Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10009)
| Target Name | Polyunsaturated fatty acid lipoxygenase ALOX15 (ALOX15) | ||||
|---|---|---|---|---|---|
| Synonyms |
12/15-lipoxygenase; Arachidonate 12-lipoxygenase, leukocyte-type; Arachidonate 15-lipoxygenase; Arachidonate omega-6 lipoxygenase ; Hepoxilin A3 synthase Alox15; Linoleate 13S-lipoxygenase
Click to Show/Hide
|
||||
| Gene Name | ALOX15 | ||||
| Sequence |
MGLYRIRVSTGASLYAGSNNQVQLWLVGQHGEAALGKRLWPARGKETELKVEVPEYLGPL
LFVKLRKRHLLKDDAWFCNWISVQGPGAGDEVRFPCYRWVEGNGVLSLPEGTGRTVGEDP QGLFQKHREEELEERRKLYRWGNWKDGLILNMAGAKLYDLPVDERFLEDKRVDFEVSLAK GLADLAIKDSLNVLTCWKDLDDFNRIFWCGQSKLAERVRDSWKEDALFGYQFLNGANPVV LRRSAHLPARLVFPPGMEELQAQLEKELEGGTLFEADFSLLDGIKANVILCSQQHLAAPL VMLKLQPDGKLLPMVIQLQLPRTGSPPPPLFLPTDPPMAWLLAKCWVRSSDFQLHELQSH LLRGHLMAEVIVVATMRCLPSIHPIFKLIIPHLRYTLEINVRARTGLVSDMGIFDQIMST GGGGHVQLLKQAGAFLTYSSFCPPDDLADRGLLGVKSSFYAQDALRLWEIIYRYVEGIVS LHYKTDVAVKDDPELQTWCREITEIGLQGAQDRGFPVSLQARDQVCHFVTMCIFTCTGQH ASVHLGQLDWYSWVPNAPCTMRLPPPTTKDATLETVMATLPNFHQASLQMSITWQLGRRQ PVMVAVGQHEEEYFSGPEPKAVLKKFREELAALDKEIEIRNAKLDMPYEYLRPSVVENSV AI Click to Show/Hide
|
||||
| Family | Lipoxygenase family | ||||
| Function |
Non-heme iron-containing dioxygenase that catalyzes the stereo-specific peroxidation of free and esterified polyunsaturated fatty acids generating a spectrum of bioactive lipid mediators. It inserts peroxyl groups at C12 or C15 of arachidonate ((5Z,8Z,11Z,14Z)-eicosatetraenoate) producing both 12-hydroperoxyeicosatetraenoate/12-HPETE and 15- hydroperoxyeicosatetraenoate/15-HPETE. It may then act on 12-HPETE to produce hepoxilins, which may show pro-inflammatory properties. Can also peroxidize linoleate ((9Z,12Z)-octadecadienoate) to 13-hydroperoxyoctadecadienoate/13-HPODE. May participate in the sequential oxidations of DHA ((4Z,7Z,10Z,13Z,16Z,19Z)-docosahexaenoate) to generate specialized pro- resolving mediators (SPMs)like resolvin D5 ((7S,17S)-diHPDHA) and (7S,14S)-diHPDHA, that actively down-regulate the immune response and have anti-aggregation properties with platelets. Can convert epoxy fatty acids to hydroperoxy-epoxides derivatives followed by an intramolecular nucleophilic substitution leading to the formation of monocyclic endoperoxides. Plays an important role during the maintenance of self-tolerance by peroxidizing membrane-bound phosphatidylethanolamine which can then signal the sorting process for clearance of apoptotic cells during inflammation and prevent an autoimmune response. In addition to its role in the immune and inflammatory responses, this enzyme may play a role in epithelial wound healing in the cornea through production of lipoxin A4 (LXA(4)) and docosahexaenoic acid-derived neuroprotectin D1 (NPD1; 10R,17S-HDHA), both lipid autacoids exhibit anti-inflammatory and neuroprotective properties. Furthermore, it may regulate actin polymerization which is crucial for several biological processes such as the phagocytosis of apoptotic cells. It is also implicated in the generation of endogenous ligands for peroxisome proliferator activated receptor (PPAR-gamma), hence modulating macrophage development and function. It may also exert a negative effect on skeletal development by regulating bone mass through this pathway. As well as participates in ER stress and downstream inflammation in adipocytes, pancreatic islets, and liver. Finally, it is also involved in the cellular response to IL13/interleukin-13.
