO1 (circRNA)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [33] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
In Vitro Model |
SiHa cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | |
| HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| Ca Ski cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | ||
| C33A cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1094 | ||
| In Vivo Model |
Male BALB/c nude mice (6 weeks old) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The mice were kept in a constant temperature (25) and pathogen-free room with free access to food and water ad libitum. The animal experiments were approved by the Ethics Committee for Animal Experimentation of The Second Affiliated Hospital and Yuying Childrens Hospital. Mice were euthanised with isoflurane inhalation. CaSki cells overexpressing circLMO1 (7 x 106 cells/100 uL PBS) were injected subcutaneously into the flank of mice. Tumor growth was measured with a caliper 3 times a week and tumor-bearing mice were euthanised at 5 weeks after inoculation.
Click to Show/Hide
|
||||
| Response Description | CircLMO1 acted as a competing endogenous RNA (ceRNA) by sponging miR-4192 to repress target gene ACSL4. CircLMO1 promoted cervical cancer cell ferroptosis through up-regulating ACSL4 expression. Overexpression of miR-4291 or knockdown of ACSL4 reversed the effect of circLMO1 on facilitating ferroptosis and repressing cervical cancer cell proliferation and invasion. | ||||
CircEXOC5 (circRNA)
Lung injury [ICD-11: NB32]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mLVECs (Mouse lung microvascular endothelial cells) | ||||
| In Vivo Model |
The sepsis mouse model was established by cecal ligation and puncture (CLP) of male C57BL/6 mouse (6-8 weeks, Guangzhou Animal Medical Center). Firstly, the mice were anesthetized by intraperitoneal injection of 4% chloral hydrate (0.1 ml/10g). The cecum was found by cutting a 1 cm longitudinally in the abdomen of the mice, and ligating the cecum from the end of the cecum to half the length of the ileocecal valve with 5-0 silk suture. Then a 21 G needle was used to puncture the ligature and the midpoint of the cecum once, and finally the wound was sutured. In the sham operation group, only laparotomy was performed, without ligation and perforation of the cecum. All animal-related experiments in this study were approved by hospital ethics committee according to following the Nation Institutes of Health Guide for the Laboratory Animals Care and Use (approval No. Med-Eth-Re [2022] 168).
Click to Show/Hide
|
||||
| Response Description | CircEXOC5 can enhance the stability of the target gene ACSL4 by binding to the RNA binding protein PTBP1 and up-regulate its expression, thereby promoting ferroptosis and exacerbating sepsis-induced acute lung injury. | ||||
Circ-Carm1 (circRNA)
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [29] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Circ-Carm1 was evidently abundant in acute cerebral infarction model cells, and knockdown of circ-Carm1 notably restored cell viability and inhibited ferroptosis in ACI model cells. Mechanistically, circ-Carm1 sponged miR-3098-3p to upregulate ACSL4 expression in ACI model cells to participate in ACI progressionin vitro. | |||
Cir93 (circRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [37] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| PC-9 cells | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| NCI-H1975 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | ||
| NCI-H358 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1559 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| In Vivo Model |
All athymic nude mice (6-week-old) were purchased from Jiesijie (Shanghai, China). To generate routine cell-derived xenograft (CDX) mouse models, established LUAD cells (initial 5 x 106) were subcutaneously injected into the bilateral dorsal flank of athymic nude mice. To generate H1975/A549 cell-implanted intrapulmonary LUAD mice, athymic nude mice were intrapulmonarily injected with cells (5 x 106) under anesthesia and then intranasally administered adeno-associated virus 5 (AAV5) particles (2 x 1012 viral particles/mL, Genomeditech, Shanghai, China) 3 weeks later. To generate patient-derived xenograft (PDX) mouse models, fresh LUAD tissues with a size of 2-3 mm3 were subcutaneously implanted into athymic nude mice. After successful passage, the PDX mice were used for further studies.
Click to Show/Hide
|
||||
| Response Description | Intracellular cir93 FABP3 interactions are critical to upregulate FABP3 to reduce global AA via reactions with taurine. The product of AA and taurine (i.e., NAT) prevents AA incorporation into the plasma membrane, thus further reducing the opportunity for PUFA peroxidation in the membrane. NAT reduces ACSL4, LPCAT3 and PLTP. Exosome and cir93 are critical to desensitize lung adenocarcinoma to ferroptosis. | ||||
CBSLR (IncRNA)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [38] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| MKN-28 cells | Gastric epithelial carcinoma | Homo sapiens | CVCL_1416 | ||
| In Vivo Model |
Female non-obese diabetic severe combined immune-deficient mice at 5 weeks of age were divided into indicated groups and injected subcutaneously at either side of flank area with indicated cell lines (1 x 106 cells) suspended in 0.1 ml phosphate-buffered saline (PBS). Tumor sizes in all groups were measured every 3 days using Vernier calipers and calculated using the following formula: (length x width2)/2. For the xenograft Cisplatin treatment assay, day 0 was designed when tumors reached around 50 mm3 in volume. DDP 7 mg/kg or carrier (PBS, 100 uL)) was injected intraperitoneally 1 time per week. 21 days after treatment, all mice were sacrificed and tumors were harvested and weighed. Representative images were presented, and all experiments were repeated at least 3 times.
Click to Show/Hide
|
||||
| Response Description | Schematic diagram showing that HIF-1 induces lncRNA-CBSLR to recruit YTHDF2 protein and CBS mRNA to form CBSLR/ YTHDF2/CBS complex, which in turn decreases CBS mRNA stability in an m6A dependent manner. The decreased CBS expression reduced methylation of ACSL4 protein, thus, the protein is degraded via the ubiquitination-proteasome pathway. Hypoxia inducible lncRNA-CBSLR modulates ferroptosis through m6A-YTHDF2-dependent modulation of CBS in gastric cancer. | ||||
ADP-ribosylation factor 6 (ARF6)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [39] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Ras signaling pathway | hsa04014 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HPDE6-C7 cells | Normal | Homo sapiens | CVCL_0P38 |
| AsPC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0152 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| BxPC-3 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | |
| SW1990 cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1723 | |
| Capan-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0237 | |
| MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | |
| Response Description | ARF6, functioned as a downstream of Kras/ERK signaling pathway, could promote proliferation and Warburg effect in pancreatic cancer cells. ARF6 decreased ACSL4 protein level and this effect endowed pancreatic cancer cells to a status that sensitized to oxidative stress. | |||
Unspecific Regulator
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [40] | |||
| Responsed Drug | Dihydrotanshinone I | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HEB (Human glial cells) | |||
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| Response Description | Dihydrotanshinone I (DHI) inhibited the proliferation of human glioma cells. Following treatment of the U251 and U87 cells with DHI, changes in the expression levels of ferroptosis-associated proteins were observed; the expression level of GPX4 decreased and that of ACSL-4 increased. DHI also increased the levels of LDH and MDA in the human glioma cells and reduced the GSH/GSSG ratio. | |||
Gastrointestinal cancer [ICD-11: 2B5B]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [41] | ||||
| Responsed Drug | Berberine | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| TMK-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4384 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
Five-week-old male BALB/c mice were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained in a pathogen-free animal facility under a 12 h light/dark cycle in a temperature (22 )- and humidity-controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following the current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval no. 12924, 5 November 2020). Animals were acclimated to their housing for seven days before the start of the experiment. For the peritoneal dissemination tumor model, CT26 cancer cells (1 x 107 in 0.2 mL per mouse) were injected into the mouse peritoneal cavity. To measure tumor weight, mice were euthanized on Day 12 and the tumors were excised, while the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, with gross removal of non-tumor tissues. The largest tumor was formed on the diaphragm, and paraffin-embedded sections of the excised diaphragmatic tumor were prepared and stained with hematoxylin-eosin. BBR was diluted with distilled water to produce a final concentration of 48 mg/mL. The solutions were ultrasonically treated for 1 h, and fully vortexed for 30 min. BBR solution was administered by free drinking. The intake calculated from the amount of water consumed was 15.2 mg/kg body weight/day.
Click to Show/Hide
|
||||
| Response Description | Berberine induces apoptosis and ferroptosis by inhibiting mitochondrial complex I and promoting autophagy, leading to combined cell death in the GIC and suppressing stemness. BBR induces cell death in gastrointestinal cancer cells accompanied by increased mitochondrial superoxide and ACSL4 levels, decreased SLC7A11, and impaired antioxidant mechanisms, indicated by decreased GPX4 expression and decreased GSH. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [42] | |||
| Responsed Drug | Atractylodin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hccm (Human hepatocellular carcinoma cells) | ||||
| Response Description | Atractylodin can inhibit the proliferation, migration, and invasion of Huh7 and Hccm liver cancer cells, and induce cell apoptosis and cell cycle arrest. In addition, atractylodin may induce ferroptosis in hepatocellular carcinoma cells by inhibiting the expression of GPX4 and FTL proteins, and up-regulating the expression of ACSL4 and TFR1 proteins. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [43] | |||
| Responsed Drug | Seco-Lupane Triterpene Derivatives | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Response Description | A new seco-lupane triterpene derivative, compound21, was found to regulate cell growth through the cell cycle and ferroptosis, which in turn inhibited the proliferation, migration, and invasion of HepG2 cells. And it was found that compound 21 significantly upregulated ACSL4 protein expression and downregulated GPX4 protein expression. It has the potential to become an effective new drug for the treatment of hepatocellular carcinoma. | |||
Lung cancer [ICD-11: 2C25]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [44] | ||||
| Responsed Drug | Curcumin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
In Vitro Model |
A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
Female C57BL/6 mice (14-18 g) were purchased from SiPeiFu (Beijing) Biotechnology. C57BL/6 mice were subcutaneously injected with a total of 6 x 105 Lewis lung carcinomas (LLC) cells on the left flank. Four days after LLC inoculation, the mice were randomly divided into two groups of five. The vehicle control and curcumin groups were given sodium carboxymethyl cellulose (CMC) or curcumin (100 mg/kg/day) by intraperitoneal injection for 15 days.
