Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10042)
| Target Name | Prostaglandin G/H synthase 2 (PTGS2) | ||||
|---|---|---|---|---|---|
| Synonyms |
Cyclooxygenase-2 ; PHS II; Prostaglandin H2 synthase 2; Prostaglandin-endoperoxide synthase 2
Click to Show/Hide
|
||||
| Gene Name | PTGS2 | ||||
| Sequence |
MLARALLLCAVLALSHTANPCCSHPCQNRGVCMSVGFDQYKCDCTRTGFYGENCSTPEFL
TRIKLFLKPTPNTVHYILTHFKGFWNVVNNIPFLRNAIMSYVLTSRSHLIDSPPTYNADY GYKSWEAFSNLSYYTRALPPVPDDCPTPLGVKGKKQLPDSNEIVEKLLLRRKFIPDPQGS NMMFAFFAQHFTHQFFKTDHKRGPAFTNGLGHGVDLNHIYGETLARQRKLRLFKDGKMKY QIIDGEMYPPTVKDTQAEMIYPPQVPEHLRFAVGQEVFGLVPGLMMYATIWLREHNRVCD VLKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNKQFQYQ NRIAAEFNTLYHWHPLLPDTFQIHDQKYNYQQFIYNNSILLEHGITQFVESFTRQIAGRV AGGRNVPPAVQKVSQASIDQSRQMKYQSFNEYRKRFMLKPYESFEELTGEKEMSAELEAL YGDIDAVELYPALLVEKPRPDAIFGETMVEVGAPFSLKGLMGNVICSPAYWKPSTFGGEV GFQIINTASIQSLICNNVKGCPFTSFSVPDPELIKTVTINASSSRSGLDDINPTVLLKER STEL Click to Show/Hide
|
||||
| Family | Prostaglandin G/H synthase family | ||||
| Function |
Dual cyclooxygenase and peroxidase in the biosynthesis pathway of prostanoids, a class of C20 oxylipins mainly derived from arachidonate ((5Z,8Z,11Z,14Z)-eicosatetraenoate, AA, C20:4(n-6)), with a particular role in the inflammatory response. The cyclooxygenase activity oxygenates AA to the hydroperoxy endoperoxide prostaglandin G2 (PGG2), and the peroxidase activity reduces PGG2 to the hydroxy endoperoxide prostaglandin H2 (PGH2), the precursor of all 2-series prostaglandins and thromboxanes. This complex transformation is initiated by abstraction of hydrogen at carbon 13 (with S-stereochemistry), followed by insertion of molecular O2 to form the endoperoxide bridge between carbon 9 and 11 that defines prostaglandins. The insertion of a second molecule of O2 (bis-oxygenase activity) yields a hydroperoxy group in PGG2 that is then reduced to PGH2 by two electrons. Similarly catalyzes successive cyclooxygenation and peroxidation of dihomo-gamma-linoleate (DGLA, C20:3(n-6)) and eicosapentaenoate (EPA, C20:5(n-3)) to corresponding PGH1 and PGH3, the precursors of 1- and 3- series prostaglandins . In an alternative pathway of prostanoid biosynthesis, converts 2-arachidonoyl lysophopholipids to prostanoid lysophopholipids, which are then hydrolyzed by intracellular phospholipases to release free prostanoids. Metabolizes 2-arachidonoyl glycerol yielding the glyceryl ester of PGH2, a process that can contribute to pain response. Generates lipid mediators from n-3 and n-6 polyunsaturated fatty acids (PUFAs) via a lipoxygenase-type mechanism. Oxygenates PUFAs to hydroperoxy compounds and then reduces them to corresponding alcohols. Plays a role in the generation of resolution phase interaction products (resolvins) during both sterile and infectious inflammation. Metabolizes docosahexaenoate (DHA, C22:6(n-3)) to 17R-HDHA, a precursor of the D- series resolvins (RvDs). As a component of the biosynthetic pathway of E-series resolvins (RvEs), converts eicosapentaenoate (EPA, C20:5(n-3)) primarily to 18S-HEPE that is further metabolized by ALOX5 and LTA4H to generate 18S-RvE1 and 18S- RvE2. In vascular endothelial cells, converts docosapentaenoate (DPA, C22:5(n-3)) to 13R-HDPA, a precursor for 13- series resolvins (RvTs) shown to activate macrophage phagocytosis during bacterial infection. In activated leukocytes, contributes to oxygenation of hydroxyeicosatetraenoates (HETE) to diHETES (5,15-diHETE and 5,11-diHETE) . Can also use linoleate (LA, (9Z,12Z)- octadecadienoate, C18:2(n-6)) as substrate and produce hydroxyoctadecadienoates (HODEs) in a regio- and stereospecific manner, being (9R)-HODE ((9R)-hydroxy-(10E,12Z)-octadecadienoate) and (13S)- HODE ((13S)-hydroxy-(9Z,11E)-octadecadienoate) its major products. During neuroinflammation, plays a role in neuronal secretion of specialized preresolving mediators (SPMs) 15R-lipoxin A4 that regulates phagocytic microglia.
Click to Show/Hide
|
||||
| Gene ID | 5743 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
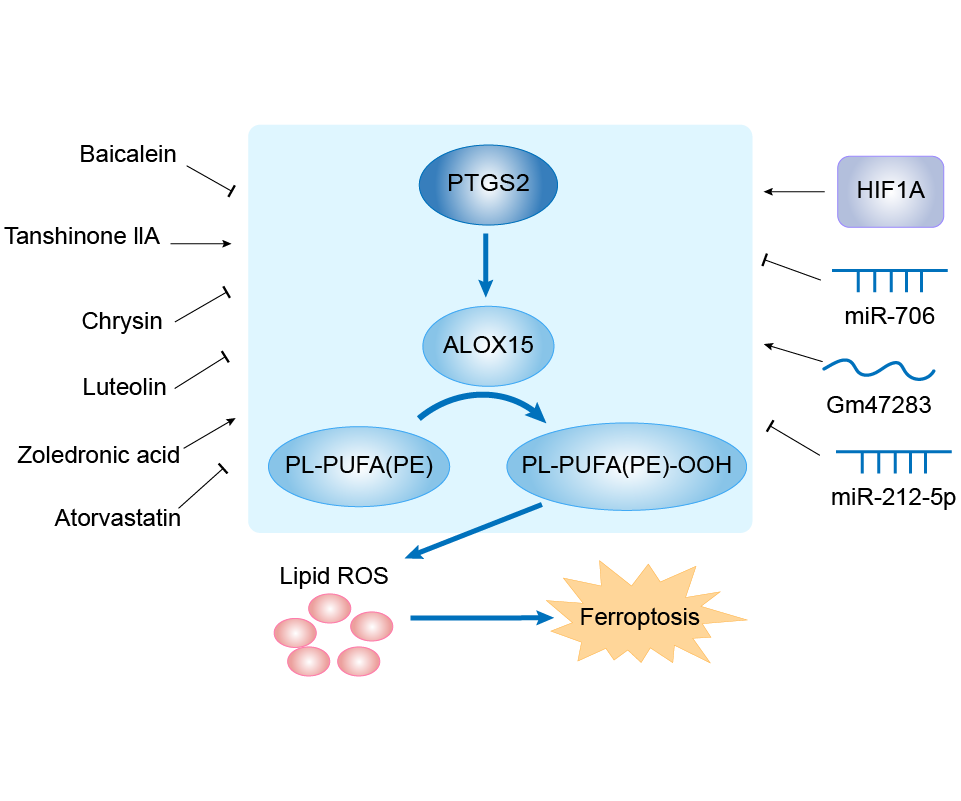
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
PTGS2 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Drug | Cadmium | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/ Sirt1 axis. | |||
Mitogen-activated protein kinase 8 (MAPK8)
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | L-F001 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | L-F001 could restore GPX4 and glutamate-cysteine ligase modifier subunit (GCLM) levels, and significantly deceased Cyclooxygenase (COX-2) levels to rescue the lipid peroxidation imbalance. And L-F001 could reduce RSL3-induced c-Jun N-terminal kinase (JNK) activation, which might be a potential drug target for for the therapy of ferroptosis-related diseases, such as cerebral ischemia. | |||
Hypoxia-inducible factor 1-alpha (HIF1A)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Atorvastatin | Investigative | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mMTs (Mouse myocardial tissues) | ||||
| In Vivo Model |
Rats were anesthetized intraperitoneally with 3% pentobarbital sodium (40 mg/kg). After mechanical ventilation, the rats were subjected to a thoracotomy to expose the hearts and ascending aorta. Finally, the ascending aorta was occluded for 10 s, and 8,000 polyester microspheres (diameter 42 um, Biosphere Medical, Rockland, MA, United States ) were injected into the left ventricle at the same time, while the sham-operated rats received the same dosage of normal saline instead. Forin vivotreatment, rats were treated with recombinant adeno-associated virus 9 (rAAV9)-GFP-shPtsg2 (Hanbio, Shanghai, China) or rAAV9-GFP-shHif1a (Genechem, Shanghai, China) at a dose of 1 x 1012 VG in 200 uL salineviaa single tail vein before CME. Deferoxamine (DFO) was purchased from Selleck (Shanghai, China) and administered intraperitoneally at a dose of 100 mg/kg for 7 days before CME. ATV (Pfizer, New York, United Kingdom) was administered intragastrically at a dose of 10 mg/kg for 7 days before CME.
