Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0116)
| Name |
Artesunate
|
||||
|---|---|---|---|---|---|
| Synonyms |
Artesunate; Artesunic acid; Arsumax; 88495-63-0; Plasmotrin; Qinghaozhi; Saphnate; Dihydroqinghasu hemsuccinate; Artesunatum; Zysunate; Arinate; Artesunato; Asumax; Gsunate Forte; Plasmotrim; CHEBI:63918; .alpha.-artesunic acid; Dihydroqinghaosu hemisuccinate; Succinyl dihydroartemisinin; LJPC-0118; 60W3249T9M; NSC-712571; Butanedioic acid, 1-[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-decahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano[4,3-j]-1,2-benzodioxepin-10-yl] ester; Arsuamoon; WR-256283; cosunate; Artesunata; Cosinate; Artesunate [USAN]; (3R,5aS,6R,8aS,9R,10S,12R,12aR)-Decahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano(4,3-j)-1,2-benzodioxepin-10-ol, hydrogen succinate; 4-oxo-4-(((3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-trimethyldecahydro-3H-3,12-epoxy[1,2]dioxepino[4,3-i]isochromen-10-yl)oxy)butanoic acid; 4-oxo-4-{[(3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-trimethyldecahydro-3,12-epoxypyrano[4,3-j][1,2]benzodioxepin-10-yl]oxy}butanoic acid; Artesunate [INN]; Butanedioic acid, mono((3R,5aS,6R,8aS,9R,10S,12R,12aR)-decahydro-3,6,9-trimethyl-3,12-epoxy-12H-pyrano(4,3-j)-1,2-benzodioxepin-10-yl) ester; Armax 200; Artesunatum [INN-Latin]; Artesunato [INN-Spanish]; DTXSID3042681; Artsuna; Nuartez; Artesunate [USAN:INN:BAN]; UNII-60W3249T9M; HSDB 7458; Quinghaosu reduced succinate ester; D95; Dihydroartemisinine-12alpha-succinate; ARTESUNATE [MI]; WR 256283; ARTESUNATE [HSDB]; ARTESUNATE [VANDF]; ARTESUNATE [MART.]; ARTESUNATE [USP-RS]; ARTESUNATE [WHO-DD]; ARTESUNATE [WHO-IP]; MLS006011590; CHEMBL361497; GTPL9956; ARTESUNATE [ORANGE BOOK]; SCHEMBL14552891; FIHJKUPKCHIPAT-AHIGJZGOSA-N; ARTESUNATUM [WHO-IP LATIN]; HY-N0193; STR09744; BDBM50248021; AKOS037515734; CS-8151; DB09274; NCGC00164600-10; NCGC00164600-15; SMR002499399; DIHYDROARTEMISININE-12.ALPHA.-SUCCINATE; EN300-6482026; Q707939; BRD-K54634444-001-05-9; WR-256283;ART;Armax 200;SM-804;HSDB-7458; 3R,5AS,6R,8AS,9R,10S,12R,12AR)-3,6,9-TRIMETHYLDECAHYDRO-3,12-EPOXY-12H-PYRANO(4,3-J)-1,2-BENZODIOXEPIN-10-YL HYDROGEN BUTANEDIOATE; 4-Oxo-4-(((3R,5aS,6R,8aS,9R,10S,12R,12aR)-3,6,9-trimethyldecahydro-3,12-epoxypyrano(4,3-j)-1,2-benzodioxepin-10-yl hydrogen butanedioate; 4-OXO-4-(3R,5AS,6R,8AS,9R,10S,12R,12AR)-3,6,9-TRIMETHYLDECAHYDRO-3,12-EPOXYPYRANO(4,3-J)-1,2-BENZODIOXEPIN-10-YL HYDROGEN BUTANEDIOATE; 4-oxo-4-[[(1R,4S,5R,8S,9R,10S,12R,13R)-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-yl]oxy]butanoic acid; 4-oxo-4-{[(1R,4S,5R,8S,9R,10S,12R,13R)-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.0?,(1)(3).0?,(1)(3)]hexadecan-10-yl]oxy}butanoic acid; 4-oxo-4-{[(1R,4S,5R,8S,9R,10S,12R,13R)-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.0^{4,13}.0^{8,13}]hexadecan-10-yl]oxy}butanoic acid; BUTANEDIOIC ACID, MONO(DECAHYDRO-3,6,9-TRIMETHYL-3,12-EPOXY-12H-PYRANO(4,3-J)-1,2-BENZODIOXEPIN-10-YL) ESTER, (3R-(3.ALPHA.,5A.BETA.,6.BETA.,8A.BETA.,9.ALPHA.,10.BETA.,12.BETA.,12AR*))-
Click to Show/Hide
|
||||
| Structure |
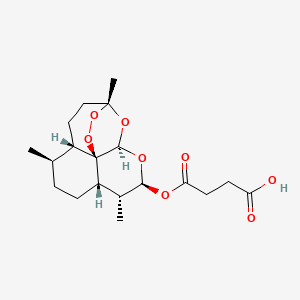 |
||||
| Formula |
C19H28O8
|
||||
| IUPAC Name |
4-oxo-4-[[(1R,4S,5R,8S,9R,10S,12R,13R)-1,5,9-trimethyl-11,14,15,16-tetraoxatetracyclo[10.3.1.04,13.08,13]hexadecan-10-yl]oxy]butanoic acid
|
||||
| Canonical SMILES |
CC1CCC2C(C(OC3C24C1CCC(O3)(OO4)C)OC(=O)CCC(=O)O)C
|
||||
| InChI |
InChI=1S/C19H28O8/c1-10-4-5-13-11(2)16(23-15(22)7-6-14(20)21)24-17-19(13)12(10)8-9-18(3,25-17)26-27-19/h10-13,16-17H,4-9H2,1-3H3,(H,20,21)/t10-,11-,12+,13+,16-,17-,18-,19-/m1/s1
|
||||
| InChIKey |