Click to Show/Hide
|
||||
| Gene ID | 246 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
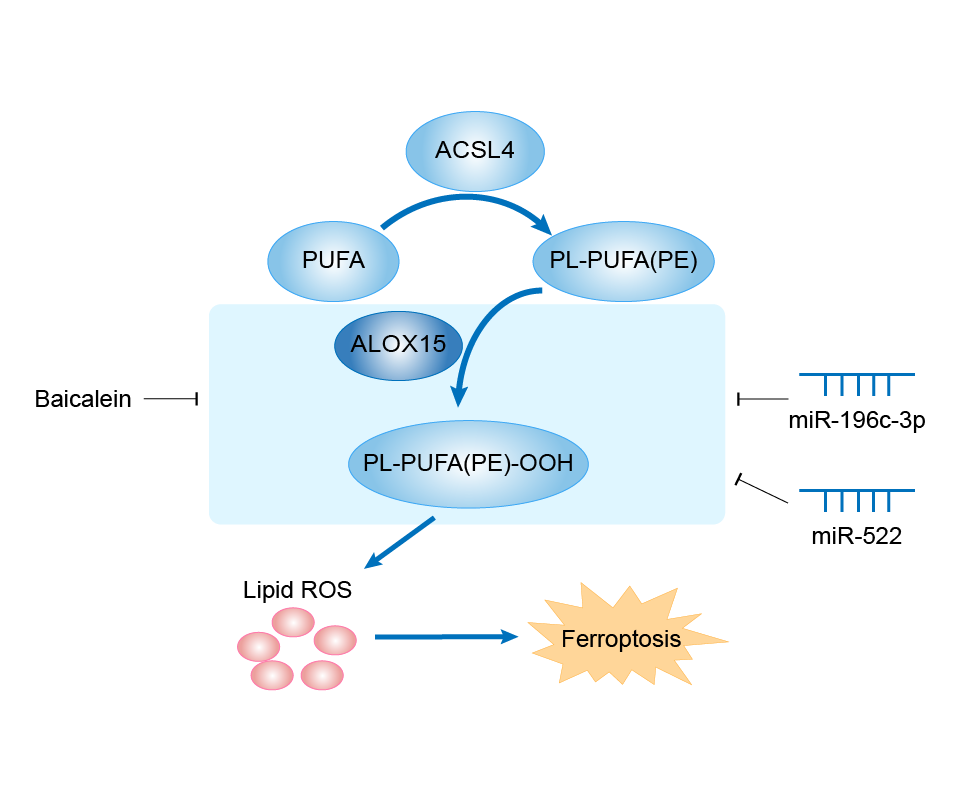
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
ALOX15 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
hsa-mir-522 (Precursor RNA)
Gastric cancer [ICD-11: 2B72]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Cisplatin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
Male nude mice (BALB/c-nu, 6B8 weeks) were housed in a pathogen free animal facility with access to water and food, and allowed to eat and drink adlibitum. 5 x 105 SGC7901 cells and CAFs were injected subcutaneously for one mouse. These tumor-implanted mice were injected with either cisplatin (5 ug/g) or saline every 5 days since the day ten, and were sacrificed and tumors were removed at the 30th Day.
Click to Show/Hide
|
||||
| Response Description | Cisplatin and paclitaxel promote miR-522 secretion from CAFs by activating USP7/hnRNPA1 axis, leading to ALOX15 suppression and decreased lipid-ROS accumulation in cancer cells, and ultimately result in decreased chemo-sensitivity. The intercellular pathway, comprising USP7, hnRNPA1, exo-miR-522 and ALOX15, reveals new mechanism of acquired chemo-resistance in gastric cancer. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Paclitaxel | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
Male nude mice (BALB/c-nu, 6B8 weeks) were housed in a pathogen free animal facility with access to water and food, and allowed to eat and drink adlibitum. 5 x 105 SGC7901 cells and CAFs were injected subcutaneously for one mouse. These tumor-implanted mice were injected with either cisplatin (5 ug/g) or saline every 5 days since the day ten, and were sacrificed and tumors were removed at the 30th Day.
