Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0155)
| Name |
Liraglutide
|
||||
|---|---|---|---|---|---|
| Synonyms |
Liraglutide; 204656-20-2; NN2211; victoza; Liraglutida; Liraglutidum; NN-2211; NN 2211; UNII-839I73S42A; HSDB 8205; Liraglutide (rDNA origin); NNC 90-1170; CHEMBL4084119; Saxenda; CHEBI:71193; DTXSID60174433; 839I73S42A; NN-9924; NNC-90-1170; N26-(Hexadecanoyl-gamma-glutamyle)-(34-arginine)GLP-1-(7-37)-peptide; N26-(Hexadecanoyl-gamma-glutamyle)-(34-arginine)glucagon-like-peptide-1-(7-37)-peptide; Arg34Lys26-(N-epsilon-(gamma-Glu(N-alpha-hexadecanoyl)))-GLP-1(7-37); Liraglutidum [INN-Latin]; N(sup 26)-(Hexadecanoyl-gamma-glutamyle)-(34-arginine)GLP-1-(7-37)-peptide; Liraglutida [INN-Spanish]; Liraglutide recombinant; Liraglutide [USAN:INN:BAN:JAN]; Liraglutidum (Latin); LIRAGLUTIDE (MART.); GTPL1133; DTXCID6096924; A10BJ02; EX-A2418; BDBM50240819; NN9924; AKOS037435224; AS-56276; A16115; XULTOPHY 100/3.6 COMPONENT LIRAGLUTIDE; LIRAGLUTIDE COMPONENT OF XULTOPHY 100/3.6; ARG(SUP 34)LYS(SUP 26)-(N-epsilon-(gamma-GLU(N-alpha-HEXADECANOYL)))-GLP-1(7-37); N(SUP epsilon26)-(N-HEXADECANOYL-L-gamma-GLUTAMYL)-(34-L-ARGININE)GLUCAGON-LIKE PEPTIDE 1-(7-37)-PEPTIDE; N26-(N-Hexadecanoyl-L-gamma-glutamyl)-(34-L-arginine)glucagon-like peptide 1-(7-37)-peptide
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
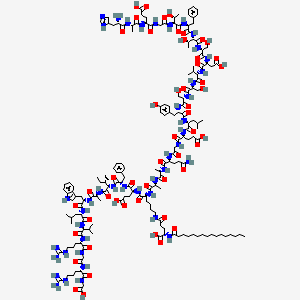 |
||||
|
3D MOL
|
|||||
| Formula |
C172H265N43O51
|
||||
| IUPAC Name |
(2S)-5-[[(5S)-5-[[(2S)-2-[[(2S)-2-[[(2S)-5-amino-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S,3R)-2-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-amino-3-(1H-imidazol-5-yl)propanoyl]amino]propanoyl]amino]-4-carboxybutanoyl]amino]acetyl]amino]-3-hydroxybutanoyl]amino]-3-phenylpropanoyl]amino]-3-hydroxybutanoyl]amino]-3-hydroxypropanoyl]amino]-3-carboxypropanoyl]amino]-3-methylbutanoyl]amino]-3-hydroxypropanoyl]amino]-3-hydroxypropanoyl]amino]-3-(4-hydroxyphenyl)propanoyl]amino]-4-methylpentanoyl]amino]-4-carboxybutanoyl]amino]acetyl]amino]-5-oxopentanoyl]amino]propanoyl]amino]propanoyl]amino]-6-[[(2S)-1-[[(2S)-1-[[(2S,3S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-1-[[(2S)-5-carbamimidamido-1-[[2-[[(2S)-5-carbamimidamido-1-(carboxymethylamino)-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-1-oxopentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]amino]-4-methyl-1-oxopentan-2-yl]amino]-3-(1H-indol-3-yl)-1-oxopropan-2-yl]amino]-1-oxopropan-2-yl]amino]-3-methyl-1-oxopentan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-4-carboxy-1-oxobutan-2-yl]amino]-6-oxohexyl]amino]-2-(hexadecanoylamino)-5-oxopentanoic acid
|
||||
| Canonical SMILES |
CCCCCCCCCCCCCCCC(=O)NC(CCC(=O)NCCCCC(C(=O)NC(CCC(=O)O)C(=O)NC(CC1=CC=CC=C1)C(=O)NC(C(C)CC)C(=O)NC(C)C(=O)NC(CC2=CNC3=CC=CC=C32)C(=O)NC(CC(C)C)C(=O)NC(C(C)C)C(=O)NC(CCCNC(=N)N)C(=O)NCC(=O)NC(CCCNC(=N)N)C(=O)NCC(=O)O)NC(=O)C(C)NC(=O)C(C)NC(=O)C(CCC(=O)N)NC(=O)CNC(=O)C(CCC(=O)O)NC(=O)C(CC(C)C)NC(=O)C(CC4=CC=C(C=C4)O)NC(=O)C(CO)NC(=O)C(CO)NC(=O)C(C(C)C)NC(=O)C(CC(=O)O)NC(=O)C(CO)NC(=O)C(C(C)O)NC(=O)C(CC5=CC=CC=C5)NC(=O)C(C(C)O)NC(=O)CNC(=O)C(CCC(=O)O)NC(=O)C(C)NC(=O)C(CC6=CN=CN6)N)C(=O)O
|
||||
| InChI |
InChI=1S/C172H265N43O51/c1-18-20-21-22-23-24-25-26-27-28-29-30-37-53-129(224)195-116(170(265)266)59-64-128(223)180-68-41-40-50-111(153(248)199-115(62-67-135(232)233)154(249)204-120(73-100-44-33-31-34-45-100)159(254)214-140(93(11)19-2)167(262)192-97(15)146(241)201-122(76-103-79-183-108-49-39-38-48-106(103)108)157(252)203-118(72-90(5)6)158(253)212-138(91(7)8)165(260)200-110(52-43-70-182-172(177)178)149(244)184-81-130(225)193-109(51-42-69-181-171(175)176)148(243)187-84-137(236)237)196-144(239)95(13)189-143(238)94(12)191-152(247)114(58-63-127(174)222)194-131(226)82-185-151(246)113(61-66