Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0178)
| Name |
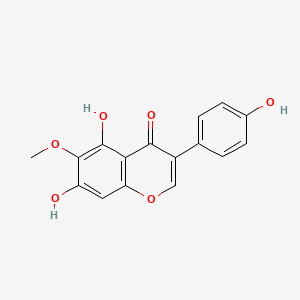
Tectorigenin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Tectorigenin; 548-77-6; Tectorigenine; 4',5,7-Trihydroxy-6-methoxyisoflavone; 5,7-dihydroxy-3-(4-hydroxyphenyl)-6-methoxy-4H-chromen-4-one; K 251T; 5,7-dihydroxy-3-(4-hydroxyphenyl)-6-methoxychromen-4-one; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-hydroxyphenyl)-6-methoxy-; 5,7,4'-Trihydroxy-6-methoxyisoflavone; 5,7-dihydroxy-3-(4-hydroxyphenyl)-6-methoxy-4H-1-benzopyran-4-one; CHEBI:9429; CHEMBL242740; 855130H9CO; 3-(4-hydroxyphenyl)-6-methoxy-5,7-bis(oxidanyl)chromen-4-one; BRN 0305601; 4',5',7-trihydroxy-6-methoxyisoflavone; UNII-855130H9CO; Spectrum_000761; SpecPlus_000145; TECTORIGENIN [MI]; KBioSS_001241; 5-18-05-00311 (Beilstein Handbook Reference); ISOFLAVONE, 4',5,7-TRIHYDROXY-6-METHOXY-; DivK1c_006241; SCHEMBL351641; GTPL9738; KBio1_001185; KBio2_001241; KBio2_003809; KBio2_006377; Tectorigenin, analytical standard; DTXSID50203286; EX-A6676; HY-N0792; BDBM50241222; LMPK12050385; MFCD00597094; s9122; AKOS015897084; CCG-267463; AS-56397; CS-0009804; FT-0688353; T3729; A870334; Q-100619; Q3517006; 5,7-Dihydroxy-3-(4-hydroxy-phenyl)-6-methoxy-chromen-4-one; R0U
Click to Show/Hide
|
||||
| Structure |
 |
||||
| Formula |
C16H12O6
|
||||
| IUPAC Name |
5,7-dihydroxy-3-(4-hydroxyphenyl)-6-methoxychromen-4-one
|
||||
| Canonical SMILES |
COC1=C(C2=C(C=C1O)OC=C(C2=O)C3=CC=C(C=C3)O)O
|
||||
| InChI |
InChI=1S/C16H12O6/c1-21-16-11(18)6-12-13(15(16)20)14(19)10(7-22-12)8-2-4-9(17)5-3-8/h2-7,17-18,20H,1H3
|
||||
| InChIKey |
OBBCRPUNCUPUOS-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
NADPH oxidase 4 (NOX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Unilateral ureteral obstruction | ICD-11: MG30 | |||
| Responsed Regulator | Mothers against decapentaplegic homolog 3 (SMAD3) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | mRTECs (Mouse renal tubular epithelial cells) | ||||
| In Vivo Model |
The male C57BL/6 mice (8 weeks old, 22-25 g body weight) used in this study were purchased from Dashuo Bio-Technique Co. Ltd. (Chengdu, China) and were maintained in 12 hr of light/12 hr of darkness. The mice were randomly divided into the following 4 groups (n = 8 per group): Sham, UUO, UUO with tectorigenin (20 mg/kg/day, dissolved in saline with 10% dimethyl sulfoxide [DMSO]) or irbesartan (IRB, a positive control, 20 mg/kg/day) treatment. After anesthesia with 1% pentobarbital, the left ureter was exposed through the lower left incision on the midline of the back and blocked by two-point ligation with 40 silk thread as previously described. The Sham-operated mice underwent the same operation but absence of ligation. The tectorigenin and IRB group were administered intraperitoneally with drug daily for 7 consecutive days since surgery. Sham-operated mice and UUO mice were received the equal volume of solvent.
Click to Show/Hide
|
||||
| Response regulation | Tectorigenin exerts as a Smad3 inhibitor to suppress Smad3 activation through an Nox4-dependent mechanism. Tectorigenin protects against unilateral ureteral obstruction by inhibiting Smad3-mediated ferroptosis and fibrosis. | ||||
