Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10038)
| Target Name | NADPH oxidase 4 (NOX4) | ||||
|---|---|---|---|---|---|
| Synonyms |
Kidney oxidase-1; Kidney superoxide-producing NADPH oxidase; Renal NAD(P)H-oxidase
Click to Show/Hide
|
||||
| Gene Name | NOX4 | ||||
| Sequence |
MAVSWRSWLANEGVKHLCLFIWLSMNVLLFWKTFLLYNQGPEYHYLHQMLGLGLCLSRAS
ASVLNLNCSLILLPMCRTLLAYLRGSQKVPSRRTRRLLDKSRTFHITCGVTICIFSGVHV AAHLVNALNFSVNYSEDFVELNAARYRDEDPRKLLFTTVPGLTGVCMVVVLFLMITASTY AIRVSNYDIFWYTHNLFFVFYMLLTLHVSGGLLKYQTNLDTHPPGCISLNRTSSQNISLP EYFSEHFHEPFPEGFSKPAEFTQHKFVKICMEEPRFQANFPQTWLWISGPLCLYCAERLY RYIRSNKPVTIISVMSHPSDVMEIRMVKENFKARPGQYITLHCPSVSALENHPFTLTMCP TETKATFGVHLKIVGDWTERFRDLLLPPSSQDSEILPFIQSRNYPKLYIDGPFGSPFEES LNYEVSLCVAGGIGVTPFASILNTLLDDWKPYKLRRLYFIWVCRDIQSFRWFADLLCMLH NKFWQENRPDYVNIQLYLSQTDGIQKIIGEKYHALNSRLFIGRPRWKLLFDEIAKYNRGK TVGVFCCGPNSLSKTLHKLSNQNNSYGTRFEYNKESFS Click to Show/Hide
|
||||
| Function |
Constitutive NADPH oxidase which generates superoxide intracellularly upon formation of a complex with CYBA/p22phox. Regulates signaling cascades probably through phosphatases inhibition. May function as an oxygen sensor regulating the KCNK3/TASK-1 potassium channel and HIF1A activity. May regulate insulin signaling cascade. May play a role in apoptosis, bone resorption and lipolysaccharide-mediated activation of NFKB. May produce superoxide in the nucleus and play a role in regulating gene expression upon cell stimulation. Isoform 3 is not functional. Isoform 5 and isoform 6 display reduced activity.; [Isoform 4]: Involved in redox signaling in vascular cells. Constitutively and NADPH-dependently generates reactive oxygen species (ROS). Modulates the nuclear activation of ERK1/2 and the ELK1 transcription factor, and is capable of inducing nuclear DNA damage. Displays an increased activity relative to isoform 1.
Click to Show/Hide
|
||||
| Gene ID | 50507 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
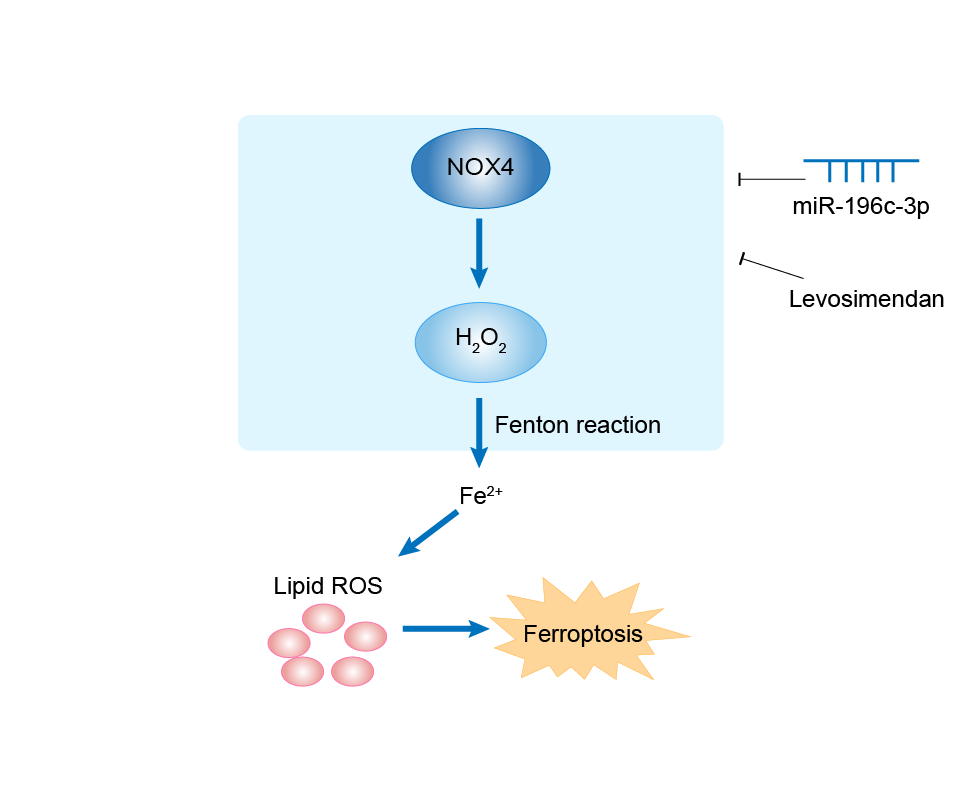
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
NOX4 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Mothers against decapentaplegic homolog 3 (SMAD3)
Unilateral ureteral obstruction [ICD-11: MG30]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Tectorigenin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
mRTECs (Mouse renal tubular epithelial cells) | ||||
| In Vivo Model |
