Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0045)
| Name |
Atorvastatin
|
||||
|---|---|---|---|---|---|
| Synonyms |
atorvastatin; 134523-00-5; Cardyl; Tozalip; Xavator; Lipitor; ATORVASTATIN CALCIUM; Torvast; Sotis; 110862-48-1; atorvastatina; atorvastatine; Atofast; Atorcor; Atorlip; Lipilou; Lipinon; Atorin; Ator; atorvastatinum; CCRIS 7159; (3R,5R)-7-(2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoic acid; HSDB 7039; rel-Atorvastatin; UNII-A0JWA85V8F; A0JWA85V8F; Liprimar; Tulip; Atorvastatin (INN); DTXSID8029868; CHEBI:39548; CI-981; Lipitor (TN); (R-(R*,R*))-2-(4-Fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-1H-pyrrole-1-heptanoic acid; CHEMBL1487; DTXCID509868; (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (betaR,deltaR)-2-(p-Fluorophenyl)-beta,delta-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoic acid; Xarator; 1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-, (R-(R*,R*))-; ATORVASTATIN [INN]; 134523-03-8; Atorvastatin [INN:BAN]; 1H-Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-, (betaR,deltaR)-; 7-[2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYRROL-1-YL]- 3,5-DIHYDROXY-HEPTANOIC ACID; Atorvastatin calcium salt; atrovastin; Lipotropic; Atorpic; Faboxim; Torvacard; Vastina; Xanator; Zurinel; Atogal; Lowden; Sincol; Lipovastatinklonal; Lipitor(TM); (3R,5R)-7-[2-(4-fluorophenyl)-5-(1-methylethyl)-3-phenyl-4-(phenylcarbamoyl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (3R,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]-3,5-dihydroxy-heptanoic acid; (3R,5R)-7-[3-(anilinocarbonyl)-5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid; 7-[2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYRROL-1-YL]-3,5-DIHYDROXY-HEPTANOIC ACID; Sortis (TN); C33H35FN2O5; NCGC00159458-03; (3R,5R)-7-(3-(anilinocarbonyl)-5-(4-fluorophenyl)-4-phenyl-2-(propan-2-yl)-1H-pyrrol-1-yl)-3,5-dihydroxyheptanoic acid; (3R,5R)-7-[3-(anilinocarbonyl)-5-(4-fluorophenyl)-4-phenyl-2-(propan-2-yl)-1H-pyrrol-1-yl]-3,5-dihydroxyheptanoic acid; 7-(2-(4-FLUORO-PHENYL)-5-ISOPROPYL-3-PHENYL-4-PHENYLCARBAMOYL-PYRROL-1-YL)-3,5-DIHYDROXY-HEPTANOIC ACID; Atorvastatin & Primycin; ATORVASTATIN [MI]; ATORVASTATIN [HSDB]; SCHEMBL3831; ATORVASTATIN [VANDF]; ATORVASTATIN [WHO-DD]; BIDD:GT0336; Atorvastatin (Relative Stereo); GTPL2949; BDBM22164; C10AA05; DTXSID60274003; XUKUURHRXDUEBC-KAYWLYCHSA-N; HMS3715L05; HMS3886C20; Lipilou; Tozalip; Torvast; Cardyl; (3S,5S)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid; HY-B0589; Tox21_302417; MFCD00899261; s5715; AKOS000281127; AC-9386; CCG-221172; DB01076; MRF-0000761; NCGC00159458-02; NCGC00159458-20; NCGC00255181-01; AS-35260; CAS-134523-00-5; C06834; D07474; A802259; A806791; A806793; EN300-18527331; Q668093; SR-01000872702; SR-01000872702-1; BRD-K69726342-001-02-6; Atorvastatin is known as an HMG-CoA reductase inhibitor.; (.BETA.R,.DELTA.R)-2-(P-FLUOROPHENYL)-.BETA.,.DELTA.