Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10067)
| Target Name | Cytochrome b-245 heavy chain (CYBB) | ||||
|---|---|---|---|---|---|
| Synonyms |
NOX2; CGD91-phox; Cytochrome b(558) subunit beta (Cytochrome b558 subunit beta); Heme-binding membrane glycoprotein gp91phox; NADPH oxidase 2; Neutrophil cytochrome b 91 kDa polypeptide; Superoxide-generating NADPH oxidase heavy chain subunit; gp91-1; gp91-phox; p22 phagocyte B-cytochrome
Click to Show/Hide
|
||||
| Gene Name | CYBB | ||||
| Sequence |
MGNWAVNEGLSIFVILVWLGLNVFLFVWYYRVYDIPPKFFYTRKLLGSALALARAPAACL
NFNCMLILLPVCRNLLSFLRGSSACCSTRVRRQLDRNLTFHKMVAWMIALHSAIHTIAHL FNVEWCVNARVNNSDPYSVALSELGDRQNESYLNFARKRIKNPEGGLYLAVTLLAGITGV VITLCLILIITSSTKTIRRSYFEVFWYTHHLFVIFFIGLAIHGAERIVRGQTAESLAVHN ITVCEQKISEWGKIKECPIPQFAGNPPMTWKWIVGPMFLYLCERLVRFWRSQQKVVITKV VTHPFKTIELQMKKKGFKMEVGQYIFVKCPKVSKLEWHPFTLTSAPEEDFFSIHIRIVGD WTEGLFNACGCDKQEFQDAWKLPKIAVDGPFGTASEDVFSYEVVMLVGAGIGVTPFASIL KSVWYKYCNNATNLKLKKIYFYWLCRDTHAFEWFADLLQLLESQMQERNNAGFLSYNIYL TGWDESQANHFAVHHDEEKDVITGLKQKTLYGRPNWDNEFKTIASQHPNTRIGVFLCGPE ALAETLSKQSISNSESGPRGVHFIFNKENF Click to Show/Hide
|
||||
| Function |
Critical component of the membrane-bound oxidase of phagocytes that generates superoxide. It is the terminal component of a respiratory chain that transfers single electrons from cytoplasmic NADPH across the plasma membrane to molecular oxygen on the exterior. Also functions as a voltage-gated proton channel that mediates the H+ currents of resting phagocytes. It participates in the regulation of cellular pH and is blocked by zinc.
Click to Show/Hide
|
||||
| Gene ID | 1536 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
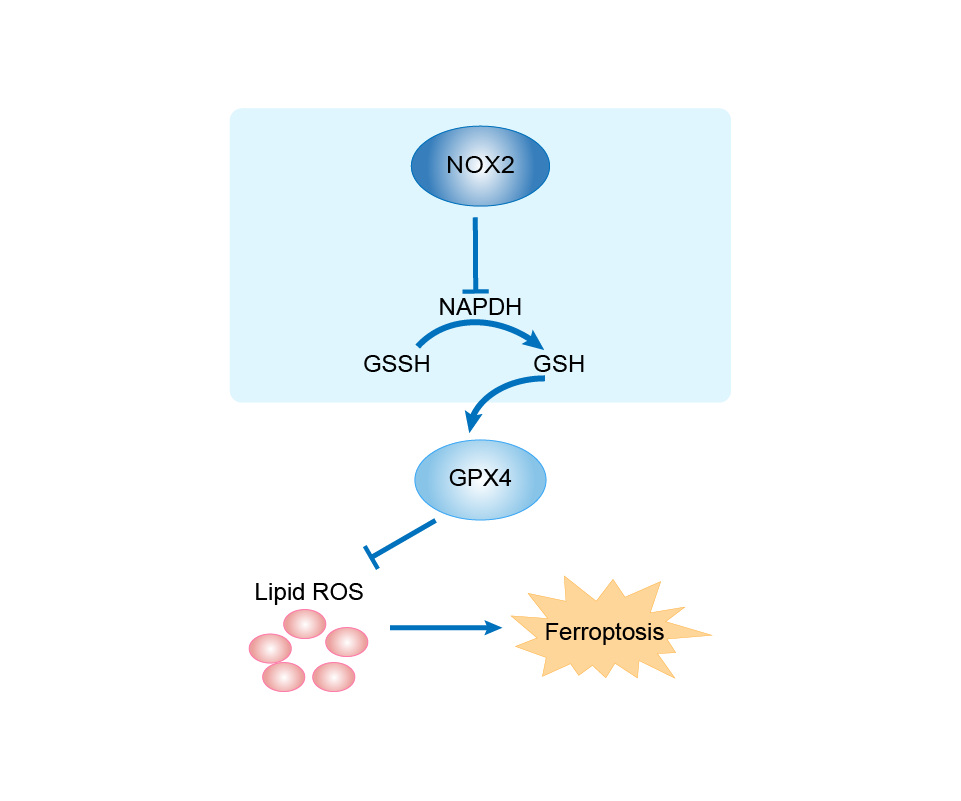
|
|||||
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
CYBB can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
WW domain-containing transcription regulator protein 1 (WWTR1)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Carboplatin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Hippo signaling pathway | hsa04390 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
Caov-2 cells | Ovarian carcinoma | Homo sapiens | CVCL_6861 |
| TOV-21G cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3613 | |
| Response Description | There is a significant correlation between the expression of ANGPTL4 and TAZ (encoded by WWTR1) in the TCGA ovarian tumor dataset. Carboplatin-treated CAOV2R cells are less sensitive to ferroptosis and have a lower level of TAZ (TAFAZZIN). TAZ promotes ferroptosis in ovarian cancers by regulating ANGPTL4 and NOX2, offering a novel therapeutic potential for ovarian tumors with TAZ activation. | |||
Aquaporin-8 (AQP8)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Hydrogen Peroxide | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SAS cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1675 | |