Click to Show/Hide
|
||||
| Response Description | Curcumin induced ferroptosis via activating autophagy in non-small-cell lung cancer (NSCLC), which enhanced the therapeutic effect of NSCLC. Meanwhile, the protein level of ACSL4 was higher and the levels of SLC7A11 and GPX4 were lower in curcumin group than that in control group. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [45] | ||||
| Responsed Drug | Borneol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell adhesion molecules | hsa04514 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
H460/CisR cells | Lung large cell carcinoma | Homo sapiens | CVCL_C5S1 | |
| In Vivo Model |
Male Balb/c nude mice (4-week-old) were purchased from SPF (Beijing) biotechnology co., LTD and maintained in the Experimental Animal Research Center of Chengdu University of TCM. After 1 week of adaptable feeding, H460/CDDP cells (5 x 106 cells in 0.1 ml phosphate-buffered saline) were subcutaneously injected into the right dorsal flank to establish tumor model. When the tumor volume grows to 100 mm3, the tumor-bearing mice were randomly divided into the following four treatment groups: a control group (Con, n = 6): intraperitoneal injection of saline once a day; vehicle group (Vehicle, n = 6): intragastric administration of 2% tween and intraperitoneal injection of saline; d-borneol low-dose group (Bor-L, n = 6): intragastric administration of d-borneol (30 mg/kg) once a day; d-borneol high-dose group (Bor-H, n = 6): intragastric administration of d-borneol (60 mg/kg) once a day; CDDP group (CDDP, n = 6): intraperitoneal injection of cisplatin (3 mg/kg) every two days; a low-dose combination treatment group (C+B-L, n = 6): intragastric administration of d-borneol (30 mg/kg) once a day and intraperitoneal injection of cisplatin (3 mg/kg) every two days; a high-dose combination treatment group (C+B-H, n = 6): intragastric administration of d-borneol (60 mg/kg) once a day and intraperitoneal injection of cisplatin (3 mg/kg) every two days. We usually first orally gavage d-borneol, and then inject cisplatin intraperitoneally half an hour later. After 14 days treatment, the samples were obtained from the mice for the further experiments.
Click to Show/Hide
|
||||
| Response Description | d-borneol in combination with cisplatin induced ferroptosisviaNCOA4-mediated ferritinophagy and also increased the expression levels of ACSL4, regulated PCBP2 and PRNP to promote the conversion of Fe3+to Fe2+, reduced the activity or expression of antioxidants enzymes (GSH and HO-1), and induced ROS accumulation and thereby promoted ferroptosis. In addition, activation of autophagy inhibited progression of the EMT and increased sensitivity to cisplatin in cisplatin-resistant lung cancer cells. | ||||
Breast cancer [ICD-11: 2C60]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [46] | ||||
| Responsed Drug | Polyphyllin III | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| HBL-100 cells | Normal | Homo sapiens | CVCL_4362 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| MDA-MB-453 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | ||
| In Vivo Model |
MDA-MB-231 xenografts were established in 5 week-old BALB/C nude mice (Shanghai SLAC Laboratory Animal Corporation) by inoculating 1 x 106 cells mixed with Matrigel (BD Biosciences) at a 1:1 ratio into the abdominal mammary fat pad. When the tumor reached 50-100 mm3, the mice were assigned randomly into different treatment groups (DMSO, PPIII, SAS, and PPIII + SAS groups), and each group consisted of 5 mice. PPIII (5 mg/kg/day) and SAS (200 mg/kg/day) were dissolved in dimethyl sulfoxide (DMSO), diluted in PBS, and then intraperitoneally injected into mice at a dose of 10 ml/kg/d once a day.
Click to Show/Hide
|
||||
| Response Description | Polyphyllin III, which is a major saponin extracted fromParis polyphyllarhizomes, exerted its proliferation-inhibitory effect on MDA-MB-231 triple-negative breast cancer cells mainly through ACSL4-mediated lipid peroxidation elevation and ferroptosis induction. Polyphyllin III treatment also induced KLF4-mediated protective upregulation of xCT(SLC7A11), which is the negative regulator of ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [47] | ||||
| Responsed Drug | Robustaflavone 7,5'-dimethyl ether | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | |
| Response Description | Molecular docking study indicated that robustaflavone 7,5'-dimethyl ether (8) is a potential strong inhibitor, which induce ferroptosis via down-regulating the expression level of ACSL4 proteins in human breast cancer MCF-7 cells. | ||||
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [48] | ||||
| Responsed Drug | Oleanolic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| In Vivo Model |
Male BALB/c Nude mice (20 ± 2g, 5 weeks old) were supplied by Hangzhou Ziyuan Experimental Animal Technology Co. LTD (SYXK-20180049) for this study. The nude mice were kept under specefic pathogen free (SPF) conditions for one week, and 5x107 Hela cells were injected under the left axillary skin after acclimatization. The tumors of Hela cell-inoculated mice were measured every 3 days after modeling, and tumor>=0.5 cm in diameter were considered successful. The tumor-bearing mice were randomly divided into control group (n = 6), 40 mg/kg OA group (n = 6) and 80 mg/kg OA group (n = 6). 40 mg/kg OA group and 80 mg/kg OA group both received subcutaneous injection modeling and control mice received saline intraperitoneal injections. The 40 mg/kg OA group and 80 mg/kg OA group received daily intraperitoneal injections of 40 mg/kg OA and 80 mg/kg OA for 15 days.
Click to Show/Hide
|
||||
| Response Description | Oleanolic acid (OA) significantly reduced the viability and proliferative capacity of Hela cells. OA activated ferroptosis in Hela cells by promoting ACSL4 expression, thereby reducing the survival rate of Hela cells. Therefore, promotion of ACSL4-dependent ferroptosis by OA may be a potential approach for the treatment of cervical cancer. | ||||
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [49] | |||
| Responsed Drug | Lycorine | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | |
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| Response Description | Lycorine could inhibit the proliferation in human renal cell carcinoma (RCC) cells. The anti-tumor effect of lycorine was associated with the induction of ferroptosis. After lycorine treatment, the expression levels of GPX4 in RCC cells decreased, whereas those of ACSL4 increased. | |||
Cognitive disorder [ICD-11: 6D71]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [50] | ||||
| Responsed Drug | Liraglutide | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
Male diabetic db/db mice and nondiabetic littermate db/m mice that were 4 weeks of age were purchased from Changzhou Cavens Experimental Animal Co., Ltd. After 1week of adaptive feeding, 20 db/db mice were randomly divided into two groups: a model group (db/db, n = 10) and a treatment group (LIRA, n = 10). Another 10 db/m mice were used as the control group (db/m, n = 10). After feeding to 10weeks old, the LIRA group was given liraglutide (CSN11311, CSNpharm, China) diluent (200 ug/kg/d) by intraperitoneal injection for 5 weeks, with equivoluminal 0.9% saline intraperitoneally administered to the other two groups.
Click to Show/Hide
|
||||
| Response Description | Liraglutide was shown to prevent ferroptosis in the hippocampus by elevating the expression of GPX4 and SLC7A11 and suppressing the excessive amount of ACSL4. LIRA can reduce oxidative stress, lipid peroxidation and iron overload in diabetes-induced cognitive dysfunction and further inhibit ferroptosis, thereby weakening the damage to hippocampal neurons and synaptic plasticity and ultimately restoring cognitive function. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [51] | ||||
| Responsed Drug | Oxidopamine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
The AB strain of wild-type zebrafish (Danio rerio) was applied in this study. Zebrafish larvae at 4 dpf (days post-fertilization) were co-incubated with 250 uM 6-OHDA or 1.5 ug/mL nomifensine (Nomi, a dopamine transporter inhibitor) in 6-well plates at a density of 30 zebrafish embryos per group for 2 days and the medium was refreshed every day. The swimming total distance of each fish was recorded for 10 min and was analyzed by an automated video tracking system.
Click to Show/Hide
|
||||
| Response Description | 6-hydroxydopamine (6-OHDA) treatment-induced ferroptosis in SH-SY5Y cells mainly by disturbing the protein expression of GPX4 and ACSL4. Collectively, the activation of the p62-Keap1-Nrf2 pathway prevents 6-OHDA-induced ferroptosis in SH-SY5Y cells, targeting this pathway in combination with a pharmacological inhibitor of ferroptosis can be a potential approach for parkinson's disease therapy. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [52] | ||||
| Responsed Drug | L. lactis MG1363-pMG36e-GLP-1 | Investigative | |||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Colon tissues (Mouse colon tissues) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
Fifty male C57BL/6 mice provided by Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China) resided in an animal house (temperature 26 ± 1 , humidity 50 ± 10%), in which the light was on for 12 h and off for 12 h. Mice were acclimatised for 1 week and allowed water and animal food with no limitations. Then, all mice were stochastically divided into 5 groups using random number tables available online (https://www.random-online.com/, accessed on 26 December 2021), including: (1) C group, a control group treated with normal saline for 7 consecutive days (n = 10); (2) M group, a model group with intraperitoneal injection of 20 mg/kg/day MPTP (Sigma-Aldrich, Taufkirchen, Germany, M0896) for 7 consecutive days (n = 10); (3) L group, treated with MPTP and 0.4 mg/kg/day liraglutide for 7 consecutive days (n = 10); (4) R group, treated with MPTP and 109 colony-forming unit (CFU) L. lactis MG1363 for 7 consecutive days via gavage (n = 10); (5) RG group, treated with MPTP and 109 CFUL. lactis MG1363-pMG36e-GLP-1 for 7 consecutive days via gavage (n = 10). All animals survived treatment and all animal experiments were administered from 9:00 to 12:00 in the morning to reduce systematic errors.
