Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0160)
| Name |
Bleomycin
|
||||
|---|---|---|---|---|---|
| Synonyms |
bleomycin a2; bleomycin; Bleomycins; 11116-31-7; Bleocin; Blenamax; Bleomycin Hexal; CHEBI:3139; NSC-125066; Bleo; Bleomycin hydrochloride; 11056-06-7; Bleo-kyowa; SCHEMBL134155; CHEMBL403664; DTXSID20872327; BLM; BDBM50547621; AKOS032960358; C06854; N(1)-[3-(dimethylsulfonio)propyl]bleomycinamide; Q26841044
Click to Show/Hide
|
||||
| Structure |
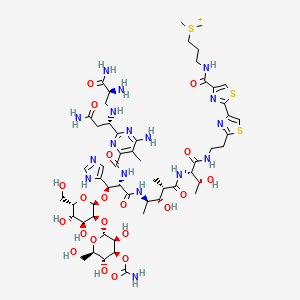 |
||||
|
3D MOL
|
|||||
| Formula |
C55H84N17O21S3+
|
||||
| IUPAC Name |
3-[[2-[2-[2-[[(2S,3R)-2-[[(2S,3S,4R)-4-[[(2S,3R)-2-[[6-amino-2-[(1S)-3-amino-1-[[(2S)-2,3-diamino-3-oxopropyl]amino]-3-oxopropyl]-5-methylpyrimidine-4-carbonyl]amino]-3-[(2R,3S,4S,5S,6S)-3-[(2R,3S,4S,5R,6R)-4-carbamoyloxy-3,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-3-(1H-imidazol-5-yl)propanoyl]amino]-3-hydroxy-2-methylpentanoyl]amino]-3-hydroxybutanoyl]amino]ethyl]-1,3-thiazol-4-yl]-1,3-thiazole-4-carbonyl]amino]propyl-dimethylsulfanium
|
||||
| Canonical SMILES |
CC1=C(N=C(N=C1N)C(CC(=O)N)NCC(C(=O)N)N)C(=O)NC(C(C2=CN=CN2)OC3C(C(C(C(O3)CO)O)O)OC4C(C(C(C(O4)CO)O)OC(=O)N)O)C(=O)NC(C)C(C(C)C(=O)NC(C(C)O)C(=O)NCCC5=NC(=CS5)C6=NC(=CS6)C(=O)NCCC[S+](C)C)O
|
||||
| InChI |
InChI=1S/C55H83N17O21S3/c1-20-33(69-46(72-44(20)58)25(12-31(57)76)64-13-24(56)45(59)82)50(86)71-35(41(26-14-61-19-65-26)91-54-43(39(80)37(78)29(15-73)90-54)92-53-40(81)42(93-55(60)88)38(79)30(16-74)89-53)51(87)66-22(3)36(77)21(2)47(83)70-34(23(4)75)49(85)63-10-8-32-67-28(18-94-32)52-68-27(17-95-52)48(84)62-9-7-11-96(5)6/h14,17-19,21-25,29-30,34-43,53-54,64,73-75,77-81H,7-13,15-16,56H2,1-6H3,(H13-,57,58,59,60,61,62,63,65,66,69,70,71,72,76,82,83,84,85,86,87,88)/p+1/t21-,22+,23+,24-,25-,29-,30+,34-,35-,36-,37+,38+,39-,40-,41-,42-,43-,53+,54-/m0/s1
|
||||
| InChIKey |
OYVAGSVQBOHSSS-UAPAGMARSA-O
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Transferrin receptor protein 1 (TFRC)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor/Driver | ||||
| Responsed Disease | Pulmonary fibrosis | ICD-11: CB03 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response regulation | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of ACSL4 but similarly decreased those of FSP1. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
Solute carrier family 40 member 1 (SLC40A1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Pulmonary fibrosis | ICD-11: CB03 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response regulation | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of TfR1 and DMT1 in a concentration- and time-dependent manner but similarly decreased those of FPN. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
Natural resistance-associated macrophage protein 2 (SLC11A2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Pulmonary fibrosis | ICD-11: CB03 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response regulation | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of TfR1 and DMT1 in a concentration- and time-dependent manner but similarly decreased those of FPN. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Pulmonary fibrosis | ICD-11: CB03 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response regulation | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of ACSL4 but similarly decreased those of FSP1. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
Ferroptosis suppressor protein 1 (AIFM2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Pulmonary fibrosis | ICD-11: CB03 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MLE-12 cells | Normal | Mus musculus | CVCL_3751 | |
| In Vivo Model |
C57BL/6 J mice (8-week old) from SLAC Laboratory Animal Co. LTD (Shanghai, China) were housed in a specific pathogen-free (SPF) barrier system at 20 with 12-h light/dark cycles. They were randomly grouped as follows: (1) intratracheal saline (control group); (2) intraperitoneal deferoxamine (DFO, Sigma-Aldrich; DFO group); (3) intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd.; BLM group); and (4) intratracheal BLM plus intraperitoneal deferoxamine (BLM + DFO group). They were intratracheally injected with 50 ul of BLM (5 mg/kg) on day 0. For the preventive anti-fibrotic treatment, DFO (50 mg/kg2 day-1) was administered from day 0 to day 20. Lung samples were collected at day 21.
Click to Show/Hide
|
||||
| Response regulation | Bleomycin (BLM) can induce the inhibition of cellular GPX4, leading to the generation of lipid ROS. Besides, BLM treatment significantly increased the expression levels of ACSL4 but similarly decreased those of FSP1. TfR1 expression was significantly increased by BLM treatment but decreased by BLM + DFO treatment. These findings indicate that iron metabolism disorder, iron deposition, and ferroptosis in ATII cells may be involved in the pathogenesis of BLM-induced pulmonary fibrosis. | ||||