| Response Description | Mitochondrial transfer upregulated the mitochondrial quality control protein prohibitin 2 (PHB2), which contributes to reduced AQPs(AQP3, AQP5, AQP8) expression. H2O2 treatment enhances AQPs expression, Fe2+ level, and lipid peroxidation, and decrease mitochondrial function by downregulating PHB2 in endocervical adenocarcinoma, and thus, is a promising modality for effective cancer treatment. Moreover, NOX2 expression is upregulated in 0 cells, and that NOX2 binds to AQP3, 5, and 8 in both HeLa and SAS cells. | |||
Aquaporin-5 (AQP5)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Hydrogen Peroxide | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SAS cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1675 | |
| Response Description | Mitochondrial transfer upregulated the mitochondrial quality control protein prohibitin 2 (PHB2), which contributes to reduced AQPs(AQP3, AQP5,AQP8) expression. H2O2 treatment enhances AQPs expression, Fe2+ level, and lipid peroxidation, and decrease mitochondrial function by downregulating PHB2 in endocervical adenocarcinoma, and thus, is a promising modality for effective cancer treatment. Moreover, NOX2 expression is upregulated in 0 cells, and that NOX2 binds to AQP3, 5, and 8 in both HeLa and SAS cells. | |||
Aquaporin-3 (AQP3)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Hydrogen Peroxide | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SAS cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1675 | |
| Response Description | Mitochondrial transfer upregulated the mitochondrial quality control protein prohibitin 2 (PHB2), which contributes to reduced AQPs( AQP3, AQP5,AQP8) expression. H2O2 treatment enhances AQPs expression, Fe2+ level, and lipid peroxidation, and decrease mitochondrial function by downregulating PHB2 in endocervical adenocarcinoma, and thus, is a promising modality for effective cancer treatment. Moreover, NOX2 expression is upregulated in 0 cells, and that NOX2 binds to AQP3, 5, and 8 in both HeLa and SAS cells. | |||
Unspecific Regulator
Rhabdomyosarcoma [ICD-11: 2B55]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | |||
| Responsed Drug | Diphenyleneiodonium | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response Description | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC-a and b-selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX, including NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2 inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell migration | |||||
| Cell invasion | |||||
In Vitro Model |
U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 | |
| In Vivo Model |
A total of four groups were formed by randomly dividing the mice: control group consisting of shScramble xenografts treated with vehicle (n = 5), shScramble xenografts treated with IKE (n = 5), shSOD2 xenografts treated with vehicle (n = 5), and shSOD2 xenografts treated with IKE (n = 5). Drug treatment was started after the tumor volume reached approximately 100 mm3 or 10 days after the injection of the tumor xenograft. Depending on the treatment group, vehicles (5 mg/kg/day) or IKE (25 mg/kg/day) were administered intraperitoneally. Treatment was administered intraperitoneally for 3 weeks, and tumor growth was observed for 6 weeks after treatment began.