Click to Show/Hide
|
||||
| Response Description | Ptgs2 was positively regulated by Hif1a and contributed to ferroptosis-dependent myocardial injury and inflammation. Furthermore, Atorvastatin protects against ferroptosis and inflammation induced by CME via the Hif1a/Ptgs2 pathway. | ||||
hsa-miR-34a-5p (miRNA)
Kidney injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Cadmium | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/Sirt1 axis. | |||
Aquaporin-11 (AQP11)
Status epilepticus [ICD-11: 8A66]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Penicillamine | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were provided by at the Centre for Animals of Central South University (Changsha, China). To prepare the seizure mouse model, the mice underwent the intrahippocampal injection of KA as described in our previous investigation. For short, mice were anesthetized with sodium phenobarbital (50 mg/kg, i.p.) and carefully placed on a stereotaxic apparatus. Then, KA (1 uL, 250 ng/uL dissolved in saline) was stereotactically injected into the hippocampus according to the following coordinates: anteroposterior -2.0 mm; lateral -1.3 mm; dorsoventral -1.2 mm. After injection, the infusion needle was kept in place for 5-10 min to avoid liquid reflux. Mice in the control group underwent the same surgical procedure but received injection with an equal volume of phosphate buffered saline (PBS) instead of KA.
Click to Show/Hide
|
||||
| Response Description | D-penicillamine (DPA) can be repurposed to cure seizure disorders such as epilepsy. Furthermore, ferroptosis-associated indices including acyl-coA synthetase long chain family member 4 (ACSL4), prostaglandin-endoperoxide synthase 2 (Ptgs2) gene and lipid peroxide (LPO) level were significantly decreased in KA mouse model after DPA treatment. The effects of DPA on ferroptosis process are dependent upon Aqp11. | ||||
Prolyl hydroxylase EGLN2 (EGLN2)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | |
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 HT1080 cells in 100 ul of phosphate-buffered saline (PBS) were injected subcutaneously at the right of the dorsal midline in 6- to 8-week-old female athymic nude mice (no. 490, Charles River Laboratories).
Click to Show/Hide
|
||||
| Response Description | The autophagy-mediated degradation of ARNTL facilitates EGLN2 expression, thus destabilizing the prosurvival factor HIF1A, ultimately favoring lipid peroxidation and cell death. And the HIF1A inhibitor chetomin enhanced the anticancer activity of RSL3, PTGS2 mRNA expression in Lung squamous cell carcinoma. | ||||
Parkinson disease protein 7 (PARK7)
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| NCI-H292 cells | Lung mucoepidermoid carcinoma | Homo sapiens | CVCL_0455 | ||
| NCI-H838 cells | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | ||
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| KHOS cells | Osteosarcoma | Homo sapiens | CVCL_2546 | ||
| A2780 cells | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Tumors were established by a subcutaneous injection of shRNA (control or DJ-1 KD) transfected H1299 cells (1,000,000/200 uL) into BALB/c female athymic nude mice (5 weeks, National Rodent Laboratory Animal Resource, Shanghai, China). Twelve days after injection, mice were randomly allocated into different groups and treated with vehicle (0.625% DMSO/99.375% HBSS (pH = 2)) or 30 mg/kg PE (tail intravenous injection, once every other day) for 16 days before the final tumor size was measured in all groups.
Click to Show/Hide
|
||||
| Response Description | KD of DJ-1 (PARK7) increases ferroptosis as PTGS2 levels increased. DJ-1 mutant neuronal cells experience high levels of ferroptosis, which might establish a potential mechanism via which DJ-1 could regulate early-onset recessive Parkinsons disease. | ||||
mmu-miR-706 (miRNA)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Male mice were used in this research. The mice were housed in a colony room at a controlled temperature (22) and humidity, under a 12-h light/dark cycle, and with food and water freely available. All surgical procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In brief, the mice were anesthetized by 3% pentobarbital sodium and then ligated the anterior descending branch of the left coronary artery (LAD) for 48 h to establish the in vivo MI models. The sham group mice were opened the chest bur not ligated with LAD. The mice were randomly divided into three groups as follows: (1) the sham group, which underwent sham operation and received vehicle (PBS, caudal vein injection); (2) the model group, which was subjected to LAD and received vehicle (PBS, caudal vein injection); and (3) the siRNA group, which were subjected to LAD and treated with siRNA of lncRNA Gm47283 (30 nM siRNA dose per mice every day for one week, caudal vein injection).
