Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0032)
| Name |
Paclitaxel
|
||||
|---|---|---|---|---|---|
| Synonyms |
33069-62-4; P88XT4IS4D; Paclitaxel; Taxol; Taxol A; Yewtaxan; Genaxol; Plaxicel; Onxol; Paxene; Abraxane; Ebetaxel; Genetaxyl; Capxol; Cyclopax; Genexol; Intaxel; Mitotax; Pacliex; TaxAlbin; OncoGel; Paxceed; EmPAC; Onxal; Zisu; Taxus stent; Taxus Liberte; ABI-007; Padexol; EndoTAG 1; LipoPac; NSC-125973; Tocosol Paclitaxel; (-)-Paclitaxel; Nanoxel; Paclitaxol; Sindaxel; Coroflex Please; Cypher select; Taxus Express; LEP-ETU; Genexol-PM; (NAB)-Paclitaxel; MBT 0206; BMS 181339-01; Infinnium; NSC 125973; Taxus; HSDB 6839; ABI 007; DHP 107; DHP-107; Abraxane I.V. Suspension; BMS-181339-01; UNII-P88XT4IS4D; DRG-0190; Paclitaxel (Taxol); NK 105; NSC125973; Paclitaxel (taxus canadensis); QW 8184; CCRIS 8143; Liposome-entrapped paclitaxel easy-to-use; DTXSID9023413; CHEBI:45863; ABI-007 COMPONENT PACLITAXEL; IG 001; NK-105; 5beta,20-Epoxy-1,2-alpha,4,7beta,10beta,13alpha-hexahydroxytax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R,3S)-N-benzoyl-3-phenylisoserine; QW-8184; CHEMBL428647; DTXCID603413; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-9-(((2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b-diyl diacetate; nab-paclitaxel; 7-epi-Paclitaxel; ORAXOL COMPONENT PACLITAXEL; Abraxane (albumin-bound suspension); ABRAXANE COMPONENT PACLITAXEL; MBT-0206; ABI 007 COMPONENT PACLITAXEL; (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alpha R*,betaS*),11alpha,12alpha,12balpha))-beta-(Benzoylamino)-alpha-hydroxybenzenepropanoic acid 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester; NCGC00164367-01; NAB-PACLITAXEL COMPONENT PACLITAXEL; MFCD00869953; PACLITAXEL (MART.); PACLITAXEL [MART.]; PACLITAXEL (USP-RS); PACLITAXEL [USP-RS]; (1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-bis(acetyloxy)-1,9-dihydroxy-15-{[(2R,3S)-2-hydroxy-3-phenyl-3-(phenylformamido)propanoyl]oxy}-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0^{3,10}.0^{4,7}]heptadec-13-en-2-yl benzoate; PACLITAXEL (EP MONOGRAPH); PACLITAXEL (USP IMPURITY); PACLITAXEL [EP MONOGRAPH]; PACLITAXEL [USP IMPURITY]; Anzatax; Cynviloq; PACLITAXEL (USP MONOGRAPH); PACLITAXEL [USP MONOGRAPH]; Xorane; Bris Taxol; Taxol, Bris; 7,11-Methano-1H-cyclodeca[3,4]benz[1,2-b]oxete, benzenepropanoic acid deriv.; SMR000857385; EndoTAG-1; SR-01000075350; paclitaxelum; Nanotaxel; Paclical; Pacligel; Paxoral; Paclitaxel,(S); Paclitaxel [USAN:USP:INN:BAN]; Abraxane (TN); (2alpha,5beta,7beta,10beta,13alpha)-4,10-bis(acetyloxy)-1,7-dihydroxy-13-({(2R,3S)-2-hydroxy-3-phenyl-3-[(phenylcarbonyl)amino]propanoyl}oxy)-9-oxo-5,20-epoxytax-11-en-2-yl benzoate; [diacetoxy-[(2R,3S)-3-benzamido-2-hydroxy-3-phenyl-propanoyl]oxy-dihydroxy-tetramethyl-oxo-[?]yl] benzoate; 4alpha,10beta-bis(acetyloxy)-13alpha-((2S,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyloxy)-1,7beta-dihydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl benzoate; 4alpha,10beta-bis(acetyloxy)-13alpha-[(2S,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyloxy]-1,7beta-dihydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl benzoate; Paclitaxel; 5beta,20-Epoxy-1,7beta-dihydroxy-9-oxotax-11-ene-2alpha,4,10beta,13alpha-tetrayl 4,10-diacetate 2-benzoate 13-[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoate]; Taxol; Docetaxel Anhydrous Impurity F; Docetaxel Impurity F; CAS-33069-62-4; PACITAXEL; BMS-181339; Paclitaxel-SSMM-VIP; P-SSMM-VIP; PACLITAXEL [MI]; PACLITAXEL [INN]; PACLITAXEL [JAN]; Prestwick3_000155; PACLITAXEL [HSDB]; PACLITAXEL [USAN]; TAXOL (TN); PACLITAXEL [VANDF]; SCHEMBL3976; 3PPC5TL76P; Nova-12005; PACLITAXEL [WHO-DD]; Paclitaxel, Taxus brevifolia; BIDD:PXR0046; BSPBio_000290; KBioGR_002509; KBioSS_002517; Paclitaxel (JAN/USP/INN); MLS002154218; MLS002695976; OAS-PAC-100; PACLITAXEL [EMA EPAR]; BPBio1_000320; GTPL2770; MEGxp0_001940; Taxol (TN) (Bristol Meyers); PACLITAXEL [GREEN BOOK]; PACLITAXEL [ORANGE BOOK]; ACon1_002231; KBio2_002509; KBio2_005077; KBio2_007645; KBio3_002987; ANX-513; DHP-208; DTS-301; L01CD01; SDP-013; cMAP_000068; RCINICONZNJXQF-MZXODVADSA-N; HMS2090D07; HMS2095O12; HMS2231A16; HMS3712O12; HY-B0015; MPI-5018; Tox21_112107; BDBM50001839; NSC745099; AKOS007930675; AKOS015969673; AKOS025312303; CCG-220155; CS-1145; DB01229; GS-6554; NSC-745099; NCGC00164367-02; NCGC00164367-03; NCGC00164367-04; NCGC00164367-05; NCGC00164367-10; Paclitaxel, From Taxus brevifolia, 95%; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro-4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano-5H-cyclodeca(3,4)benz(1,2-b)oxet-5-one 6,12b-diacetate, 12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserine; NCI60_000601; Paclitaxel, from Taxus yannanensis, powder; PACLITAXEL IMPURITY L [EP IMPURITY]; AB00513812; D00491; EN300-117275; M02242; N88686; AB00513812-02; AB00513812-03; Paclitaxel, Antibiotic for Culture Media Use Only; Q423762; 7,4]benz[1,2-b]oxete,benzenepropanoic acid deriv.; Q-201533; SR-01000075350-1; SR-01000075350-3; SR-01000075350-6; SR-01000075350-7; SR-01000075350-9; BRD-K62008436-001-03-1; BRD-K62008436-001-05-6; BRD-K62008436-001-22-1; Paclitaxel, from semisynthetic (from Taxus sp.), >=97%; Paclitaxel, European Pharmacopoeia (EP) Reference Standard; Paclitaxel, from Taxus brevifolia, >=95% (HPLC), powder; Paclitaxel, United States Pharmacopeia (USP) Reference Standard; 12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserine; Paclitaxel, Pharmaceutical Secondary Standard; Certified Reference Material; Paclitaxel natural for peak identification, European Pharmacopoeia (EP) Reference Standard; (1S,2S,3R,4S,5R,7S,8S,10R,13S)-4,10-Diacetoxy-2-benzoyloxy-5,20-epoxy-1,7-dihydroxy-9-oxotax-11-en-13-yl (2R,3S)-3-benzoylamino-2-hydroxy-3-phenylpropionate; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-1,2a,3,4,4a,6,9,10,11,12,12a,12b-Dodecahydro 4,6,9,11,12,12b-hexahydroxy-4a,8,13,13-tetramethyl-7,11-methano 5Hcyclodeca(3,4)benz(1,2-b)oxet-5-one 6,12b-diacetate,; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-4,6,12b-Tris(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl (alphaR,betaS)-beta-(benzoylamino)-alpha-hydroxybenzenepropanoate; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl (aR,bS)-b-(benzoylamino)-a-hydroxybenzenepropanoate; (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-9-(((2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl)oxy)-12-(benzoyloxy)-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-3,4,4a,5,6,9,10,11,12,12a-decahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxete-6,12b(2aH)-diyl diacetate; (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alpha R*,betaS*),11alpha,12alpha,12balpha))-beta-(Benzoylamino)-alpha-hydroxybenzenepropanoic acid 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12; (2beta,5beta,7alpha,8alpha,10alpha,13alpha)-4,10-bis(acetyloxy)-1,7-dihydroxy-13-({(2R,3S)-2-hydroxy-3-phenyl-3-[(phenylcarbonyl)amino]propanoyl}oxy)-9-oxo-5,20-epoxytax-11-en-2-yl benzoate; ,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester; ,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (alphaR,betaS)- (9CI); -cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, [2aR-[2aalpha,4beta,4abeta,6beta,9alpha(aR*,betaS*),11alpha,12alpha,12aalpha,12balpha]]-; [(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-diacetyloxy-15-[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy-1,9-dihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate; [(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-diacetyloxy-15-[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy-1,9-dihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl]benzoate; 