Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10066)
| Target Name | Ferroptosis suppressor protein 1 (AIFM2) | ||||
|---|---|---|---|---|---|
| Synonyms |
FSP1; AMID; PRG3; Apoptosis-inducing factor homologous mitochondrion-associated inducer of death; p53-responsive gene 3 protein
Click to Show/Hide
|
||||
| Gene Name | AIFM2 | ||||
| Sequence |
MGSQVSVESGALHVVIVGGGFGGIAAASQLQALNVPFMLVDMKDSFHHNVAALRASVETG
FAKKTFISYSVTFKDNFRQGLVVGIDLKNQMVLLQGGEALPFSHLILATGSTGPFPGKFN EVSSQQAAIQAYEDMVRQVQRSRFIVVVGGGSAGVEMAAEIKTEYPEKEVTLIHSQVALA DKELLPSVRQEVKEILLRKGVQLLLSERVSNLEELPLNEYREYIKVQTDKGTEVATNLVI LCTGIKINSSAYRKAFESRLASSGALRVNEHLQVEGHSNVYAIGDCADVRTPKMAYLAGL HANIAVANIVNSVKQRPLQAYKPGALTFLLSMGRNDGVGQISGFYVGRLMVRLTKSRDLF VSTSWKTMRQSPP Click to Show/Hide
|
||||
| Family | FAD-dependent oxidoreductase family | ||||
| Function |
A NAD(P)H-dependent oxidoreductase that acts as a key inhibitor of ferroptosis. At the plasma membrane, catalyzes reduction of coenzyme Q/ubiquinone-10 to ubiquinol-10, a lipophilic radical-trapping antioxidant that prevents lipid oxidative damage and consequently ferroptosis. Acts in parallel to GPX4 to suppress phospholipid peroxidation and ferroptosis. This anti-ferroptotic function is independent of cellular glutathione levels. Also acts as a potent radical-trapping antioxidant by mediating warfarin-resistant vitamin K reduction in the canonical vitamin K cycle: catalyzes NAD(P)H-dependent reduction of vitamin K (phylloquinone, menaquinone-4 and menadione) to hydroquinone forms. Hydroquinones act as potent radical-trapping antioxidants inhibitor of phospholipid peroxidation and ferroptosis.
Click to Show/Hide
|
||||
| Gene ID | 84883 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
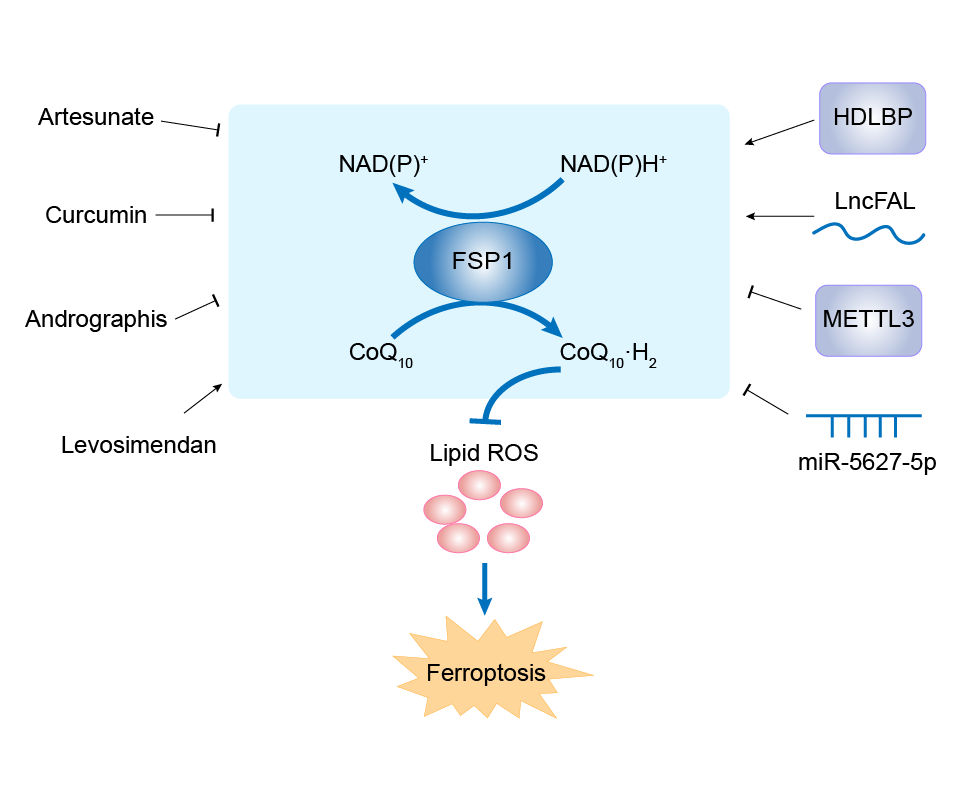
|
|||||
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
AIFM2 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Protein Mdm4 (MDM4)
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | NCS207895 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| Response Description | Inhibition of MDM2 (Nutlin-3) or MDMX (NCS207895) leads to increased levels of FSP1 protein and a consequent increase in the levels of coenzyme Q10, an endogenous lipophilic antioxidant. This suggests that MDM2 and MDMX normally prevent cells from mounting an adequate defense against lipid peroxidation and thereby promote ferroptosis in Colon carcinoma. | |||
E3 ubiquitin-protein ligase Mdm2 (MDM2)
Colon cancer [ICD-11: 2B90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Drug | Nutlin-3 | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| Response Description | Inhibition of MDM2 (Nutlin-3) or MDMX (NCS207895) leads to increased levels of FSP1 protein and a consequent increase in the levels of coenzyme Q10, an endogenous lipophilic antioxidant. This suggests that MDM2 and MDMX normally prevent cells from mounting an adequate defense against lipid peroxidation and thereby promote ferroptosis in Colon carcinoma. | |||
Vigilin (HDLBP)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| SNU-387 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0250 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| In Vivo Model |
