Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0011)
| Name |
Curcumin
|
||||
|---|---|---|---|---|---|
| Synonyms |
curcumin; 458-37-7; Diferuloylmethane; Natural yellow 3; Indian saffron; Kacha haldi; Curcuma; Gelbwurz; Haldar; Curcumin I; Souchet; Haidr; Halad; Halud; Yellow Ginger; Terra Merita; (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione; Safran d'Inde; Yo-Kin; C.I. Natural Yellow 3; Hydrastis; Yellow puccoon; Golden seal; Diferaloylmethane; Curcumine; Tumeric yellow; CI Natural Yellow 3; Kurkumin [Czech]; Kurkumin; C.I. 75300; 8024-37-1; Zlut prirodni 3; Jianghuangsu; Zlut prirodni 3 [Czech]; Cucurmin; Curcumin (synthetic); NanoCurc; E 100; Tumeric oleoresin; 94875-80-6; CI 75300; NSC32982; NSC 32982; 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; CCRIS 3257; CHEBI:3962; Curcurmin; Lipocurc; Kurkum; HSDB 4334; 1,5-Di(vanillyliden)acetylaceton; NCI-C61325; 1,5-Divanillyliden-2,4-pentandion; EINECS 207-280-5; UNII-IT942ZTH98; MFCD00008365; NSC-32982; Curcumin e100; NSC 687842; trans,trans-Curcumin; BRN 2306965; IT942ZTH98; 1,9-Bis(4-hydroxy-3-methoxyphenyl)-2,7-nonadiene-4,6-dione; E 100 (Dye); MLS000069631; DTXSID8031077; 1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione; (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (E,E)-; CHEMBL140; NSC-687842; (E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; SMR000058237; 1,6-Heptadiene-3,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-, (1E,6E)-; DTXCID901421; INS NO. 100(I); INS-100(I); 4-08-00-03697 (Beilstein Handbook Reference); NSC687842; curouma; NCGC00017159-05; kachs haldi; 2,7-Nonadiene-4,6-dione, 1,9-bis(4-hydroxy-3-methoxyphenyl)-; safra d'inde; CURCUMIN (MART.); CURCUMIN [MART.]; (1E,6E)-1,7-bis(4-hydroxy-3-methoxy-phenyl)hepta-1,6-diene-3,5-dione; (1E,6E)-1,7-bis[4-hydroxy-3-(methyloxy)phenyl]hepta-1,6-diene-3,5-dione; Tumeric; Ukon; 1,7-BIS-(4-HYDROXY-3-METHOXYPHENYL)-HEPTA-1,6-DIENE-3,5-DIONE; CAS-458-37-7; Phytosome, Curcumin; Ukon (dye); FEMA No. 3085; FEMA No. 3086; CCRIS 5804; C Yellow 15; SR-01000000149; Curcuminoids; Curcumin,(S); (E/Z)-Curcumin; starbld0017234; CURCUMIN [HSDB]; CURCUMIN [INCI]; CURCUMIN [MI]; Opera_ID_1627; CURCUMIN [USP-RS]; CURCUMIN [WHO-DD]; SCHEMBL8440; SCHEMBL8441; Curcumin, analytical standard; MLS001148449; BIDD:ER0479; CU-01000001305-2; cid_969516; GTPL7000; SCHEMBL13521974; SCHEMBL23884885; SCHEMBL23884886; SCHEMBL23884892; SCHEMBL23884893; BDBM29532; cid_5281767; cMAP_000052; CI 75300 [INCI]; HMS2233K04; HMS3649K06; AMY33436; BCP04695; Tox21_110803; Tox21_111505; Tox21_201116; BBL027711; BDBM50067040; BDBM50140172; CCG-36020; CCG-36107; STL371943; AKOS001305497; BCP9000557; DB11672; NCGC00017159-04; NCGC00017159-06; NCGC00017159-07; NCGC00017159-09; NCGC00017159-10; NCGC00017159-11; NCGC00017159-12; NCGC00023332-03; NCGC00023332-04; NCGC00023332-05; NCGC00258668-01; AC-24238; AS-72202; BP-25396; CURCUMIN (CONSTITUENT OF TURMERIC); BCP0726000035; WLN: 1OR BQ E1U1V1V1U1R DQ CO1; C-230; C2302; CS-0149275; EN300-21494; F21478; K00009; Curcumin, Curcuma longa L. - CAS 458-37-7; A826902; Curcumin, primary pharmaceutical reference standard; Q312266; 1,5-dione, 1,7-bis(4-hydroxy-3-methoxyphenyl)-; SR-01000000149-2; SR-01000000149-5; BRD-K07572174-001-02-2; BRD-K07572174-001-19-6; BRD-K07572174-001-22-0; Z104500108; Curcumin, >=94% (curcuminoid content), >=80% (Curcumin); 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadien-3,5-dione; 1,7-bis(4-hydroxy-3-methoxyphenyl)1,6-heptadiene-3,5-dione; Curcumin, matrix substance for MALDI-MS, >=99.5% (HPLC); Curcumin, United States Pharmacopeia (USP) Reference Standard; 1,7-BIS(4-HYDROXYMETHOXYPHENYL)-1,6-HEPTADIENE-3,5-DIONE; 1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione; 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; '(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione'; ((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione); (1E,6E)-1,7-bis(3-methoxy-4-oxidanyl-phenyl)hepta-1,6-diene-3,5-dione; (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione #; (1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione.; (1E,6E)-1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione; (1Z,6E)-1,7-Bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,6-diene-3,5-dione; 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione, (E,E)-; 5-hydroxy-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,4,6-heptatrien-3-one; 5-Hydroxy-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,4,6-trien-3-one; Curcumin; 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; (1E,4Z,6E)-5-Hydroxy-1,7-bis-(4-hydroxy-3-methoxy-phenyl)-hepta-1,4,6-trien-3-one
Click to Show/Hide
|
||||
| Structure |
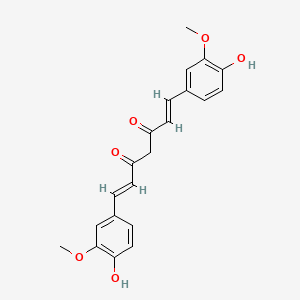 |
||||
| Formula |
C21H20O6
|
||||
| IUPAC Name |
(1E,6E)-1,7-bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione
|
||||
| Canonical SMILES |
COC1=C(C=CC(=C1)C=CC(=O)CC(=O)C=CC2=CC(=C(C=C2)O)OC)O
|
||||
| InChI |
InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3/b7-3+,8-4+
|
||||
| InChIKey |
VFLDPWHFBUODDF-FCXRPNKRSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 9 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Responsed Regulator | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) | Suppressor | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | |
| Response regulation | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/Akt/mTOR signaling pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Responsed Regulator | Serine/threonine-protein kinase mTOR (MTOR) | Suppressor | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | |
| Response regulation | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/Akt/ mTOR signaling pathway. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Responsed Regulator | RAC-alpha serine/threonine-protein kinase (AKT1) | Suppressor | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | |
| Response regulation | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/ Akt/mTOR signaling pathway. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Responsed Regulator | Serine/threonine-protein kinase mTOR (MTOR) | Suppressor | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | |
| Response regulation | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/Akt/mTOR signaling pathway. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Responsed Regulator | RAC-alpha serine/threonine-protein kinase (AKT1) | Suppressor | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HCT-8 cells | Ileocecal adenocarcinoma | Homo sapiens | CVCL_2478 | |
| Response regulation | Treating HCT-8 cells with curcumin significantly downregulated GSH, SLC7A11, and GPX4, while significantly increasing levels of iron, MDA, and ROS. Curcumin triggers ferroptosis and suppresses proliferation of colorectal cancer cells by inhibiting the PI3K/Akt/mTOR signaling pathway. | ||||
| Experiment 6 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response regulation | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
| Experiment 7 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Thyroid cancer | ICD-11: 2D10 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | |
| Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | ||
| FTC 238 cells | Thyroid gland follicular carcinoma | Homo sapiens | CVCL_2447 | ||
| Response regulation | Knockdown of HO-1 inhibits ferroptosis by upregulating the GPX4 expression in follicular thyroid cancer cells. We conclude that curcumin inhibits the tumorigenesis of follicular thyroid cancer via HO-1-induced activation of the ferroptosis signalling pathway. | ||||