Click to Show/Hide
|
||||
| Response Description | Stem cell membrane coated siRNA of lncRNA Gm47283 inhibits cardiomyocyte ferroptosis in myocardial infarction rat. Stem cell membrane-coated siRNA of lncRNA Gm47283 increases miR-706, and the miR-706 suppresses the expression of Ptgs2 to reduce lipid peroxidation toxicity, and then inhibits cardiomyocyte ferroptosis. Over-expression of lncRNA Gm47283 significantly increased the expression of Ptgs2 and Alox15 and repressed the expression of Gpx4. | ||||
hsa-miR-212-5p (miRNA)
Traumatic brain injury [ICD-11: NA07]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| Neuro-2a cells | Neuroblastoma | Mus musculus | CVCL_0470 | ||
| In Vivo Model |
Adult male C57BL/6J mice, aged 10-12 weeks and weighing 20 to 24 g. Briefly, following anesthesia with isoflurane (4% for induction and 12% for maintenance), mice were mounted on a stereotaxic frame. A midsagittal incision was performed in the scalp under sterile conditions and a 4.5 mm diameter circular craniotomy was made over the left parietotemporal cortex with a burr drill. Then, the skullcap was gently removed and a 3.0 mm diameter round and flat tip was carefully placed vertically to the dural surface. The electromagnetic controlled cortical impact device was set to 5.0 m/s for strike velocity, 2.0 mm for strike depth and 100 ms for dwell time. A sterile plastic film covered the bone window and intermittent sutures of the skin, and disinfection with iodophor were performed. The entire procedure required 15-30 min per mouse.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis, a newly discovered form of iron-dependent regulated cell death, has been implicated in traumatic brain injury (TBI). Overexpression of miR-212-5p attenuated ferroptosis while downregulation of miR-212-5p promoted ferroptotic cell death partially by targeting prostaglandin-endoperoxide synthase-2 (Ptgs2) in HT-22 and Neuro-2a cell lines. | ||||
hsa-miR-137-3p (miRNA)
Cerebral ischaemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Arachidonic acid metabolism | hsa00590 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 |
| Response Description | MiR-137 is reported to regulate ferroptosis and to be involved in the neuroprotection against ischemic stroke. MiR-137overexpression boosts the neuroprotective effects of EPC-EXs against apoptosis and mitochondrial dysfunction in oxyHb-treated SH-SY5Y cells. Furthermore, EXsmiR-137 rather than EXs can restore the decrease in miR-137 levels and inhibit ferroptosis, and the protection mechanism might involve the MiR-137-COX2/PGE2 signaling pathway. | |||
High mobility group protein B1 (HMGB1)
Myeloid leukaemia [ICD-11: 2B33]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
In Vitro Model |
HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| NB4 cells | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | ||
| KG-1 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0374 | ||
| U-937 cells | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | ||
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| In Vivo Model |
Seven- to eight-week male NOD/SCID (non-obese diabetic/severe combined immunodeficient) mice that weighed about 20 g were purchased from Xiangya Medical College Animal Laboratory (Changsha, China). Indicated HL-60/NRASQ61L cells were subcutaneously injected into the dorsal flanks right of the midline in NOD/SCID mice (weight, approximately 20 g). At day seven, mice were intraperitoneal injected with erastin (20 mg/kg i.v., three times a week) for two weeks. Erastin was dissolved in the vehicle (2% DMSO and 98% PBS) and prepared by Ultrasonic Cleaner (Fisher Scientific, Hampton, NH). A final volume of 300 uL of erastin was applied via intraperitoneal injection.
Click to Show/Hide
|
||||
| Response Description | HMGB1 could be a potential drug target for therapeutic interventions in leukemia.Importantly, these data were further supported by our in vivo experiment, in which xenografts formed by HMGB1 knockdown HL-60/NRASQ61L cells had lower PTGS2 and TfR1 expression than that in control mice. | ||||
Gm47283 (IncRNA)
Acute myocardial infarction [ICD-11: BA41]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HL-1 cells | Normal | Mus musculus | CVCL_0303 | |
| In Vivo Model |
Male mice were used in this research. The mice were housed in a colony room at a controlled temperature (22) and humidity, under a 12-h light/dark cycle, and with food and water freely available. All surgical procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. In brief, the mice were anesthetized by 3% pentobarbital sodium and then ligated the anterior descending branch of the left coronary artery (LAD) for 48 h to establish the in vivo MI models. The sham group mice were opened the chest bur not ligated with LAD. The mice were randomly divided into three groups as follows: (1) the sham group, which underwent sham operation and received vehicle (PBS, caudal vein injection); (2) the model group, which was subjected to LAD and received vehicle (PBS, caudal vein injection); and (3) the siRNA group, which were subjected to LAD and treated with siRNA of lncRNA Gm47283 (30 nM siRNA dose per mice every day for one week, caudal vein injection).
Click to Show/Hide
|
||||
| Response Description | Stem cell membrane coated siRNA of lncRNA Gm47283 inhibits cardiomyocyte ferroptosis in myocardial infarction rat. Stem cell membrane-coated siRNA of lncRNA Gm47283 increases miR-706, and the miR-706 suppresses the expression of Ptgs2 to reduce lipid peroxidation toxicity, and then inhibits cardiomyocyte ferroptosis. Over-expression of lncRNA Gm47283 significantly increased the expression of Ptgs2 and Alox15 and repressed the expression of Gpx4. | ||||
Basic helix-loop-helix ARNT-like protein 1 (BMAL1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
Calu-1 cells | Lung squamous cell carcinoma | Homo sapiens | CVCL_0608 | |
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | ||
| HL-60 cells | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 HT1080 cells in 100 ul of phosphate-buffered saline (PBS) were injected subcutaneously at the right of the dorsal midline in 6- to 8-week-old female athymic nude mice (no. 490, Charles River Laboratories).
Click to Show/Hide
|
||||
| Response Description | The autophagy-mediated degradation of ARNTL facilitates EGLN2 expression, thus destabilizing the prosurvival factor HIF1A, ultimately favoring lipid peroxidation and cell death. And the HIF1A inhibitor chetomin enhanced the anticancer activity of RSL3, PTGS2 mRNA expression in Lung squamous cell carcinoma. | ||||
Unspecific Regulator
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | |||
| Responsed Drug | Zoledronic acid | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| MNNG/HOS Cl #5 cells | Osteosarcoma | Homo sapiens | CVCL_0439 | |
| Response Description | Zoledronic acid treatment decreased cell viability and promoted the increase in lipid peroxide content and PTGS2 expression. Our results indicate that zoledronic acid induces ferroptosis by decreasing ubiquinone content and promoting HMOX1 expression in osteosarcoma cells. | |||
Gastric cancer [ICD-11: 2B72]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Responsed Drug | Tanshinone IIA | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| NCI-N87 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1603 | ||
| In Vivo Model |
All mice were housed under a setting of 12-h light/dark cycle at 22 ± 1, 55% humidity and fed with water and food provided at regular time. During the entire maintenance period, all mice were permitted free cage activity without joint immobilization. The initial body weights of the mice were between 20 and 23 grams. After subcutaneous injection of 2 x 106 BGC-823 gastric cancer cells into the back of NOD-SCID mice, the mice were treated with or without Tan IIA (50 mg/kg) or Tan IIA in combination with Fer-1 (50 mg/kg). Tan IIA was diluted in DMSO:Methanol:Hydroxypropyl-b-cydodextrin (HP-b-CD) = 1:1:1. Fer-1 was also dissolved in DMSO:Methanol:HP-b-CD. Seven days after BGC-823 gastric cancer cells injection, intraperitoneal injection with Tan IIA was carried out every other day followed by killing at day 22 of tumor cell inoculation. All mice were killed by dislocation of the cervical vertebrae. Before killing, the tumor volume was measured every 3 days. All experiments were carried out using six mice each group in three independent experiments of a time-dependent manner with three time points.