-DIHYDROXY-5-ISOPROPYL-3-PHENYL-4-(PHENYLCARBAMOYL)PYRROLE-1-HEPTANOIC ACID; (3R,5R)-7-[2-(4-FLUOROPHENYL)-3-PHENYL-4-(PHENYLCARBAMOYL)-5-PROPAN-2-YL-PYRROL-1-YL]-3,5-DIHYDROXY-HEPTANOIC ACID; (3R,5R)-7-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-phenylcarbamoyl-pyrrol-1-yl]-3,5-dihydroxy-heptanoic acid; (3R,5R)-7-[2-(4-Fluorophenyl)-5-isopropyl-3-phenyl-4-phenylcarbamoylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid; (betaR,deltaR)-2-(4-Fluorophenyl)-beta,delta-dihydroxy-5-(1-methylethyl)-3-phenyl-4-((phenylamino)carbonyl)-1H-pyrrole-1-heptanoic Acid; (betaR,deltaR)-2-(p-Fluorophenyl)-beta,delta-dihydroxy-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)-pyrrole-1-heptanoic Acid; 1H-PYRROLE-1-HEPTANOIC ACID, 2-(4-FLUOROPHENYL)-.BETA.,.DELTA.-DIHYDROXY-5-(1-METHYLETHYL)-3-PHENYL-4-((PHENYLAMINO)CARBONYL)-, (R-(R*,R*))-; sodium 7-[5-(4-fluorophenyl)-2-isopropyl-4-phenyl-3-(phenylcarbamoyl)-2,3-dihydropyrrol-1-yl]-3,5-dihydroxy-heptanoate
Click to Show/Hide
|
||||
| Structure |
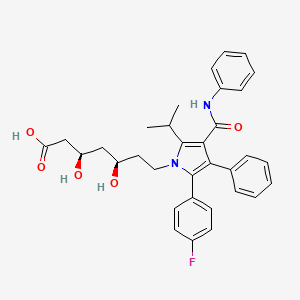 |
||||
| Formula |
C33H35FN2O5
|
||||
| IUPAC Name |
(3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl)-5-propan-2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoic acid
|
||||
| Canonical SMILES |
CC(C)C1=C(C(=C(N1CCC(CC(CC(=O)O)O)O)C2=CC=C(C=C2)F)C3=CC=CC=C3)C(=O)NC4=CC=CC=C4
|
||||
| InChI |
InChI=1S/C33H35FN2O5/c1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40/h3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40)/t26-,27-/m1/s1
|
||||
| InChIKey |
XUKUURHRXDUEBC-KAYWLYCHSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Prostaglandin G/H synthase 2 (PTGS2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker | ||||
| Responsed Disease | Acute myocardial infarction | ICD-11: BA41 | |||
| Responsed Regulator | Hypoxia-inducible factor 1-alpha (HIF1A) | Driver | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mMTs (Mouse myocardial tissues) | ||||
| In Vivo Model |
Rats were anesthetized intraperitoneally with 3% pentobarbital sodium (40 mg/kg). After mechanical ventilation, the rats were subjected to a thoracotomy to expose the hearts and ascending aorta. Finally, the ascending aorta was occluded for 10 s, and 8,000 polyester microspheres (diameter 42 um, Biosphere Medical, Rockland, MA, United States ) were injected into the left ventricle at the same time, while the sham-operated rats received the same dosage of normal saline instead. Forin vivotreatment, rats were treated with recombinant adeno-associated virus 9 (rAAV9)-GFP-shPtsg2 (Hanbio, Shanghai, China) or rAAV9-GFP-shHif1a (Genechem, Shanghai, China) at a dose of 1 x 1012 VG in 200 uL salineviaa single tail vein before CME. Deferoxamine (DFO) was purchased from Selleck (Shanghai, China) and administered intraperitoneally at a dose of 100 mg/kg for 7 days before CME. ATV (Pfizer, New York, United Kingdom) was administered intragastrically at a dose of 10 mg/kg for 7 days before CME.