Click to Show/Hide
|
||||
| Response Description | L. lactis MG1363-pMG36e-GLP-1 exerts neurotrophic effects via activating the Keap1/Nrf2/GPX4 signalling pathway to down-regulate ACSL4 and up-regulate FSP1 to suppress ferroptosis. These results indicated that the neurotrophic effects of the next-generation probiotics L. lactis MG1363-pMG36e-GLP-1 against MPTP-induced Parkinsonism are mediated by modulating oxidative stress, inhibiting ferroptosis, and redressing dysbiosis. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [53] | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
The mice (23-25 g, 8-10 weeks old) were subjected to transientmiddle cerebral artery occlusion (tMCAO) to induce cerebral ischemia as previously described protocol . Briefly, mice were anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg) and subcutaneous injection of meloxicam (10mg/kg) during tMCAO operation. Monofilament with a silicon coating on the tip and a diameter of 0.12 mm (A5-122, Beijing Cinontech Co. Ltd., China) was inserted into the ICA from CCA to occlude the middle cerebral artery (MCA) for 1.5 h. The suture was then removed to restore blood flow for another 22.5 h reperfusion. Sham control mice were subjected to similar surgical operations without MCA occlusion. Specifically, the monofilament was inserted only 5 mm above the carotid bifurcation and withdrew immediately in the Sham group.
Click to Show/Hide
|
||||
| Response Description | Baicalein inhibited the ferroptosis by regulating on the expression levels of GPX4, ACSL4 and ACSL3 in OGD/R cells, tMCAO mice and RSL3-stimulated HT22 cells. Our findings demonstrated that baicalein reversed the cerebral ischemia-reperfusion injury via anti-ferroptosis, which was regulated by GPX4/ACSL4/ACSL3 axis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [54] | ||||
| Responsed Drug | Chrysin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [55] | ||||
| Responsed Drug | Carthamin yellow | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| NF-kappa B signaling pathway | hsa04064 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
A total of 32 male Sprague-Dawley rats (aged 6-8 weeks; 250-280 g) were purchased from Shanghai Sipper-BK Lab Animal Co., Ltd. Animals were randomly divided into the following four groups (n = 8 per group): i) Sham; ii) MCAO; iii) CY (20 mg/kg); and iv) CY (40 mg/kg). CY was administered intragastrically to rats once daily for 2 weeks. At 60 min after the last administration, MCAO surgery was performed as previously described. At 24 h post-reperfusion, neurological scores, brain water content and infarct volume were determined.
Click to Show/Hide
|
||||
| Response Description | Carthamin yellow (CY) treatment inhibited Fe2+ and reactive oxygen species accumulation, and reversed acylCoA synthetase longchain family member 4, transferrin receptor 1, glutathione peroxidase 4 and ferritin heavy chain 1 protein expression levels in the brain. Collectively, the results of the present study demonstrated that CY protected rats against ischemic stroke, which was associated with mitigation of inflammation and ferroptosis. | ||||
Nervous system disease [ICD-11: 8E7Z]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [56] | |||
| Responsed Drug | N2L | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | N2L recovered glutathione peroxidase 4 (GPX4) expression and blocked the increase of Cyclooxygenase-2 (cox-2) and acyl-CoA synthetase long-chain family member 4 (ACSL4) protein expressions. Moreover, N2L also significantly prevented Ferritin Heavy Chain 1 (FTH1) from downregulation and maintained iron homeostasis. And N2L could be a ferroptosis inhibitor for the therapy of ferroptosis-related neurodegenerative diseases, such as Alzheimer's disease. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [57] | |||
| Responsed Drug | Sertaconazole | Approved | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
LUHMES cells | Normal | Homo sapiens | CVCL_B056 |
| Response Description | Sertaconazole is the most potent ACSL4 inhibitor identified. In addition, sertaconazole significantly reduced lipid peroxidation and ferroptosis in human differentiated dopaminergic neurons (Lund human mesencephalic LUHMES cells), demonstrating that it is a valuable chemical tool for further investigating the role of ACSL4 in nervous system disease. | |||
Pulmonary fibrosis [ICD-11: CB03]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [58] | ||||
| Responsed Drug | Bleomycin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response Description | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of ACSL4 but similarly decreased those of FSP1. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [59] | ||||
| Responsed Drug | Epigallocatechin Gallate | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
After adaptive feeding, mice were randomly assigned to five groups (n = 10 per group). The details of the groups are as follows: 1) the normal diet (ND) group in which mice were fed ND (18% calories from fat); 2) the HFD group in which mice were fed HFD (60% calories from fat); 3) the HFD-EGCG/L group in which mice received 20 mg/kgbw EGCG by oral gavage daily during HFD feeding; 4) the HFD-EGCG/H group in which mice received 100 mg/kgbw EGCG by oral gavage daily during HFD feeding; and 5) the HFD-Fer-1 group in which mice received intraperitoneal injection of Fer-1 at 1 mg/kg. bw every 3 days during HFD feeding. Mice in the EGCG treatment groups were supplemented with EGCG (20 and 100 mg/kgbw) for 12 weeks. Meanwhile, mice in the ND group and the HFD group were orally gavaged with deionized water daily.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation and Fer-1 treatment apparently increased the protein expression of GPX4 and markedly decreased the protein expression of COX-2 and ACSL4 in the livers of HFD-fed mice. Epigallocatechin gallate may exert protective effects on hepatic lipotoxicity by inhibiting mitochondrial reactive oxygen species-mediated hepatic ferroptosis. Findings from our study provide new insight into prevention and treatment strategies for non-alcoholic fatty liver disease pathological processes. | ||||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 7 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [60] | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [61] | ||||
| Responsed Drug | Baicalin | Terminated | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague-Dawley rats, 260-280 g, were provided by Beijing Vital River Laboratory Animal Technology CO., Ltd. (Beijing, China). Rats were randomly divided into five groups (n = 15 per group): control (sham operation + saline), I/R (I/R + saline), baicalin 100 mg/kg (BA-100, I/R + baicalin 100 mg/kg), baicalin 200 mg/kg (BA-200, I/R + baicalin 200 mg/kg), and diltiazem 20 mg/kg (DI-20, I/R + diltiazem 20 mg/kg). Drugs were given by oral gavage once daily (8 a.m.) for 6 days. At day 6, myocardial ischemia was induced 1 h after drug was administered.
Click to Show/Hide
|
||||
| Response Description | Baicalin prevents against myocardial ischemia/reperfusion injury via suppressing ACSL4-controlled ferroptosis. In addition, enhanced lipid peroxidation and significant iron accumulation along with activated transferrin receptor protein 1 (TfR1) signal and nuclear receptor coactivator 4 (NCOA4)-medicated ferritinophagy were observed in in vivo and in vitro models, which were reversed by baicalin treatment. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [62] | ||||
| Responsed Drug | Curcumin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| rPTs (Rat pancreas tissues) | |||||
| rHTs (Rat hippocampal tissues) | |||||
| In Vivo Model |
Forty female albino Wistar rats weighing 180-220 g were used in the study. Eight rats in each group were randomly assigned to five different groups: Group I (Sham); Group II (IR); Group III (IR + DMSO); Group IV (IR + Curcumin 100 mg/kg); and Group V (IR + 2 ug/kg LoxBlock-1) were determined. The animals were maintained at a temperature of 21 ± 2 and regulated humidity conditions (50 ± 5%) with a twelve-hour light/dark cycle. Throughout the experiment, the animals were fed standard commercial rat pellets and given tap water. All surgical and anesthesia procedures were performed understerile conditions. In addition, in a case of abnormal symptoms, the animals would be removed from the group and sacrificed under deep anesthesia.
Click to Show/Hide
|
||||
| Response Description | Curcumin attenuates liver, pancreas and cardiac ferroptosis, oxidative stress and injury in ischemia/reperfusion-damaged rats by facilitating ACSL/GPx4 signaling. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Disease Response of This Regulator | [63] | ||||
| Responsed Drug | Gossypol acetic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
A total of 55 adult male Sprague-Dawley rat (350-450 g) were anesthetized with urethane (1.5 g/kg, i.p.), then the hearts were perfused in a Langendorff system. After 30 min of stabilization, hearts were subjected to 30 min of global no-flow ischemia by stopping the perfusion. Reperfusion was followed with Krebs Henseleit (KH) buffer and GAA together for 2 h. A thermoregulated chamber kept the heart at 37 throughout the experiment. Control hearts were not subjected to I/R. The heart slices were sectioned at a thickness of 2 mm and stained with triphenyltetrazolium chloride (25 mg/100 mL) for 10 min and then fixed with 4% formaldehyde solution for 48 h to enhance color contrast.
Click to Show/Hide
|
||||
| Response Description | Gossypol acetic acid significantly attenuated myocardial infarct size, reduced lipid peroxidation, decreased the mRNA levels of the ferroptosis markers Ptgs2 and Acsl4, decreased the protein levels of ACSL4 and NRF2, and increased the protein levels of GPX4 in I/R-induced ex vivo rat hearts. Thus, GAA may play a cytoprotectant role in ferroptosis-induced cardiomyocyte death and myocardial ischemia/reperfusion-induced ferroptotic cell death. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Disease Response of This Regulator | [64] | ||||
| Responsed Drug | Xanthohumol | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Rats were anesthetized with urethane (1.5 g/kg, i.p.), then hearts were excised and arrested in Krebs Henseleit (KH) buffer as previously described. Following 30 min equilibration, ischemia was induced by halting perfusion for 45 min. Reperfusion was followed with KH buffer and XN (5 or 10 uM) together for 60 min. Control hearts were not subjected to I/R.
Click to Show/Hide
|
||||
| Response Description | Xanthohumol can prevent ferroptosis during cardiac ischemia-reperfusion by reducing the expression of Acsl4 and Ptgs2 mRNA, reducing the expression of ACSL4 and NRF2 protein, and modulating the expression of GPX4 protein. | ||||
| Experiment 6 Reporting the Ferroptosis-centered Disease Response of This Regulator | [60] | ||||
| Responsed Drug | Luteolin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
| Experiment 7 Reporting the Ferroptosis-centered Disease Response of This Regulator | [65] | ||||
| Responsed Drug | Nobiletin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague-Dawley (SD) rats (4 weeks old, 90-110 g) were obtained from the Vital River Biological company. Rats were kept in a specific-pathogen-free (SPF) environment, with access to food and tap water at an ambient temperature of 20-22 . All institutional and national guidelines for the care and use of laboratory animals were followed. The protocols were reviewed and approved by the Institution of Animal Care and Use Committee of Renmin Hospital of Wuhan University (IACUC, license no. 20200303).