Click to Show/Hide
|
||||
| Response Description | Tanshinone IIA increased lipid peroxidation and up-regulated Ptgs2 and Chac1 expression, two markers of ferroptosis. In addition, Tan IIA also up-regulated p53 expression and down-regulated xCT expression. Therefore, Tan IIA could suppress the proliferation of gastric cancer via inducing p53 upregulation-mediated ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Responsed Drug | XN4 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | ||
| Response Description | The pro-ferroptotic role of XN4 in gastric cancer (GC) might enable it to become a promising drug for GC treatment in the future despite the need for extensive research. Moreover, GPX4 levels decreased, but NOX4 and ferroptosis-related protein PTGS2 levels increased in GC cells following XN4 treatment, which was nullified by NOX4 knockdown. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | ||||
| Responsed Drug | Paraquat | Investigative | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| Ferroptosis | hsa04216 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Twenty male C57BL/6 mice at 12 weeks old were purchased from Hebei Medical University Experimental Animal Center. 10 mice of the experimental group were intraperitoneally injected with PQ (10 mg PQ (salt)/kg/dose) three times a week for 3 weeks according to the previous report. Ten mice of the control group were intraperitoneally injected with the same dose of normal saline. Once the experimental schedule was completed, firstly, the animals were used for behavioral tests. Then, the mice were anesthetized with 0.4% pentobarbital sodium (1 mL/100 g) solution and perfused. The substantia nigra tissue was exfoliated for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Paraquat (PQ) significantly caused the iron accumulation in cytoplasm and mitochondria through ferritinophagy pathway induced by NCOA4. Iron overload initiated lipid peroxidation through 12Lox, further inducing ferroptosis by producing lipid ROS. PQ downregulated SLC7A11 and GPX4 expression and upregulated Cox2 expression. Bcl2/Bax and P-p38/p38 pathways mediated the cross-talk between ferroptosis and apoptosis induced by PQ. These data further demonstrated the complexity of Parkinson's disease occurrence. | ||||
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | ||||
| Responsed Drug | Chrysin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
Nervous system disease [ICD-11: 8E7Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | |||
| Responsed Drug | N2L | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | N2L recovered glutathione peroxidase 4 (GPX4) expression and blocked the increase of Cyclooxygenase-2 (cox-2) and acyl-CoA synthetase long-chain family member 4 (ACSL4) protein expressions. Moreover, N2L also significantly prevented Ferritin Heavy Chain 1 (FTH1) from downregulation and maintained iron homeostasis. And N2L could be a ferroptosis inhibitor for the therapy of ferroptosis-related neurodegenerative diseases, such as Alzheimer's disease. | |||
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [17] | |||
| Responsed Drug | Berberine | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | Berberine (BBR) inhibited ferroptosis via reducing ROS generation and reducing lipid peroxidation in erastin and RSL3-treated cardiac cells.Furthermore, quantitative polymerase chain reaction results showed that Ptgs2 mRNA was reduced in BBR-treated cells. BBR has the potential to treat ferroptosis-induced cardiomyopathy. | |||
Congestive heart failure [ICD-11: BD10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | ||||
| Responsed Drug | Atorvastatin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
8-week C57BL/6J male mice purchased from Comparative Medicine Center of Yangzhou University were retained with unrestricted access to sterilized diet and water at standard bio-clean laboratory settings (Experimental Animal Center of College of Veterinary Medicine of Yangzhou University). Animals were randomly divided into four groups(n = 6-8 mice per group): control group or ISO group: injected with saline or ISO (5 mg/kg) subcutaneously for 14 days and, meanwhile, received vehicle saline via gavage for 14 days respectively; ATV (Pfizer,USA) group or ISO + ATV group: injected with saline or 5 mg/kg ISO (Sigma, USA) subcutaneously for 14 days and, meanwhile, received 20 mg/kg ATV via gavage for 14 days respectively.
Click to Show/Hide
|
||||
| Response Description | Atorvastatin showed significantly protective effects through suppressing the activation of ferroptosis related signaling, as evidenced by decreasing the mRNA levels of PTGS2 (a marker of ferroptosis), contents of malonaldehyde and protein levels of NOX4 and increasing the contents of glutathione (GSH), the ratio of GSH/GSSG and protein levels of GPX4 and SLC7A11. ATV reduced cardiac hypertrophy and fibrosis and accumulation of iron in heart failure. | ||||
Aortic aneurysm [ICD-11: BD50]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Responsed Drug | Acrolein | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rVSMCs (Rat vascular smooth muscle cells) | ||||
| A7r5 cells | Normal | Rattus norvegicus | CVCL_0137 | ||
| EAhy926 cells | Normal | Homo sapiens | CVCL_3901 | ||
| C3H/10T1/2 cells | Normal | Mus musculus | CVCL_0190 | ||
| In Vivo Model |
C57BL/6J wild-type mice (8-10 wk old, male) were purchased from Japan SLC (Tokyo, Japan). Mice were housed (4/cage, RAIR HD-ventilated micro-isolator animal housing systems; Laboratory Products, Seaford, DE) in an environment maintained at 23 ± 2 with ad libitum access to food and water under a 12-h:12-h light-dark cycle, with lights on from 0800 to 2000. A total of 13 mice were used. The aorta was harvested immediately after mice were euthanized by inhalation of carbon dioxide, washed in 0.1% antibiotics/PBS, and cut into 3-4-mm rings. The aortic rings were cultured in 10% FCS/DMEM for 3 h and then treated with CSE (0.8 mg/mL) for the indicated periods.
Click to Show/Hide
|
||||
| Response Description | VSMC ferroptosis was induced by acrolein and methyl vinyl ketone, major constituents of CSE. CSE also induced the upregulation of Ptgs2 mRNA, lipid peroxidation, and intracellular GSH depletion, which are key features of ferroptosis. These findings suggest that ferroptosis is a potential therapeutic target for preventing aortic aneurysm and dissection. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Responsed Drug | Methyl vinyl ketone | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rVSMCs (Rat vascular smooth muscle cells) | ||||
| A7r5 cells | Normal | Rattus norvegicus | CVCL_0137 | ||
| EAhy926 cells | Normal | Homo sapiens | CVCL_3901 | ||
| C3H/10T1/2 cells | Normal | Mus musculus | CVCL_0190 | ||
| In Vivo Model |
C57BL/6J wild-type mice (8-10 wk old, male) were purchased from Japan SLC (Tokyo, Japan). Mice were housed (4/cage, RAIR HD-ventilated micro-isolator animal housing systems; Laboratory Products, Seaford, DE) in an environment maintained at 23 ± 2 with ad libitum access to food and water under a 12-h:12-h light-dark cycle, with lights on from 0800 to 2000. A total of 13 mice were used. The aorta was harvested immediately after mice were euthanized by inhalation of carbon dioxide, washed in 0.1% antibiotics/PBS, and cut into 3-4-mm rings. The aortic rings were cultured in 10% FCS/DMEM for 3 h and then treated with CSE (0.8 mg/mL) for the indicated periods.
Click to Show/Hide
|
||||
| Response Description | VSMC ferroptosis was induced by acrolein and methyl vinyl ketone, major constituents of CSE. CSE also induced the upregulation of Ptgs2 mRNA, lipid peroxidation, and intracellular GSH depletion, which are key features of ferroptosis. These findings suggest that ferroptosis is a potential therapeutic target for preventing aortic aneurysm and dissection. | ||||
Nonalcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Responsed Drug | Epigallocatechin Gallate | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
After adaptive feeding, mice were randomly assigned to five groups (n = 10 per group). The details of the groups are as follows: 1) the normal diet (ND) group in which mice were fed ND (18% calories from fat); 2) the HFD group in which mice were fed HFD (60% calories from fat); 3) the HFD-EGCG/L group in which mice received 20 mg/kgbw EGCG by oral gavage daily during HFD feeding; 4) the HFD-EGCG/H group in which mice received 100 mg/kgbw EGCG by oral gavage daily during HFD feeding; and 5) the HFD-Fer-1 group in which mice received intraperitoneal injection of Fer-1 at 1 mg/kg. bw every 3 days during HFD feeding. Mice in the EGCG treatment groups were supplemented with EGCG (20 and 100 mg/kgbw) for 12 weeks. Meanwhile, mice in the ND group and the HFD group were orally gavaged with deionized water daily.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation and Fer-1 treatment apparently increased the protein expression of GPX4 and markedly decreased the protein expression of COX-2 and ACSL4 in the livers of HFD-fed mice. Epigallocatechin gallate may exert protective effects on hepatic lipotoxicity by inhibiting mitochondrial reactive oxygen species-mediated hepatic ferroptosis. Findings from our study provide new insight into prevention and treatment strategies for non-alcoholic fatty liver disease pathological processes. | ||||
Drug-induced or toxic liver disease [ICD-11: DB95]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | ||||
| Responsed Drug | Nickel Chloride | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hLCs (Liver cells) | ||||
| In Vivo Model |
Totally 128 7-week-old ICR male mice (22-25 g) were provided by Dashuo Biological Technology (Chengdu, China). The animals were divided into four groups (32 mice per group) randomly. The mice in the three experimental groups were gavage administered with Ni (NiCl2·6H2O) at doses of 7.5, 15, and 30 mg/kg body weight respectively, while those in the control group were given distilled water. The Ni dose adopted here was determined according to the value of median lethal dose (LD50, 306.11 mg/kg) attained in the research on acute oral toxicity of male mice. We selected 1/40, 1/20 and 1/10 LD50 (306.11 mg/kg) of NiCl2 in this study.