The male C57BL/6 mice (8 weeks old, 22-25 g body weight) used in this study were purchased from Dashuo Bio-Technique Co. Ltd. (Chengdu, China) and were maintained in 12 hr of light/12 hr of darkness. The mice were randomly divided into the following 4 groups (n = 8 per group): Sham, UUO, UUO with tectorigenin (20 mg/kg/day, dissolved in saline with 10% dimethyl sulfoxide [DMSO]) or irbesartan (IRB, a positive control, 20 mg/kg/day) treatment. After anesthesia with 1% pentobarbital, the left ureter was exposed through the lower left incision on the midline of the back and blocked by two-point ligation with 40 silk thread as previously described. The Sham-operated mice underwent the same operation but absence of ligation. The tectorigenin and IRB group were administered intraperitoneally with drug daily for 7 consecutive days since surgery. Sham-operated mice and UUO mice were received the equal volume of solvent.
Click to Show/Hide
|
||||
| Response Description | Tectorigenin exerts as a Smad3 inhibitor to suppress Smad3 activation through an Nox4-dependent mechanism. Tectorigenin protects against unilateral ureteral obstruction by inhibiting Smad3-mediated ferroptosis and fibrosis. | ||||
Cyclic AMP-dependent transcription factor ATF-3 (ATF3)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Brucine | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U118 cells | Astrocytoma | Homo sapiens | CVCL_0633 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| In Vivo Model |
The athymic BALB/c nude mice (4 weeks; 20-22 g; Beijing Vital River Laboratory Animal Technology Company, China) were housed in a specific pathogen-free environment under a 12-h lightdark cycle with free access to food and water. The animals were allowed to acclimatize to their surroundings for 3 days. U87 cells (1 x 106) in the logarithmic growth phase in 100 uL PBS were subcutaneously injected into the right flank. Therapeutic experiments were started when the tumor reached around 150 mm3 after about 10 days. Mice were allocated to receive intraperitoneal injections of vehicle (control group, n = 6) or 40 mg/kg bodyweight (n = 6) in the same volume (50 uL) once a day for 13 times.
Click to Show/Hide
|
||||
| Response Description | Brucine inhibited glioma cell growth in vitro and in vivo, and brucine induced ATF3 upregulation and translocation into nuclei via activation of ER stress. ATF3 promoted brucine-induced H2O2 accumulation via upregulating NOX4 and SOD1 to generate H2O2 on one hand, and downregulating catalase and xCT to prevent H2O2 degradation on the other hand. | ||||
Toll-like receptor 4 (TLR4)
Congestive heart failure [ICD-11: BD10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
Present animal studies used male Sprague Dawley rats (80-100 g) to create a HF model induced by the descending aortic banding (AB) procedure. The sham-operated (SO) group was defined as rats subjected to a similar procedure with the exception of arterial ligation. Echocardiography was applied to confirm the arterial banding immediately after the surgical procedure.