Click to Show/Hide
|
||||
| Response Description | Cisplatin and paclitaxel promote miR-522 secretion from CAFs by activating USP7/hnRNPA1 axis, leading to ALOX15 suppression and decreased lipid-ROS accumulation in cancer cells, and ultimately result in decreased chemo-sensitivity. The intercellular pathway, comprising USP7, hnRNPA1, exo-miR-522 and ALOX15, reveals new mechanism of acquired chemo-resistance in gastric cancer. | ||||
rno-miR-196c-3p (miRNA)
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Wild-type SD rats were kept in the Animal Experiment Center of Southeast University. Experimental rats were divided into 4 groups (n = 6 per group). The method of establishing the I/R model was provided in supplementary material. Then, we covered the ligation with gel. In order to fully cover the infarcted area of the heart, we chose to inject about 300 uL of mimics + Gel at 23 mm below the left atrial appendage (about the ligation). In order to prevent excessive irradiation of tissue burns, we selected each irradiation for 2 min to control the body surface temperature for a total of 10 min of irradiation.
Click to Show/Hide
|
||||
| Response Description | The mir-196c-3p mimic (mimics) and photothermal nanoparticles (BTN) were co-encapsulated in an injectable Gel (mimics + Gel/BTN) with NIR-II light-triggered release. Consequently, declined ferroptosis in cardiomyocytes and improved cardiac function, survival rate in rats was achieved through the controlled release of Gel/BTN mimics in cerebral ischemia-reperfusion injury model to simultaneously inhibit ferroptosis hub genes NOX4, P53, and ALOX15 expression. | ||||
Perilipin-2 (PLIN2)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| In Vivo Model |
SGC7901 cell line transfected with OvPLIN2, ShPLIN2 and Control were injected subcutaneously into the nude mice (BALB/c nu/nu, female, 5 weeks old, Beijing Huafukang Biotechnology Co. Ltd. China) which were anaesthetized with 1% Sodium pentobarbital. The long diameter a and short diameter b of mouse tumor and the weights were measured every 4-5 days, and the relative tumor volumes (RTV) were calculated according to formula 0.5 x a x b x b.
Click to Show/Hide
|
||||
| Response Description | Overexpression and knockdown of PLIN2 augmented the proliferation and apoptosis of gastric carcinoma cell lines SGC7901 and MGC803, respectively. PLIN2 modulated Ferroptosis pathway through regulating transcription factors-PRDM11 and IPO7:ACSL3 was a critical gene involved in abnormal lipid metabolism, ALOX15 facilitated apoptosis and necrosis. | ||||
mmu-miR-706 (miRNA)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Male mice were used in this research. The mice were housed in a colony room at a controlled temperature (22) and humidity, under a 12-h light/dark cycle, and with food and water freely available. All surgical procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In brief, the mice were anesthetized by 3% pentobarbital sodium and then ligated the anterior descending branch of the left coronary artery (LAD) for 48 h to establish the in vivo MI models. The sham group mice were opened the chest bur not ligated with LAD. The mice were randomly divided into three groups as follows: (1) the sham group, which underwent sham operation and received vehicle (PBS, caudal vein injection); (2) the model group, which was subjected to LAD and received vehicle (PBS, caudal vein injection); and (3) the siRNA group, which were subjected to LAD and treated with siRNA of lncRNA Gm47283 (30 nM siRNA dose per mice every day for one week, caudal vein injection).
Click to Show/Hide
|
||||
| Response Description | Stem cell membrane coated siRNA of lncRNA Gm47283 inhibits cardiomyocyte ferroptosis in myocardial infarction rat. Stem cell membrane-coated siRNA of lncRNA Gm47283 increases miR-706, and the miR-706 suppresses the expression of Ptgs2 to reduce lipid peroxidation toxicity, and then inhibits cardiomyocyte ferroptosis. Over-expression of lncRNA Gm47283 significantly increased the expression of Ptgs2 and Alox15 and repressed the expression of Gpx4. | ||||
Gm47283 (IncRNA)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Male mice were used in this research. The mice were housed in a colony room at a controlled temperature (22) and humidity, under a 12-h light/dark cycle, and with food and water freely available. All surgical procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In brief, the mice were anesthetized by 3% pentobarbital sodium and then ligated the anterior descending branch of the left coronary artery (LAD) for 48 h to establish the in vivo MI models. The sham group mice were opened the chest bur not ligated with LAD. The mice were randomly divided into three groups as follows: (1) the sham group, which underwent sham operation and received vehicle (PBS, caudal vein injection); (2) the model group, which was subjected to LAD and received vehicle (PBS, caudal vein injection); and (3) the siRNA group, which were subjected to LAD and treated with siRNA of lncRNA Gm47283 (30 nM siRNA dose per mice every day for one week, caudal vein injection).