FIHJKUPKCHIPAT-AHIGJZGOSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Solute carrier family 40 member 1 (SLC40A1)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response regulation | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 ( MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response regulation | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 (MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response regulation | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 ( MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response regulation | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 (MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Intracerebral hemorrhage | ICD-11: 8B00 | |||
| Responsed Regulator | 5'-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1) | Driver | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BV-2 cells | Normal | Mus musculus | CVCL_0182 | |
| In Vivo Model |
Rats were anaesthetised through intraperitoneal injection of pentobarbital (40 mg/kg) and placed onto a stereotaxic instrument (RWD Life Science Co., Ltd.). A 1-cm midline incision was performed in the rat scalp to expose the intersection point. Then, a hole 3.2 mm lateral and 1.4 mm anterior to the right bregma was produced. Next, 1.0 ul collagenase type IV (0.25 IU/ul; C5138; Sigma-Aldrich, USA) was injected into the basal ganglia via a microinjection pump (4.2 mm depth below the endocranium) at a rate of 0.2 ul/min. The needle was maintained for 5 min after injection to prevent backflow. Thereafter, the skin incision was closed using sutures. Rats in the sham group received 1.0 ul saline instead of collagenase type IV.
Click to Show/Hide
|
||||
| Response regulation | Artesunate alleviates intracerebral haemorrhage secondary injury by inducing ferroptosis in M1-polarized microglia and suppressing inflammation through AMPK/mTORC1/GPX4 pathway | ||||
Nuclear receptor coactivator 4 (NCOA4)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response regulation | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 ( MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response regulation | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 (MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
Natural resistance-associated macrophage protein 2 (SLC11A2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response regulation | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 ( MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma | ICD-11: 2A00 | |||
| Responsed Regulator | Mitogen-activated protein kinase 14 (MAPK14) | Driver | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response regulation | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 (MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
Glutathione-specific gamma-glutamylcyclotransferase 1 (CHAC1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Marker/Driver | ||||
| Responsed Disease | Burkitt lymphoma | ICD-11: 2A85 | |||
| Responsed Regulator | Cyclic AMP-dependent transcription factor ATF-4 (ATF4) | Driver | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Daudi cells | Burkitt lymphoma | Homo sapiens | CVCL_0008 | |
| CA46 cells | Burkitt lymphoma | Homo sapiens | CVCL_1101 | ||
| In Vivo Model |
Four -week-old NOD/SCID mice were purchased from Chu Shang Technology (Kunming, China). CA-46 cells were collected and re-suspended in PBS at a concentration of 1-5 x 107 cells/mL. Totally, 0.2 mL cells were inoculated subcutaneously in the middle and posterior armpits of mice. When the transplanted tumor was established, the mice were injected the artesunate solution intraperitoneally in accordance with the body weight (200 mg/kg) daily.