Click to Show/Hide
|
||||
| Response Description | TLR4 or NOX4 knock-down significantly improved left ventricular remodeling and reduced myocytes death. Simultaneously, activated autophagy and ferroptosis in rats with heart failure (HF) were remarkably retarded by either TLR4 and NOX4 knock-down, suggesting TLR4-NOX4 as a potential therapeutic target for HF through inhibiting autophagy- and ferroptosis-mediated cell death. | ||||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Adult male C57BL6 (C57) mice (8-12 weeks, 20-25 g) were purchased from the Animal Experiment Center of Wuhan University. All 64 mice were randomly divided into various groups by different treatments (n = 8). In sham group, after the right kidney excised, the left renal pedicles were without any treatment. In IRI group, the pedicle of the left kidney was clamped for 30 min followed by various reperfusion periods (6, 12, 24 h). To study the effects of LSD1, TCP (MedChemExpress) was injected intraperitoneally at different doses (2.5, 5, 10 mg/kg) before IRI model establishment, once a day for 1 week. TCP powder was dissolved in dimethyl sulfoxide (DMSO). In the vehicle control group, equal amount of DMSO was injected intraperitoneally.
Click to Show/Hide
|
||||
| Response Description | LSD1 (KDM1A) inhibition blocked ferroptosis and oxidative stress caused by renal IRI through the TLR4/NOX4 pathway, indicating that LSD1 could be a potential therapeutic target for renal ischaemia reperfusion injury (IRI). | ||||
Tafazzin (TAFAZZIN)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
RCC4 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0498 | |
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| In Vivo Model |
One million 786O cells with or without shTAZ were implanted subcutaneously into the healthy 8-week-old JAX NOD.CB17-PrkdcSCID-J mice; both male and female mice were used. Once tumor volume reached 120 mm3, mice were randomized into control or erastin treatment group. The vehicle (ORA-plus) or erastin (0.1 ml of 4 mg/ml erastin) was administrated by oral gavage twice daily for 20 days.
Click to Show/Hide
|
||||
| Response Description | Cell density-regulated ferroptosis is mediated by TAZ through the regulation of EMP1-NOX4, suggesting its therapeutic potential for renal cell carcinoma (RCC) and other TAZ-activated tumors. | ||||
rno-miR-196c-3p (miRNA)
Cerebral ischemia [ICD-11: 8B10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Wild-type SD rats were kept in the Animal Experiment Center of Southeast University. Experimental rats were divided into 4 groups (n = 6 per group). The method of establishing the I/R model was provided in supplementary material. Then, we covered the ligation with gel. In order to fully cover the infarcted area of the heart, we chose to inject about 300 uL of mimics + Gel at 23 mm below the left atrial appendage (about the ligation). In order to prevent excessive irradiation of tissue burns, we selected each irradiation for 2 min to control the body surface temperature for a total of 10 min of irradiation.
Click to Show/Hide
|
||||
| Response Description | The mir-196c-3p mimic (mimics) and photothermal nanoparticles (BTN) were co-encapsulated in an injectable Gel (mimics + Gel/BTN) with NIR-II light-triggered release. Consequently, declined ferroptosis in cardiomyocytes and improved cardiac function, survival rate in rats was achieved through the controlled release of Gel/BTN mimics in cerebral ischemia-reperfusion injury model to simultaneously inhibit ferroptosis hub genes NOX4, P53, and ALOX15 expression. | ||||
Lysine-specific histone demethylase 1A (KDM1A)
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Adult male C57BL6 (C57) mice (8-12 weeks, 20-25 g) were purchased from the Animal Experiment Center of Wuhan University. All 64 mice were randomly divided into various groups by different treatments (n = 8). In sham group, after the right kidney excised, the left renal pedicles were without any treatment. In IRI group, the pedicle of the left kidney was clamped for 30 min followed by various reperfusion periods (6, 12, 24 h). To study the effects of LSD1, TCP (MedChemExpress) was injected intraperitoneally at different doses (2.5, 5, 10 mg/kg) before IRI model establishment, once a day for 1 week. TCP powder was dissolved in dimethyl sulfoxide (DMSO). In the vehicle control group, equal amount of DMSO was injected intraperitoneally.