Six-week-old female BALB/c nude mice were purchased from Byrness Weil Biotechnology Ltd. (Chengdu, China) and housed in a specific pathogen-free environment with a 12-h light/dark cycle and controlled temperature and humidity, and food and water were provided ad libitum. Three million designated treated PLC5 cells were collected and injected subcutaneously into mice. At least 4 mice were used in each group in each experiment. Once the tumours reached a mean volume of 200 mm3, the mice were treated intraperitoneally with sorafenib every 3 days. The mice were then euthanized at the indicated time after injection. Each tumour was dissected, fixed with 4% formaldehyde, and embedded in paraffin. The tumour growth was monitored weekly by calliper measurements.
Click to Show/Hide
|
||||
| Response Description | LncFAL reduced ferroptosis vulnerability by directly binding to ferroptosis suppressor protein 1 (FSP1) and competitively abolishing Trim69-dependent FSP1 polyubiquitination degradation. Collectively, our results provide a clinically promising demonstration that HDLBP stabilizes lncFAL, which mediates a FSP1-dependent anti-ferroptosis mechanism in Hepatocellular carcinoma. | ||||
Transcriptional coactivator YAP1 (YAP1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hMPs (Human macrophages) | ||||
| In Vivo Model |
C57BL/6 mice (female, 6-8 weeks old, 20-30 g weight) and SPF-grade SD rats (female, 180-230 g weight) were used to detect the toxicity of nanoparticles. Different cells (5 x 106) cells were grafted in the left flank; 5 days after engraftation, the stimulated TAMs (1 x 106) were injected into NSG mice through the tail vein. Different treatments were given and recorded as day 0.
Click to Show/Hide
|
||||
| Response Description | The NF2- YAP signaling axis modulated the expression of ferroptosis suppressor protein 1 (FSP1) and CD24 in CD24 high cells. This system achieved dual antitumor effects, ultimately promoting cell death and thus inhibiting triple-negative breast cancer (TNBC) tumor growth, with some tumors even disappearing. | ||||
rno-miR-672-3p (miRNA)
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
AGE1.HN cells | Normal | Homo sapiens | CVCL_DF60 | |
| PC12 cells | Adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | ||
| In Vivo Model |
Adult male Sprague-Dawley rats weighing 200-220 g were purchased from the Animal Center of the Medical Department of Xi'an Jiaotong University. The rats were anesthetized by intraperitoneal injection of 1% pentobarbital sodium (50 mg/kg), and the spines of the rats were fixed. The skin of T9-12 level was incised, and laminectomy was performed at the T10 level to expose the spinal cord. The rats were then placed in the appropriate position of the impactor so that 10 g of rod fell freely at a height of 3 cm and hit the center of the T10 level of the spinal cord. The signs of successful establishment of the model were the appearance of hind limb extension and tail-flick reflex in rats. Laminectomy was performed only in the sham-operated group. The rats in the SCI group received an artificial bladder massage twice a day to assist in urination until they were able to urinate.