Click to Show/Hide
|
||||
| Response Description | Stem cell membrane coated siRNA of lncRNA Gm47283 inhibits cardiomyocyte ferroptosis in myocardial infarction rat. Stem cell membrane-coated siRNA of lncRNA Gm47283 increases miR-706, and the miR-706 suppresses the expression of Ptgs2 to reduce lipid peroxidation toxicity, and then inhibits cardiomyocyte ferroptosis. Over-expression of lncRNA Gm47283 significantly increased the expression of Ptgs2 and Alox15 and repressed the expression of Gpx4. | ||||
Dihydroorotate dehydrogenase (quinone), mitochondrial (DHODH)
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In Vivo Model |
Forty female Sprague-Dawley rats (200-300 g, 8 weeks old) were purchased from the Animal Experiment Center of Fudan University. Forty rats were randomly divided into four groups: sham operation group (n = 10), SCI group (n = 10), SCI + ferroptosis inhibitor group (SCI + ferrostatin1) (n = 10), and SCI + DHODH Inhibitor group (SCI + teriflunomide) (n = 10). Ten rats in the sham group only received laminectomy without SCI. To induce spinal cord injury, spinal cord injury surgery was performed in the middle thoracic region of rats (T8-T9).
Click to Show/Hide
|
||||
| Response Description | The application of DHODH is a potential treatment for spinal cord injury (SCI). DHODH can reduce the ferroptosis of neurons after spinal cord injury by regulating the P53/ALOX15 signaling pathway, thereby alleviating spinal cord injury. | ||||
Cellular tumor antigen p53 (TP53)
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In Vivo Model |
Forty female Sprague-Dawley rats (200-300 g, 8 weeks old) were purchased from the Animal Experiment Center of Fudan University. Forty rats were randomly divided into four groups: sham operation group (n = 10), SCI group (n = 10), SCI + ferroptosis inhibitor group (SCI + ferrostatin1) (n = 10), and SCI + DHODH Inhibitor group (SCI + teriflunomide) (n = 10). Ten rats in the sham group only received laminectomy without SCI. To induce spinal cord injury, spinal cord injury surgery was performed in the middle thoracic region of rats (T8-T9).
Click to Show/Hide
|
||||
| Response Description | The application of DHODH is a potential treatment for spinal cord injury (SCI). DHODH can reduce the ferroptosis of neurons after spinal cord injury by regulating the P53/ALOX15 signaling pathway, thereby alleviating spinal cord injury. | ||||
Unspecific Regulator
Status epilepticus [ICD-11: 8A66]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
All adult male C57/BL6 mice weighing 18-22g were obtained from the Experimental Animal Center of Central South University, China. Animals were randomly divided into six groups as follows: 1) control group (n = 6) was given vehicle intracranial injection (PBS 5 ul); 2) FeCl3 group (n = 6) was given 5 ul 50 mM FeCl3; 3) and 4) baicalein groups were pretreated with baicalein 50 mg/kg (n = 6) and 100 mg/kg (n = 6) 30 min prior to FeCl3 administration, respectively; 5) ferroptosis inhibitor group (n = 6) was administered continuously with 10 mg/kg Lipo-1 3 d prior to FeCl3 administration; and 6) baicalein administration group (n = 6) was given 100 mg/kg baicalein once by intraperitoneal injection 30 min prior to 5 ul PBS administration.
Click to Show/Hide
|
||||
| Response Description | Baicalein, as a naturel bioactive compound, could ameliorate behavioral seizures and play a key neuroprotective role in FeCl3-induced posttraumatic epileptic seizures through inhibiting ferroptosis and its neuroprotection might be related to suppression of 12-LOX/15-LOX. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Responsed Drug | Alpha-Tocopherol | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Sixty-four male Sprague-Dawley (SD) rats (5-6 weeks old) were provided by Shandong Jinan Pengyue Experimental Animal Breeding Co. Ltd. Rats were randomly divided into four groups (n = 16/group): (i) Control group rats received normal saline (NS) administered intraperitoneally (i.p.); (ii) PTZ group rats received PTZ (35 mg/kg, i.p.; Sigma-Aldrich, USA) [7]; (iii) Vitamin E+PTZ group rats received vitamin E (200 mg/kg, i.p.; Sigma-Aldrich, St. Louis, MO, USA) 30 min before PTZ injection; (iv) Fer-1+PTZ group rats received Fer-1 (2.5 umol/g, i.p.; Selleck, Houston, TX, USA) 30 min before PTZ injection. All drugs were administered every other day for a total of 15 injections.