Click to Show/Hide
|
||||
| Response Description | LSD1 (KDM1A) inhibition blocked ferroptosis and oxidative stress caused by renal IRI through the TLR4/NOX4 pathway, indicating that LSD1 could be a potential therapeutic target for renal ischaemia reperfusion injury (IRI). | ||||
hsa-mir-132 (Precursor RNA)
Atherosclerosis [ICD-11: BD40]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HUVECs (Human umbilical vein endothelial cells) | |||
| Response Description | MiR-132 promotes atherosclerosis by inducing mitochondrial oxidative stress-mediated ferroptosis, which may serve as a promising therapeutic target for atherosclerosis. The key iron death protein GPX4 was significantly down-regulated and the oxidized protein NOX4 was significantly increased in miR-132-overexpressing HUVECs (P < 0.001). | |||
Forkhead box protein O4 (FOXO4)
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS |
| Response Description | ENPP2 was transcriptionally regulated by FoxO4 to protect cardiomyocytes from Doxorubicininduced cardiotoxicity by inhibiting ferroptosis. In addition, the inhibitory effects of ENPP2 on Dox-induced ferroptosis were significantly reduced by FoxO4 overexpression, as demonstrated by increased Fe2+ and lipid ROS activity levels, decreased SLC7A11, GPX4 and FPN1 expression, and increased NOX4 expression, which were observed following FoxO4 overexpression. | |||
Epithelial membrane protein 1 (EMP1)
Hereditary Leiomyomatosis [ICD-11: 2C90]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Hippo signaling pathway | hsa04390 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
RCC4 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0498 | |
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| MDA-MB-231 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| In Vivo Model |
One million 786O cells with or without shTAZ were implanted subcutaneously into the healthy 8-week-old JAX NOD.CB17-PrkdcSCID-J mice; both male and female mice were used. Once tumor volume reached 120 mm3, mice were randomized into control or erastin treatment group. The vehicle (ORA-plus) or erastin (0.1 ml of 4 mg/ml erastin) was administrated by oral gavage twice daily for 20 days.
Click to Show/Hide
|
||||
| Response Description | Cell density-regulated ferroptosis is mediated by TAZ through the regulation of EMP1-NOX4, suggesting its therapeutic potential for renal cell carcinoma (RCC) and other TAZ-activated tumors. | ||||
Epidermal growth factor receptor (EGFR)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hTERT-HME1 cells | Normal | Homo sapiens | CVCL_3383 | |
| H1650-ER1 cells | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_4V01 | ||
| In Vivo Model |
2.5 x 105 NCI-H1650 cells were inoculated 1:1 in Matrigel: PBS (100 mL) by subcutaneous injection into eight non-obese diabetic (NOD) severe combined immunodeficiency (SCID) gamma male mice. Tumors were allowed to engraft and grow for 30 days (tumor volume averaged ~200 mm3) and mice treated by intraperitoneal (i.p.) injection with 100 mg/kg cyst(e)inase or 100 mg/kg heat-inactivated cyst(e)inase (n = 4 ea.) on day 30, with a second dose given on day 33. Mice were necropsied 24 hr after the second dose.
