Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0110)
| Name |
Epigallocatechin Gallate
|
||||
|---|---|---|---|---|---|
| Synonyms |
(-)-Epigallocatechin gallate; EGCG; 989-51-5; Epigallocatechin gallate; Epigallocatechin 3-gallate; Tea catechin; Epigallocatechin-3-gallate; Teavigo; Epigallocatechin-3-monogallate; (-)-Epigallocatechin-3-o-gallate; (-)-epigallocatechin 3-gallate; PF-EGCg 90; (-)-Epigallocatechol gallate; NVP-XAA 723; CCRIS 3729; (2R,3R)-5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl 3,4,5-trihydroxybenzoate; Catechin deriv.; UNII-BQM438CTEL; BQM438CTEL; CHEBI:4806; Epigallocatechingallate; Epigallocatechin-gallate; Epigallocatechol, 3-gallate, (-)-; (2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl 3,4,5-trihydroxybenzoate; CHEMBL297453; DTXSID1029889; [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl] 3,4,5-trihydroxybenzoate; [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate; Benzoic acid, 3,4,5-trihydroxy-, (2R,3R)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester; C22H18O11; (2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate; EGCG cpd; Gallic acid, 3-ester with epigallocatechol, (-)-; DTXCID80567; epigallo-catechin gallate; epigallocatechin-3-O-gallate; Benzoic acid, 3,4,5-trihydroxy-, 3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester, (2R-cis)-; CAS-989-51-5; SMR000449288; SR-01000759328; (-)-EPIGALLOCATECHIN-3-O-GALLATE (USP-RS); (-)-EPIGALLOCATECHIN-3-O-GALLATE [USP-RS]; L-Epigallocatechin gallate; Epigallocate; Sunphenon; EPIGALOCATECHIN GALLATE; (-)-EGCG; Epigallocic acid; Teatannin II; 2kdh; 3oob; 4awm; (-)-cis-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-gallate; (-)-epigallocatechin 3-O-gallate; KDH; Epigallocatcchin Gallate; Epigallocatechol Gallate; Spectrum_000316; SpecPlus_000277; Spectrum2_000168; Spectrum3_000244; Spectrum4_001541; Spectrum5_000102; Galloyl-L-epigallocatechol; EGCG [WHO-DD]; EGCG [MI]; 3-O-Galloylepigallocatechin; (-)-Epigallocatechin gallat; (-)-Epigallocatehin gallate; SCHEMBL35258; BSPBio_001628; epigallocatechin-gallate-(-); KBioGR_002002; KBioSS_000796; SPECTRUM210239; cid_65064; MLS000758300; MLS001424000; DivK1c_006373; SPBio_000035; Epigallocatechin monogallate, B; GTPL7002; MEGxp0_001166; (-)-Epigallocatechin-3-gallate; ACon1_001054; KBio1_001317; KBio2_000796; KBio2_003364; KBio2_005932; KBio3_001128; (-)-cis-3,3',4',5,5',7-Hexahydroxy-flavane-3-gallate; HMS2051K21; HMS3649E08; 3-O-Galloyl-(-)-epigallocatechin; EPIGALLOCATECHIN 3-O-GALLATE; Tox21_201468; Tox21_303457; BDBM50070942; CCG-38378; FR-109; LMPK12030005; s2250; AKOS015918182; CS-1258; DB12116; DS-9030; EPIGALLOCATECHIN GALLATE [INCI]; NC00078; SDCCGMLS-0066550.P001; (-)-Epigallocatechin gallate, >=95%; (-)-Epigallocatechin-3-gallate; EGCG; NCGC00164319-01; NCGC00164319-02; NCGC00164319-03; NCGC00164319-04; NCGC00164319-06; NCGC00257243-01; NCGC00259019-01; (-)-Epigallocatechin gallate (85% (-)-epigallocatechin gallate, 10% (-)-epigallocatechin, 5% (-)- epicatechin gallate); AC-34075; BP-30205; HY-13653; E0694; SW197458-3; C09731; M01719; (-)-Epigallocatechin gallate, >=97.0% (HPLC); (-)-Epigallocatechin gallate, analytical standard; A845931; Q393339; SR-01000946601; Q-100914; SR-01000759328-5; SR-01000759328-6; SR-01000946601-1; Epigallocatechin-3-gallate 1000 microg/mL in Acetonitrile; (-)-Epigallocatechin gallate, >=80% (HPLC), from green tea; Epigallocatechin gallate, primary pharmaceutical reference standard; ((2R,3R)-2-(3,4,5-trihydroxyphenyl)-5,7-dihydroxy-chroman-3-yl) 3,4,5-trihydroxybenzoate; (-)-Epigallocatechin-3-O-gallate, United States Pharmacopeia (USP) Reference Standard; (2R,3R)-5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl3,4,5-trihydroxybenzoate; [5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl] 2,3,4-trihydroxybenzoate; Epigallocatechin gallate, Pharmaceutical Secondary Standard; Certified Reference Material; (-)-cis-3,4-Dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-1(2H)-benzopyran-3-yl Gallate; (-)-EPIGALLOCATECHIN GALLATE (85% (-)-EPIGALLOCATECHIN GALLATE, 10% (-)-EPIGALLOCATECHIN, 5% (-)-EPICATECHIN GALLATE); (-)-EPIGALLOCATECHIN-3-O-GALLATE (EGCG) (CONSTITUENT OF POWDERED DECAFFEINATED GREEN TEA EXTRACT); (2R,3R)-5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl-3,4,5-trihydroxybenzoate; (2R-cis)-3,4-Dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl 3,4,5-Trihydroxybenzoate; [(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl]3,4,5-trihydroxybenzoate; 3,4,5-Trihydroxybenzoic acid (2R,3R)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester; 3,4-Dihydro-5,7-dihydroxy-2R-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3R-yl-3,4,5-trihydroxybenzoate; Benzoic acid, 3,4,5-trihydroxy-,(2R,3R)-3,4-dihydro-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3-yl ester
