Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0310)
| Name |
Nutlin-3
|
||||
|---|---|---|---|---|---|
| Synonyms |
Nutlin-3; 548472-68-0; 890090-75-2; nutlin 3; 4-(4,5-bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydro-1H-imidazole-1-carbonyl)piperazin-2-one; (Rac)-Nutlin-3; (+/-)-Nutlin3; 4-[[4,5-Bis(4-chlorophenyl)-4,5-dihydro-2-[4-methoxy-2-(1-methylethoxy)phenyl]-1H-imidazol-1-yl]carbonyl]-2-piperazinone; 4-[4,5-bis(4-chlorophenyl)-2-(4-methoxy-2-propan-2-yloxyphenyl)-4,5-dihydroimidazole-1-carbonyl]piperazin-2-one; CHEMBL211045; (+)-Nutlin-3; (?)-Nutlin-3; MFCD14636430; Nutlin 3(Random Configuration); Nutlin3; NSC-732664; Rac-Nutlin-3; Nutln 3A; MFCD07784509; 4-({4,5-bis(4-chlorophenyl)-2-[4-methoxy-2-(propan-2-yloxy)phenyl]-4,5-dihydro-1H-imidazol-1-yl}carbonyl)piperazin-2-one; the p53/MDM2 Agonist; SCHEMBL2458627; BDBM31197; CHEBI:93777; EX-A851; HMS3651G03; HMS3653F08; HMS3653J08; HMS3750A11; AMY39899; BCP02265; BCP05161; BCP29278; MFCD11977784; NSC732664; s1061; AKOS005146527; CCG-264800; SB19406; SB19407; NCGC00165848-01; NCGC00165848-02; AC-35939; AS-10121; SY283510; SY289992; FT-0700329; FT-0713388; FT-0771774; FT-0773561; SW220144-1; H10266; A861337; J-514247; J-523776; BRD-A12230535-001-01-8; Q27165473; Z2159890360; (+-)-4-[4,5-dihydro-imidazole-1-carbonyl]piperazin-2-one; (-)-4-(4,5-bis-(4-Chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydroimidazole-1-carbonyl)piperazine-2-one; (??)-4-[4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxy-phenyl)-4,5-dihydro-imidazole-1-carbonyl]-piperazin-2-one; 4-{[(4S,5R)-4,5-Bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydro-1H-imidazol-1-yl]carbonyl}-2-piperazinone; RG7112;R7112;rel-4-((4R,5S)-4,5-bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydro-1H-imidazole-1-carbonyl)piperazin-2-one
Click to Show/Hide
|
||||
| Structure |
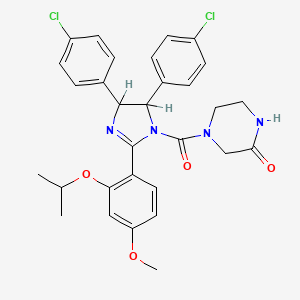 |
||||
| Formula |
C30H30Cl2N4O4
|
||||
| IUPAC Name |
4-[4,5-bis(4-chlorophenyl)-2-(4-methoxy-2-propan-2-yloxyphenyl)-4,5-dihydroimidazole-1-carbonyl]piperazin-2-one
|
||||
| Canonical SMILES |
CC(C)OC1=C(C=CC(=C1)OC)C2=NC(C(N2C(=O)N3CCNC(=O)C3)C4=CC=C(C=C4)Cl)C5=CC=C(C=C5)Cl
|
||||
| InChI |
InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)
|
||||
| InChIKey |
BDUHCSBCVGXTJM-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Ferroptosis suppressor protein 1 (AIFM2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Colon cancer | ICD-11: 2B90 | ||
| Responsed Regulator | E3 ubiquitin-protein ligase Mdm2 (MDM2) | Driver | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| SK-HEP-1 cells | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| Response regulation | Inhibition of MDM2 (Nutlin-3) or MDMX (NCS207895) leads to increased levels of FSP1 protein and a consequent increase in the levels of coenzyme Q10, an endogenous lipophilic antioxidant. This suggests that MDM2 and MDMX normally prevent cells from mounting an adequate defense against lipid peroxidation and thereby promote ferroptosis in Colon carcinoma. | |||