| Experiment 8 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Two-month-old male New Zealand rabbits purchased from the Medical Experimental Animal Center of Bengbu Medical College were used as experimental subjects. Streptozotocin was dissolved in sterile saline and intraperitoneally injected into the rabbits at a dose of 80 mg/kg. The rabbits were allowed to eat freely after receiving the injection. The fasting blood glucose levels of the rabbits were monitored regularly. The diabetic rabbit model was considered successfully established when the fasting blood glucose level was measured as 11 mmol/L twice or 14 mmol/L once. Following successful modelling, grouping was performed as follows: blank control group (Con-Group), diabetic rabbit group (DM-Group), diabetic rabbit + every other day curcumin administration group (Qod-Group), and diabetic rabbit + daily administration group (Qd-Group).
Click to Show/Hide
|
||||
| Response regulation | Curcumin can promote the nuclear translocation of Nrf2, increase the expression of oxidative scavenging factors, such as HO-1, reduce excessive Gpx4 loss, and inhibit glucose-induced ferroptosis in cardiomyocytes. This highlights a potentially new therapeutic route for investigation for the treatment diabetic cardiomyopathy. | ||||
| Experiment 9 Reporting the Ferroptosis-centered Drug Act on This Target | [6] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| rPTs (Rat pancreas tissues) | |||||
| rHTs (Rat hippocampal tissues) | |||||
| In Vivo Model |
Forty female albino Wistar rats weighing 180-220 g were used in the study. Eight rats in each group were randomly assigned to five different groups: Group I (Sham); Group II (IR); Group III (IR + DMSO); Group IV (IR + Curcumin 100 mg/kg); and Group V (IR + 2 ug/kg LoxBlock-1) were determined. The animals were maintained at a temperature of 21 ± 2 and regulated humidity conditions (50 ± 5%) with a twelve-hour light/dark cycle. Throughout the experiment, the animals were fed standard commercial rat pellets and given tap water. All surgical and anesthesia procedures were performed understerile conditions. In addition, in a case of abnormal symptoms, the animals would be removed from the group and sacrificed under deep anesthesia.
Click to Show/Hide
|
||||
| Response regulation | Curcumin attenuates liver, pancreas and cardiac ferroptosis, oxidative stress and injury in ischemia/reperfusion-damaged rats by facilitating ACSL/GPx4 signaling. | ||||
Ferritin heavy chain (FTH1)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | |||
| Target for Ferroptosis | Marker/Suppressor | |||
| Responsed Disease | Hereditary Leiomyomatosis | ICD-11: 2C90 | ||
| Responsed Regulator | A disintegrin and metalloproteinase with thrombospondin motifs 18 (ADAMTS18) | Driver | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell proliferation | ||||
| In Vitro Model | A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 |
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | |
| Response regulation | Curcumin induces ferroptosis in tumor cells by upregulating the expression of ADAMTS18, thereby enhancing the sensitivity of clear cell renal cell carcinoma (ccRCC) to sunitinib. And Curcumin can significantly inhibit FTH1 and FTL1 gene expression in tumor tissues of nude mice. | |||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [7] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Intracerebral hemorrhage | ICD-11: 8B00 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MDCK cells | Normal | Canis lupus familiaris | CVCL_0422 | |
| HT22 cells | Normal | Mus musculus | CVCL_0321 | ||
| In Vivo Model |
Male C57BL/6 mice (8-10 weeks old) were obtained from the Experimental Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China). Briefly, mice were anesthetized and placed in a prone position with head stabilization in a stereotaxic frame. A dental drill was then utilized to generate a 1 mm burr hole at 2.0 mm to the lateral right of the bregma and 3.5 mm deep of the brain. Next, acute ICH was induced by slowly injecting 0.1U of type IV collagenase into this hole.