Click to Show/Hide
|
||||
| Response Description | Nickel chloride caused hepatic ferroptosis accompanied by increased iron content in the liver and up-regulation of cyclooxygenase 2 (COX-2) protein and mRNA expression levels, down-regulation of glutathione eroxidase 4 (GPX4), ferritin heavy chain 1 (FTH1) and nuclear receptor coactivator 4 (NCOA4) protein and mRNA expression levels. Altogether, Mitochondria damage and ferroptosis involved in Ni-induced hepatotoxicity in mice. | ||||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | ||||
| Responsed Drug | Baicalein | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Responsed Drug | Histochrome | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mCMs (Mouse cardiomyocytes) | ||||
| In Vivo Model |
Male Fischer 344 rats (8 weeks old and 160 to 180 g; KOATECH, Pyeongtaek-si, Korea) were anesthetized by inhalation with 2% isoflurane and intubated using an 18-gauge intravenous catheter. The rats were mechanically ventilated with medical-grade oxygen. Surgery was performed on a 37 heating pad to prevent the body from getting cold. A left thoracotomy was performed after the chest was shaved to prevent contamination during surgery.
Click to Show/Hide
|
||||
| Response Description | Histochrome treatment significantly increased GPx4 and free GSH levels, but decreased Cox-2 level. HC treatment significantly decreased intracellular and mitochondrial ROS levels by upregulating the expression of Nrf2 and antioxidant genes. The substantial cardioprotective effects of HC against myocardia I/R injury by reducing ferroptosis-associated myocardial injury. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | ||||
| Responsed Drug | Luteolin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | ||||
| Responsed Drug | Aspirin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
These mice were on eight weeks old male DBA/2J background (n = 36, HFK Bioscience, Beijing, China). They were randomized one of the six groups: control normal mice group (NC); diabetic mice group (DM); diabetic mice group (Fer-1), who intraperitoneal injected Fer-1 (Selleck, Houston, TX, USA); diabetic mice group (vehicle-P), who intraperitoneal injected 1% dimethyl sulfoxide (DMSO); diabetic mice group (As), who intragastric administrated Aspirin (Solarbio, Beijing, China); diabetic mice group (vehicle-G), who intragastric administrated 0.5% sodium carboxymethyl cellulose (Na-CMC; Solarbio, Beijing, China). Diabetes models were induced with 5 consecutive days of a single intraperitoneal injection of streptozotocin 40 mg/kg (dissolved in 0.1 M citrate buffer, pH 4.5; SigmaAldrich, St Louis, MO, USA). Control mice only was injected the same volume of citrate buffer. In the Fer-1 or vehicle-P groups, the diabetic mice were treated respectively with Fer-1 (2.5 umol/kg, dissolved in 1% DMSO) or 1% DMSO during the duration of treatment for 12-week every day. And in the AS and vehicle-G groups, the diabetic mice were treated respectively with aspirin (50 mg/kg, dissolved in 0.5% Na-CMC) or 0.5% Na-CMC for 12-week every day.
Click to Show/Hide
|
||||
| Response Description | Aspirin can upregulate SLC7A11 and GPX4 expression by suppressing COX2. Our results demonstrated that ferroptosis in renal tubular cells contributes to Diabetic kidney disease (DKD) development and that diabetes-related ferroptosis was inhibited through the downregulation of COX2 by aspirin, thus retarding the progression of DKD. | ||||
Traumatic brain injury [ICD-11: NA07]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [25] | ||||
| Responsed Drug | Ruxolitinib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
hBCs (Brain cells) | ||||
| In Vivo Model |
Adult male C57BL/6J mice (6-8 weeks, weighting 20-25 g) were used for all experiments. Mice were housed in pairs in a cage with access to food and water ad libitum. Mice were anesthetized with 4% chloral hydrate (0.4 mg/g) and mounted in a stereotaxic system (David Kopf Instruments, Tujunga, California). A midline skin incision was performed on the scalp to expose the skull, and a 5-mm craniotomy was made lateral to the sagittal suture and centered between bregma and lambda. The skull cap was then removed carefully to avoid destroying the dura mater.
Click to Show/Hide
|
||||
| Response Description | Ruxolitinib exerts neuroprotection via repressing ferroptosis in a mouse model of traumatic brain injury. Ruxo significantly inhibited the expressions of COX2 and TfR1. In addition, Ruxo also reversed the lower expression of GPX4 caused by Traumatic brain injury. | ||||
Neurotoxicity [ICD-11: NE61]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | ||||
| Responsed Drug | Formaldehyde | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Sprague-Dawley (SD, weight: 200-220 g) rats were obtained from Hunan SJA laboratory animal company (Changsha, Hunan, China). SD rats were anaesthetized by sodium pentobarbital (45 mg/kg, i.p.) and secured in a stereotaxic frame. The aseptically cannula was implanted into lateral ventricle according to the following coordinates: AP: -1mm; ML: 2 mm; DV: 4 mm. During experiment, the unilateral ventricle of rats was received 2.5 ul FA (0.1, 1, 10 umol) according to the experiment scheme in vivo.
Click to Show/Hide
|
||||
| Response Description | Formaldehyde (FA)-induced neurotoxicity is implicated in neuronal ferroptosis. FA induced cell death in HT22 cells, and upregulated the ferroptosis-associated genes, including Ptgs2 (prostaglandin-endoperoxide synthase 2), GLS2 (glutaminase 2), solute carrier family 1 member 5 (SLC1A5), and solute carrier family 38 member 1 (SLC38A1) in HT22 cells, indicating the inductive role of FA in the ferroptosis of HT22 cells for the formaldehyde-induced neurotoxicity. | ||||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Cadmium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Responsed Disease | Kidney injury [ICD-11: NB92] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/ Sirt1 axis. | |||
Mitogen-activated protein kinase 8 (MAPK8)
L-F001
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | L-F001 could restore GPX4 and glutamate-cysteine ligase modifier subunit (GCLM) levels, and significantly deceased Cyclooxygenase (COX-2) levels to rescue the lipid peroxidation imbalance. And L-F001 could reduce RSL3-induced c-Jun N-terminal kinase (JNK) activation, which might be a potential drug target for for the therapy of ferroptosis-related diseases, such as cerebral ischemia. | |||
Hypoxia-inducible factor 1-alpha (HIF1A)
Atorvastatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Acute myocardial infarction [ICD-11: BA41] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mMTs (Mouse myocardial tissues) | ||||
| In Vivo Model |
Rats were anesthetized intraperitoneally with 3% pentobarbital sodium (40 mg/kg). After mechanical ventilation, the rats were subjected to a thoracotomy to expose the hearts and ascending aorta. Finally, the ascending aorta was occluded for 10 s, and 8,000 polyester microspheres (diameter 42 um, Biosphere Medical, Rockland, MA, United States ) were injected into the left ventricle at the same time, while the sham-operated rats received the same dosage of normal saline instead. Forin vivotreatment, rats were treated with recombinant adeno-associated virus 9 (rAAV9)-GFP-shPtsg2 (Hanbio, Shanghai, China) or rAAV9-GFP-shHif1a (Genechem, Shanghai, China) at a dose of 1 x 1012 VG in 200 uL salineviaa single tail vein before CME. Deferoxamine (DFO) was purchased from Selleck (Shanghai, China) and administered intraperitoneally at a dose of 100 mg/kg for 7 days before CME. ATV (Pfizer, New York, United Kingdom) was administered intragastrically at a dose of 10 mg/kg for 7 days before CME.