Click to Show/Hide
|
||||
| Response Description | Vitamin E treatment was associated with decreased epileptic grade, seizure latency, and number of seizures in the PTZ-kindled epileptic model. Vitamin E treatment also decreased 15-LOX expression, inhibited MDA and iron accumulation, and increased GPX4 and GSH expression. In conclusion, vitamin E can reduce neuronal ferroptosis and seizures by inhibiting 15-LOX expression. | ||||
Traumatic brain injury [ICD-11: NA07]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rRHs (Rat right hemispheres) | ||||
| In Vivo Model |
Healthy male Sprague-Dawley rats (weighing 370 g-420 g) were purchased from the Laboratory Animal Center of Sun Yat-sen University. The rats were randomized into five groups. Rats in sham group (n = 6) underwent the same anesthetic and surgical procedures, excluding cardiac arrest and CPR. All of the remaining four groups were given the interventions within 10 minutes of ROSC. Rats in CPR group (n = 6) received an intraperitoneal injection of 0.9% saline (1 mL/kg). Rats in baicalein group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein at the same time, based on previous studies. Rats in tunicamycin group (n = 6) were intraperitoneally injected with tunicamycin (2 mg/kg body weight) (22-24). Rats in baicalein + tunicamycin group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein and 2 mg/kg (body weight) tunicamycin. All groups were given the same volume of normal saline solvent (2 mL/kg).
Click to Show/Hide
|
||||
| Response Description | Baicalein inhibited ferroptosis after ROSC by targeting ALOX15. Iron content, and MDA were reduced. More importantly, baicalein alleviated ER stress by inhibiting the expression of GRP78, ATF4, and CHOP. Baicalein is a potential drug to relieve brain injury after ROSC. | ||||
Phosphatidylethanolamine-binding protein 1 (PEBP1)
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hAECs (Human airway epithelial cells) | ||||
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| HK-2 cells | Normal | Homo sapiens | CVCL_0302 | ||
| In Vivo Model |
Male 20 weeks old CD-1 mice were obtained from Charles River Laboratories. The mice were randomly assigned (using random number generator in Excel) to receive 5 mg/kg Fer-1 (Abcam, cat #ab146169) or 1.5% DMSO (vehicle) 60 minutes before injection of folic acid (Sigma-Aldrich) of 250 mg/kg in 0.3 mol/L sodium bicarbonate intraperitoneally. Mice were euthanized 48 h later.
Click to Show/Hide
|
||||
| Response Description | PEBP1, a scaffold protein inhibitor of protein kinase cascades, complexes with two 15LO isoforms, 15LO1 (ALOX15) and 15LO2 (ALOX15B) , and changes their substrate competence to generate hydroperoxy-PE. Inadequate reduction of hydroperoxy-PE due to insufficiency or dysfunction of a selenoperoxidase, GPX4, leads to ferroptosis. | ||||
hsa-mir-522 (Precursor RNA)
Cisplatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
Male nude mice (BALB/c-nu, 6B8 weeks) were housed in a pathogen free animal facility with access to water and food, and allowed to eat and drink adlibitum. 5 x 105 SGC7901 cells and CAFs were injected subcutaneously for one mouse. These tumor-implanted mice were injected with either cisplatin (5 ug/g) or saline every 5 days since the day ten, and were sacrificed and tumors were removed at the 30th Day.
Click to Show/Hide
|
||||
| Response Description | Cisplatin and paclitaxel promote miR-522 secretion from CAFs by activating USP7/hnRNPA1 axis, leading to ALOX15 suppression and decreased lipid-ROS accumulation in cancer cells, and ultimately result in decreased chemo-sensitivity. The intercellular pathway, comprising USP7, hnRNPA1, exo-miR-522 and ALOX15, reveals new mechanism of acquired chemo-resistance in gastric cancer. | ||||
Paclitaxel
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
Male nude mice (BALB/c-nu, 6B8 weeks) were housed in a pathogen free animal facility with access to water and food, and allowed to eat and drink adlibitum. 5 x 105 SGC7901 cells and CAFs were injected subcutaneously for one mouse. These tumor-implanted mice were injected with either cisplatin (5 ug/g) or saline every 5 days since the day ten, and were sacrificed and tumors were removed at the 30th Day.
