Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0261)
| Name |
Diphenyleneiodonium
|
||||
|---|---|---|---|---|---|
| Synonyms |
Diphenyleneiodonium; Dibenziodolium; 244-54-2; 2,2'-Biphenylyleneiodonium; dibenzo[b,d]iodolium; (1,1'-Biphenyl)-2,2'-diyliodonium; Dibenzo[b,d]iodol-5-ium; diphenyl-iodonium hydrochloride; Diphenylene iodonium; CHEBI:77986; 6HJ411TU98; 8-iodoniatricyclo[7.4.0.02,7]trideca-1(13),2,4,6,9,11-hexaene; CHEMBL397686; UNII-6HJ411TU98; Tocris-0504; 1010-76-0; Lopac-D-2926; Lopac0_000367; BSPBio_001027; KBioGR_000367; KBioSS_000367; SCHEMBL219548; CHEMBL365739; KBio2_000367; KBio2_002935; KBio2_005503; KBio3_000713; KBio3_000714; QFXKXRXFBRLLPQ-UHFFFAOYSA-; DTXSID00924595; Bio1_000428; Bio1_000917; Bio1_001406; Bio2_000344; Bio2_000824; HMS1362C09; HMS1792C09; HMS1990C09; HMS3403C09; BDBM50206334; CCG-204462; IDI1_002099; QTL1_000031; NCGC00015334-01; NCGC00015334-02; NCGC00015334-03; NCGC00015334-04; NCGC00015334-05; NCGC00015334-06; NCGC00015334-07; NCGC00015334-13; NCGC00024620-01; NCGC00024620-02; NCGC00024620-03; NCGC00024620-04; BRD-K65814004-001-02-3; BRD-K65814004-003-01-1; Q27147567; 5-METHYL-1-(2-METHYLPHENYL)-1H-PYRAZOLE-4-CARBONYLCHLORIDE; InChI=1/C12H8I/c1-3-7-11-9(5-1)10-6-2-4-8-12(10)13-11/h1-8H/q+1
Click to Show/Hide
|
||||
| Structure |
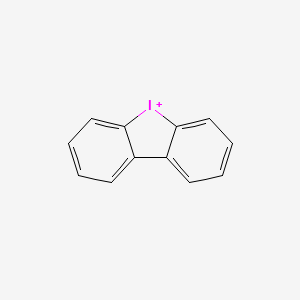 |
||||
|
3D MOL
|
|||||
| Formula |
C12H8I+
|
||||
| IUPAC Name |
8-iodoniatricyclo[7.4.0.02,7]trideca-1(13),2,4,6,9,11-hexaene
|
||||
| Canonical SMILES |
C1=CC=C2C(=C1)C3=CC=CC=C3[I+]2
|
||||
| InChI |
InChI=1S/C12H8I/c1-3-7-11-9(5-1)10-6-2-4-8-12(10)13-11/h1-8H/q+1
|
||||
| InChIKey |
QFXKXRXFBRLLPQ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 4 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Responsed Disease | Rhabdomyosarcoma | ICD-11: 2B55 | ||
| Responsed Regulator | Dual oxidase 2 (DUOX2) | Driver | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response regulation | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC-a and b-selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX, including NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Responsed Disease | Rhabdomyosarcoma | ICD-11: 2B55 | ||
| Responsed Regulator | Dual oxidase 1 (DUOX1) | Driver | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response regulation | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC-a and b-selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX, including NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Responsed Disease | Rhabdomyosarcoma | ICD-11: 2B55 | ||
| Responsed Regulator | NADPH oxidase 3 (NOX3) | Driver | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response regulation | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC-a and b-selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX, including NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
| Experiment 4 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Responsed Disease | Rhabdomyosarcoma | ICD-11: 2B55 | ||
| Responsed Regulator | NADPH oxidase 5 (NOX5) | Driver | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response regulation | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC-a and b-selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX, including NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
NADPH oxidase 4 (NOX4)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Driver | |||
| Responsed Disease | Rhabdomyosarcoma | ICD-11: 2B55 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response regulation | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC- and -selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
NADPH oxidase 1 (NOX1)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Driver | |||
| Responsed Disease | Rhabdomyosarcoma | ICD-11: 2B55 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response regulation | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC- and -selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX) inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
Cytochrome b-245 heavy chain (CYBB)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Driver | |||
| Responsed Disease | Rhabdomyosarcoma | ICD-11: 2B55 | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response regulation | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC-a and b-selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX, including NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2 inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