Click to Show/Hide
|
||||
| Response Description | miR-672-3p exerts a neural restoration effectin vivo and in vitro by inhibiting ferroptosis via the FSP1 pathway. In addition, miR-672-3p improved locomotor function in spinal cord injury rats, suggesting its potential as a target for the development of therapeutics for SCI. | ||||
N6-adenosine-methyltransferase catalytic subunit (METTL3)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| 16HBE14o- cells | Normal | Homo sapiens | CVCL_0112 | ||
| PG-CL3 cells | Lung giant cell carcinoma | Homo sapiens | CVCL_4391 | ||
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
BALB/c nude mice (male, 4 weeks old) were purchased from the Animal Center of Nanjing University with free access to water and food. A549 cells (106 cells per mouse) transfected with miR-4443 mimic or mimic-NC were injected subcutaneously to generate subcutaneous tumors. Tumor volume was recorded.
Click to Show/Hide
|
||||
| Response Description | METTL3 was confirmed as a direct target gene of miR-4443. Further mechanistic analysis showed that miR-4443 regulated the expression of FSP1 in an m6A manner via METLL3. A high level of exosomal miR-4443 conferred cisplatin resistance in non-small cell lung carcinoma (NSCLC) via METTL3/FSP1-mediated ferroptosis. | ||||
mmu-miR-5627-5p (miRNA)
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Male C57BL/6 (7-8 weeks) were purchased from the Animal Center of Nanjing University (Nanjing, China) and housed in the condition with controlled temperature and humidity under a 12-h light/dark circadian rhythm. For determining the effects of MSCs-exo, the animals were derived into three groups: sham (n = 10), ASCI (n = 10), ASCI + MSCs (n = 10); for determining the effects of exosomal lncGm36569, the animals were divided into five groups: sham (n = 10), ASCI (n = 10), ASCI + MSC-Exo (ctrl) (n = 10), ASCI + MSCs-Exo (lnc-OE) (n =10), ASCI + MSCs-Exo (si-lnc) (n = 10).
Click to Show/Hide
|
||||
| Response Description | Exosomes derived from mesenchymal stem cells (MSCs) have been considered as an alternative for cell therapy of acute spinal cord injury (ASCI). MSCs-exosomes lncGm36569 inhibited neuronal cell ferroptosis through miR-5627-5p/FSP1 axis, thereby attenuating neuronal dysfunction. | ||||
Merlin (NF2)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
hMPs (Human macrophages) | ||||
| In Vivo Model |
C57BL/6 mice (female, 6-8 weeks old, 20-30 g weight) and SPF-grade SD rats (female, 180-230 g weight) were used to detect the toxicity of nanoparticles. Different cells (5 x 106) cells were grafted in the left flank; 5 days after engraftation, the stimulated TAMs (1 x 106) were injected into NSG mice through the tail vein. Different treatments were given and recorded as day 0.
Click to Show/Hide
|
||||
| Response Description | The NF2-YAP signaling axis modulated the expression of ferroptosis suppressor protein 1 (FSP1) and CD24 in CD24 high cells. This system achieved dual antitumor effects, ultimately promoting cell death and thus inhibiting triple-negative breast cancer (TNBC) tumor growth, with some tumors even disappearing. | ||||
lncGm36569 (IncRNA)
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Male C57BL/6 (7-8 weeks) were purchased from the Animal Center of Nanjing University (Nanjing, China) and housed in the condition with controlled temperature and humidity under a 12-h light/dark circadian rhythm. For determining the effects of MSCs-exo, the animals were derived into three groups: sham (n = 10), ASCI (n = 10), ASCI + MSCs (n = 10); for determining the effects of exosomal lncGm36569, the animals were divided into five groups: sham (n = 10), ASCI (n = 10), ASCI + MSC-Exo (ctrl) (n = 10), ASCI + MSCs-Exo (lnc-OE) (n =10), ASCI + MSCs-Exo (si-lnc) (n = 10).