Click to Show/Hide
|
||||
| Response Description | Cisplatin and paclitaxel promote miR-522 secretion from CAFs by activating USP7/hnRNPA1 axis, leading to ALOX15 suppression and decreased lipid-ROS accumulation in cancer cells, and ultimately result in decreased chemo-sensitivity. The intercellular pathway, comprising USP7, hnRNPA1, exo-miR-522 and ALOX15, reveals new mechanism of acquired chemo-resistance in gastric cancer. | ||||
Unspecific Regulator
Baicalein
[Investigative]
| In total 2 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
All adult male C57/BL6 mice weighing 18-22g were obtained from the Experimental Animal Center of Central South University, China. Animals were randomly divided into six groups as follows: 1) control group (n = 6) was given vehicle intracranial injection (PBS 5 ul); 2) FeCl3 group (n = 6) was given 5 ul 50 mM FeCl3; 3) and 4) baicalein groups were pretreated with baicalein 50 mg/kg (n = 6) and 100 mg/kg (n = 6) 30 min prior to FeCl3 administration, respectively; 5) ferroptosis inhibitor group (n = 6) was administered continuously with 10 mg/kg Lipo-1 3 d prior to FeCl3 administration; and 6) baicalein administration group (n = 6) was given 100 mg/kg baicalein once by intraperitoneal injection 30 min prior to 5 ul PBS administration.
Click to Show/Hide
|
||||
| Response Description | Baicalein, as a naturel bioactive compound, could ameliorate behavioral seizures and play a key neuroprotective role in FeCl3-induced posttraumatic epileptic seizures through inhibiting ferroptosis and its neuroprotection might be related to suppression of 12-LOX/15-LOX. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Responsed Disease | Traumatic brain injury [ICD-11: NA07] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rRHs (Rat right hemispheres) | ||||
| In Vivo Model |
Healthy male Sprague-Dawley rats (weighing 370 g-420 g) were purchased from the Laboratory Animal Center of Sun Yat-sen University. The rats were randomized into five groups. Rats in sham group (n = 6) underwent the same anesthetic and surgical procedures, excluding cardiac arrest and CPR. All of the remaining four groups were given the interventions within 10 minutes of ROSC. Rats in CPR group (n = 6) received an intraperitoneal injection of 0.9% saline (1 mL/kg). Rats in baicalein group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein at the same time, based on previous studies. Rats in tunicamycin group (n = 6) were intraperitoneally injected with tunicamycin (2 mg/kg body weight) (22-24). Rats in baicalein + tunicamycin group (n = 6) were intraperitoneally injected with 50 mg/kg (body weight) baicalein and 2 mg/kg (body weight) tunicamycin. All groups were given the same volume of normal saline solvent (2 mL/kg).
Click to Show/Hide
|
||||
| Response Description | Baicalein inhibited ferroptosis after ROSC by targeting ALOX15. Iron content, and MDA were reduced. More importantly, baicalein alleviated ER stress by inhibiting the expression of GRP78, ATF4, and CHOP. Baicalein is a potential drug to relieve brain injury after ROSC. | ||||
Alpha-Tocopherol
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [7] | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Sixty-four male Sprague-Dawley (SD) rats (5-6 weeks old) were provided by Shandong Jinan Pengyue Experimental Animal Breeding Co. Ltd. Rats were randomly divided into four groups (n = 16/group): (i) Control group rats received normal saline (NS) administered intraperitoneally (i.p.); (ii) PTZ group rats received PTZ (35 mg/kg, i.p.; Sigma-Aldrich, USA) [7]; (iii) Vitamin E+PTZ group rats received vitamin E (200 mg/kg, i.p.; Sigma-Aldrich, St. Louis, MO, USA) 30 min before PTZ injection; (iv) Fer-1+PTZ group rats received Fer-1 (2.5 umol/g, i.p.; Selleck, Houston, TX, USA) 30 min before PTZ injection. All drugs were administered every other day for a total of 15 injections.
Click to Show/Hide
|
||||
| Response Description | Vitamin E treatment was associated with decreased epileptic grade, seizure latency, and number of seizures in the PTZ-kindled epileptic model. Vitamin E treatment also decreased 15-LOX expression, inhibited MDA and iron accumulation, and increased GPX4 and GSH expression. In conclusion, vitamin E can reduce neuronal ferroptosis and seizures by inhibiting 15-LOX expression. | ||||
References