Click to Show/Hide
|
||||
| Response Description | Both ferrostain-1 and nobiletin decreased the expression of ferroptosis-related proteins including Acyl-CoA synthetase long chain family member 4 (ACSL4) and nuclear receptor coactivator 4 (NCOA4) but not glutathione peroxidase 4 (GPX4) in rats with mature T2DM and cells with HFHG and H/R injury. Nobiletin has therapeutic potential for alleviating myocardial ischemia-reperfusion injury associated with ACSL4- and NCOA4-related ferroptosis. | ||||
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [66] | ||||
| Responsed Drug | Vitamin K1 | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
NIH3T3 cells | Normal | Mus musculus | CVCL_0594 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| MCT (Murine proximal tubular epithelial cells) | |||||
| In Vivo Model |
All mice used in our in vivo studies were 8-week-old males of the C57BL/6J background. Kidneys were exposed via a midline abdominal incision and bilateral renal pedicle clamping for 35 min using microaneurysm clamps (Aesculap Inc., Center Valley, PA, USA). Throughout the surgical procedure, the mice were kept under isoflurane narcosis, and their body temperature was maintained at 36-37 by continuous monitoring using a temperature-controlled, self-regulated heating system (Fine Science Tools, Heidelberg, Germany). After clamps were removed, kidney reperfusion was confirmed visually before the abdomen was closed in two layers using standard 6-0 sutures. To maintain fluid balance, all mice were supplemented with 1 ml of prewarmed PBS administered intraperitoneally directly after surgery. After 48 h of reperfusion, the mice were sacrificed, blood samples were obtained by retrobulbar puncture, and kidneys were collected for analysis.
Click to Show/Hide
|
||||
| Response Description | Renal expression of ACSL4 was markedly enhanced by IRI and reduced by vitamin K1. Vitamin K1 as a potent inhibitor of ferroptosis, and hence, it represents a potential drug for the treatment of pathological cell death processes during acute kidney injury in humans. | ||||
Traumatic brain injury [ICD-11: NA07]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [67] | ||||
| Responsed Drug | Sevoflurane | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Sixty-four male Sprague-Dawley rats (2 weeks), weighing 20-30 g. All animals were brought from the Institute of Medical Laboratory Animals at the Chinese Academy of Medical Sciences and were kept in the same unit in a temperature-controlled environment [(22 ± 1) ]. The rats were fasted for 12 h before the experiment and drank water freely. After being numbered according to body weight, the rats were randomly divided into four groups using the random number table. The number of rats in each group was 16. The experimental groups were as follows: sham-operated group (S group, n = 16), the model group receiving HIR (HIR group, n = 16), sevoflurane group treated (HIR + Sev group, n = 16), and desferrioxamine treated group [deferoxamine (HIR + Sev + DFO) group, n = 16]. In HIR+Sev and HIR + Sev + DFO groups, rats were placed in an anesthetizing chamber and exposed to 3.6% sevoflurane (Cayman, 23996, USA) with complete oxygen for 2 h, and sham and HIR group rats were conducted with the same procedure without sevoflurane exposure. DFO (100 mg/kg, MCE, HY-B0988, China) was administered continuously daily for 6 days before surgery in the HIR + Sev + DFO group. Other groups were given equal amounts of saline.
Click to Show/Hide
|
||||
| Response Description | TFRC levels and ACSL4 levels were elevated after sevoflurane administration, suggesting that ferroptosis occurs in whole-brain regions of young rats after HIR and that sevoflurane aggravates the extent of ferroptosis. The results suggest that ferroptosis may mediate sevoflurane-aggravated young rats' brain injury induced by liver transplantation. | ||||
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [68] | ||||
| Responsed Drug | Edaravone | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rSCTs (Rat spinal cord tissues) | ||||
| In Vivo Model |
The rats were initially anesthetized with 5% isoflurane (RWD life science, Shenzhen, China) and then maintained with 22.5% isoflurane. A 1-cm midline incision was made over the thoracic vertebrae, and laminectomy on T10 and the caudal half of T9 vertebrae was performed. Spinal cord contusion injury was conducted by NYU Impactor Model III (W.M. Keck Center for Collaborative Neuroscience Rutgers, The State University of New Jersey, United States) using a 10-g node dropping freely from a height of 2.5 cm and muscles and skin sutured in layers. Sham controls underwent laminectomy without the contusion. To prevent infection at the incision, cefuroxime sodium was applied for 3 days after injury. The bladders were emptied manually twice daily in the first week after injury.
Click to Show/Hide
|
||||
| Response Description | Edaravone not only rescues the ferroptosis negative regulators, xCT and GPX4, but also downregulates those pro-ferroptosis factors, ACSL4 and 5-LOX. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. And in the acute phase of spinal cord injury (SCI), edaravone reduced neuronal cell death and neuroinflammation. | ||||
Signal transducer and activator of transcription 3 (STAT3)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [70] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | |
| SUM159PT cells | Breast pleomorphic carcinoma | Homo sapiens | CVCL_5590 | ||
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| In Vivo Model |
PDX models of triple-negative breast cancer were obtained from the Dana-Farber Cancer Institute and propagated in NSG mice. Tumors were harvested and digested using collagenase at 37. Once digested, the cells were filtered using a cell strainer (40 um), washed twice with PBS, and plated in DMEM/F12 (containing 10% FBS).
Click to Show/Hide
|
||||
| Response Description | 64-mediated activation of Src and STAT3 suppresses expression of ACSL4, an enzyme that enriches membranes with long polyunsaturated fatty acids and is required for ferroptosis. | ||||
Proto-oncogene tyrosine-protein kinase Src (SRC)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [70] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
In Vitro Model |
MCF-10A cells | Normal | Homo sapiens | CVCL_0598 | |
| SUM159PT cells | Breast pleomorphic carcinoma | Homo sapiens | CVCL_5590 | ||
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| In Vivo Model |
PDX models of triple-negative breast cancer were obtained from the Dana-Farber Cancer Institute and propagated in NSG mice. Tumors were harvested and digested using collagenase at 37. Once digested, the cells were filtered using a cell strainer (40 um), washed twice with PBS, and plated in DMEM/F12 (containing 10% FBS).
Click to Show/Hide
|
||||
| Response Description | 64-mediated activation of Src and STAT3 suppresses expression of ACSL4, an enzyme that enriches membranes with long polyunsaturated fatty acids and is required for ferroptosis. | ||||
hsa-miR-92 (miRNA)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | The upregulated expression of individual miRNAs, miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92 were determined by qRT-PCR. This study revealed a novel mechanism through which miR-17-92 protects endothelial cells from erastin-induced ferroptosis by targeting the A20-ACSL4 axis. | |||
hsa-mir-20a (Precursor RNA)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | The upregulated expression of individual miRNAs, miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92 were determined by qRT-PCR. This study revealed a novel mechanism through which miR-17-92 protects endothelial cells from erastin-induced ferroptosis by targeting the A20-ACSL4 axis. | |||
hsa-mir-19a (Precursor RNA)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | The upregulated expression of individual miRNAs, miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92 were determined by qRT-PCR. This study revealed a novel mechanism through which miR-17-92 protects endothelial cells from erastin-induced ferroptosis by targeting the A20-ACSL4 axis. | |||
hsa-mir-18a (Precursor RNA)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | The upregulated expression of individual miRNAs, miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92 were determined by qRT-PCR. This study revealed a novel mechanism through which miR-17-92 protects endothelial cells from erastin-induced ferroptosis by targeting the A20-ACSL4 axis. | |||
hsa-mir-17 (Precursor RNA)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [69] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | The upregulated expression of individual miRNAs, miR-17, miR-18a, miR-19a, miR-20a, miR-19b and miR-92 were determined by qRT-PCR. This study revealed a novel mechanism through which miR-17-92 protects endothelial cells from erastin-induced ferroptosis by targeting the A20-ACSL4 axis. | |||
Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (ENPP2)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [71] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | ENPP2 overexpression causes upregulation of GPX4 in H9c2 cells. In erastin-induced ferroptosis of H9c2 cells, both NRF2 and ACSL4 are increased, whereas ENPP2 overexpression reduces their expression in erastin-treated H9c2 cells. | |||
Tumor necrosis factor alpha-induced protein 3 (TNFAIP3)
Erastin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
Cisplatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response Description | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
Transcription factor Sp1 (SP1)
Sevoflurane
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Adult male SD rats (250-300 g) were purchased from Charies River (Beijing, China). The animals were placed in laboratory cages, kept on a 12-h light-dark cycle, and had free access to food and water throughout the study. The rats were randomly assigned to the sham (only the left neck was exposed without ligation) group, MACO group, and sevo + MACO (2.5% sevoflurane before refusion) group. The MCAO model was made by a modified nylon suture method. After 1 h of ischemia, the suture was gently pulled to the beginning of the external carotid artery and re-perfused for 24 h. For sevoflurane postconditioning, rats were stabilized in a gas-tight anesthesia chamber with sevoflurane inhalation for 1 h at the onset of blood refusion. Sevoflurane (AbbVie, Japan) was delivered at a concentration of 2.5% through a vaporizer (Vapor 2000, Germany). In the sham or MCAO group, rats were only exposed to the mixed gas (95% O2 and 5% CO2).
Click to Show/Hide
|
||||
| Response Description | Sevoflurane treatment inhibits ferroptosis and increases apoptosis events by inhibiting the SP1/ASCL4 axis, thereby reducing cerebral ischemia-reperfusion injury damage. | ||||
RAF proto-oncogene serine/threonine-protein kinase (RAF1)
Tetraarsenic tetrasulfide
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| In Vitro Model | NCI-H23 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1547 |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | |
| Response Description | On H23 cells treated with realgar, the expression of GPX4, SCL7A11 decreased while ACSL4 expression increased; this effect could also be amplified by Sorafenib. In conclusion, the present study indicated that realgar may induce ferroptosis by regulating the Raf, and hence plays a role in antiKRAS mutant lung cancer. | |||
Protein C-ets-1 (ETS1)
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MHCC97-L cells | Hepatocellular carcinoma | Homo sapiens | CVCL_4973 | |
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| HEK293 FT cells | Normal | Homo sapiens | CVCL_6911 | ||
| In Vivo Model |
Parental MHCC97L cells (2 x 106 cells/mouse) were subcutaneously injected into the 4-to-5-week-old NOD-SCID mice. When the tumours reached a volume of around 50-100 mm3 (calculated by the formula 4/3(D/2)(d/2)2, where D and d represent the minor and major axis of the tumour, respectively), the maximum tolerated dose of sorafenib (50 mg/kg) was given to the mice by oral gavage daily until the drug resistance occurred, denoted as the drug resistant group. For the control, the wild type group was treated with the vehicle (0.5% CMC-Na). The tumour size and body weight were measured every 3 days.