Click to Show/Hide
|
||||
| Structure |
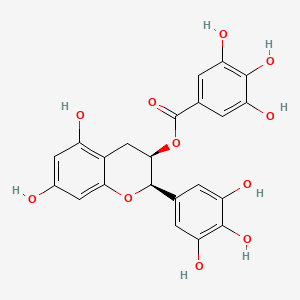 |
||||
| Formula |
C22H18O11
|
||||
| IUPAC Name |
[(2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate
|
||||
| Canonical SMILES |
C1C(C(OC2=CC(=CC(=C21)O)O)C3=CC(=C(C(=C3)O)O)O)OC(=O)C4=CC(=C(C(=C4)O)O)O
|
||||
| InChI |
InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1
|
||||
| InChIKey |
WMBWREPUVVBILR-WIYYLYMNSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | 5'-AMP-activated protein kinase catalytic subunit alpha-2 (PRKAA2) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | ||||
| mTOR signaling pathway | hsa04150 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Mice were reseparated into 5 groups: control, Dox, Dox + Fer-1, Dox + EGCG, and Dox + EGCG+compound C groups. The Dox group mice were administered 6 intraperitoneal (ip) injections of 2.5 mg/kg Dox over 3 weeks for a cumulative dose of 15 mg/kg. Mice in the Dox + Fer-1 group were ip injected with 1 mg/kg/d Fer-1 for 2 weeks as in the Dox group. Mice in the Dox + EGCG group were intragastrically (ig) injected 20 mg/kg/d EGCG (dissolved in normal saline) for six consecutive weeks; Dox was administered 1 h prior to this as in the Dox group. Mice in the Dox + EGCG + compound C group were treated using the same method as in the Dox + EGCG group for four consecutive weeks, followed by ip injections of 10 mg/kg/d compound C for 2 weeks. Mice in the control groups were administered an equal volume of normal saline via gavage for 6 weeks.
Click to Show/Hide
|
||||
| Response regulation | Epigallocatechin-3-gallate pretreatment upregulated the expression and phosphorylation of AMPK2 and activated adaptive autophagy, thus decreasing iron accumulation, inhibiting excess ROS generation and abnormal lipid metabolism, increasing energy supply, and maintaining mitochondrial function, ultimately protecting the myocardium against Dox-induced cardiotoxicity (DIC)-induced ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Spinal cord injury | ICD-11: ND51 | |||
| Responsed Regulator | Polycystin-1 (PKD1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rCGNs (Rat cerebellar granule neurons) | ||||
| Response regulation | (-)-Epigallocatechin-3-gallate (EGCG) upregulated PKD1 phosphorylation levels and inhibited ferroptosis to reduce the cell death of CGNs under oxidative stress and to promote functional recovery and ERK phosphorylation in rats following complete ST. Together, EGCG is a novel strategy for the treatment of spinal cord injury (SCI) related to PKD1 phosphorylation and ferroptosis. | ||||
Solute carrier family 40 member 1 (SLC40A1)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | |||
| Target for Ferroptosis | Marker/Suppressor | |||
| Responsed Disease | Parkinson disease | ICD-11: 8A00 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Response regulation | Epigallocatechin-3-Gallate (EGCG) pretreatment counteracted 6-OHDA-induced increased expression of divalent metal transporter-1 (DMT1) and hepcidin and decreased expression of the iron-export protein ferroportin 1 (Fpn1), leading to a 28% reduction in Fe2+ uptake. EGCG inhibits iron overload, decreased LPO, and increased GSH levels in Parkinson disease models, which are the three major hallmarks of ferroptosis. | |||
Prostaglandin G/H synthase 2 (PTGS2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Nonalcoholic fatty liver disease | ICD-11: DB92 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
After adaptive feeding, mice were randomly assigned to five groups (n = 10 per group). The details of the groups are as follows: 1) the normal diet (ND) group in which mice were fed ND (18% calories from fat); 2) the HFD group in which mice were fed HFD (60% calories from fat); 3) the HFD-EGCG/L group in which mice received 20 mg/kgbw EGCG by oral gavage daily during HFD feeding; 4) the HFD-EGCG/H group in which mice received 100 mg/kgbw EGCG by oral gavage daily during HFD feeding; and 5) the HFD-Fer-1 group in which mice received intraperitoneal injection of Fer-1 at 1 mg/kg. bw every 3 days during HFD feeding. Mice in the EGCG treatment groups were supplemented with EGCG (20 and 100 mg/kgbw) for 12 weeks. Meanwhile, mice in the ND group and the HFD group were orally gavaged with deionized water daily.