Click to Show/Hide
|
||||
| Response Description | Ptgs2 was positively regulated by Hif1a and contributed to ferroptosis-dependent myocardial injury and inflammation. Furthermore, Atorvastatin protects against ferroptosis and inflammation induced by CME via the Hif1a/Ptgs2 pathway. | ||||
hsa-miR-34a-5p (miRNA)
Cadmium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Kidney injury [ICD-11: NB92] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| Response Description | CdCl2-initiated injury was found to result from the induction of not only apoptosis but also ferroptosis, as evidenced by the increased iron content, ROS production, and mitochondrial membrane potential along with changes in the expressions of iron death-related genes (FTH1, GPX4, ASCL4, PTGS2, and NOX1) and levels of caspase9, Bax, and Bcl-2 proteins. It is possible that the damage caused by cadmium results from the induced ferroptosis and apoptosis via the miR-34a-5p/Sirt1 axis. | |||
Aquaporin-11 (AQP11)
Penicillamine
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Status epilepticus [ICD-11: 8A66] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were provided by at the Centre for Animals of Central South University (Changsha, China). To prepare the seizure mouse model, the mice underwent the intrahippocampal injection of KA as described in our previous investigation. For short, mice were anesthetized with sodium phenobarbital (50 mg/kg, i.p.) and carefully placed on a stereotaxic apparatus. Then, KA (1 uL, 250 ng/uL dissolved in saline) was stereotactically injected into the hippocampus according to the following coordinates: anteroposterior -2.0 mm; lateral -1.3 mm; dorsoventral -1.2 mm. After injection, the infusion needle was kept in place for 5-10 min to avoid liquid reflux. Mice in the control group underwent the same surgical procedure but received injection with an equal volume of phosphate buffered saline (PBS) instead of KA.
Click to Show/Hide
|
||||
| Response Description | D-penicillamine (DPA) can be repurposed to cure seizure disorders such as epilepsy. Furthermore, ferroptosis-associated indices including acyl-coA synthetase long chain family member 4 (ACSL4), prostaglandin-endoperoxide synthase 2 (Ptgs2) gene and lipid peroxide (LPO) level were significantly decreased in KA mouse model after DPA treatment. The effects of DPA on ferroptosis process are dependent upon Aqp11. | ||||
Unspecific Regulator
Zoledronic acid
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HEK-293T cells | Normal | Homo sapiens | CVCL_0063 |
| MG-63 cells | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| U2OS cells | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| MNNG/HOS Cl #5 cells | Osteosarcoma | Homo sapiens | CVCL_0439 | |
| Response Description | Zoledronic acid treatment decreased cell viability and promoted the increase in lipid peroxide content and PTGS2 expression. Our results indicate that zoledronic acid induces ferroptosis by decreasing ubiquinone content and promoting HMOX1 expression in osteosarcoma cells. | |||
Tanshinone IIA
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| NCI-N87 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1603 | ||
| In Vivo Model |
All mice were housed under a setting of 12-h light/dark cycle at 22 ± 1, 55% humidity and fed with water and food provided at regular time. During the entire maintenance period, all mice were permitted free cage activity without joint immobilization. The initial body weights of the mice were between 20 and 23 grams. After subcutaneous injection of 2 x 106 BGC-823 gastric cancer cells into the back of NOD-SCID mice, the mice were treated with or without Tan IIA (50 mg/kg) or Tan IIA in combination with Fer-1 (50 mg/kg). Tan IIA was diluted in DMSO:Methanol:Hydroxypropyl-b-cydodextrin (HP-b-CD) = 1:1:1. Fer-1 was also dissolved in DMSO:Methanol:HP-b-CD. Seven days after BGC-823 gastric cancer cells injection, intraperitoneal injection with Tan IIA was carried out every other day followed by killing at day 22 of tumor cell inoculation. All mice were killed by dislocation of the cervical vertebrae. Before killing, the tumor volume was measured every 3 days. All experiments were carried out using six mice each group in three independent experiments of a time-dependent manner with three time points.
Click to Show/Hide
|
||||
| Response Description | Tanshinone IIA increased lipid peroxidation and up-regulated Ptgs2 and Chac1 expression, two markers of ferroptosis. In addition, Tan IIA also up-regulated p53 expression and down-regulated xCT expression. Therefore, Tan IIA could suppress the proliferation of gastric cancer via inducing p53 upregulation-mediated ferroptosis. | ||||
XN4
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [13] | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 |
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | |
| Response Description | The pro-ferroptotic role of XN4 in gastric cancer (GC) might enable it to become a promising drug for GC treatment in the future despite the need for extensive research. Moreover, GPX4 levels decreased, but NOX4 and ferroptosis-related protein PTGS2 levels increased in GC cells following XN4 treatment, which was nullified by NOX4 knockdown. | |||
Paraquat
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [14] | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Apoptosis | hsa04210 | |||
| Ferroptosis | hsa04216 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Twenty male C57BL/6 mice at 12 weeks old were purchased from Hebei Medical University Experimental Animal Center. 10 mice of the experimental group were intraperitoneally injected with PQ (10 mg PQ (salt)/kg/dose) three times a week for 3 weeks according to the previous report. Ten mice of the control group were intraperitoneally injected with the same dose of normal saline. Once the experimental schedule was completed, firstly, the animals were used for behavioral tests. Then, the mice were anesthetized with 0.4% pentobarbital sodium (1 mL/100 g) solution and perfused. The substantia nigra tissue was exfoliated for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Paraquat (PQ) significantly caused the iron accumulation in cytoplasm and mitochondria through ferritinophagy pathway induced by NCOA4. Iron overload initiated lipid peroxidation through 12Lox, further inducing ferroptosis by producing lipid ROS. PQ downregulated SLC7A11 and GPX4 expression and upregulated Cox2 expression. Bcl2/Bax and P-p38/p38 pathways mediated the cross-talk between ferroptosis and apoptosis induced by PQ. These data further demonstrated the complexity of Parkinson's disease occurrence. | ||||
Chrysin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [15] | ||||
| Responsed Disease | Cerebral ischemia [ICD-11: 8B10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male SD rats were randomly divided into a sham group, a model group, high-, medium-, and low-dose chrysin groups (200, 100, and 50 mg/kg), and a positive drug group (Ginaton, 21.6 mg/kg). The CIRI model was induced in rats by transient middle cerebral artery occlusion (tMCAO). The indexes were evaluated and the samples were taken 24 h after the operation.