Click to Show/Hide
|
||||
| Response Description | Sorafenib treatment triggered ferroptosis via lipid ROS production and chelatable iron accumulation. The ETS1 upregulated by sorafenib was a key transcription factor of miR-23a-3p that directly enhanced miR-23a-3p expression. MiR-23a-3p recognized and bound to ACSL4 3UTR to limit lipid ROS production, thus attenuating sorafenib-induced ferroptotic cell death in hepatocellular carcinoma. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Icariin
[Phase 3]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Supraventricular tachycardia [ICD-11: BC81] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response Description | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Cadmium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Kidney injury [ICD-11: NB92] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/ Sirt1 axis. | |||
mmu-miR-7a-5p (miRNA)
Bromelain
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
Mitofusin-2 (MFN2)
Arsenic
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Nonalcoholic fatty liver disease [ICD-11: DB92] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (300 g-350 g, specific pathogen free) were obtained from Institute of Genome Engineered Animal Models for Human Disease of Dalian Medical University (Dalian, China). To explore the influence of NaAsO2 (CAS No.7784-46-5, Sigma-Aldrich, USA) on the liver, the rats were subjected to NaAsO2 at the dosage of 0, 2.5, and 5 mg/kg by gavage for 9 months. The control group was gavaged with distilled water as vehicle.
Click to Show/Hide
|
||||
| Response Description | Arsenic induces rat liver nonalcoholic steatohepatitis (NASH) and Ferroptosis via interacting between Mitofusin-2 with IRE1. NaAsO2 increases IRE1 and Mfn2 expression, subsequently led to upregulated ACSL4 expression and 5-HETE via the directly combination Mfn2 with IRE1, ultimately induced ferroptotic cell death. | ||||
Sodium arsenite
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Nonalcoholic fatty liver disease [ICD-11: DB92] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
Adult male Sprague-Dawley rats (300 g-350 g, specific pathogen free) were obtained from Institute of Genome Engineered Animal Models for Human Disease of Dalian Medical University (Dalian, China). To explore the influence of NaAsO2 (CAS No.7784-46-5, Sigma-Aldrich, USA) on the liver, the rats were subjected to NaAsO2 at the dosage of 0, 2.5, and 5 mg/kg by gavage for 9 months. The control group was gavaged with distilled water as vehicle.
Click to Show/Hide
|
||||
| Response Description | Arsenic induces rat liver nonalcoholic steatohepatitis (NASH) and Ferroptosis via interacting between Mitofusin-2 with IRE1. NaAsO2 increases IRE1 and Mfn2 expression, subsequently led to upregulated ACSL4 expression and 5-HETE via the directly combination Mfn2 with IRE1, ultimately induced ferroptotic cell death. | ||||
Hydroxymethylglutaryl-CoA synthase, cytoplasmic (HMGCS1)
Itraconazole
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Squamous cell carcinoma of skin [ICD-11: 2C31] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-431 cells | Skin squamous cell carcinoma | Homo sapiens | CVCL_0037 | |
| COLO 16 cells | Skin squamous cell carcinoma | Homo sapiens | CVCL_D607 | ||
| In Vivo Model |
Female BALB/c nude mice (6weeks old and 18-22 g weight) were purchased from the Model Animal Research Center of Nanjing University. A431 cells (5 x 106) in cold DMEM (50 ul) were mixed with Matrigel (50 ul) and injected into mice subcutaneously. After 6 days, the tumor volume was measured and the mice were assigned to three groups. Mice were treated with either normal saline or itraconazole (40 mg/kg oral twice daily; 80 mg/kg oral twice daily).
Click to Show/Hide
|
||||
| Response Description | Itraconazole inhibited the cell proliferation, induced apoptosis and blocked cell cycle of Cutaneous squamous cell carcinoma cells. And 3-hydroxy-3-methylglutaryl-CoA synthase 1 (HMGCS1) and acyl-CoA synthetase long-chain family member 4 (ACSL4) were significantly upregulated in A431 cells treated with itraconazole. Itraconazole may induce ferroptosis via HMGCS1/ACSL4 axis in A431 cells. | ||||
Hydroxycarboxylic acid receptor 1 (HCAR1)
Lactate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| AMPK signaling pathway | hsa04152 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | CAF cells | Normal | Carassius auratus | CVCL_R883 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| In Vivo Model |
Female mice aged around 6-7 weeks were used for this study, which were purchased through Laboratory Animal Center of Chongqing Medical University from Vital River Co. Ltd (Beijing, China).After one week, each mouse was injected subcutaneously with 100 uL of Huh-7 cell suspension (5 x 106 units) to establish the tumor model. The mice were grouped randomly, and then subjected to different treatments after subcutaneous tumors became visually detectable.
Click to Show/Hide
|
||||
| Response Description | Lactate regulates the ferroptosis of hepatocellular carcinoma cells. And blocking the lactate uptake via hydroxycarboxylic acid receptor 1 (HCAR1)/MCT1 inhibition promotes ferroptosis by activating the AMPK to downregulate SCD1, which may synergize with its acyl-coenzyme A synthetase 4 (ACSL4)-promoting effect to amplify the ferroptotic susceptibility. | ||||
hsa-miR-34a-5p (miRNA)
Cadmium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Kidney injury [ICD-11: NB92] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/Sirt1 axis. | |||
hsa-miR-23a-3p (miRNA)
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MHCC97-L cells | Hepatocellular carcinoma | Homo sapiens | CVCL_4973 | |
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| HEK293 FT cells | Normal | Homo sapiens | CVCL_6911 | ||
| In Vivo Model |
Parental MHCC97L cells (2 x 106 cells/mouse) were subcutaneously injected into the 4-to-5-week-old NOD-SCID mice. When the tumours reached a volume of around 50-100 mm3 (calculated by the formula 4/3(D/2)(d/2)2, where D and d represent the minor and major axis of the tumour, respectively), the maximum tolerated dose of sorafenib (50 mg/kg) was given to the mice by oral gavage daily until the drug resistance occurred, denoted as the drug resistant group. For the control, the wild type group was treated with the vehicle (0.5% CMC-Na). The tumour size and body weight were measured every 3 days.
Click to Show/Hide
|
||||
| Response Description | Sorafenib treatment triggered ferroptosis via lipid ROS production and chelatable iron accumulation. The ETS1 upregulated by sorafenib was a key transcription factor of miR-23a-3p that directly enhanced miR-23a-3p expression. MiR-23a-3p recognized and bound to ACSL4 3UTR to limit lipid ROS production, thus attenuating sorafenib-induced ferroptotic cell death in hepatocellular carcinoma. | ||||
hsa-miR-19b-3p (miRNA)
Bromelain
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
hsa-miR-17-5p (miRNA)
Bromelain
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
hsa-miR-16-5p (miRNA)
Bromelain
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
hsa-miR-150-5p (miRNA)
Bromelain
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
hsa-miR-144-3p (miRNA)
Bromelain
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
hsa-miR-130a-3p (miRNA)
Bromelain
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | NCI-H508 cells | Cecum adenocarcinoma | Homo sapiens | CVCL_1564 | |
| HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| G13D (Human colorectal cancer cells) | |||||
| DLD-1 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| G12D (Human colorectal cancer cells) | |||||
| CCD-18Co cells | Normal | Homo sapiens | CVCL_2379 | ||
| In Vivo Model |
Animals (n = 7) were given 2.5% DSS in drinking water for 5 days and then no treatment for 14 days as one cycle; this process was repeated for three cycles. In the last cycle, 2% DSS water treated to each group and no treatment for 14 days. During the three DSS cycle, 3 mg/kg bromelain were injected daily intraperitoneally and colon and spleen tissues were harvested after three DSS cycle in 57 days to study polyp burden and to perform histological staining.
Click to Show/Hide
|
||||
| Response Description | Elevated miR-19b-3p, -130a-3p, -150-5p, -144-3p, -16-5p, -7a-5p, and -17-5p in bromelain-treated CaCO2cells compared to in DLD-1 cells potentially targeted ACSL-4 and resulted in suppression of ACSL-4. Overall, bromelain inhibits proliferation of Kras mutant Colorectal Cancer (CRC) effectively via ACSL-4. | ||||
HOTAIR (IncRNA)
Paeonol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Intracerebral hemorrhage [ICD-11: 8B00] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
C57BL/6 mice (aged 8-10 weeks, Vital River, Beijing, China) were housed in SPF conditions. The animal study was performed according to the National Institutes of Health Guide and approved by the Ethics Committees of Affiliated Jiangmen Traditional Chinese Medicine Hospital of Jinan University.
Click to Show/Hide
|
||||
| Response Description | Paeonol (PAN) inhibits the progression of intracerebral haemorrhage via the HOTAIR/UPF1/ACSL4 signalling pathway. Thus, PAN could act as a new agent for the treatment of ferroptosis in intracerebral haemorrhage. | ||||
Elongation of very long chain fatty acids protein 6 (ELOVL6)
Apatinib
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| HIEC-6 cells | Normal | Homo sapiens | CVCL_6C21 | |
| Response Description | ACSL4, a vital regulator of ferroptosis, could interact with ELOVL6 directly. Apatinib promoted ferroptosis in colorectal cancer (CRC) cells by targeting ELOVL6/ACSL4, providing a new mechanism support for apatinib application in the clinical treatment of CRC. | |||
Aquaporin-11 (AQP11)
Penicillamine
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [13] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were provided by at the Centre for Animals of Central South University (Changsha, China). To prepare the seizure mouse model, the mice underwent the intrahippocampal injection of KA as described in our previous investigation. For short, mice were anesthetized with sodium phenobarbital (50 mg/kg, i.p.) and carefully placed on a stereotaxic apparatus. Then, KA (1 uL, 250 ng/uL dissolved in saline) was stereotactically injected into the hippocampus according to the following coordinates: anteroposterior -2.0 mm; lateral -1.3 mm; dorsoventral -1.2 mm. After injection, the infusion needle was kept in place for 5-10 min to avoid liquid reflux. Mice in the control group underwent the same surgical procedure but received injection with an equal volume of phosphate buffered saline (PBS) instead of KA.
