Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10023)
| Target Name | Ferritin light chain (FTL) | ||||
|---|---|---|---|---|---|
| Gene Name | FTL | ||||
| Sequence |
MSSQIRQNYSTDVEAAVNSLVNLYLQASYTYLSLGFYFDRDDVALEGVSHFFRELAEEKR
EGYERLLKMQNQRGGRALFQDIKKPAEDEWGKTPDAMKAAMALEKKLNQALLDLHALGSA RTDPHLCDFLETHFLDEEVKLIKKMGDHLTNLHRLGGPEAGLGEYLFERLTLKHD Click to Show/Hide
|
||||
| Family | Ferritin family | ||||
| Function |
Stores iron in a soluble, non-toxic, readily available form. Important for iron homeostasis. Iron is taken up in the ferrous form and deposited as ferric hydroxides after oxidation. Also plays a role in delivery of iron to cells. Mediates iron uptake in capsule cells of the developing kidney.
Click to Show/Hide
|
||||
| Gene ID | 2512 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
FTL can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Transcription regulator protein BACH1 (BACH1)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell metastasis | |||||
In Vitro Model |
mEFs (Mouse embryonic fibroblasts) | ||||
| In Vivo Model |
The generation of Bach1-/-mice on the C57BL/6J background was described previously. Mice 13 weeks of age were analyzed for models of AMI. The mice were subjected to ligation of the proximal LAD to induce AMI. They were randomly assigned to sham or AMI, DMSO, or DFX groups.
Click to Show/Hide
|
||||
| Response Description | BACH1 accelerates ferroptosis by suppressing labile iron metabolism. And ferritin genes (Fth1 and Ftl1) and the ferroportin gene (Slc40a1) were dramatically up-regulated in Bach1-/- MEFs. BACH1 controls the threshold of ferroptosis induction and may represent a therapeutic target for alleviating ferroptosis-related diseases, including myocardial infarction. | ||||
Unspecific Regulator
Osteosarcoma [ICD-11: 2B51]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Responsed Drug | Ursolic Acid | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
In Vitro Model |
HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| 143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | ||
| In Vivo Model |
NU/NU mice (the Fourth Military Medical University, Shaanxi, China) were injected with 143B cells (100 uL, 5 x 107 cells/mL, i.h.). Seven days after the injection, the mice were divided into 6 different groups (n= 3) and intraperitoneally injected with different drugs twice a week. Then, on day 28, the mice were sacrificed, and the tumours in the different groups were weighed. Body weight and tumour size were measured every 3 days from day 7 to day 28. The tumour tissue was fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 4 um thick sections for haematoxylin-eosin (H&E) and immunofluorescence staining.
Click to Show/Hide
|
||||
| Response Description | Ursolic acid inhibited tumour cell proliferation and promoted the apoptosis of a variety of osteosarcoma cells. Mechanistic studies showed that ursolic acid degraded ferritin by activating autophagy and induced intracellular overload of ferrous ions, leading to ferroptosis. Ferritin, which includes ferritin light peptide 1 (FTL1) and ferritin heavy peptide 1 (FTH1). | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Responsed Drug | Bavachin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | ||
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | |||
| Responsed Drug | Atractylodin | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hccm (Human hepatocellular carcinoma cells) | ||||
| Response Description | Atractylodin can inhibit the proliferation, migration, and invasion of Huh7 and Hccm liver cancer cells, and induce cell apoptosis and cell cycle arrest. In addition, atractylodin may induce ferroptosis in hepatocellular carcinoma cells by inhibiting the expression of GPX4 and FTL proteins, and up-regulating the expression of ACSL4 and TFR1 proteins. | |||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
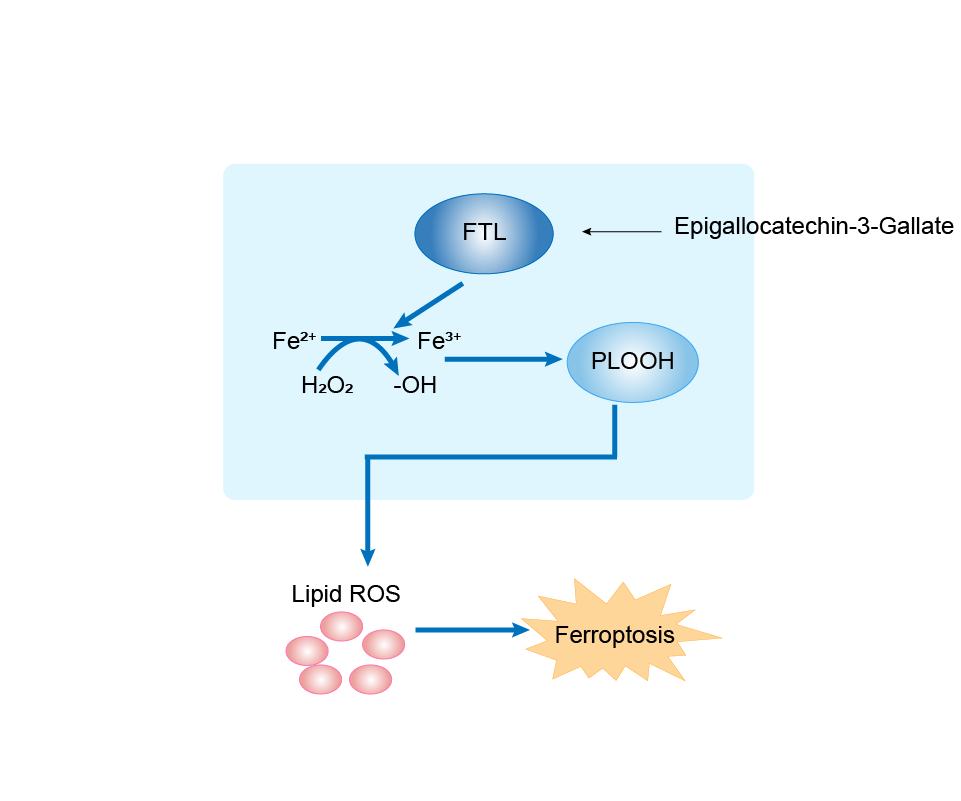
| Responsed Drug | Epigallocatechin Gallate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | |||
| Responsed Drug | JQ1 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hDFs (Human dermal fibroblasts) | |||