1203669-79-7; 4,7beta,10beta-tris(acetyloxy)-13alpha-[[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy]-1-hydroxy-9-oxo-5beta,20-epoxytax-11-en-2alpha-yl benzoate; 5-BETA,20-EPOXY-1,2-ALPHA,4,7-BETA,10-BETA,13-ALPHA-HEXAHYDROXY-TAX-11-EN-9-ONE 4,10-DIACETATE 2-BENZOATE 13-ESTER WITH (2R,3S)-N-BENZOYL-3-PHENYL-ISOSERINE; 5beta,20-Epoxy-1,2 alpha, 4,7beta, 10beta, 13alpha-hexahydroxy tax-11-en-9-one 4,10-diacetate 2-benzoate 13-ester with (2R, 3S)-N-benzoyl-3-phenylisoserine; BENZENEPROPANOIC ACID, .BETA.-(BENZOYLAMINO)-.ALPHA.-HYDROXY-, (2AR,4S,4AS,6R,9S,11S,12S,12AR,12BS)-6,12B-BIS(ACETYLOXY)-12-(BENZOYLOXY)-2A,3,4,4A,5,6,9,10,11,12,12A,12B-DODECAHYDRO-4,11-DIHYDROXY-4A,8,13,13-TETRAMETHYL-5-OXO-7,11-METHANO-1H-CYCLODECA(3,4)BENZ(1,2-B)OXET-9-YL ESTER, (.ALPHA.R,.BETA.S)-; Benzenepropanoic acid, 6,12b-bis(acetyl oxy)-12-(benzoyloxy)- 2a,3,4,4a,5,6,9,10,11,12,12a,12b,- dodecahydro-4,11- dihydroxy-4a,8,13,13-tetramethyl-5-oxo- 7,11-methano- 1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, [2aR- [2a.alpha.,4.beta.,4a.beta.,6.beta.,9.alpha.(alpha. R*,.beta.S*),11.alpha.,12.alpha.,12a.alpha.,12b.alpha.]]-; Benzenepropanoic acid, b-(benzoylamino)-.alpha.-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (aR,bS)-; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-4,6,12b-tris(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-11-hydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca[3,4]benz[1,2-b]oxet-9-yl ester, (alphaR,betaS)-; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, (2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxe
Click to Show/Hide
|
||||
| Structure |
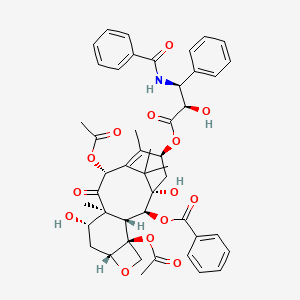 |
||||
|
3D MOL
|
|||||
| Formula |
C47H51NO14
|
||||
| IUPAC Name |
[(1S,2S,3R,4S,7R,9S,10S,12R,15S)-4,12-diacetyloxy-15-[(2R,3S)-3-benzamido-2-hydroxy-3-phenylpropanoyl]oxy-1,9-dihydroxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.03,10.04,7]heptadec-13-en-2-yl] benzoate
|
||||
| Canonical SMILES |
CC1=C2C(C(=O)C3(C(CC4C(C3C(C(C2(C)C)(CC1OC(=O)C(C(C5=CC=CC=C5)NC(=O)C6=CC=CC=C6)O)O)OC(=O)C7=CC=CC=C7)(CO4)OC(=O)C)O)C)OC(=O)C
|
||||
| InChI |
InChI=1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)5/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)/t31-,32-,33+,35-,36+,37+,38-,40-,45+,46-,47+/m0/s1
|
||||
| InChIKey |
RCINICONZNJXQF-MZXODVADSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Polyunsaturated fatty acid lipoxygenase ALOX15 (ALOX15)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Responsed Regulator | hsa-mir-522 (Precursor RNA) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| MGC-803 cells | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| In Vivo Model |
Male nude mice (BALB/c-nu, 6B8 weeks) were housed in a pathogen free animal facility with access to water and food, and allowed to eat and drink adlibitum. 5 x 105 SGC7901 cells and CAFs were injected subcutaneously for one mouse. These tumor-implanted mice were injected with either cisplatin (5 ug/g) or saline every 5 days since the day ten, and were sacrificed and tumors were removed at the 30th Day.
Click to Show/Hide
|
||||
| Response regulation | Cisplatin and paclitaxel promote miR-522 secretion from CAFs by activating USP7/hnRNPA1 axis, leading to ALOX15 suppression and decreased lipid-ROS accumulation in cancer cells, and ultimately result in decreased chemo-sensitivity. The intercellular pathway, comprising USP7, hnRNPA1, exo-miR-522 and ALOX15, reveals new mechanism of acquired chemo-resistance in gastric cancer. | ||||