Click to Show/Hide
|
||||
| Response Description | D-penicillamine can be repurposed to cure seizure disorders such as epilepsy. D-penicillamine reveals the amelioration of seizure-induced neuronal injury via inhibiting Aqp11-dependent ferroptosis. Furthermore, ferroptosis-associated indices including acyl-coA synthetase long chain family member 4 (ACSL4), prostaglandin-endoperoxide synthase 2 (Ptgs2) gene and lipid peroxide (LPO) level were significantly decreased in KA mouse model after DPA treatment. | ||||
Unspecific Regulator
Dihydrotanshinone I
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [40] | |||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | HEB (Human glial cells) | |||
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| Response Description | Dihydrotanshinone I (DHI) inhibited the proliferation of human glioma cells. Following treatment of the U251 and U87 cells with DHI, changes in the expression levels of ferroptosis-associated proteins were observed; the expression level of GPX4 decreased and that of ACSL-4 increased. DHI also increased the levels of LDH and MDA in the human glioma cells and reduced the GSH/GSSG ratio. | |||
Berberine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [41] | ||||
| Responsed Disease | Gastrointestinal cancer [ICD-11: 2B5B] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | HT29 cells | Colon cancer | Mus musculus | CVCL_A8EZ | |
| TMK-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_4384 | ||
| CT26 cells | Colon adenocarcinoma | Mus musculus | CVCL_7254 | ||
| In Vivo Model |
Five-week-old male BALB/c mice were purchased from SLC Japan (Shizuoka, Japan). The animals were maintained in a pathogen-free animal facility under a 12 h light/dark cycle in a temperature (22 )- and humidity-controlled environment, in accordance with the institutional guidelines approved by the Committee for Animal Experimentation of Nara Medical University, Kashihara, Japan, following the current regulations and standards of the Japanese Ministry of Health, Labor and Welfare (approval no. 12924, 5 November 2020). Animals were acclimated to their housing for seven days before the start of the experiment. For the peritoneal dissemination tumor model, CT26 cancer cells (1 x 107 in 0.2 mL per mouse) were injected into the mouse peritoneal cavity. To measure tumor weight, mice were euthanized on Day 12 and the tumors were excised, while the peritoneal tumors were dissected from the intestine, mesenterium, diaphragm, and abdominal wall, with gross removal of non-tumor tissues. The largest tumor was formed on the diaphragm, and paraffin-embedded sections of the excised diaphragmatic tumor were prepared and stained with hematoxylin-eosin. BBR was diluted with distilled water to produce a final concentration of 48 mg/mL. The solutions were ultrasonically treated for 1 h, and fully vortexed for 30 min. BBR solution was administered by free drinking. The intake calculated from the amount of water consumed was 15.2 mg/kg body weight/day.
Click to Show/Hide
|
||||
| Response Description | Berberine induces apoptosis and ferroptosis by inhibiting mitochondrial complex I and promoting autophagy, leading to combined cell death in the GIC and suppressing stemness. BBR induces cell death in gastrointestinal cancer cells accompanied by increased mitochondrial superoxide and ACSL4 levels, decreased SLC7A11, and impaired antioxidant mechanisms, indicated by decreased GPX4 expression and decreased GSH. | ||||
Atractylodin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [42] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
| In Vitro Model | Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hccm (Human hepatocellular carcinoma cells) | ||||
| Response Description | Atractylodin can inhibit the proliferation, migration, and invasion of Huh7 and Hccm liver cancer cells, and induce cell apoptosis and cell cycle arrest. In addition, atractylodin may induce ferroptosis in hepatocellular carcinoma cells by inhibiting the expression of GPX4 and FTL proteins, and up-regulating the expression of ACSL4 and TFR1 proteins. | |||
Seco-Lupane Triterpene Derivatives
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [43] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Response Description | A new seco-lupane triterpene derivative, compound21, was found to regulate cell growth through the cell cycle and ferroptosis, which in turn inhibited the proliferation, migration, and invasion of HepG2 cells. And it was found that compound 21 significantly upregulated ACSL4 protein expression and downregulated GPX4 protein expression. It has the potential to become an effective new drug for the treatment of hepatocellular carcinoma. | |||
Curcumin
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [44] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
Female C57BL/6 mice (14-18 g) were purchased from SiPeiFu (Beijing) Biotechnology. C57BL/6 mice were subcutaneously injected with a total of 6 x 105 Lewis lung carcinomas (LLC) cells on the left flank. Four days after LLC inoculation, the mice were randomly divided into two groups of five. The vehicle control and curcumin groups were given sodium carboxymethyl cellulose (CMC) or curcumin (100 mg/kg/day) by intraperitoneal injection for 15 days.
Click to Show/Hide
|
||||
| Response Description | Curcumin induced ferroptosis via activating autophagy in non-small-cell lung cancer (NSCLC), which enhanced the therapeutic effect of NSCLC. Meanwhile, the protein level of ACSL4 was higher and the levels of SLC7A11 and GPX4 were lower in curcumin group than that in control group. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [62] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| rPTs (Rat pancreas tissues) | |||||
| rHTs (Rat hippocampal tissues) | |||||
| In Vivo Model |
Forty female albino Wistar rats weighing 180-220 g were used in the study. Eight rats in each group were randomly assigned to five different groups: Group I (Sham); Group II (IR); Group III (IR + DMSO); Group IV (IR + Curcumin 100 mg/kg); and Group V (IR + 2 ug/kg LoxBlock-1) were determined. The animals were maintained at a temperature of 21 ± 2 and regulated humidity conditions (50 ± 5%) with a twelve-hour light/dark cycle. Throughout the experiment, the animals were fed standard commercial rat pellets and given tap water. All surgical and anesthesia procedures were performed understerile conditions. In addition, in a case of abnormal symptoms, the animals would be removed from the group and sacrificed under deep anesthesia.
Click to Show/Hide
|
||||
| Response Description | Curcumin attenuates liver, pancreas and cardiac ferroptosis, oxidative stress and injury in ischemia/reperfusion-damaged rats by facilitating ACSL/GPx4 signaling. | ||||
Borneol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [45] | ||||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell adhesion molecules | hsa04514 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | H460/CisR cells | Lung large cell carcinoma | Homo sapiens | CVCL_C5S1 | |
| In Vivo Model |
Male Balb/c nude mice (4-week-old) were purchased from SPF (Beijing) biotechnology co., LTD and maintained in the Experimental Animal Research Center of Chengdu University of TCM. After 1 week of adaptable feeding, H460/CDDP cells (5 x 106 cells in 0.1 ml phosphate-buffered saline) were subcutaneously injected into the right dorsal flank to establish tumor model. When the tumor volume grows to 100 mm3, the tumor-bearing mice were randomly divided into the following four treatment groups: a control group (Con, n = 6): intraperitoneal injection of saline once a day; vehicle group (Vehicle, n = 6): intragastric administration of 2% tween and intraperitoneal injection of saline; d-borneol low-dose group (Bor-L, n = 6): intragastric administration of d-borneol (30 mg/kg) once a day; d-borneol high-dose group (Bor-H, n = 6): intragastric administration of d-borneol (60 mg/kg) once a day; CDDP group (CDDP, n = 6): intraperitoneal injection of cisplatin (3 mg/kg) every two days; a low-dose combination treatment group (C+B-L, n = 6): intragastric administration of d-borneol (30 mg/kg) once a day and intraperitoneal injection of cisplatin (3 mg/kg) every two days; a high-dose combination treatment group (C+B-H, n = 6): intragastric administration of d-borneol (60 mg/kg) once a day and intraperitoneal injection of cisplatin (3 mg/kg) every two days. We usually first orally gavage d-borneol, and then inject cisplatin intraperitoneally half an hour later. After 14 days treatment, the samples were obtained from the mice for the further experiments.
Click to Show/Hide
|
||||
| Response Description | d-borneol in combination with cisplatin induced ferroptosisviaNCOA4-mediated ferritinophagy and also increased the expression levels of ACSL4, regulated PCBP2 and PRNP to promote the conversion of Fe3+to Fe2+, reduced the activity or expression of antioxidants enzymes (GSH and HO-1), and induced ROS accumulation and thereby promoted ferroptosis. In addition, activation of autophagy inhibited progression of the EMT and increased sensitivity to cisplatin in cisplatin-resistant lung cancer cells. | ||||
Polyphyllin III
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [46] | ||||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| Hs-578T cells | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | ||
| MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 | ||
| T-47D cells | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| HBL-100 cells | Normal | Homo sapiens | CVCL_4362 | ||
| BT-549 cells | Invasive breast carcinoma | Homo sapiens | CVCL_1092 | ||
| MDA-MB-453 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | ||
| In Vivo Model |
MDA-MB-231 xenografts were established in 5 week-old BALB/C nude mice (Shanghai SLAC Laboratory Animal Corporation) by inoculating 1 x 106 cells mixed with Matrigel (BD Biosciences) at a 1:1 ratio into the abdominal mammary fat pad. When the tumor reached 50-100 mm3, the mice were assigned randomly into different treatment groups (DMSO, PPIII, SAS, and PPIII + SAS groups), and each group consisted of 5 mice. PPIII (5 mg/kg/day) and SAS (200 mg/kg/day) were dissolved in dimethyl sulfoxide (DMSO), diluted in PBS, and then intraperitoneally injected into mice at a dose of 10 ml/kg/d once a day.