Click to Show/Hide
|
||||
| Response Description | In non-small-cell lung cancer (NSCLC) cells, active MAPK signaling downstream of active EGFR can sensitize cells to ferroptosis upon cystine depletion. Sensitization involves both impaired detoxification of lipid peroxides, due to reduced expression of GPX4, and generation of hydrogen peroxide, via NOX4. | ||||
Unspecific Regulator
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Responsed Drug | Pseudolaric acid B | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-87MG cells | Glioblastoma | Homo sapiens | CVCL_0022 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| SHG-44 cells | Astrocytoma | Homo sapiens | CVCL_6728 | ||
| In Vivo Model |
Twenty athymic BALB/c nude mice (aged 4 weeks, weight 20-22 g, from Shanghai laboratory animal Center, Shanghai, China) were housed in a specific pathogen-free environment. A total of 1 x 106 logarithmically growing C6 cells in 100 uL of PBS were subcutaneously injected into the right flank of each mouse. Therapeutic experiments were started when the tumor reached about 150 mm3 after about 7 days. The mice were allocated to receive intraperitoneal injections of vehicle (control group, n = 5/group), PAB at the dosage of 10 mg/kg body weight (n = 10/group) and 20 mg/kg body weight (n = 10/group) in the same volume 50 uL once a days for 8 times.
Click to Show/Hide
|
||||
| Response Description | Pseudolaric acid B (PAB) improved intracellular iron by upregulation of transferrin receptor. The increased iron activated Nox4, which resulted in overproduction of H2O2and lipid peroxides. Moreover, PAB depleted intracellular GSH via p53-mediated xCT pathway, which further exacerbated accumulation of H2O2and lipid peroxides. Thus, PAB triggers ferroptosis in glioma cells and is a potential medicine for glioma treatment. | ||||
Rhabdomyosarcoma [ICD-11: 2B55]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | |||
| Responsed Drug | Diphenyleneiodonium | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response Description | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC- and -selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | |||
| Responsed Drug | GKT137831 | Phase 2 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response Description | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC- and -selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | |||
| Responsed Drug | XN4 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 |
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | |
| Response Description | The pro-ferroptotic role of XN4 in gastric cancer (GC) might enable it to become a promising drug for GC treatment in the future despite the need for extensive research. Moreover, GPX4 levels decreased, but NOX4 and ferroptosis-related protein PTGS2 levels increased in GC cells following XN4 treatment, which was nullified by NOX4 knockdown. | |||
Congestive heart failure [ICD-11: BD10]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Responsed Drug | Atorvastatin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
8-week C57BL/6J male mice purchased from Comparative Medicine Center of Yangzhou University were retained with unrestricted access to sterilized diet and water at standard bio-clean laboratory settings (Experimental Animal Center of College of Veterinary Medicine of Yangzhou University). Animals were randomly divided into four groups(n = 6-8 mice per group): control group or ISO group: injected with saline or ISO (5 mg/kg) subcutaneously for 14 days and, meanwhile, received vehicle saline via gavage for 14 days respectively; ATV (Pfizer,USA) group or ISO + ATV group: injected with saline or 5 mg/kg ISO (Sigma, USA) subcutaneously for 14 days and, meanwhile, received 20 mg/kg ATV via gavage for 14 days respectively.
Click to Show/Hide
|
||||
| Response Description | Atorvastatin showed significantly protective effects through suppressing the activation of ferroptosis related signaling, as evidenced by decreasing the mRNA levels of PTGS2 (a marker of ferroptosis), contents of malonaldehyde and protein levels of NOX4 and increasing the contents of glutathione (GSH), the ratio of GSH/GSSG and protein levels of GPX4 and SLC7A11. ATV reduced cardiac hypertrophy and fibrosis and accumulation of iron in heart failure. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | ||||
| Responsed Drug | Puerarin | Phase 2 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague Dawley ratsweighing 80-100 g were used to make the HF model induced by descending aortic banding (AB) procedure. Rats receiving a similar procedure except for the arterial ligation were defined as the sham-operated (SO) group. After the procedure, echocardiography was immediately applied to confirm the arterial banding. Rats receiving subcutaneous injections of low- or high-dose puerarin (100 mg/kg/day and 200 mg/kg/day, respectively) after the AB procedure were respectively defined as the Pue1 and Pue2 groups. An equal volume of normal saline was injected into the rats of the SO and AB groups.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis is involved in the loss of myocytes during heart failure. Puerarin exerted protective effects against heart failure through inhibition of ferroptosis. And puerarin exerted protective effects against heart failure through inhibition of ferroptosis. Regulation of Nox4 signaling might be involved in puerarin inhibiting ferroptosis. | ||||