Click to Show/Hide
|
||||
| Response Description | Exosomes derived from mesenchymal stem cells (MSCs) have been considered as an alternative for cell therapy of acute spinal cord injury (ASCI). MSCs-exosomes lncGm36569 inhibited neuronal cell ferroptosis through miR-5627-5p/FSP1 axis, thereby attenuating neuronal dysfunction. | ||||
lncFAL (IncRNA)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| SNU-387 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0250 | ||
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| In Vivo Model |
Six-week-old female BALB/c nude mice were purchased from Byrness Weil Biotechnology Ltd. (Chengdu, China) and housed in a specific pathogen-free environment with a 12-h light/dark cycle and controlled temperature and humidity, and food and water were provided ad libitum. Three million designated treated PLC5 cells were collected and injected subcutaneously into mice. At least 4 mice were used in each group in each experiment. Once the tumours reached a mean volume of 200 mm3, the mice were treated intraperitoneally with sorafenib every 3 days. The mice were then euthanized at the indicated time after injection. Each tumour was dissected, fixed with 4% formaldehyde, and embedded in paraffin. The tumour growth was monitored weekly by calliper measurements.
Click to Show/Hide
|
||||
| Response Description | LncFAL reduced ferroptosis vulnerability by directly binding to ferroptosis suppressor protein 1 (FSP1) and competitively abolishing Trim69-dependent FSP1 polyubiquitination degradation. Collectively, our results provide a clinically promising demonstration that HDLBP stabilizes lncFAL, which mediates a FSP1-dependent anti-ferroptosis mechanism in Hepatocellular carcinoma. | ||||
hsa-miR-4443 (miRNA)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | |
| 16HBE14o- cells | Normal | Homo sapiens | CVCL_0112 | ||
| PG-CL3 cells | Lung giant cell carcinoma | Homo sapiens | CVCL_4391 | ||
| NCI-H460 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
BALB/c nude mice (male, 4 weeks old) were purchased from the Animal Center of Nanjing University with free access to water and food. A549 cells (106 cells per mouse) transfected with miR-4443 mimic or mimic-NC were injected subcutaneously to generate subcutaneous tumors. Tumor volume was recorded.
Click to Show/Hide
|
||||
| Response Description | METTL3 was confirmed as a direct target gene of miR-4443. Further mechanistic analysis showed that miR-4443 regulated the expression of FSP1 in an m6A manner via METLL3. A high level of exosomal miR-4443 conferred cisplatin resistance in non-small cell lung carcinoma (NSCLC) via METTL3/FSP1-mediated ferroptosis. | ||||
hsa-miR-1228-3p (miRNA)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell infiltration | |||||
| Cell migration | |||||
In Vitro Model |
SK-BR-3 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | |
| BT-474 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0179 | ||
| In Vivo Model |
Female BALB/c nude mice aged 4 weeks were administered subcutaneously with 2 x 106 cells (five mice/group). After that, the mice were given intratumoural inoculation of 40 uL si-circGFRA1 or si-NC every 4 days. To detect lung metastases, we intravenously inoculated 1 x 105 cells into the tail veins of mice (six mice/group). After the elapse of 8 weeks, we anaesthetized the mice, harvested their lungs and visually counted the metastatic nodules in the lungs, followed by validation via H&E staining and counting under a microscope.
Click to Show/Hide
|
||||
| Response Description | Knockdown of circGFRA1 could attenuate HER-2-positive HER-2-positive breast cancer progression by inhibiting the proliferation, infiltration and migratory ability of HER-2-positive BC cells. Through ceRNA mechanism, circGFRA1 could bind to miR-1228 and alleviate inhibitory activity of miR-1228 on targeted gene AIFM2. | ||||
CircGFRA1 (circRNA)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell infiltration | |||||
| Cell migration | |||||
In Vitro Model |
SK-BR-3 cells | Breast adenocarcinoma | Homo sapiens | CVCL_0033 | |
| BT-474 cells | Invasive breast carcinoma | Homo sapiens | CVCL_0179 | ||
| In Vivo Model |
Female BALB/c nude mice aged 4 weeks were administered subcutaneously with 2 x 106 cells (five mice/group). After that, the mice were given intratumoural inoculation of 40 uL si-circGFRA1 or si-NC every 4 days. To detect lung metastases, we intravenously inoculated 1 x 105 cells into the tail veins of mice (six mice/group). After the elapse of 8 weeks, we anaesthetized the mice, harvested their lungs and visually counted the metastatic nodules in the lungs, followed by validation via H&E staining and counting under a microscope.