Click to Show/Hide
|
||||
| Response regulation | Epigallocatechin-3-Gallate (EGCG) supplementation and Fer-1 treatment apparently increased the protein expression of GPX4 and markedly decreased the protein expression of COX-2 and ACSL4 in the livers of HFD-fed mice. Epigallocatechin gallate may exert protective effects on hepatic lipotoxicity by inhibiting mitochondrial reactive oxygen species-mediated hepatic ferroptosis. Findings from our study provide new insight into prevention and treatment strategies for non-alcoholic fatty liver disease pathological processes. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Nonalcoholic fatty liver disease | ICD-11: DB92 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
After adaptive feeding, mice were randomly assigned to five groups (n = 10 per group). The details of the groups are as follows: 1) the normal diet (ND) group in which mice were fed ND (18% calories from fat); 2) the HFD group in which mice were fed HFD (60% calories from fat); 3) the HFD-EGCG/L group in which mice received 20 mg/kgbw EGCG by oral gavage daily during HFD feeding; 4) the HFD-EGCG/H group in which mice received 100 mg/kgbw EGCG by oral gavage daily during HFD feeding; and 5) the HFD-Fer-1 group in which mice received intraperitoneal injection of Fer-1 at 1 mg/kg. bw every 3 days during HFD feeding. Mice in the EGCG treatment groups were supplemented with EGCG (20 and 100 mg/kgbw) for 12 weeks. Meanwhile, mice in the ND group and the HFD group were orally gavaged with deionized water daily.
Click to Show/Hide
|
||||
| Response regulation | Epigallocatechin-3-Gallate (EGCG) supplementation and Fer-1 treatment apparently increased the protein expression of GPX4 and markedly decreased the protein expression of COX-2 and ACSL4 in the livers of HFD-fed mice. Epigallocatechin gallate may exert protective effects on hepatic lipotoxicity by inhibiting mitochondrial reactive oxygen species-mediated hepatic ferroptosis. Findings from our study provide new insight into prevention and treatment strategies for non-alcoholic fatty liver disease pathological processes. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response regulation | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response regulation | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
Natural resistance-associated macrophage protein 2 (SLC11A2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | |||
| Target for Ferroptosis | Driver | |||
| Responsed Disease | Parkinson disease | ICD-11: 8A00 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Response regulation | Epigallocatechin-3-Gallate (EGCG) pretreatment counteracted 6-OHDA-induced increased expression of divalent metal transporter-1 (DMT1) and hepcidin and decreased expression of the iron-export protein ferroportin 1 (Fpn1), leading to a 28% reduction in Fe2+ uptake. EGCG inhibits iron overload, decreased LPO, and increased GSH levels in Parkinson disease models, which are the three major hallmarks of ferroptosis. | |||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Nonalcoholic fatty liver disease | ICD-11: DB92 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
After adaptive feeding, mice were randomly assigned to five groups (n = 10 per group). The details of the groups are as follows: 1) the normal diet (ND) group in which mice were fed ND (18% calories from fat); 2) the HFD group in which mice were fed HFD (60% calories from fat); 3) the HFD-EGCG/L group in which mice received 20 mg/kgbw EGCG by oral gavage daily during HFD feeding; 4) the HFD-EGCG/H group in which mice received 100 mg/kgbw EGCG by oral gavage daily during HFD feeding; and 5) the HFD-Fer-1 group in which mice received intraperitoneal injection of Fer-1 at 1 mg/kg. bw every 3 days during HFD feeding. Mice in the EGCG treatment groups were supplemented with EGCG (20 and 100 mg/kgbw) for 12 weeks. Meanwhile, mice in the ND group and the HFD group were orally gavaged with deionized water daily.
Click to Show/Hide
|
||||
| Response regulation | Epigallocatechin-3-Gallate (EGCG) supplementation and Fer-1 treatment apparently increased the protein expression of GPX4 and markedly decreased the protein expression of COX-2 and ACSL4 in the livers of HFD-fed mice. Epigallocatechin gallate may exert protective effects on hepatic lipotoxicity by inhibiting mitochondrial reactive oxygen species-mediated hepatic ferroptosis. Findings from our study provide new insight into prevention and treatment strategies for non-alcoholic fatty liver disease pathological processes. | ||||
Ferritin light chain (FTL)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response regulation | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
Ferritin heavy chain (FTH1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response regulation | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
References