Left ventricular failure [ICD-11: BD11]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | ||||
| Responsed Drug | Levosimendan | Approved | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response Description | Levosimendan reversed mitochondrial malfunction in heart failure with preserved ejection fraction (HFpEF) mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan reverses cardiac malfunction and cardiomyocyte ferroptosis during heart failure with preserved ejection fraction via connexin 43 signaling activation. | ||||
Mothers against decapentaplegic homolog 3 (SMAD3)
Tectorigenin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Unilateral ureteral obstruction [ICD-11: MG30] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mRTECs (Mouse renal tubular epithelial cells) | ||||
| In Vivo Model |
The male C57BL/6 mice (8 weeks old, 22-25 g body weight) used in this study were purchased from Dashuo Bio-Technique Co. Ltd. (Chengdu, China) and were maintained in 12 hr of light/12 hr of darkness. The mice were randomly divided into the following 4 groups (n = 8 per group): Sham, UUO, UUO with tectorigenin (20 mg/kg/day, dissolved in saline with 10% dimethyl sulfoxide [DMSO]) or irbesartan (IRB, a positive control, 20 mg/kg/day) treatment. After anesthesia with 1% pentobarbital, the left ureter was exposed through the lower left incision on the midline of the back and blocked by two-point ligation with 40 silk thread as previously described. The Sham-operated mice underwent the same operation but absence of ligation. The tectorigenin and IRB group were administered intraperitoneally with drug daily for 7 consecutive days since surgery. Sham-operated mice and UUO mice were received the equal volume of solvent.
Click to Show/Hide
|
||||
| Response Description | Tectorigenin exerts as a Smad3 inhibitor to suppress Smad3 activation through an Nox4-dependent mechanism. Tectorigenin protects against unilateral ureteral obstruction by inhibiting Smad3-mediated ferroptosis and fibrosis. | ||||
Cyclic AMP-dependent transcription factor ATF-3 (ATF3)
Brucine
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U118 cells | Astrocytoma | Homo sapiens | CVCL_0633 | |
| U87 MG-Red-Fluc cells | Glioblastoma | Homo sapiens | CVCL_5J12 | ||
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| In Vivo Model |
The athymic BALB/c nude mice (4 weeks; 20-22 g; Beijing Vital River Laboratory Animal Technology Company, China) were housed in a specific pathogen-free environment under a 12-h lightdark cycle with free access to food and water. The animals were allowed to acclimatize to their surroundings for 3 days. U87 cells (1 x 106) in the logarithmic growth phase in 100 uL PBS were subcutaneously injected into the right flank. Therapeutic experiments were started when the tumor reached around 150 mm3 after about 10 days. Mice were allocated to receive intraperitoneal injections of vehicle (control group, n = 6) or 40 mg/kg bodyweight (n = 6) in the same volume (50 uL) once a day for 13 times.
Click to Show/Hide
|
||||
| Response Description | Brucine inhibited glioma cell growth in vitro and in vivo, and brucine induced ATF3 upregulation and translocation into nuclei via activation of ER stress. ATF3 promoted brucine-induced H2O2 accumulation via upregulating NOX4 and SOD1 to generate H2O2 on one hand, and downregulating catalase and xCT to prevent H2O2 degradation on the other hand. | ||||
Unspecific Regulator
Pseudolaric acid B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [10] | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-87MG cells | Glioblastoma | Homo sapiens | CVCL_0022 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| SHG-44 cells | Astrocytoma | Homo sapiens | CVCL_6728 | ||
| In Vivo Model |
Twenty athymic BALB/c nude mice (aged 4 weeks, weight 20-22 g, from Shanghai laboratory animal Center, Shanghai, China) were housed in a specific pathogen-free environment. A total of 1 x 106 logarithmically growing C6 cells in 100 uL of PBS were subcutaneously injected into the right flank of each mouse. Therapeutic experiments were started when the tumor reached about 150 mm3 after about 7 days. The mice were allocated to receive intraperitoneal injections of vehicle (control group, n = 5/group), PAB at the dosage of 10 mg/kg body weight (n = 10/group) and 20 mg/kg body weight (n = 10/group) in the same volume 50 uL once a days for 8 times.