Click to Show/Hide
|
||||
| Response Description | Knockdown of circGFRA1 could attenuate HER-2-positive HER-2-positive breast cancer progression by inhibiting the proliferation, infiltration and migratory ability of HER-2-positive BC cells. Through ceRNA mechanism, circGFRA1 could bind to miR-1228 and alleviate inhibitory activity of miR-1228 on targeted gene AIFM2. | ||||
Unspecific Regulator
Colorectal cancer [ICD-11: 2B91]
| In total 2 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Responsed Drug | Curcumin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response Description | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Responsed Drug | Andrographis | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response Description | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
Ovarian dysfunction [ICD-11: 5A80]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | ||||
| Responsed Drug | N-acetylcysteine | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell differentiation | |||||
In Vitro Model |
hPTs (Human placental tissues) | ||||
| hPTs (Human placental tissues) | |||||
| In Vivo Model |
Adult Sprague-Dawley rats (70 days old) of both sexes were purchased from the Laboratory Animal Centre of Harbin Medical University, Harbin, China. Before the experiment, female rats were allowed to acclimatize for a minimum of 1 week and then were monitored daily by vaginal lavage to determine the stage of the estrous cycle as previously described (Zhanget al., 2016). Pregnancy was achieved by housing female rats on the night of proestrus with fertile males of the same strain at a 2:1 ratio. Confirmation of mating was performed the morning after by the presence of a vaginal plug, and this was considered as GD 0.5. The rats were sacrificed between 8:00 a.m. and 9:00 a.m. hours on GD 14.5.
Click to Show/Hide
|
||||
| Response Description | Previous studies demonstrate that increased uterine and placental ferroptosis is associated with oxidative stress-induced fetal loss in a pre-clinical polycystic ovary syndrome (PCOS)-like rat model. N-acetylcysteine treatment results in increased mRNA expression of Aifm2, a negative regulator of GPX4-independent ferroptosis in the placenta. Moreover, NAC reverses HAIR-induced uterine and placental ferroptosis through activation of the Slc7a11/GSH/GPX4 axis. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Responsed Drug | L. lactis MG1363-pMG36e-GLP-1 | Investigative | |||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
Colon tissues (Mouse colon tissues) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
Fifty male C57BL/6 mice provided by Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China) resided in an animal house (temperature 26 ± 1 , humidity 50 ± 10%), in which the light was on for 12 h and off for 12 h. Mice were acclimatised for 1 week and allowed water and animal food with no limitations. Then, all mice were stochastically divided into 5 groups using random number tables available online (https://www.random-online.com/, accessed on 26 December 2021), including: (1) C group, a control group treated with normal saline for 7 consecutive days (n = 10); (2) M group, a model group with intraperitoneal injection of 20 mg/kg/day MPTP (Sigma-Aldrich, Taufkirchen, Germany, M0896) for 7 consecutive days (n = 10); (3) L group, treated with MPTP and 0.4 mg/kg/day liraglutide for 7 consecutive days (n = 10); (4) R group, treated with MPTP and 109 colony-forming unit (CFU) L. lactis MG1363 for 7 consecutive days via gavage (n = 10); (5) RG group, treated with MPTP and 109 CFUL. lactis MG1363-pMG36e-GLP-1 for 7 consecutive days via gavage (n = 10). All animals survived treatment and all animal experiments were administered from 9:00 to 12:00 in the morning to reduce systematic errors.
