Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0188)
| Name |
Sertaconazole
|
||||
|---|---|---|---|---|---|
| Synonyms |
Sertaconazole; 99592-32-2; Sertaconazol [Spanish]; Sertaconazolum [Latin]; Sertaconazol; Sertaconazolum; FI-7045; Sertaconazole [INN]; Demofix; 1-[2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole; 1-(2-((7-Chlorobenzo[b]thiophen-3-yl)methoxy)-2-(2,4-dichlorophenyl)ethyl)-1H-imidazole; Sertaconazole (INN); CHEBI:83682; 1-(2-((7-Chlorobenzo(b)thien-3-yl)methoxy)-2-(2,4-dichlorophenyl)ethyl)-1H-imidazole; 72W71I16EG; 1-{2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}-1H-imidazole; 7-Chloro-3-(1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethoxy-methyl)benzo(b)thiophene; 1H-Imidazole, 1-(2-((7-chlorobenzo(b)thien-3-yl)methoxy)-2-(2,4-dichlorophenyl)ethyl)-; 7-Chloro-3-[1-(2,4-dichlorophenyl)-2-(1H-imidazol-1-yl)ethoxy-methyl]benzo[b]thiophene; Sertaconazole [INN:BAN]; BRN 5385663; UNII-72W71I16EG; SR-05000001439; 1-{2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl}imidazole; 1H-Imidazole, 1-[2-[(7-chlorobenzo[b]thien-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-; 7-Cloro-3-(1-(2,4-diclorofenil)-2-(1H-imidazol-1-il)etoxi-metil)benzo(b)tiofeno [Spanish]; Prestwick0_001045; Prestwick1_001045; Prestwick2_001045; Prestwick3_001045; SERTACONAZOLE [MI]; (+-)-1-(2,4-Dichloro-beta-((7-chlorobenzo(b)thien-3-yl)methoxy)phenethyl)imidazole; 7-Cloro-3-(1-(2,4-diclorofenil)-2-(1H-imidazol-1-il)etoxi-metil)benzo(b)tiofeno; SCHEMBL66043; BSPBio_000970; SERTACONAZOLE [VANDF]; SPBio_002905; SERTACONAZOLE [WHO-DD]; BPBio1_001068; CHEMBL1201196; DTXSID0048551; JLGKQTAYUIMGRK-UHFFFAOYSA-N; HMS2089M16; BCP12180; HY-B0736; BDBM50051842; DL-208; STL452922; AKOS015850283; AC-6939; DB01153; 1-[2-[(7-chlorobenzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole; NCGC00179356-01; NCGC00179356-09; AB00514018; CS-0009631; FT-0631003; FT-0674559; FT-0674560; FT-0674561; D06883; AB00514018-07; AB00514018_08; EN300-6481081; Q3479994; SR-05000001439-1; BRD-A95939040-008-03-3; Z2194031487; 1-[2-[(7-chlorobenzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole; nitric acid; SZ
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
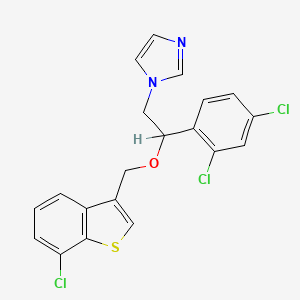 |
||||
| Formula |
C20H15Cl3N2OS
|
||||
| IUPAC Name |
1-[2-[(7-chloro-1-benzothiophen-3-yl)methoxy]-2-(2,4-dichlorophenyl)ethyl]imidazole
|
||||
| Canonical SMILES |
C1=CC2=C(C(=C1)Cl)SC=C2COC(CN3C=CN=C3)C4=C(C=C(C=C4)Cl)Cl
|
||||
| InChI |
InChI=1S/C20H15Cl3N2OS/c21-14-4-5-16(18(23)8-14)19(9-25-7-6-24-12-25)26-10-13-11-27-20-15(13)2-1-3-17(20)22/h1-8,11-12,19H,9-10H2
|
||||
| InChIKey |
JLGKQTAYUIMGRK-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Driver | |||
| Responsed Disease | Nervous system disease | ICD-11: 8E7Z | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | LUHMES cells | Normal | Homo sapiens | CVCL_B056 |
| Response regulation | Sertaconazole is the most potent ACSL4 inhibitor identified. In addition, sertaconazole significantly reduced lipid peroxidation and ferroptosis in human differentiated dopaminergic neurons (Lund human mesencephalic LUHMES cells), demonstrating that it is a valuable chemical tool for further investigating the role of ACSL4 in nervous system disease. | |||