Click to Show/Hide
|
||||
| Response Description | Pseudolaric acid B (PAB) improved intracellular iron by upregulation of transferrin receptor. The increased iron activated Nox4, which resulted in overproduction of H2O2and lipid peroxides. Moreover, PAB depleted intracellular GSH via p53-mediated xCT pathway, which further exacerbated accumulation of H2O2and lipid peroxides. Thus, PAB triggers ferroptosis in glioma cells and is a potential medicine for glioma treatment. | ||||
Diphenyleneiodonium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | |||
| Responsed Disease | Rhabdomyosarcoma [ICD-11: 2B55] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response Description | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC- and -selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
GKT137831
[Phase 2]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | |||
| Responsed Disease | Rhabdomyosarcoma [ICD-11: 2B55] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response Description | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC- and -selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
XN4
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | SGC-7901 cells | Gastric carcinoma | Homo sapiens | CVCL_0520 |
| BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | |
| Response Description | The pro-ferroptotic role of XN4 in gastric cancer (GC) might enable it to become a promising drug for GC treatment in the future despite the need for extensive research. Moreover, GPX4 levels decreased, but NOX4 and ferroptosis-related protein PTGS2 levels increased in GC cells following XN4 treatment, which was nullified by NOX4 knockdown. | |||
Atorvastatin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [13] | ||||
| Responsed Disease | Congestive heart failure [ICD-11: BD10] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
8-week C57BL/6J male mice purchased from Comparative Medicine Center of Yangzhou University were retained with unrestricted access to sterilized diet and water at standard bio-clean laboratory settings (Experimental Animal Center of College of Veterinary Medicine of Yangzhou University). Animals were randomly divided into four groups(n = 6-8 mice per group): control group or ISO group: injected with saline or ISO (5 mg/kg) subcutaneously for 14 days and, meanwhile, received vehicle saline via gavage for 14 days respectively; ATV (Pfizer,USA) group or ISO + ATV group: injected with saline or 5 mg/kg ISO (Sigma, USA) subcutaneously for 14 days and, meanwhile, received 20 mg/kg ATV via gavage for 14 days respectively.
Click to Show/Hide
|
||||
| Response Description | Atorvastatin showed significantly protective effects through suppressing the activation of ferroptosis related signaling, as evidenced by decreasing the mRNA levels of PTGS2 (a marker of ferroptosis), contents of malonaldehyde and protein levels of NOX4 and increasing the contents of glutathione (GSH), the ratio of GSH/GSSG and protein levels of GPX4 and SLC7A11. ATV reduced cardiac hypertrophy and fibrosis and accumulation of iron in heart failure. | ||||
Puerarin
[Phase 2]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [14] | ||||
| Responsed Disease | Congestive heart failure [ICD-11: BD10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague Dawley ratsweighing 80-100 g were used to make the HF model induced by descending aortic banding (AB) procedure. Rats receiving a similar procedure except for the arterial ligation were defined as the sham-operated (SO) group. After the procedure, echocardiography was immediately applied to confirm the arterial banding. Rats receiving subcutaneous injections of low- or high-dose puerarin (100 mg/kg/day and 200 mg/kg/day, respectively) after the AB procedure were respectively defined as the Pue1 and Pue2 groups. An equal volume of normal saline was injected into the rats of the SO and AB groups.
Click to Show/Hide
|
||||
| Response Description | Ferroptosis is involved in the loss of myocytes during heart failure. Puerarin exerted protective effects against heart failure through inhibition of ferroptosis. And puerarin exerted protective effects against heart failure through inhibition of ferroptosis. Regulation of Nox4 signaling might be involved in puerarin inhibiting ferroptosis. | ||||
Levosimendan
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [15] | ||||
| Responsed Disease | Left ventricular failure [ICD-11: BD11] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response Description | Levosimendan reversed mitochondrial malfunction in heart failure with preserved ejection fraction (HFpEF) mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan reverses cardiac malfunction and cardiomyocyte ferroptosis during heart failure with preserved ejection fraction via connexin 43 signaling activation. | ||||
References