Click to Show/Hide
|
||||
| Response regulation | Ptgs2 was positively regulated by Hif1a and contributed to ferroptosis-dependent myocardial injury and inflammation. Furthermore, Atorvastatin protects against ferroptosis and inflammation induced by CME via the Hif1a/Ptgs2 pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Marker | ||||
| Responsed Disease | Congestive heart failure | ICD-11: BD10 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
8-week C57BL/6J male mice purchased from Comparative Medicine Center of Yangzhou University were retained with unrestricted access to sterilized diet and water at standard bio-clean laboratory settings (Experimental Animal Center of College of Veterinary Medicine of Yangzhou University). Animals were randomly divided into four groups(n = 6-8 mice per group): control group or ISO group: injected with saline or ISO (5 mg/kg) subcutaneously for 14 days and, meanwhile, received vehicle saline via gavage for 14 days respectively; ATV (Pfizer,USA) group or ISO + ATV group: injected with saline or 5 mg/kg ISO (Sigma, USA) subcutaneously for 14 days and, meanwhile, received 20 mg/kg ATV via gavage for 14 days respectively.
Click to Show/Hide
|
||||
| Response regulation | Atorvastatin showed significantly protective effects through suppressing the activation of ferroptosis related signaling, as evidenced by decreasing the mRNA levels of PTGS2 (a marker of ferroptosis), contents of malonaldehyde and protein levels of NOX4 and increasing the contents of glutathione (GSH), the ratio of GSH/GSSG and protein levels of GPX4 and SLC7A11. ATV reduced cardiac hypertrophy and fibrosis and accumulation of iron in heart failure. | ||||
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Responsed Regulator | Mothers against decapentaplegic homolog 7 (SMAD7) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
A total of 18 Wistar rats (250~300 g) were purchased from Hunan slake Jingda experimental animal Co., Ltd. The rats were randomly divided into the Sham group, I/R group, and I/R + ATV group (n = 6/group).They received standard diet and water before myocardial I/R. Rats in the I/R + ATV group were orally treated with ATV (10 mg/kg/d) for 2 weeks before myocardial I/R (9).The Sham and I/R model rats were constructed as follows: The rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal injection), ligation of the left anterior descending branch with 4-0 silk thread for 30 min, and then reperfusion for 180 min. In the sham control group, the entire procedure was performed with silk thread passing below the coronary artery, but the LAD coronary artery was not ligated. At the end of reperfusion, the rats were given excessive isoflurane for 10 min and sacrificed by bloodletting. Then the rat myocardial tissues were isolated for subsequent detection.
Click to Show/Hide
|
||||
| Response regulation | Atorvastatin intervention blocked erastin or H/R-induced ferroptosis in H9C2 cells by activating SMAD7 expression and thereby down-regulating the hepcidin/FPN1 pathway. The in vivo study also demonstrated that ATV inhibited ferroptosis in ischemia-reperfusion cardiomyopathy rat myocardium through the SMAD7/hepcidin pathway. | ||||
Solute carrier family 40 member 1 (SLC40A1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
A total of 18 Wistar rats (250~300 g) were purchased from Hunan slake Jingda experimental animal Co., Ltd. The rats were randomly divided into the Sham group, I/R group, and I/R + ATV group (n = 6/group).They received standard diet and water before myocardial I/R. Rats in the I/R + ATV group were orally treated with ATV (10 mg/kg/d) for 2 weeks before myocardial I/R (9).The Sham and I/R model rats were constructed as follows: The rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneal injection), ligation of the left anterior descending branch with 4-0 silk thread for 30 min, and then reperfusion for 180 min. In the sham control group, the entire procedure was performed with silk thread passing below the coronary artery, but the LAD coronary artery was not ligated. At the end of reperfusion, the rats were given excessive isoflurane for 10 min and sacrificed by bloodletting. Then the rat myocardial tissues were isolated for subsequent detection.
Click to Show/Hide
|
||||
| Response regulation | Atorvastatin (ATV) intervention blocked erastin or H/R-induced ferroptosis in H9C2 cells by activating SMAD7 expression and thereby down-regulating the hepcidin/FPN1 pathway. The in vivo study also demonstrated that ATV inhibited ferroptosis in ischemia-reperfusion rat myocardium through the SMAD7/hepcidin pathway. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Obesity | ICD-11: 5B81 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell senescence | |||||
| In Vivo Model |
Mice were sacrificed by cervical dislocation. As described previously, epidydimal adipose tissues (EAT) were isolated and minced into ~5-mg pieces in DMEM containing 10% FBS. After 2 h of incubation, 50 mg of small pieces were placed in serum-free DMEM and exposed to 1 umol/L atorvastatin for 18 h, and 0.1% DMSO served as a control. In specific experiments, EAT explants were also treated with GGPP (50 uM; GlpBio), or ferrostatin-1 (Fer-1, 8 uM), and added to the culture medium at the same time as was atorvastatin. Group animal size was n = 6-8 per group. The exact group size is specially described in the Figure legends.