-134(230)231)198-155(250)117(71-89(3)4)202-156(251)119(75-102-54-56-105(221)57-55-102)205-162(257)124(85-216)208-164(259)126(87-218)209-166(261)139(92(9)10)213-161(256)123(78-136(234)235)206-163(258)125(86-217)210-169(264)142(99(17)220)215-160(255)121(74-101-46-35-32-36-47-101)207-168(263)141(98(16)219)211-132(227)83-186-150(245)112(60-65-133(228)229)197-145(240)96(14)190-147(242)107(173)77-104-80-179-88-188-104/h31-36,38-39,44-49,54-57,79-80,88-99,107,109-126,138-142,183,216-221H,18-30,37,40-43,50-53,58-78,81-87,173H2,1-17H3,(H2,174,222)(H,179,188)(H,180,223)(H,184,244)(H,185,246)(H,186,245)(H,187,243)(H,189,238)(H,190,242)(H,191,247)(H,192,262)(H,193,225)(H,194,226)(H,195,224)(H,196,239)(H,197,240)(H,198,250)(H,199,248)(H,200,260)(H,201,241)(H,202,251)(H,203,252)(H,204,249)(H,205,257)(H,206,258)(H,207,263)(H,208,259)(H,209,261)(H,210,264)(H,211,227)(H,212,253)(H,213,256)(H,214,254)(H,215,255)(H,228,229)(H,230,231)(H,232,233)(H,234,235)(H,236,237)(H,265,266)(H4,175,176,181)(H4,177,178,182)/t93-,94-,95-,96-,97-,98+,99+,107-,109-,110-,111-,112-,113-,114-,115-,116-,117-,118-,119-,120-,121-,122-,123-,124-,125-,126-,138-,139-,140-,141-,142-/m0/s1
|
||||
| InChIKey |
YSDQQAXHVYUZIW-QCIJIYAXSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Cognitive disorder | ICD-11: 6D71 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
Male diabetic db/db mice and nondiabetic littermate db/m mice that were 4 weeks of age were purchased from Changzhou Cavens Experimental Animal Co., Ltd. After 1week of adaptive feeding, 20 db/db mice were randomly divided into two groups: a model group (db/db, n = 10) and a treatment group (LIRA, n = 10). Another 10 db/m mice were used as the control group (db/m, n = 10). After feeding to 10weeks old, the LIRA group was given liraglutide (CSN11311, CSNpharm, China) diluent (200 ug/kg/d) by intraperitoneal injection for 5 weeks, with equivoluminal 0.9% saline intraperitoneally administered to the other two groups.
Click to Show/Hide
|
||||
| Response regulation | Liraglutide was shown to prevent ferroptosis in the hippocampus by elevating the expression of GPX4 and SLC7A11 and suppressing the excessive amount of ACSL4. LIRA can reduce oxidative stress, lipid peroxidation and iron overload in diabetes-induced cognitive dysfunction and further inhibit ferroptosis, thereby weakening the damage to hippocampal neurons and synaptic plasticity and ultimately restoring cognitive function. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cognitive disorder | ICD-11: 6D71 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mHNs (Mouse hippocampal neurons) | ||||
| In Vivo Model |
Male diabetic db/db mice and nondiabetic littermate db/m mice that were 4 weeks of age were purchased from Changzhou Cavens Experimental Animal Co., Ltd. After 1week of adaptive feeding, 20 db/db mice were randomly divided into two groups: a model group (db/db, n = 10) and a treatment group (LIRA, n = 10). Another 10 db/m mice were used as the control group (db/m, n = 10). After feeding to 10weeks old, the LIRA group was given liraglutide (CSN11311, CSNpharm, China) diluent (200 ug/kg/d) by intraperitoneal injection for 5 weeks, with equivoluminal 0.9% saline intraperitoneally administered to the other two groups.
Click to Show/Hide
|
||||
| Response regulation | Liraglutide was shown to prevent ferroptosis in the hippocampus by elevating the expression of GPX4 and SLC7A11 and suppressing the excessive amount of ACSL4. LIRA can reduce oxidative stress, lipid peroxidation and iron overload in diabetes-induced cognitive dysfunction and further inhibit ferroptosis, thereby weakening the damage to hippocampal neurons and synaptic plasticity and ultimately restoring cognitive function. | ||||