Click to Show/Hide
|
||||
| Response Description | The chrysin groups showed reduced content of total iron, lipid peroxide, and malondialdehyde in brain tissues and serum, increased mRNA and protein expression levels of SLC7A11 and GPX4, and decreased mRNA and protein expression levels of TFR1, PTGS2, and ACSL4. Chrysin may regulate iron metabolism via regulating the related targets of ferroptosis and inhibit neuronal ferroptosis induced by cerebral ischemia-reperfusion injury. | ||||
N2L
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [16] | |||
| Responsed Disease | Nervous system disease [ICD-11: 8E7Z] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| MAPK signaling pathway | hsa04010 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | N2L recovered glutathione peroxidase 4 (GPX4) expression and blocked the increase of Cyclooxygenase-2 (cox-2) and acyl-CoA synthetase long-chain family member 4 (ACSL4) protein expressions. Moreover, N2L also significantly prevented Ferritin Heavy Chain 1 (FTH1) from downregulation and maintained iron homeostasis. And N2L could be a ferroptosis inhibitor for the therapy of ferroptosis-related neurodegenerative diseases, such as Alzheimer's disease. | |||
Berberine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [17] | |||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | Berberine (BBR) inhibited ferroptosis via reducing ROS generation and reducing lipid peroxidation in erastin and RSL3-treated cardiac cells.Furthermore, quantitative polymerase chain reaction results showed that Ptgs2 mRNA was reduced in BBR-treated cells. BBR has the potential to treat ferroptosis-induced cardiomyopathy. | |||
Atorvastatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [18] | ||||
| Responsed Disease | Congestive heart failure [ICD-11: BD10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
8-week C57BL/6J male mice purchased from Comparative Medicine Center of Yangzhou University were retained with unrestricted access to sterilized diet and water at standard bio-clean laboratory settings (Experimental Animal Center of College of Veterinary Medicine of Yangzhou University). Animals were randomly divided into four groups(n = 6-8 mice per group): control group or ISO group: injected with saline or ISO (5 mg/kg) subcutaneously for 14 days and, meanwhile, received vehicle saline via gavage for 14 days respectively; ATV (Pfizer,USA) group or ISO + ATV group: injected with saline or 5 mg/kg ISO (Sigma, USA) subcutaneously for 14 days and, meanwhile, received 20 mg/kg ATV via gavage for 14 days respectively.
Click to Show/Hide
|
||||
| Response Description | Atorvastatin showed significantly protective effects through suppressing the activation of ferroptosis related signaling, as evidenced by decreasing the mRNA levels of PTGS2 (a marker of ferroptosis), contents of malonaldehyde and protein levels of NOX4 and increasing the contents of glutathione (GSH), the ratio of GSH/GSSG and protein levels of GPX4 and SLC7A11. ATV reduced cardiac hypertrophy and fibrosis and accumulation of iron in heart failure. | ||||
Acrolein
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [19] | ||||
| Responsed Disease | Aortic aneurysm [ICD-11: BD50] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rVSMCs (Rat vascular smooth muscle cells) | ||||
| A7r5 cells | Normal | Rattus norvegicus | CVCL_0137 | ||
| EAhy926 cells | Normal | Homo sapiens | CVCL_3901 | ||
| C3H/10T1/2 cells | Normal | Mus musculus | CVCL_0190 | ||
| In Vivo Model |
C57BL/6J wild-type mice (8-10 wk old, male) were purchased from Japan SLC (Tokyo, Japan). Mice were housed (4/cage, RAIR HD-ventilated micro-isolator animal housing systems; Laboratory Products, Seaford, DE) in an environment maintained at 23 ± 2 with ad libitum access to food and water under a 12-h:12-h light-dark cycle, with lights on from 0800 to 2000. A total of 13 mice were used. The aorta was harvested immediately after mice were euthanized by inhalation of carbon dioxide, washed in 0.1% antibiotics/PBS, and cut into 3-4-mm rings. The aortic rings were cultured in 10% FCS/DMEM for 3 h and then treated with CSE (0.8 mg/mL) for the indicated periods.
Click to Show/Hide
|
||||
| Response Description | VSMC ferroptosis was induced by acrolein and methyl vinyl ketone, major constituents of CSE. CSE also induced the upregulation of Ptgs2 mRNA, lipid peroxidation, and intracellular GSH depletion, which are key features of ferroptosis. These findings suggest that ferroptosis is a potential therapeutic target for preventing aortic aneurysm and dissection. | ||||
Methyl vinyl ketone
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [19] | ||||
| Responsed Disease | Aortic aneurysm [ICD-11: BD50] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rVSMCs (Rat vascular smooth muscle cells) | ||||
| A7r5 cells | Normal | Rattus norvegicus | CVCL_0137 | ||
| EAhy926 cells | Normal | Homo sapiens | CVCL_3901 | ||
| C3H/10T1/2 cells | Normal | Mus musculus | CVCL_0190 | ||
| In Vivo Model |
C57BL/6J wild-type mice (8-10 wk old, male) were purchased from Japan SLC (Tokyo, Japan). Mice were housed (4/cage, RAIR HD-ventilated micro-isolator animal housing systems; Laboratory Products, Seaford, DE) in an environment maintained at 23 ± 2 with ad libitum access to food and water under a 12-h:12-h light-dark cycle, with lights on from 0800 to 2000. A total of 13 mice were used. The aorta was harvested immediately after mice were euthanized by inhalation of carbon dioxide, washed in 0.1% antibiotics/PBS, and cut into 3-4-mm rings. The aortic rings were cultured in 10% FCS/DMEM for 3 h and then treated with CSE (0.8 mg/mL) for the indicated periods.
Click to Show/Hide
|
||||
| Response Description | VSMC ferroptosis was induced by acrolein and methyl vinyl ketone, major constituents of CSE. CSE also induced the upregulation of Ptgs2 mRNA, lipid peroxidation, and intracellular GSH depletion, which are key features of ferroptosis. These findings suggest that ferroptosis is a potential therapeutic target for preventing aortic aneurysm and dissection. | ||||
Epigallocatechin Gallate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [20] | ||||
| Responsed Disease | Nonalcoholic fatty liver disease [ICD-11: DB92] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | L-02 cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| In Vivo Model |
After adaptive feeding, mice were randomly assigned to five groups (n = 10 per group). The details of the groups are as follows: 1) the normal diet (ND) group in which mice were fed ND (18% calories from fat); 2) the HFD group in which mice were fed HFD (60% calories from fat); 3) the HFD-EGCG/L group in which mice received 20 mg/kgbw EGCG by oral gavage daily during HFD feeding; 4) the HFD-EGCG/H group in which mice received 100 mg/kgbw EGCG by oral gavage daily during HFD feeding; and 5) the HFD-Fer-1 group in which mice received intraperitoneal injection of Fer-1 at 1 mg/kg. bw every 3 days during HFD feeding. Mice in the EGCG treatment groups were supplemented with EGCG (20 and 100 mg/kgbw) for 12 weeks. Meanwhile, mice in the ND group and the HFD group were orally gavaged with deionized water daily.
Click to Show/Hide
|
||||
| Response Description | Epigallocatechin-3-Gallate (EGCG) supplementation and Fer-1 treatment apparently increased the protein expression of GPX4 and markedly decreased the protein expression of COX-2 and ACSL4 in the livers of HFD-fed mice. Epigallocatechin gallate may exert protective effects on hepatic lipotoxicity by inhibiting mitochondrial reactive oxygen species-mediated hepatic ferroptosis. Findings from our study provide new insight into prevention and treatment strategies for non-alcoholic fatty liver disease pathological processes. | ||||
Nickel Chloride
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [21] | ||||
| Responsed Disease | Drug-induced or toxic liver disease [ICD-11: DB95] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| In Vivo Model |
Totally 128 7-week-old ICR male mice (22-25 g) were provided by Dashuo Biological Technology (Chengdu, China). The animals were divided into four groups (32 mice per group) randomly. The mice in the three experimental groups were gavage administered with Ni (NiCl2·6H2O) at doses of 7.5, 15, and 30 mg/kg body weight respectively, while those in the control group were given distilled water. The Ni dose adopted here was determined according to the value of median lethal dose (LD50, 306.11 mg/kg) attained in the research on acute oral toxicity of male mice. We selected 1/40, 1/20 and 1/10 LD50 (306.11 mg/kg) of NiCl2 in this study.