Click to Show/Hide
|
||||
| Response Description | Polyphyllin III, which is a major saponin extracted fromParis polyphyllarhizomes, exerted its proliferation-inhibitory effect on MDA-MB-231 triple-negative breast cancer cells mainly through ACSL4-mediated lipid peroxidation elevation and ferroptosis induction. Polyphyllin III treatment also induced KLF4-mediated protective upregulation of xCT(SLC7A11), which is the negative regulator of ferroptosis. | ||||
Robustaflavone 7,5'-dimethyl ether
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [47] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MCF-7 cells | Breast carcinoma | Homo sapiens | CVCL_0031 |
| Response Description | Molecular docking study indicated that robustaflavone 7,5'-dimethyl ether (8) is a potential strong inhibitor, which induce ferroptosis via down-regulating the expression level of ACSL4 proteins in human breast cancer MCF-7 cells. | |||
Oleanolic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [48] | ||||
| Responsed Disease | Cervical cancer [ICD-11: 2C77] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| In Vivo Model |
Male BALB/c Nude mice (20 ± 2g, 5 weeks old) were supplied by Hangzhou Ziyuan Experimental Animal Technology Co. LTD (SYXK-20180049) for this study. The nude mice were kept under specefic pathogen free (SPF) conditions for one week, and 5x107 Hela cells were injected under the left axillary skin after acclimatization. The tumors of Hela cell-inoculated mice were measured every 3 days after modeling, and tumor>=0.5 cm in diameter were considered successful. The tumor-bearing mice were randomly divided into control group (n = 6), 40 mg/kg OA group (n = 6) and 80 mg/kg OA group (n = 6). 40 mg/kg OA group and 80 mg/kg OA group both received subcutaneous injection modeling and control mice received saline intraperitoneal injections. The 40 mg/kg OA group and 80 mg/kg OA group received daily intraperitoneal injections of 40 mg/kg OA and 80 mg/kg OA for 15 days.
Click to Show/Hide
|
||||
| Response Description | Oleanolic acid (OA) significantly reduced the viability and proliferative capacity of Hela cells. OA activated ferroptosis in Hela cells by promoting ACSL4 expression, thereby reducing the survival rate of Hela cells. Therefore, promotion of ACSL4-dependent ferroptosis by OA may be a potential approach for the treatment of cervical cancer. | ||||
Lycorine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [49] | |||
| Responsed Disease | Hereditary Leiomyomatosis [ICD-11: 2C90] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 |
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | |
| Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| Response Description | Lycorine could inhibit the proliferation in human renal cell carcinoma (RCC) cells. The anti-tumor effect of lycorine was associated with the induction of ferroptosis. After lycorine treatment, the expression levels of GPX4 in RCC cells decreased, whereas those of ACSL4 increased. | |||
Liraglutide
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [50] | ||||
| Responsed Disease | Cognitive disorder [ICD-11: 6D71] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
Male diabetic db/db mice and nondiabetic littermate db/m mice that were 4 weeks of age were purchased from Changzhou Cavens Experimental Animal Co., Ltd. After 1week of adaptive feeding, 20 db/db mice were randomly divided into two groups: a model group (db/db, n = 10) and a treatment group (LIRA, n = 10). Another 10 db/m mice were used as the control group (db/m, n = 10). After feeding to 10weeks old, the LIRA group was given liraglutide (CSN11311, CSNpharm, China) diluent (200 ug/kg/d) by intraperitoneal injection for 5 weeks, with equivoluminal 0.9% saline intraperitoneally administered to the other two groups.
Click to Show/Hide
|
||||
| Response Description | Liraglutide was shown to prevent ferroptosis in the hippocampus by elevating the expression of GPX4 and SLC7A11 and suppressing the excessive amount of ACSL4. LIRA can reduce oxidative stress, lipid peroxidation and iron overload in diabetes-induced cognitive dysfunction and further inhibit ferroptosis, thereby weakening the damage to hippocampal neurons and synaptic plasticity and ultimately restoring cognitive function. | ||||
Oxidopamine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [51] | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
The AB strain of wild-type zebrafish (Danio rerio) was applied in this study. Zebrafish larvae at 4 dpf (days post-fertilization) were co-incubated with 250 uM 6-OHDA or 1.5 ug/mL nomifensine (Nomi, a dopamine transporter inhibitor) in 6-well plates at a density of 30 zebrafish embryos per group for 2 days and the medium was refreshed every day. The swimming total distance of each fish was recorded for 10 min and was analyzed by an automated video tracking system.
Click to Show/Hide
|
||||
| Response Description | 6-hydroxydopamine (6-OHDA) treatment-induced ferroptosis in SH-SY5Y cells mainly by disturbing the protein expression of GPX4 and ACSL4. Collectively, the activation of the p62-Keap1-Nrf2 pathway prevents 6-OHDA-induced ferroptosis in SH-SY5Y cells, targeting this pathway in combination with a pharmacological inhibitor of ferroptosis can be a potential approach for parkinson's disease therapy. | ||||
L. lactis MG1363-pMG36e-GLP-1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [52] | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Colon tissues (Mouse colon tissues) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
Fifty male C57BL/6 mice provided by Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China) resided in an animal house (temperature 26 ± 1 , humidity 50 ± 10%), in which the light was on for 12 h and off for 12 h. Mice were acclimatised for 1 week and allowed water and animal food with no limitations. Then, all mice were stochastically divided into 5 groups using random number tables available online (https://www.random-online.com/, accessed on 26 December 2021), including: (1) C group, a control group treated with normal saline for 7 consecutive days (n = 10); (2) M group, a model group with intraperitoneal injection of 20 mg/kg/day MPTP (Sigma-Aldrich, Taufkirchen, Germany, M0896) for 7 consecutive days (n = 10); (3) L group, treated with MPTP and 0.4 mg/kg/day liraglutide for 7 consecutive days (n = 10); (4) R group, treated with MPTP and 109 colony-forming unit (CFU) L. lactis MG1363 for 7 consecutive days via gavage (n = 10); (5) RG group, treated with MPTP and 109 CFUL. lactis MG1363-pMG36e-GLP-1 for 7 consecutive days via gavage (n = 10). All animals survived treatment and all animal experiments were administered from 9:00 to 12:00 in the morning to reduce systematic errors.
Click to Show/Hide
|
||||
| Response Description | L. lactis MG1363-pMG36e-GLP-1 exerts neurotrophic effects via activating the Keap1/Nrf2/GPX4 signalling pathway to down-regulate ACSL4 and up-regulate FSP1 to suppress ferroptosis. These results indicated that the neurotrophic effects of the next-generation probiotics L. lactis MG1363-pMG36e-GLP-1 against MPTP-induced Parkinsonism are mediated by modulating oxidative stress, inhibiting ferroptosis, and redressing dysbiosis. | ||||
Baicalein
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [53] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
The mice (23-25 g, 8-10 weeks old) were subjected to transientmiddle cerebral artery occlusion (tMCAO) to induce cerebral ischemia as previously described protocol . Briefly, mice were anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg) and subcutaneous injection of meloxicam (10mg/kg) during tMCAO operation. Monofilament with a silicon coating on the tip and a diameter of 0.12 mm (A5-122, Beijing Cinontech Co. Ltd., China) was inserted into the ICA from CCA to occlude the middle cerebral artery (MCA) for 1.5 h. The suture was then removed to restore blood flow for another 22.5 h reperfusion. Sham control mice were subjected to similar surgical operations without MCA occlusion. Specifically, the monofilament was inserted only 5 mm above the carotid bifurcation and withdrew immediately in the Sham group.
Click to Show/Hide
|
||||
| Response Description | Baicalein inhibited the ferroptosis by regulating on the expression levels of GPX4, ACSL4 and ACSL3 in OGD/R cells, tMCAO mice and RSL3-stimulated HT22 cells. Our findings demonstrated that baicalein reversed the cerebral ischemia-reperfusion injury via anti-ferroptosis, which was regulated by GPX4/ACSL4/ACSL3 axis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [60] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
Chrysin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [54] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
Carthamin yellow
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [55] | ||||
| Responsed Disease | Cerebral ischaemic stroke [ICD-11: 8B11] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| NF-kappa B signaling pathway | hsa04064 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
A total of 32 male Sprague-Dawley rats (aged 6-8 weeks; 250-280 g) were purchased from Shanghai Sipper-BK Lab Animal Co., Ltd. Animals were randomly divided into the following four groups (n = 8 per group): i) Sham; ii) MCAO; iii) CY (20 mg/kg); and iv) CY (40 mg/kg). CY was administered intragastrically to rats once daily for 2 weeks. At 60 min after the last administration, MCAO surgery was performed as previously described. At 24 h post-reperfusion, neurological scores, brain water content and infarct volume were determined.
