Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0056)
| Name |
N-acetylcysteine
|
||||
|---|---|---|---|---|---|
| Synonyms |
N-Acetyl-L-cysteine; acetylcysteine; 616-91-1; N-Acetylcysteine; mercapturic acid; Acetadote; Broncholysin; Mucomyst; L-Acetylcysteine; Fluimucetin; Fluimucil; Parvolex; N-Acetyl-cysteine; Fluprowit; Respaire; Acetein; Airbron; Fabrol; Mucosil; Flumucetin; Mucosolvin; Brunac; L-Cysteine, N-acetyl-; Fluimicil Infantil; N-Acetyl cysteine; Acetilcisteina; Acetylcysteinum; Syntemucol; Lysomucil; Mucofilin; acetyl cysteine; Exomuc; Inspir; Tixair; Mucolyticum Lappe; Mucolytikum Lappe; N-Acetyl-3-mercaptoalanine; (R)-2-Acetamido-3-mercaptopropanoic acid; Mucolyticum; Cetylev; Neo-fluimucil; Ac-Cys-OH; Cysteine, N-acetyl-, L-; Mucolyticum-Lappe; N-Acetyl-L-(+)-cysteine; NAC-TB; Acetyl-L-cysteine; component of Naxid; Mercapturic acid, (R)-; Fluatox; Fluimicil; Mucolator; Flumil; Mucret; (R)-mercapturic acid; Muco sanigen; Oristar nalc; (2R)-2-acetamido-3-sulfanylpropanoic acid; MUCOSIL-10; MUCOSIL-20; N-acetyi-l-cysteine; cysteine, N-acetyl-; L-alpha-Acetamido-beta-mercaptopropionic acid; CCRIS 3764; Mucocedyl; N-acetyl-(R)-cysteine; NSC 111180; UNII-WYQ7N0BPYC; WYQ7N0BPYC; HSDB 3003; EINECS 210-498-3; (2R)-2-Acetamido-3-sulfanyl-propanoic acid; Ilube (eye drops); N-A-C Sustain; NSC-111180; (r)-n-acetylcysteine; DTXSID5020021; CHEBI:28939; N-Acetyl-L-cysteine hydrochloride; RK-0202; DTXCID4021; MLS000028419; (R)-2-acetylamino-3-mercaptopropanoic acid; (2R)-2-acetylamino-3-sulfanylpropanoic acid; NSC111180; NCGC00022304-05; LNAC; SMR000058377; MFCD00004880; ACETYLCYSTEINE (II); ACETYLCYSTEINE [II]; ACETYLCYSTEINE (MART.); ACETYLCYSTEINE [MART.]; Ilube; N-acetylcystein; ACETYLCYSTEINE (EP MONOGRAPH); ACETYLCYSTEINE [EP MONOGRAPH]; N Acetylcysteine; ACETYLCYSTEINE (USP MONOGRAPH); ACETYLCYSTEINE [USP MONOGRAPH]; Acid, Mercapturic; Acetylcysteinum [INN-Latin]; CAS-616-91-1; Acetilcisteina [INN-Spanish]; N Acetyl L cysteine; N-Acetyl-N-Cysteine; SR-01000075439; DTXSID8048105; AcCys; N-acetyl-l-cys; Sodium 2-acetamido-3-mercaptopropionate; SC2; N-acetyl-L-cystein; Naxid (Salt/Mix); N-Acety-L-Cysteine; Acetyl Cysteine,(S); Acetylcysteine [USAN:USP:INN:BAN:JAN]; Opera_ID_452; MUCOMYST (TN); Acetylcysteine Ph. Eur.; Spectrum2_000086; Spectrum3_000287; Spectrum4_000137; Spectrum5_000764; CHEMBL600; NAC & TNF; ACETYLCYSTEINE [MI]; SCHEMBL5292; ACETYLCYSTEINE [INN]; ACETYLCYSTEINE [JAN]; Lopac0_000081; ACETYLCYSTEINE [HSDB]; ACETYLCYSTEINE [USAN]; BSPBio_001794; KBioGR_000554; MLS001076125; MLS006011563; ACETYLCYSTEINE [VANDF]; SPECTRUM1500105; SPBio_000012; ACETYL CYSTEINE [INCI]; ACETYLCYSTEINE [USP-RS]; ACETYLCYSTEINE [WHO-DD]; Acetylcysteine(N-acetylcysteine); BRONCHOLYSIN (MUCOLYTIC); DTXCID6028076; CHEBI:22198; GTPL10945; KBio3_001294; N-Acetyl-L-cysteine, USP grade; R05CB01; S01XA08; V03AB23; Acetylcysteine (JP17/USP/INN); HMS1920A11; HMS2091G11; HMS2234J22; HMS3260A04; HMS3655G11; HMS3715D03; HMS3884E04; HY-B0215; 2-Acetylamino-3-mercapto-propionate; ACETYLCYSTEINE [ORANGE BOOK]; Tox21_110877; Tox21_201078; Tox21_500081; acetylcysteine (n-acetyl-l-cysteine); BDBM50420190; CCG-38902; s1623; AKOS015841009; Tox21_110877_1; CCG-204176; DB06151; GS-3121; LP00081; SDCCGSBI-0050069.P002; (R)-2-Acetamido-3-mercaptopropanoicacid; NCGC00015086-04; NCGC00022304-03; NCGC00022304-04; NCGC00022304-06; NCGC00022304-07; NCGC00022304-08; NCGC00022304-17; NCGC00022304-23; NCGC00258631-01; NCGC00260766-01; AC-16071; AC-24117; BP-12854; SBI-0051272.P003; A0905; AM20100502; EU-0100081; SW199597-2; (2R)-2-acetylamino-3-mercapto-propionic acid; EN300-72028; A 7250; A-1100; C06809; D00221; L-Cysteine, N-acetyl- & Tumor necrosis factor; N-Acetyl-L-cysteine, BioXtra, >=99% (TLC); AB00051908_02; AB00382974-12; AB00382974_13; L-.alpha.-Acetamido-.beta.-mercaptopropionic acid; Q375613; J-507685; N-Acetyl-L-cysteine & Tumor necrosis factor (TNF); N-Acetyl-L-cysteine 100 microg/mL in Acetonitrile; SR-01000075439-1; SR-01000075439-3; SR-01000075439-5; BRD-K59058747-001-20-9; N-Acetyl-L-cysteine, cell culture tested, BioReagent; N-Acetyl-L-cysteine, Vetec(TM) reagent grade, 98%; F1905-7178; Z1143441555; CABC898A-E48B-4E13-9F72-98D0609A1854; N-Acetyl-L-cysteine, SAJ special grade, 98.0-102.0%; N-Acetyl-L-cysteine, Sigma Grade, >=99% (TLC), powder; Acetylcysteine, British Pharmacopoeia (BP) Reference Standard; Acetylcysteine, European Pharmacopoeia (EP) Reference Standard; Acetylcysteine, United States Pharmacopeia (USP) Reference Standard; N-Acetyl-L-cysteine, Pharmaceutical Secondary Standard; Certified Reference Material