Click to Show/Hide
|
||||
| Response Description | L. lactis MG1363-pMG36e-GLP-1 exerts neurotrophic effects via activating the Keap1/Nrf2/GPX4 signalling pathway to down-regulate ACSL4 and up-regulate FSP1 to suppress ferroptosis. These results indicated that the neurotrophic effects of the next-generation probiotics L. lactis MG1363-pMG36e-GLP-1 against MPTP-induced Parkinsonism are mediated by modulating oxidative stress, inhibiting ferroptosis, and redressing dysbiosis. | ||||
Extraocular muscles disorder [ICD-11: 9C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | |||
| Responsed Drug | Artesunate | Investigative | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
hOFs (Human ocular fibroblasts) | |||
| Response Description | Expression of mitochondrial GPX4 but no other forms of GPX4 was decreased after artesunate treatment and that mitochondrial GPX4 overexpression rescued artesunate-induced lipid peroxidation and ferroptosis. Other cellular ferroptosis defense mechanisms, including cellular FSP1 and Nrf2, were also inhibited by artesunate. In conclusion, our study demonstrated that artesunate protects against fibrosis through abrogation of fibroblast activation and induction of mitochondria-dependent ferroptosis in ocular fibrosis, which may offer a potential treatment for ocular fibrosis. | |||
Left ventricular failure [ICD-11: BD11]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Responsed Drug | Levosimendan | Approved | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response Description | Levosimendan reversed mitochondrial malfunction in heart failure with preserved ejection fraction (HFpEF) mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan reverses cardiac malfunction and cardiomyocyte ferroptosis during heart failure with preserved ejection fraction via connexin 43 signaling activation. | ||||
Pulmonary fibrosis [ICD-11: CB03]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Responsed Drug | Bleomycin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response Description | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of ACSL4 but similarly decreased those of FSP1. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
Protein Mdm4 (MDM4)
NCS207895
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Colon cancer [ICD-11: 2B90] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| Response Description | Inhibition of MDM2 (Nutlin-3) or MDMX (NCS207895) leads to increased levels of FSP1 protein and a consequent increase in the levels of coenzyme Q10, an endogenous lipophilic antioxidant. This suggests that MDM2 and MDMX normally prevent cells from mounting an adequate defense against lipid peroxidation and thereby promote ferroptosis in Colon carcinoma. | |||
E3 ubiquitin-protein ligase Mdm2 (MDM2)
Nutlin-3
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | |||
| Regulator for Ferroptosis | Driver | |||
| Responsed Disease | Colon cancer [ICD-11: 2B90] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| Response Description | Inhibition of MDM2 (Nutlin-3) or MDMX (NCS207895) leads to increased levels of FSP1 protein and a consequent increase in the levels of coenzyme Q10, an endogenous lipophilic antioxidant. This suggests that MDM2 and MDMX normally prevent cells from mounting an adequate defense against lipid peroxidation and thereby promote ferroptosis in Colon carcinoma. | |||
Unspecific Regulator
Curcumin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response Description | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
Andrographis
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [8] | ||||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response Description | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
N-acetylcysteine
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [9] | ||||
| Responsed Disease | Ovarian dysfunction [ICD-11: 5A80] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell differentiation | |||||
| In Vitro Model | hPTs (Human placental tissues) | ||||
| hPTs (Human placental tissues) | |||||
| In Vivo Model |
Adult Sprague-Dawley rats (70 days old) of both sexes were purchased from the Laboratory Animal Centre of Harbin Medical University, Harbin, China. Before the experiment, female rats were allowed to acclimatize for a minimum of 1 week and then were monitored daily by vaginal lavage to determine the stage of the estrous cycle as previously described (Zhanget al., 2016). Pregnancy was achieved by housing female rats on the night of proestrus with fertile males of the same strain at a 2:1 ratio. Confirmation of mating was performed the morning after by the presence of a vaginal plug, and this was considered as GD 0.5. The rats were sacrificed between 8:00 a.m. and 9:00 a.m. hours on GD 14.5.
Click to Show/Hide
|
||||
| Response Description | Previous studies demonstrate that increased uterine and placental ferroptosis is associated with oxidative stress-induced fetal loss in a pre-clinical polycystic ovary syndrome (PCOS)-like rat model. N-acetylcysteine treatment results in increased mRNA expression of Aifm2, a negative regulator of GPX4-independent ferroptosis in the placenta. Moreover, NAC reverses HAIR-induced uterine and placental ferroptosis through activation of the Slc7a11/GSH/GPX4 axis. | ||||
L. lactis MG1363-pMG36e-GLP-1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [10] | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Pathways in cancer | hsa05200 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Colon tissues (Mouse colon tissues) | ||||
| hBCs (Brain cells) | |||||
| In Vivo Model |
Fifty male C57BL/6 mice provided by Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China) resided in an animal house (temperature 26 ± 1 , humidity 50 ± 10%), in which the light was on for 12 h and off for 12 h. Mice were acclimatised for 1 week and allowed water and animal food with no limitations. Then, all mice were stochastically divided into 5 groups using random number tables available online (https://www.random-online.com/, accessed on 26 December 2021), including: (1) C group, a control group treated with normal saline for 7 consecutive days (n = 10); (2) M group, a model group with intraperitoneal injection of 20 mg/kg/day MPTP (Sigma-Aldrich, Taufkirchen, Germany, M0896) for 7 consecutive days (n = 10); (3) L group, treated with MPTP and 0.4 mg/kg/day liraglutide for 7 consecutive days (n = 10); (4) R group, treated with MPTP and 109 colony-forming unit (CFU) L. lactis MG1363 for 7 consecutive days via gavage (n = 10); (5) RG group, treated with MPTP and 109 CFUL. lactis MG1363-pMG36e-GLP-1 for 7 consecutive days via gavage (n = 10). All animals survived treatment and all animal experiments were administered from 9:00 to 12:00 in the morning to reduce systematic errors.
