Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0244)
| Name |
Cisplatin
|
||||
|---|---|---|---|---|---|
| Synonyms |
cisplatin; 14913-33-8; trans-Dichlorodiamineplatinum(II); azane;dichloroplatinum; Platinum, diamminedichloro-, (SP-4-1)-; trans-Diamminedichloro platinum(II); trans-Platinum(II)diammine dichloride; MFCD00011623; trans-Dichlorodiamineplatinum(II);Transplatin; SR-05000001586; IA call; Trans-Platinum diammine dichloride; Cisplatin in water; Cisplatin,(S); Cisplatin in DMSO; DDP-H; PLATINOL (TN); SPECTRUM1502107; Cisplatin (JP17/USP/INN); cis-Diaminodichloroplatinum(II); HMS502M12; HMS1921J18; HMS2092D04; BCP01792; BCP18182; CCG-39901; AKOS005137969; NCGC00094962-01; NCGC00094962-02; NCGC00094962-03; FT-0777996; C06911; D00275; cis-Diammineplatinum(II) dichloride, crystalline; Q412415; SR-01000076254; SR-01000076254-1; SR-05000001586-1; SR-05000001586-3; Z3182781436; Cisplatin, European Pharmacopoeia (EP) Reference Standard; Cisplatin, United States Pharmacopeia (USP) Reference Standard; cis-Diamineplatinum(II) dichloride, >=99.9% trace metals basis; Cisplatin impurity A, European Pharmacopoeia (EP) Reference Standard; Transplatin, United States Pharmacopeia (USP) Reference Standard; Cisplatin, Pharmaceutical Secondary Standard; Certified Reference Material
Click to Show/Hide
|
||||
| Structure |
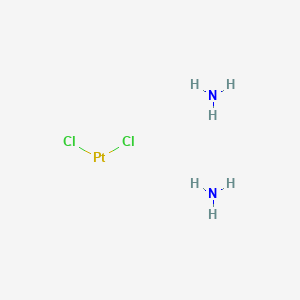 |
||||
|
3D MOL
|
|||||
| Formula |
Cl2H6N2Pt
|
||||
| IUPAC Name |
azane;dichloroplatinum
|
||||
| Canonical SMILES |
N.N.Cl[Pt]Cl
|
||||
| InChI |
InChI=1S/2ClH.2H3N.Pt/h2*1H;2*1H3;/q;;;;+2/p-2
|
||||
| InChIKey |
LXZZYRPGZAFOLE-UHFFFAOYSA-L
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Polyunsaturated fatty acid lipoxygenase ALOX15 (ALOX15)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Responsed Regulator | hsa-mir-522 (Precursor RNA) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
Male nude mice (BALB/c-nu, 6B8 weeks) were housed in a pathogen free animal facility with access to water and food, and allowed to eat and drink adlibitum. 5 x 105 SGC7901 cells and CAFs were injected subcutaneously for one mouse. These tumor-implanted mice were injected with either cisplatin (5 ug/g) or saline every 5 days since the day ten, and were sacrificed and tumors were removed at the 30th Day.
Click to Show/Hide
|
||||
| Response regulation | Cisplatin and paclitaxel promote miR-522 secretion from CAFs by activating USP7/hnRNPA1 axis, leading to ALOX15 suppression and decreased lipid-ROS accumulation in cancer cells, and ultimately result in decreased chemo-sensitivity. The intercellular pathway, comprising USP7, hnRNPA1, exo-miR-522 and ALOX15, reveals new mechanism of acquired chemo-resistance in gastric cancer. | ||||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Responsed Regulator | Tumor necrosis factor alpha-induced protein 3 (TNFAIP3) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response regulation | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Responsed Regulator | Tumor necrosis factor alpha-induced protein 3 (TNFAIP3) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In Vivo Model |
An in vivo tumor transplantation model of immunodeficient mice was used to evaluate the effect of SENP1 on tumor growth in vivo. There were six mice in each group. A total of 2 x 106 cells were seeded subcutaneously into 6-week-old BALB/ C-Nu Male mice. Tumor width (W) and length (L) at different experimental time points were measured with calipers, and tumor growth was monitored.
Click to Show/Hide
|
||||
| Response regulation | SENP1 overexpression protected lung cancer cells from ferroptosis induced by erastin or cisplatin. SENP1 was identified as a suppressor of ferroptosis through a novel network of A20 ( TNFAIP3) SUMOylation links ACSL4 and SLC7A11 in lung cancer cells. SENP1 inhibition promotes ferroptosis and apoptosis and represents a novel therapeutic target for lung cancer therapy. | ||||
References