Click to Show/Hide
|
||||
| Response regulation | Atorvastatin decreased the level of GPX4 and depleted GGPP production, but not Fer-1. Atorvastatin was able to induce ferroptosis in adipose tissue, which was due to increased ROS and an increase in cellular senescence. Moreover, this effect could be reversed by the supplement of GGPP. Taken together, our results suggest that the induction of ferroptosis contributed to statin-induced cell senescence in adipose tissue and may contributed to obesity disease. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | |||
| Target for Ferroptosis | Marker/Suppressor | |||
| Responsed Disease | Muscle injury | ICD-11: ND36 | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hCMs (Human cardiomyocytes) | |||
| C2C12 cells | Normal | Mus musculus | CVCL_0188 | |
| HUVECs (Human umbilical vein endothelial cells) | ||||
| Response regulation | Atorvastatin suppressed the Nrf2, which would, in turn, inhibit the expression of System xc-(SLC7A11)and GPX4 (especially the mitochondrial GPX4), leading to a severe damage to the antioxidant system of ferroptosis.The datas point toward ferroptosis as an essential molecular mechanism leading to statin-induced muscle damage. | |||
NADPH oxidase 4 (NOX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Congestive heart failure | ICD-11: BD10 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
8-week C57BL/6J male mice purchased from Comparative Medicine Center of Yangzhou University were retained with unrestricted access to sterilized diet and water at standard bio-clean laboratory settings (Experimental Animal Center of College of Veterinary Medicine of Yangzhou University). Animals were randomly divided into four groups(n = 6-8 mice per group): control group or ISO group: injected with saline or ISO (5 mg/kg) subcutaneously for 14 days and, meanwhile, received vehicle saline via gavage for 14 days respectively; ATV (Pfizer,USA) group or ISO + ATV group: injected with saline or 5 mg/kg ISO (Sigma, USA) subcutaneously for 14 days and, meanwhile, received 20 mg/kg ATV via gavage for 14 days respectively.
Click to Show/Hide
|
||||
| Response regulation | Atorvastatin showed significantly protective effects through suppressing the activation of ferroptosis related signaling, as evidenced by decreasing the mRNA levels of PTGS2 (a marker of ferroptosis), contents of malonaldehyde and protein levels of NOX4 and increasing the contents of glutathione (GSH), the ratio of GSH/GSSG and protein levels of GPX4 and SLC7A11. ATV reduced cardiac hypertrophy and fibrosis and accumulation of iron in heart failure. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Congestive heart failure | ICD-11: BD10 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
8-week C57BL/6J male mice purchased from Comparative Medicine Center of Yangzhou University were retained with unrestricted access to sterilized diet and water at standard bio-clean laboratory settings (Experimental Animal Center of College of Veterinary Medicine of Yangzhou University). Animals were randomly divided into four groups(n = 6-8 mice per group): control group or ISO group: injected with saline or ISO (5 mg/kg) subcutaneously for 14 days and, meanwhile, received vehicle saline via gavage for 14 days respectively; ATV (Pfizer,USA) group or ISO + ATV group: injected with saline or 5 mg/kg ISO (Sigma, USA) subcutaneously for 14 days and, meanwhile, received 20 mg/kg ATV via gavage for 14 days respectively.
Click to Show/Hide
|
||||
| Response regulation | Atorvastatin showed significantly protective effects through suppressing the activation of ferroptosis related signaling, as evidenced by decreasing the mRNA levels of PTGS2 (a marker of ferroptosis), contents of malonaldehyde and protein levels of NOX4 and increasing the contents of glutathione (GSH), the ratio of GSH/GSSG and protein levels of GPX4 and SLC7A11. ATV reduced cardiac hypertrophy and fibrosis and accumulation of iron in heart failure. | ||||
References