Click to Show/Hide
|
||||
| Response Description | L. lactis MG1363-pMG36e-GLP-1 exerts neurotrophic effects via activating the Keap1/Nrf2/GPX4 signalling pathway to down-regulate ACSL4 and up-regulate FSP1 to suppress ferroptosis. These results indicated that the neurotrophic effects of the next-generation probiotics L. lactis MG1363-pMG36e-GLP-1 against MPTP-induced Parkinsonism are mediated by modulating oxidative stress, inhibiting ferroptosis, and redressing dysbiosis. | ||||
Artesunate
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [11] | |||
| Responsed Disease | Extraocular muscles disorder [ICD-11: 9C82] | |||
| Pathway Response | Ferroptosis | hsa04216 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hOFs (Human ocular fibroblasts) | |||
| Response Description | Expression of mitochondrial GPX4 but no other forms of GPX4 was decreased after artesunate treatment and that mitochondrial GPX4 overexpression rescued artesunate-induced lipid peroxidation and ferroptosis. Other cellular ferroptosis defense mechanisms, including cellular FSP1 and Nrf2, were also inhibited by artesunate. In conclusion, our study demonstrated that artesunate protects against fibrosis through abrogation of fibroblast activation and induction of mitochondria-dependent ferroptosis in ocular fibrosis, which may offer a potential treatment for ocular fibrosis. | |||
Levosimendan
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | ||||
| Responsed Disease | Left ventricular failure [ICD-11: BD11] | ||||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rHTs (Rat hippocampal tissues) | ||||
| In Vivo Model |
We purchased forty-eight 3-week-old male C57BL/6N mice from Beijing HFK Bioscience Co. Ltd. and gave a twelve-hour light and dark cycle starting from 06:00 (am) to 18:00 (pm). Mice were randomly assigned into three groups after 2 weeks of adaptive feeding as follows. (1) The control group (n = 16): mice were provided with normal drinking water, a normal diet and intraperitoneal administration of solvent (5% DMSO + 40% Peg300 + 5% Tween 80 + 50% ddH2O) 3 mL/kg once a week aged 13 to 17 weeks. (2) The HFpEF group (n = 16): a double-hit model was designed, in which metabolic and mechanical stress worked together and resulted in HFpEF. Briefly, C57BL/6N mice had unrestricted access to a high-fat diet (HFD, D12492, Research Diet) starting from 5 weeks old. Meanwhile, a nitric oxide synthase inhibitor, N (gamma)-nitro-L-arginine methyl ester (L-NAME) (N5751, Sigma) was supplied in drinking water (0.5 g/L) for HFpEF groups, and the pH of the drinking water was adjusted to 7.4. The above placebo solvent was administrated in the same manner. (3) The HFpEF + Levo group (n = 16): according to the previous study, HFpEF mice received 3 mg/kg levosimendan (S2446, Selleck) (Dissolve 1 mg of levosimendan in 50 uL of DMSO, subsequently dilute to 1 mg/mL with the above solvent) intraperitoneally once a week from week 13 to 17.
Click to Show/Hide
|
||||
| Response Description | Levosimendan reversed mitochondrial malfunction in heart failure with preserved ejection fraction (HFpEF) mice, as evidenced by increased mitofilin and decreased ROS, superoxide anion, NOX4, and cytochrome C levels. Interestingly, after levosimendan administration, myocardial tissue from HFpEF mice showed restricted ferroptosis, indicated by an increased GSH/GSSG ratio; upregulated GPX4, xCT, and FSP-1 expression; and reduced intracellular ferrous ion, MDA, and 4-HNE levels. Levosimendan reverses cardiac malfunction and cardiomyocyte ferroptosis during heart failure with preserved ejection fraction via connexin 43 signaling activation. | ||||
Bleomycin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [13] | ||||
| Responsed Disease | Pulmonary fibrosis [ICD-11: CB03] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response Description | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of ACSL4 but similarly decreased those of FSP1. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
References
