Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0207)
| Name |
Icariin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Icariin; 489-32-7; Ieariline; 5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one; CHEBI:78420; MFCD00210516; VNM47R2QSQ; Icariine; 3-[(6-deoxy-alpha-L-mannopyranosyl)oxy]-5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-4-oxo-4H-chromen-7-yl beta-D-glucopyranoside; 5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one; SMR000466309; UNII-VNM47R2QSQ; -Anhydroicaritin; 3-((6-Deoxymannopyranosyl)oxy)-7-(glucopyranosyloxy)-5-hydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-butenyl)-4H-1-benzopyran-4-one; Epimedii herba icariin; Spectrum2_001695; Spectrum3_001130; Spectrum4_001975; Spectrum5_000933; Icariin, analytical standard; BSPBio_002599; KBioGR_002475; MLS000759413; MLS001424083; MLS006011789; SCHEMBL312615; SPECTRUM1505257; SPBio_001650; CHEMBL553204; Icariin, >=94% (HPLC); KBio3_002099; DTXSID00964133; TZJALUIVHRYQQB-XLRXWWTNSA-N; HMS2051J13; HMS2235I20; BCP18807; EPIMEDII HERBA ICARIIN [MI]; EX-A6783; HY-N0014; BBL010487; BDBM50027363; CCG-38780; STK801622; AKOS005614005; AM85785; CCG-100955; CS-3675; DB12052; NC00205; SDCCGMLS-0066754.P001; NCGC00178583-01; 4H-1-Benzopyran-4-one, 3-((6-deoxy-alpha-L-mannopyranosyl)oxy)-7-(beta-D-glucopyranosyloxy)-5-hydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-butenyl)-; VS-02526; C17555; AB00639912-06; A827628; SR-01000759346; Q-100549; Q5985057; SR-01000759346-4; BRD-K65639003-001-02-5; BRD-K65639003-001-05-8; BRD-K65639003-001-09-0; 3-[(6-Deoxy-alpha-L-mannopyranosyl)oxy]-7-(beta-D-glucopyranosyloxy)-5-hydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-buten-1-yl)-4H-1-benzopyran-4-one; 4H-1-BENZOPYRAN-4-ONE, 3-((6-DEOXY-.ALPHA.-L-MANNOPYRANOSYL)OXY)-7-(.BETA.-D-GLUCOPYRANOSYLOXY)-5-HYDROXY-2-(4-METHOXYPHENYL)-8-(3-METHYL-2-BUTEN-1-YL)-; 5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-(((2S,4S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-3-(((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one; 5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4H-chromen-4-one; 5-HYDROXY-2-(4-METHOXYPHENYL)-8-(3-METHYLBUT-2-EN-1-YL)-7-{[3,4,5-TRIHYDROXY-6-(HYDROXYMETHYL)OXAN-2-YL]OXY}-3-[(3,4,5-TRIHYDROXY-6-METHYLOXAN-2-YL)OXY]-4H-CHROMEN-4-ONE; 5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yloxy)-3-((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yloxy)-4H-chromen-4-one
Click to Show/Hide
|
||||
| Status |
Phase 3
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
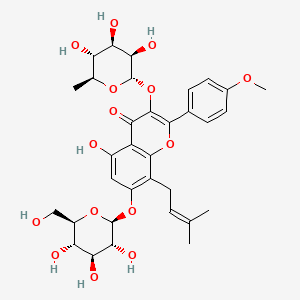 |
||||
| Formula |
C33H40O15
|
||||
| IUPAC Name |
5-hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxychromen-4-one
|
||||
| Canonical SMILES |
CC1C(C(C(C(O1)OC2=C(OC3=C(C2=O)C(=CC(=C3CC=C(C)C)OC4C(C(C(C(O4)CO)O)O)O)O)C5=CC=C(C=C5)OC)O)O)O
|
||||
| InChI |
InChI=1S/C33H40O15/c1-13(2)5-10-17-19(45-33-28(42)26(40)23(37)20(12-34)46-33)11-18(35)21-24(38)31(48-32-27(41)25(39)22(36)14(3)44-32)29(47-30(17)21)15-6-8-16(43-4)9-7-15/h5-9,11,14,20,22-23,25-28,32-37,39-42H,10,12H2,1-4H3/t14-,20+,22-,23+,25+,26-,27+,28+,32-,33+/m0/s1
|
||||
| InChIKey |
TZJALUIVHRYQQB-XLRXWWTNSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Supraventricular tachycardia | ICD-11: BC81 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response regulation | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Supraventricular tachycardia | ICD-11: BC81 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response regulation | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Supraventricular tachycardia | ICD-11: BC81 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response regulation | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Supraventricular tachycardia | ICD-11: BC81 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Adult male mice (C57BL6) aged 12 weeks were purchased from HUAFUKANG Bioscience Co, Ltd (Beijing, China) and housed in controlled temperature with free access to water and standard pellet chow. The animal studies were approved by the General Hospital of Northern Theatre Command Animal Care Committee. All experiments were carried out in accordance with institutional regulations and in adherence with the Guide for the Care and Use of Laboratory Animals issued by the US National Institutes of Health (NIH Publication, 8th Edition, 2011). Additionally, the study was reported in accordance with ARRIVE guidelines. After an accommodation period of 7 days, the mice were randomly assigned into the following groups (n = 18/group): control group, control + Ferrostatin-1 (Fer-1)/Erastin/EX527 group, ethanol (EtOH) group, EtOH + Fer-1 group, EtOH + Icar group, EtOH + Icar + Erastin group, EtOH + Icar + EX527 group.
Click to Show/Hide
|
||||
| Response regulation | Icariin activated atrial SIRT1-Nrf-2-HO-1 signaling pathway, while EX527 not only reversed these effects, but also abolished the therapeutic effects of icariin. Moreover, the stimulatory effects on GPX4, SLC7A11 and the suppressive effects on ACSL4, P53 conferred by icariin were blunted by EX527 treatment. These data demonstrate that ferroptosis plays a causative role in the pathogenesis of ethanol-induced atrial remodeling and susceptibility to atrial fibrillation. | ||||