Click to Show/Hide
|
||||
| Response Description | Carthamin yellow (CY) treatment inhibited Fe2+ and reactive oxygen species accumulation, and reversed acylCoA synthetase longchain family member 4, transferrin receptor 1, glutathione peroxidase 4 and ferritin heavy chain 1 protein expression levels in the brain. Collectively, the results of the present study demonstrated that CY protected rats against ischemic stroke, which was associated with mitigation of inflammation and ferroptosis. | ||||
N2L
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [56] | |||
| Responsed Disease | Nervous system disease [ICD-11: 8E7Z] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | N2L recovered glutathione peroxidase 4 (GPX4) expression and blocked the increase of Cyclooxygenase-2 (cox-2) and acyl-CoA synthetase long-chain family member 4 (ACSL4) protein expressions. Moreover, N2L also significantly prevented Ferritin Heavy Chain 1 (FTH1) from downregulation and maintained iron homeostasis. And N2L could be a ferroptosis inhibitor for the therapy of ferroptosis-related neurodegenerative diseases, such as Alzheimer's disease. | |||
Sertaconazole
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [57] | |||
| Responsed Disease | Nervous system disease [ICD-11: 8E7Z] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | LUHMES cells | Normal | Homo sapiens | CVCL_B056 |
| Response Description | Sertaconazole is the most potent ACSL4 inhibitor identified. In addition, sertaconazole significantly reduced lipid peroxidation and ferroptosis in human differentiated dopaminergic neurons (Lund human mesencephalic LUHMES cells), demonstrating that it is a valuable chemical tool for further investigating the role of ACSL4 in nervous system disease. | |||
Bleomycin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [58] | ||||
| Responsed Disease | Pulmonary fibrosis [ICD-11: CB03] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response Description | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of ACSL4 but similarly decreased those of FSP1. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
Epigallocatechin Gallate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [59] | ||||
| Responsed Disease | Nonalcoholic fatty liver disease [ICD-11: DB92] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
After adaptive feeding, mice were randomly assigned to five groups (n = 10 per group). The details of the groups are as follows: 1) the normal diet (ND) group in which mice were fed ND (18% calories from fat); 2) the HFD group in which mice were fed HFD (60% calories from fat); 3) the HFD-EGCG/L group in which mice received 20 mg/kgbw EGCG by oral gavage daily during HFD feeding; 4) the HFD-EGCG/H group in which mice received 100 mg/kgbw EGCG by oral gavage daily during HFD feeding; and 5) the HFD-Fer-1 group in which mice received intraperitoneal injection of Fer-1 at 1 mg/kg. bw every 3 days during HFD feeding. Mice in the EGCG treatment groups were supplemented with EGCG (20 and 100 mg/kgbw) for 12 weeks. Meanwhile, mice in the ND group and the HFD group were orally gavaged with deionized water daily.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation and Fer-1 treatment apparently increased the protein expression of GPX4 and markedly decreased the protein expression of COX-2 and ACSL4 in the livers of HFD-fed mice. Epigallocatechin gallate may exert protective effects on hepatic lipotoxicity by inhibiting mitochondrial reactive oxygen species-mediated hepatic ferroptosis. Findings from our study provide new insight into prevention and treatment strategies for non-alcoholic fatty liver disease pathological processes. | ||||
Baicalin
[Terminated]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [61] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague-Dawley rats, 260-280 g, were provided by Beijing Vital River Laboratory Animal Technology CO., Ltd. (Beijing, China). Rats were randomly divided into five groups (n = 15 per group): control (sham operation + saline), I/R (I/R + saline), baicalin 100 mg/kg (BA-100, I/R + baicalin 100 mg/kg), baicalin 200 mg/kg (BA-200, I/R + baicalin 200 mg/kg), and diltiazem 20 mg/kg (DI-20, I/R + diltiazem 20 mg/kg). Drugs were given by oral gavage once daily (8 a.m.) for 6 days. At day 6, myocardial ischemia was induced 1 h after drug was administered.
Click to Show/Hide
|
||||
| Response Description | Baicalin prevents against myocardial ischemia/reperfusion injury via suppressing ACSL4-controlled ferroptosis. In addition, enhanced lipid peroxidation and significant iron accumulation along with activated transferrin receptor protein 1 (TfR1) signal and nuclear receptor coactivator 4 (NCOA4)-medicated ferritinophagy were observed in in vivo and in vitro models, which were reversed by baicalin treatment. | ||||
Gossypol acetic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [63] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
A total of 55 adult male Sprague-Dawley rat (350-450 g) were anesthetized with urethane (1.5 g/kg, i.p.), then the hearts were perfused in a Langendorff system. After 30 min of stabilization, hearts were subjected to 30 min of global no-flow ischemia by stopping the perfusion. Reperfusion was followed with Krebs Henseleit (KH) buffer and GAA together for 2 h. A thermoregulated chamber kept the heart at 37 throughout the experiment. Control hearts were not subjected to I/R. The heart slices were sectioned at a thickness of 2 mm and stained with triphenyltetrazolium chloride (25 mg/100 mL) for 10 min and then fixed with 4% formaldehyde solution for 48 h to enhance color contrast.
Click to Show/Hide
|
||||
| Response Description | Gossypol acetic acid significantly attenuated myocardial infarct size, reduced lipid peroxidation, decreased the mRNA levels of the ferroptosis markers Ptgs2 and Acsl4, decreased the protein levels of ACSL4 and NRF2, and increased the protein levels of GPX4 in I/R-induced ex vivo rat hearts. Thus, GAA may play a cytoprotectant role in ferroptosis-induced cardiomyocyte death and myocardial ischemia/reperfusion-induced ferroptotic cell death. | ||||
Xanthohumol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [64] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Rats were anesthetized with urethane (1.5 g/kg, i.p.), then hearts were excised and arrested in Krebs Henseleit (KH) buffer as previously described. Following 30 min equilibration, ischemia was induced by halting perfusion for 45 min. Reperfusion was followed with KH buffer and XN (5 or 10 uM) together for 60 min. Control hearts were not subjected to I/R.
Click to Show/Hide
|
||||
| Response Description | Xanthohumol can prevent ferroptosis during cardiac ischemia-reperfusion by reducing the expression of Acsl4 and Ptgs2 mRNA, reducing the expression of ACSL4 and NRF2 protein, and modulating the expression of GPX4 protein. | ||||
Luteolin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [60] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
Nobiletin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [65] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague-Dawley (SD) rats (4 weeks old, 90-110 g) were obtained from the Vital River Biological company. Rats were kept in a specific-pathogen-free (SPF) environment, with access to food and tap water at an ambient temperature of 20-22 . All institutional and national guidelines for the care and use of laboratory animals were followed. The protocols were reviewed and approved by the Institution of Animal Care and Use Committee of Renmin Hospital of Wuhan University (IACUC, license no. 20200303).
Click to Show/Hide
|
||||
| Response Description | Both ferrostain-1 and nobiletin decreased the expression of ferroptosis-related proteins including Acyl-CoA synthetase long chain family member 4 (ACSL4) and nuclear receptor coactivator 4 (NCOA4) but not glutathione peroxidase 4 (GPX4) in rats with mature T2DM and cells with HFHG and H/R injury. Nobiletin has therapeutic potential for alleviating myocardial ischemia-reperfusion injury associated with ACSL4- and NCOA4-related ferroptosis. | ||||
Vitamin K1
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [66] | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | NIH3T3 cells | Normal | Mus musculus | CVCL_0594 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| MCT (Murine proximal tubular epithelial cells) | |||||
| In Vivo Model |
All mice used in our in vivo studies were 8-week-old males of the C57BL/6J background. Kidneys were exposed via a midline abdominal incision and bilateral renal pedicle clamping for 35 min using microaneurysm clamps (Aesculap Inc., Center Valley, PA, USA). Throughout the surgical procedure, the mice were kept under isoflurane narcosis, and their body temperature was maintained at 36-37 by continuous monitoring using a temperature-controlled, self-regulated heating system (Fine Science Tools, Heidelberg, Germany). After clamps were removed, kidney reperfusion was confirmed visually before the abdomen was closed in two layers using standard 6-0 sutures. To maintain fluid balance, all mice were supplemented with 1 ml of prewarmed PBS administered intraperitoneally directly after surgery. After 48 h of reperfusion, the mice were sacrificed, blood samples were obtained by retrobulbar puncture, and kidneys were collected for analysis.
Click to Show/Hide
|
||||
| Response Description | Renal expression of ACSL4 was markedly enhanced by IRI and reduced by vitamin K1. Vitamin K1 as a potent inhibitor of ferroptosis, and hence, it represents a potential drug for the treatment of pathological cell death processes during acute kidney injury in humans. | ||||
Sevoflurane
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [67] | ||||
| Responsed Disease | Traumatic brain injury [ICD-11: NA07] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Sixty-four male Sprague-Dawley rats (2 weeks), weighing 20-30 g. All animals were brought from the Institute of Medical Laboratory Animals at the Chinese Academy of Medical Sciences and were kept in the same unit in a temperature-controlled environment [(22 ± 1) ]. The rats were fasted for 12 h before the experiment and drank water freely. After being numbered according to body weight, the rats were randomly divided into four groups using the random number table. The number of rats in each group was 16. The experimental groups were as follows: sham-operated group (S group, n = 16), the model group receiving HIR (HIR group, n = 16), sevoflurane group treated (HIR + Sev group, n = 16), and desferrioxamine treated group [deferoxamine (HIR + Sev + DFO) group, n = 16]. In HIR+Sev and HIR + Sev + DFO groups, rats were placed in an anesthetizing chamber and exposed to 3.6% sevoflurane (Cayman, 23996, USA) with complete oxygen for 2 h, and sham and HIR group rats were conducted with the same procedure without sevoflurane exposure. DFO (100 mg/kg, MCE, HY-B0988, China) was administered continuously daily for 6 days before surgery in the HIR + Sev + DFO group. Other groups were given equal amounts of saline.
Click to Show/Hide
|
||||
| Response Description | TFRC levels and ACSL4 levels were elevated after sevoflurane administration, suggesting that ferroptosis occurs in whole-brain regions of young rats after HIR and that sevoflurane aggravates the extent of ferroptosis. The results suggest that ferroptosis may mediate sevoflurane-aggravated young rats' brain injury induced by liver transplantation. | ||||
Edaravone
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [68] | ||||
| Responsed Disease | Spinal cord injury [ICD-11: ND51] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rSCTs (Rat spinal cord tissues) | ||||
| In Vivo Model |
The rats were initially anesthetized with 5% isoflurane (RWD life science, Shenzhen, China) and then maintained with 22.5% isoflurane. A 1-cm midline incision was made over the thoracic vertebrae, and laminectomy on T10 and the caudal half of T9 vertebrae was performed. Spinal cord contusion injury was conducted by NYU Impactor Model III (W.M. Keck Center for Collaborative Neuroscience Rutgers, The State University of New Jersey, United States) using a 10-g node dropping freely from a height of 2.5 cm and muscles and skin sutured in layers. Sham controls underwent laminectomy without the contusion. To prevent infection at the incision, cefuroxime sodium was applied for 3 days after injury. The bladders were emptied manually twice daily in the first week after injury.
Click to Show/Hide
|
||||
| Response Description | Edaravone not only rescues the ferroptosis negative regulators, xCT and GPX4, but also downregulates those pro-ferroptosis factors, ACSL4 and 5-LOX. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. And in the acute phase of spinal cord injury (SCI), edaravone reduced neuronal cell death and neuroinflammation. | ||||
References