Click to Show/Hide
|
||||
| Response regulation | Curcumin in NPs (Cur-NPs) were shown to suppress erastin-induced ferroptosis in HT22 murine hippocampal cells. Cur-NPs effectively regulated the expression levels of HMOX1 and NFE2L2, which indicated that it might inhibit the ROS production through regulating the NRF2/HO-1 pathway. Cur-NPs served as an effective treatment for Intracerebral hemorrhage owing to their ability to inhibit ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Two-month-old male New Zealand rabbits purchased from the Medical Experimental Animal Center of Bengbu Medical College were used as experimental subjects. Streptozotocin was dissolved in sterile saline and intraperitoneally injected into the rabbits at a dose of 80 mg/kg. The rabbits were allowed to eat freely after receiving the injection. The fasting blood glucose levels of the rabbits were monitored regularly. The diabetic rabbit model was considered successfully established when the fasting blood glucose level was measured as 11 mmol/L twice or 14 mmol/L once. Following successful modelling, grouping was performed as follows: blank control group (Con-Group), diabetic rabbit group (DM-Group), diabetic rabbit + every other day curcumin administration group (Qod-Group), and diabetic rabbit + daily administration group (Qd-Group).
Click to Show/Hide
|
||||
| Response regulation | Curcumin can promote the nuclear translocation of Nrf2, increase the expression of oxidative scavenging factors, such as HO-1, reduce excessive Gpx4 loss, and inhibit glucose-induced ferroptosis in cardiomyocytes. This highlights a potentially new therapeutic route for investigation for the treatment diabetic cardiomyopathy. | ||||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [8] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
| In Vitro Model | A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 cells | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In Vivo Model |
Female C57BL/6 mice (14-18 g) were purchased from SiPeiFu (Beijing) Biotechnology. C57BL/6 mice were subcutaneously injected with a total of 6 x 105 Lewis lung carcinomas (LLC) cells on the left flank. Four days after LLC inoculation, the mice were randomly divided into two groups of five. The vehicle control and curcumin groups were given sodium carboxymethyl cellulose (CMC) or curcumin (100 mg/kg/day) by intraperitoneal injection for 15 days.
Click to Show/Hide
|
||||
| Response regulation | Curcumin induced ferroptosis via activating autophagy in non-small-cell lung cancer (NSCLC), which enhanced the therapeutic effect of NSCLC. Meanwhile, the protein level of ACSL4 was higher and the levels of SLC7A11 and GPX4 were lower in curcumin group than that in control group. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [6] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Ischemia/reperfusion injury | ICD-11: DB98 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hLCs (Liver cells) | ||||
| rPTs (Rat pancreas tissues) | |||||
| rHTs (Rat hippocampal tissues) | |||||
| In Vivo Model |
Forty female albino Wistar rats weighing 180-220 g were used in the study. Eight rats in each group were randomly assigned to five different groups: Group I (Sham); Group II (IR); Group III (IR + DMSO); Group IV (IR + Curcumin 100 mg/kg); and Group V (IR + 2 ug/kg LoxBlock-1) were determined. The animals were maintained at a temperature of 21 ± 2 and regulated humidity conditions (50 ± 5%) with a twelve-hour light/dark cycle. Throughout the experiment, the animals were fed standard commercial rat pellets and given tap water. All surgical and anesthesia procedures were performed understerile conditions. In addition, in a case of abnormal symptoms, the animals would be removed from the group and sacrificed under deep anesthesia.