Click to Show/Hide
|
||||
| Response Description | CYBB captured ferroptosis resilience in mesenchymal glioblastoma multiforme (GBM). The downstream compensatory activity of CYBB via the Nrf2/SOD2 axis is exploitable through erastin-induced ferroptosis to overcome TMZ resistance. | ||||
WW domain-containing transcription regulator protein 1 (WWTR1)
Carboplatin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Hippo signaling pathway | hsa04390 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | Caov-2 cells | Ovarian carcinoma | Homo sapiens | CVCL_6861 |
| TOV-21G cells | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3613 | |
| Response Description | There is a significant correlation between the expression of ANGPTL4 and TAZ (encoded by WWTR1) in the TCGA ovarian tumor dataset. Carboplatin-treated CAOV2R cells are less sensitive to ferroptosis and have a lower level of TAZ (TAFAZZIN). TAZ promotes ferroptosis in ovarian cancers by regulating ANGPTL4 and NOX2, offering a novel therapeutic potential for ovarian tumors with TAZ activation. | |||
Aquaporin-8 (AQP8)
Hydrogen Peroxide
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Cervical cancer [ICD-11: 2C77] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SAS cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1675 | |
| Response Description | Mitochondrial transfer upregulated the mitochondrial quality control protein prohibitin 2 (PHB2), which contributes to reduced AQPs(AQP3, AQP5, AQP8) expression. H2O2 treatment enhances AQPs expression, Fe2+ level, and lipid peroxidation, and decrease mitochondrial function by downregulating PHB2 in endocervical adenocarcinoma, and thus, is a promising modality for effective cancer treatment. Moreover, NOX2 expression is upregulated in 0 cells, and that NOX2 binds to AQP3, 5, and 8 in both HeLa and SAS cells. | |||
Aquaporin-5 (AQP5)
Hydrogen Peroxide
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Cervical cancer [ICD-11: 2C77] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SAS cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1675 | |
| Response Description | Mitochondrial transfer upregulated the mitochondrial quality control protein prohibitin 2 (PHB2), which contributes to reduced AQPs(AQP3, AQP5,AQP8) expression. H2O2 treatment enhances AQPs expression, Fe2+ level, and lipid peroxidation, and decrease mitochondrial function by downregulating PHB2 in endocervical adenocarcinoma, and thus, is a promising modality for effective cancer treatment. Moreover, NOX2 expression is upregulated in 0 cells, and that NOX2 binds to AQP3, 5, and 8 in both HeLa and SAS cells. | |||
Aquaporin-3 (AQP3)
Hydrogen Peroxide
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Cervical cancer [ICD-11: 2C77] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SAS cells | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1675 | |
| Response Description | Mitochondrial transfer upregulated the mitochondrial quality control protein prohibitin 2 (PHB2), which contributes to reduced AQPs( AQP3, AQP5,AQP8) expression. H2O2 treatment enhances AQPs expression, Fe2+ level, and lipid peroxidation, and decrease mitochondrial function by downregulating PHB2 in endocervical adenocarcinoma, and thus, is a promising modality for effective cancer treatment. Moreover, NOX2 expression is upregulated in 0 cells, and that NOX2 binds to AQP3, 5, and 8 in both HeLa and SAS cells. | |||
Unspecific Regulator
Diphenyleneiodonium
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | |||
| Responsed Disease | Rhabdomyosarcoma [ICD-11: 2B55] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | RD cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1649 |
| Rh18 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_1659 | |
| Rh30 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_0041 | |
| Rh36 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_M599 | |
| Rh41 cells | Alveolar rhabdomyosarcoma | Homo sapiens | CVCL_2176 | |
| T 174 cells | Rhabdomyosarcoma | Homo sapiens | CVCL_U955 | |
| TE 381.T cells | Rhabdomyosarcoma | Homo sapiens | CVCL_1751 | |
| KYM-1 cells | Embryonal rhabdomyosarcoma | Homo sapiens | CVCL_3007 | |
| Response Description | Rhabdomyosarcoma (RMS) cells might be vulnerable to oxidative stress-induced cell death. The broad-spectrum protein kinase C (PKC) inhibitor Bisindolylmaleimide I as well as the PKC-a and b-selective inhibitor G6976 significantly reduced Erastin-induced cell death. Furthermore, the broad-spectrum nicotinamide adenine dinucleotide phosphate-oxidase (NOX, including NOX1, NOX2, NOX3, NOX4, NOX5, DUOX1 and DUOX2 inhibitor Diphenyleneiodonium and the selective NOX1/4 isoform inhibitor GKT137831 significantly decreased Erastin-stimulated ROS, lipid ROS and cell death. | |||
References