| Response Description | JQ1 treatment reduced the expression of ferroptosis-resistance genes in senescent cells. And the treatment with JQ1 for 48 h showed decreased mRNA expressions of FTH and FTL. JQ1 treatment induced lipid peroxidation in senescent cells but not in non-senescent cells. | |||
Unspecific Regulator
Ursolic Acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| In Vitro Model | HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| 143B cells | Osteosarcoma | Homo sapiens | CVCL_2270 | ||
| In Vivo Model |
NU/NU mice (the Fourth Military Medical University, Shaanxi, China) were injected with 143B cells (100 uL, 5 x 107 cells/mL, i.h.). Seven days after the injection, the mice were divided into 6 different groups (n= 3) and intraperitoneally injected with different drugs twice a week. Then, on day 28, the mice were sacrificed, and the tumours in the different groups were weighed. Body weight and tumour size were measured every 3 days from day 7 to day 28. The tumour tissue was fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 4 um thick sections for haematoxylin-eosin (H&E) and immunofluorescence staining.
Click to Show/Hide
|
||||
| Response Description | Ursolic acid inhibited tumour cell proliferation and promoted the apoptosis of a variety of osteosarcoma cells. Mechanistic studies showed that ursolic acid degraded ferritin by activating autophagy and induced intracellular overload of ferrous ions, leading to ferroptosis. Ferritin, which includes ferritin light peptide 1 (FTL1) and ferritin heavy peptide 1 (FTH1). | ||||
Bavachin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 |
| HOS cells | Osteosarcoma | Homo sapiens | CVCL_0312 | |
| Response Description | Bavachin could induce Osteosarcoma cell ferroptosis. Furthermore, bavachin elevated intracellular ferrous iron levels by increasing TFRC and DMT1 expression and decreasing FTH and FTL expressions. Bavachin also reduced SLC7A11 and GPX4 expression and promoted ROS and MDA accumulation by downregulating p-STAT3 to upregulate P53 expression. | |||
Atractylodin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| Cell proliferation | ||||
| Cell migration | ||||
| Cell invasion | ||||
| In Vitro Model | Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| Hccm (Human hepatocellular carcinoma cells) | ||||
| Response Description | Atractylodin can inhibit the proliferation, migration, and invasion of Huh7 and Hccm liver cancer cells, and induce cell apoptosis and cell cycle arrest. In addition, atractylodin may induce ferroptosis in hepatocellular carcinoma cells by inhibiting the expression of GPX4 and FTL proteins, and up-regulating the expression of ACSL4 and TFR1 proteins. | |||
Epigallocatechin Gallate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [5] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
All mice were randomly divided into a 2 x 2 factorial arrangement, fed diets containing 40 mg/kg or 5000 mg/kg FeSO4 (the basis of the diet was AIN-93), and gavaged with PBS or 50 mg EGCG/kg body weight per day, respectively. The experiment lasted for 6 weeks, including a 1-week adaptation and a 3-week EGCG gavage; then, all mice were euthanized.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation alleviated the liver oxidative damage caused by iron overload by inhibiting ferroptosis. EGCG addition increased NRF2 and GPX4 expression and elevated antioxidant capacity in iron overload mice. EGCG administration attenuates iron metabolism disorders by upregulating FTH/FTL expression. Through these two mechanisms, EGCG can effectively inhibit iron overload-induced ferroptosis. | ||||
JQ1
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [6] | |||
| Responsed Disease | Health [ICD-11: N.A.] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hDFs (Human dermal fibroblasts) | |||
| Response Description | JQ1 treatment reduced the expression of ferroptosis-resistance genes in senescent cells. And the treatment with JQ1 for 48 h showed decreased mRNA expressions of FTH and FTL. JQ1 treatment induced lipid peroxidation in senescent cells but not in non-senescent cells. | |||
References