Click to Show/Hide
|
||||
| Response regulation | Curcumin attenuates liver, pancreas and cardiac ferroptosis, oxidative stress and injury in ischemia/reperfusion-damaged rats by facilitating ACSL/GPx4 signaling. | ||||
Heme oxygenase 1 (HMOX1)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Thyroid cancer | ICD-11: 2D10 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | |
| Nthy-ori3-1 cells | Normal | Homo sapiens | CVCL_2659 | ||
| FTC 238 cells | Thyroid gland follicular carcinoma | Homo sapiens | CVCL_2447 | ||
| Response regulation | Knockdown of HO-1 inhibits ferroptosis by upregulating the GPX4 expression in follicular thyroid cancer cells. We conclude that curcumin inhibits the tumorigenesis of follicular thyroid cancer via HO-1-induced activation of the ferroptosis signalling pathway. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cardiomyopathy | ICD-11: BC43 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Two-month-old male New Zealand rabbits purchased from the Medical Experimental Animal Center of Bengbu Medical College were used as experimental subjects. Streptozotocin was dissolved in sterile saline and intraperitoneally injected into the rabbits at a dose of 80 mg/kg. The rabbits were allowed to eat freely after receiving the injection. The fasting blood glucose levels of the rabbits were monitored regularly. The diabetic rabbit model was considered successfully established when the fasting blood glucose level was measured as 11 mmol/L twice or 14 mmol/L once. Following successful modelling, grouping was performed as follows: blank control group (Con-Group), diabetic rabbit group (DM-Group), diabetic rabbit + every other day curcumin administration group (Qod-Group), and diabetic rabbit + daily administration group (Qd-Group).
Click to Show/Hide
|
||||
| Response regulation | Curcumin can promote the nuclear translocation of Nrf2, increase the expression of oxidative scavenging factors, such as HO-1, reduce excessive Gpx4 loss, and inhibit glucose-induced ferroptosis in cardiomyocytes. This highlights a potentially new therapeutic route for investigation for the treatment diabetic cardiomyopathy. | ||||
Ferroptosis suppressor protein 1 (AIFM2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HCT 116 cells | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 cells | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| In Vivo Model |
Briefly, surgically resected tumors were maintained in DMEM-F12 (Gibco) supplemented with 1% HEPES (Sigma-Aldrich), 1% L-glutamine (Gibco), 10% FBS (Gibco), 2% penicillin/streptomycin (Sigma-Aldrich), and 10 uM Y-27632 (R&D Systems). Tumors were digested with collagenase solution (5 mL of the above medium with 75 uL collagenase, 124 ug/mL dispase type II, and 0.2% Primocen) for 30 min and then filtered through a 70 um filter (Corning). An organoid pellet was obtained by centrifugation (200x g for 10 min). Organoids were suspended in Matrigel (Corning, Tehama County, CA) with IntestiCult Organoid Growth Medium (#06010, STEMCELL Technologies) and seeded in 12-well plates. Approximately 750 uL of IntestiCult Organoid Growth Medium was added to each well. Organoids were divided into five groups of control, curcumin (3.0 ug/mL), andrographis (30.0 ug/mL), their combination (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL), and their combination plus ferrostatin-1 (curcumin; 3.0 ug/mL, andrographis; 30.0 ug/mL; ferrostatin-1; 20 uM). Following forty-eight hours of treatment, the numbers of organoids (<100 um) and their mean sizes were examined using Image J software.
Click to Show/Hide
|
||||
| Response regulation | In conclusion, our study revealed that combined treatment with curcumin and andrographis exhibited anti-tumorigenic effects in colorectal cancer cells through activation of ferroptosis and by dual suppression of GPX-4 and FSP-1, which have significant potential implications for the adjunctive treatment of CRC patients. This combination treatment resulted in cancer cell death via both forms of cell death: apoptosis and ferroptosis. | ||||
References
