Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0177)
| Name |
Levosimendan
|
||||
|---|---|---|---|---|---|
| Synonyms |
LEVOSIMENDAN; 141505-33-1; Simdax; (R)-Simendan; Levosimedan; (-)-OR-1259; OR-1259; Simendan, (r)-; CHEBI:50567; NSC-759644; 2-[[4-[(4R)-4-methyl-6-oxo-4,5-dihydro-1H-pyridazin-3-yl]phenyl]hydrazinylidene]propanedinitrile; DTXSID9046445; C6T4514L4E; OR1259; (R)-((4-(1,4,5,6-Tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono) propanedintrile; (R)-N-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)carbonohydrazonoyl dicyanide; Levosimendan [INN]; Mesoxalonitrile (p-((R)-1,4,5,6-tetrahydro-4-methyl-6-oxo-pyridazinyl)phenyl)hydrazone; DTXCID7026445; levosimendanum; ((4-(1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazono)propanedinitrile; Simsndan;OR-1259; 1-cyano-N-{4-[(4R)-4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl]phenyl}methanecarbohydrazonoyl cyanide; SMR002529692; Simdax (TN); CAS-141505-33-1; Levosimendan (USAN/INN); Levosimendan [USAN:INN]; OR 1259; UNII-C6T4514L4E; Levosimendan- Bio-X; LEVOSIMENDAN [MI]; LEVOSIMENDAN [USAN]; Mesoxalonitrile (-)-(p((R)-1,4,5,6-tetrahydro-4-methyl-6-oxo-3-pyridazinyl)phenyl)hydrazone; SCHEMBL83243; LEVOSIMENDAN [MART.]; MLS003899227; MLS006010741; LEVOSIMENDAN [WHO-DD]; CHEMBL2051955; Levosimendan, >=98% (HPLC); WHXMKTBCFHIYNQ-SECBINFHSA-N; HMS3264G03; HMS3884N17; KUC109648N; Pharmakon1600-01502356; BCP07048; Tox21_112191; Tox21_113768; BDBM50469700; MFCD00867135; NSC759644; s2446; AKOS015895214; Tox21_112191_1; AC-1752; AM84381; CCG-213048; DB00922; DS-8918; NSC 759644; ({4-[(4R)-4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl]phenyl}hydrazono)propanedintrile; 2-[[4-[(4R)-4-methyl-6-oxo-4,5-dihydro-1H-pyridazin-3-yl]phenyl]hydrazono]propanedinitrile; KSC-210-010; NCGC00253641-01; NCGC00263564-01; NCGC00263564-02; BM164625; HY-14286; L0320; SW219172-1; A11874; D04720; N12889; AB01562970_01; AB01562970_02; A807767; EN300-18567987; Q162541; SR-01000931342; SR-01000931342-2; 1-beta-D-Ribofuranose-1H-1,2,4-triazole-3-methylcarbonate; (R)-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)carbonohydrazonoyl dicyanide; (R)-N-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)carbonohydrazonoyldicyanide; 2-(2-(4-((4R)-1,4,5,6-TETRAHYDRO-4-METHYL-6-OXO-3-PYRIDAZINYL)PHENYL)HYDRAZINYLIDENE)PROPANEDINITRILE; Mesoxalonitrile (-)-(p((R)-1,4,5,6-tetrahydro-4-methyl-6- oxo-3-pyridazinyl)phenyl)hydrazone; PROPANEDINITRILE, ((4-(1,4,5,6-TETRAHYDRO-4-METHYL-6-OXO-3-PYRIDAZINYL)PHENYL)HYDRAZONO)-, (R)-; PROPANEDINITRILE, 2-(2-(4-((4R)-1,4,5,6-TETRAHYDRO-4-METHYL-6-OXO-3-PYRIDAZINYL)PHENYL)HYDRAZINYLIDENE)-
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
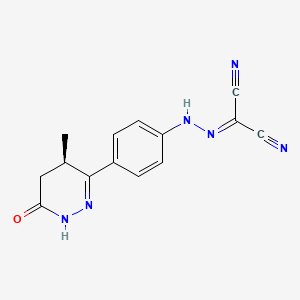 |
||||
| Formula |
C14H12N6O
|
||||
| IUPAC Name |
2-[[4-[(4R)-4-methyl-6-oxo-4,5-dihydro-1H-pyridazin-3-yl]phenyl]hydrazinylidene]propanedinitrile
|
||||
| Canonical SMILES |
CC1CC(=O)NN=C1C2=CC=C(C=C2)NN=C(C#N)C#N
|
||||
| InChI |
InChI=1S/C14H12N6O/c1-9-6-13(21)19-20-14(9)10-2-4-11(5-3-10)17-18-12(7-15)8-16/h2-5,9,17H,6H2,1H3,(H,19,21)/t9-/m1/s1
|
||||
| InChIKey |
WHXMKTBCFHIYNQ-SECBINFHSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Left ventricular failure | ICD-11: BD11 | |||
| Responsed Regulator | Gap junction alpha-1 protein (GJA1) | Suppressor | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response regulation | Levosimendan reversed mitochondrial malfunction in HFpEF mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan Reverses Cardiac Malfunction and Cardiomyocyte Ferroptosis During Heart Failure with Preserved Ejection Fraction via Connexin 43 Signaling Activation. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Left ventricular failure | ICD-11: BD11 | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response regulation | Levosimendan reversed mitochondrial malfunction in heart failure with preserved ejection fraction (HFpEF) mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan reverses cardiac malfunction and cardiomyocyte ferroptosis during heart failure with preserved ejection fraction via connexin 43 signaling activation. | ||||
NADPH oxidase 4 (NOX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Left ventricular failure | ICD-11: BD11 | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response regulation | Levosimendan reversed mitochondrial malfunction in heart failure with preserved ejection fraction (HFpEF) mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan reverses cardiac malfunction and cardiomyocyte ferroptosis during heart failure with preserved ejection fraction via connexin 43 signaling activation. | ||||
Ferroptosis suppressor protein 1 (AIFM2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Left ventricular failure | ICD-11: BD11 | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response regulation | Levosimendan reversed mitochondrial malfunction in heart failure with preserved ejection fraction (HFpEF) mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan reverses cardiac malfunction and cardiomyocyte ferroptosis during heart failure with preserved ejection fraction via connexin 43 signaling activation. | ||||