Click to Show/Hide
|
||||
| Response Description | Nickel chloride caused hepatic ferroptosis accompanied by increased iron content in the liver and up-regulation of cyclooxygenase 2 (COX-2) protein and mRNA expression levels, down-regulation of glutathione eroxidase 4 (GPX4), ferritin heavy chain 1 (FTH1) and nuclear receptor coactivator 4 (NCOA4) protein and mRNA expression levels. Altogether, Mitochondria damage and ferroptosis involved in Ni-induced hepatotoxicity in mice. | ||||
Baicalein
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [22] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
Histochrome
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mCMs (Mouse cardiomyocytes) | ||||
| In Vivo Model |
Male Fischer 344 rats (8 weeks old and 160 to 180 g; KOATECH, Pyeongtaek-si, Korea) were anesthetized by inhalation with 2% isoflurane and intubated using an 18-gauge intravenous catheter. The rats were mechanically ventilated with medical-grade oxygen. Surgery was performed on a 37 heating pad to prevent the body from getting cold. A left thoracotomy was performed after the chest was shaved to prevent contamination during surgery.
Click to Show/Hide
|
||||
| Response Description | Histochrome treatment significantly increased GPx4 and free GSH levels, but decreased Cox-2 level. HC treatment significantly decreased intracellular and mitochondrial ROS levels by upregulating the expression of Nrf2 and antioxidant genes. The substantial cardioprotective effects of HC against myocardia I/R injury by reducing ferroptosis-associated myocardial injury. | ||||
Luteolin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [22] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
In this study, 30 male Sprague Dawley rats (325-375 g) anesthetized using pentobarbital (1.5 g/kg, i.p.) were used for heart infarct studies,Western blot analysis, and qPCR. The isolated hearts were perfused in a Langendorff system. A water-filled latex balloon was inserted into the left ventricle cavity via mitral valve and linked to a physiological pressure transducer (AD Instruments, MLT884) for continuous monitoring of left ventricular systolic pressure (LVSP) and end diastolic pressure (LVEDP). Left ventricular developed pressure (LVDP) was calculated as the difference between LVSP and LVEDP (LVDP = LVSP-LVEDP). Measurements were recorded using PowerLab system and Chart 8 software (ADInstrument, Bella Vista, New South Wales, Australia). The hearts were stable for 30 min, and then subjected to 45 min of global ischemia by halting perfusion, followed by 1 h of reperfusion with Krebs-Henseleit (KH) bicarbonate buffer gassed with 95% O2, 5% CO2 at 37 (pH 7.4). The infarcted myocardium was measured using triphenyltetrazolium chloride(TTC, 25 mg/mL) staining. The KH buffer containing 118 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO 31.3 mM CaCl2, and 11 mM glucose was filtered through a 0.22 uM pore before use.
Click to Show/Hide
|
||||
| Response Description | Baicalein and luteolin protected cardiomyocytes against ferroptosis caused by ferroptosis inducers and I/R. Moreover, both baicalein and luteolin decreased ROS and malondialdehyde (MDA) generation and the protein levels of ferroptosis markers, and restored Glutathione peroxidase 4 (GPX4) protein levels in cardiomyocytes reduced by ferroptosis inducers. Baicalein and luteolin reduced the ischemia/reperfusion-induced myocardium infarction and decreased the levels of Acsl4 and Ptgs2 mRNA. | ||||
Aspirin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [24] | ||||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
These mice were on eight weeks old male DBA/2J background (n = 36, HFK Bioscience, Beijing, China). They were randomized one of the six groups: control normal mice group (NC); diabetic mice group (DM); diabetic mice group (Fer-1), who intraperitoneal injected Fer-1 (Selleck, Houston, TX, USA); diabetic mice group (vehicle-P), who intraperitoneal injected 1% dimethyl sulfoxide (DMSO); diabetic mice group (As), who intragastric administrated Aspirin (Solarbio, Beijing, China); diabetic mice group (vehicle-G), who intragastric administrated 0.5% sodium carboxymethyl cellulose (Na-CMC; Solarbio, Beijing, China). Diabetes models were induced with 5 consecutive days of a single intraperitoneal injection of streptozotocin 40 mg/kg (dissolved in 0.1 M citrate buffer, pH 4.5; SigmaAldrich, St Louis, MO, USA). Control mice only was injected the same volume of citrate buffer. In the Fer-1 or vehicle-P groups, the diabetic mice were treated respectively with Fer-1 (2.5 umol/kg, dissolved in 1% DMSO) or 1% DMSO during the duration of treatment for 12-week every day. And in the AS and vehicle-G groups, the diabetic mice were treated respectively with aspirin (50 mg/kg, dissolved in 0.5% Na-CMC) or 0.5% Na-CMC for 12-week every day.
Click to Show/Hide
|
||||
| Response Description | Aspirin can upregulate SLC7A11 and GPX4 expression by suppressing COX2. Our results demonstrated that ferroptosis in renal tubular cells contributes to Diabetic kidney disease (DKD) development and that diabetes-related ferroptosis was inhibited through the downregulation of COX2 by aspirin, thus retarding the progression of DKD. | ||||
Ruxolitinib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [25] | ||||
| Responsed Disease | Traumatic brain injury [ICD-11: NA07] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Adult male C57BL/6J mice (6-8 weeks, weighting 20-25 g) were used for all experiments. Mice were housed in pairs in a cage with access to food and water ad libitum. Mice were anesthetized with 4% chloral hydrate (0.4 mg/g) and mounted in a stereotaxic system (David Kopf Instruments, Tujunga, California). A midline skin incision was performed on the scalp to expose the skull, and a 5-mm craniotomy was made lateral to the sagittal suture and centered between bregma and lambda. The skull cap was then removed carefully to avoid destroying the dura mater.
Click to Show/Hide
|
||||
| Response Description | Ruxolitinib exerts neuroprotection via repressing ferroptosis in a mouse model of traumatic brain injury. Ruxo significantly inhibited the expressions of COX2 and TfR1. In addition, Ruxo also reversed the lower expression of GPX4 caused by Traumatic brain injury. | ||||
Formaldehyde
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [26] | ||||
| Responsed Disease | Neurotoxicity [ICD-11: NE61] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Sprague-Dawley (SD, weight: 200-220 g) rats were obtained from Hunan SJA laboratory animal company (Changsha, Hunan, China). SD rats were anaesthetized by sodium pentobarbital (45 mg/kg, i.p.) and secured in a stereotaxic frame. The aseptically cannula was implanted into lateral ventricle according to the following coordinates: AP: -1mm; ML: 2 mm; DV: 4 mm. During experiment, the unilateral ventricle of rats was received 2.5 ul FA (0.1, 1, 10 umol) according to the experiment scheme in vivo.
Click to Show/Hide
|
||||
| Response Description | Formaldehyde (FA)-induced neurotoxicity is implicated in neuronal ferroptosis. FA induced cell death in HT22 cells, and upregulated the ferroptosis-associated genes, including Ptgs2 (prostaglandin-endoperoxide synthase 2), GLS2 (glutaminase 2), solute carrier family 1 member 5 (SLC1A5), and solute carrier family 38 member 1 (SLC38A1) in HT22 cells, indicating the inductive role of FA in the ferroptosis of HT22 cells for the formaldehyde-induced neurotoxicity. | ||||
References