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
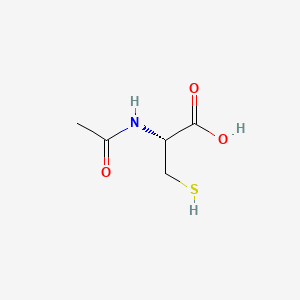 |
||||
| Formula |
C5H9NO3S
|
||||
| IUPAC Name |
(2R)-2-acetamido-3-sulfanylpropanoic acid
|
||||
| Canonical SMILES |
CC(=O)NC(CS)C(=O)O
|
||||
| InChI |
InChI=1S/C5H9NO3S/c1-3(7)6-4(2-10)5(8)9/h4,10H,2H2,1H3,(H,6,7)(H,8,9)/t4-/m0/s1
|
||||
| InChIKey |
PWKSKIMOESPYIA-BYPYZUCNSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Ferroptosis suppressor protein 1 (AIFM2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ovarian dysfunction | ICD-11: 5A80 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell differentiation | |||||
| In Vitro Model | hPTs (Human placental tissues) | ||||
| hPTs (Human placental tissues) | |||||
| In Vivo Model |
Adult Sprague-Dawley rats (70 days old) of both sexes were purchased from the Laboratory Animal Centre of Harbin Medical University, Harbin, China. Before the experiment, female rats were allowed to acclimatize for a minimum of 1 week and then were monitored daily by vaginal lavage to determine the stage of the estrous cycle as previously described (Zhanget al., 2016). Pregnancy was achieved by housing female rats on the night of proestrus with fertile males of the same strain at a 2:1 ratio. Confirmation of mating was performed the morning after by the presence of a vaginal plug, and this was considered as GD 0.5. The rats were sacrificed between 8:00 a.m. and 9:00 a.m. hours on GD 14.5.
Click to Show/Hide
|
||||
| Response regulation | Previous studies demonstrate that increased uterine and placental ferroptosis is associated with oxidative stress-induced fetal loss in a pre-clinical polycystic ovary syndrome (PCOS)-like rat model. N-acetylcysteine treatment results in increased mRNA expression of Aifm2, a negative regulator of GPX4-independent ferroptosis in the placenta. Moreover, NAC reverses HAIR-induced uterine and placental ferroptosis through activation of the Slc7a11/GSH/GPX4 axis. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ovarian dysfunction | ICD-11: 5A80 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell differentiation | |||||
| In Vitro Model | hPTs (Human placental tissues) | ||||
| In Vivo Model |
Adult Sprague-Dawley rats (70 days old) of both sexes were purchased from the Laboratory Animal Centre of Harbin Medical University, Harbin, China. Before the experiment, female rats were allowed to acclimatize for a minimum of 1 week and then were monitored daily by vaginal lavage to determine the stage of the estrous cycle as previously described (Zhanget al., 2016). Pregnancy was achieved by housing female rats on the night of proestrus with fertile males of the same strain at a 2:1 ratio. Confirmation of mating was performed the morning after by the presence of a vaginal plug, and this was considered as GD 0.5. The rats were sacrificed between 8:00 a.m. and 9:00 a.m. hours on GD 14.5.
Click to Show/Hide
|
||||
| Response regulation | Previous studies demonstrate that increased uterine and placental ferroptosis is associated with oxidative stress-induced fetal loss in a pre-clinical polycystic ovary syndrome (PCOS)-like rat model. N-acetylcysteine treatment results in increased mRNA expression of Aifm2, a negative regulator of GPX4-independent ferroptosis in the placenta. Moreover, NAC reverses HAIR-induced uterine and placental ferroptosis through activation of the Slc7a11/GSH/GPX4 axis. | ||||