Click to Show/Hide
|
||||
| Response regulation | Artesunate induced ferroptosis in different types of Burkitt's lymphoma cells, and caused a significant ERS response in tumor cells. The activation of the ATF4-CHOP-CHAC1 pathway up-regulated the expression of CHAC1 and degraded intracellular GSH, thus weakening the ability of lymphoma cells to resist ferroptosis. | ||||
Unspecific Target
| In total 7 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Responsed Disease | Diffuse large B-cell lymphoma | ICD-11: 2A81 | |||
| Responsed Regulator | Signal transducer and activator of transcription 3 (STAT3) | Suppressor | |||
| Pathway Response | JAK-STAT signaling pathway | hsa04630 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell autophagy | |||||
| Cell proliferation | |||||
| In Vitro Model | U-2932 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1896 | |
| SU-DHL-2 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_9550 | ||
| SU-DHL-4 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_0539 | ||
| SU-DHL-6 cells | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_2206 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
Female NOD/SCID mice (age, 6 weeks; body weight, 20 ± 2 g) were purchased from Beijing Huafukang Biotechnology Co., Ltd., maintained under pathogen-free conditions and allowed free access to sterilized food and water. After a week of adaptation to their surroundings, 1 x 107 U2932 cells were subcutaneously injected into the right flank near the hind leg of each mouse. Following the growth of palpable tumors (tumor volume of 50-100 mm3), the mice were randomly divided into two groups (n = 5 mice/group) and treated with 100 ul normal saline (NS) or ART (120 mg/kg/day) via intraperitoneal injection.
Click to Show/Hide
|
||||
| Response regulation | Artesunate (ART) was found to exert its effects via inhibition of STAT3 activation. ART may induce apoptosis and cell cycle arrest to inhibit cell proliferation, and regulate autophagy and ferroptosis via impairing the STAT3 signaling pathway in diffuse large B cell lymphoma (DLBCL) cells. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Responsed Disease | Pancreatic cancer | ICD-11: 2C10 | |||
| Responsed Regulator | Endoplasmic reticulum chaperone BiP (HSPA5) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | PaTu 8988t cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1847 | |
| AsPC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0152 | ||
| In Vivo Model |
AsPC-1 cells (1 x 106) with a control or GRP78 shRNA transfection were injected into right subcutaneous flank of nude mice (five mice per group). The nude mice were randomized into two groups and treated with DMSO or artesunate (30 mg/kg/i.p.), respectively. Artesunate was administered every two days. The tumor growth speed and volume were monitored every two days until the end point at day 35. All the tumor size and weight in the artesunate-treated groups were measured by using a caliper and an electronic balance.
Click to Show/Hide
|
||||
| Response regulation | Artesunate increased the mRNA and protein levels of GRP78 in a concentration-dependent manner in AsPC-1 and PaTU8988 cells. Knockdown GRP78 (HSPA5) enhanced artesunate-induced ferroptosis of pancreatic cancer cells in vitro and in vivo. Combining artesunate with GRP78 inhibition may be a novel maneuver for effective killing of KRAS mutant pancreatic ductal adenocarcinoma cells. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [6] | ||||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12 | |||
| Responsed Regulator | Cathepsin B (CTSB) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| SNU-182 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0090 | ||
| SNU-449 cells | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | ||
| In Vivo Model |
A total of 20 male Balb/c nude mice aged 6-8 weeks were purchased from Hunan SJA Laboratory Animal Co., Ltd. (Changsha, China). Five million Huh7 cells were inoculated into the right flanks of the mice. When the tumor size reached 80-100 mm3, the mice were randomly divided into four groups and administered artesunate (30 mg/kg mouse weight) alone, sorafenib (20 mg/kg mouse weight) alone, a combination of artesunate and sorafenib, or the same volume of PBS by gavage every other day.
Click to Show/Hide
|
||||
| Response regulation | Sorafenib at low dose mainly caused oxidative stress through mitochondrial impairments and SLC7A11-invovled glutathione depletion. Artesunate-induced lysosome activation synergized with sorafenib-mediated pro-oxidative effects by promoting sequential reactions including lysosomal cathepsin B/L activation, ferritin degradation, lipid peroxidation, and consequent ferroptosis. Taken together, artesunate could be repurposed to sensitize sorafenib in hepatocellular carcinoma treatment. | ||||
| Experiment 4 Reporting the Ferroptosis-centered Drug Act on This Target | [7] | ||||
| Responsed Disease | Hereditary Leiomyomatosis | ICD-11: 2C90 | |||
| Responsed Regulator | Cellular tumor antigen p53 (TP53) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Caki-1 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | |
| 786-O cells | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| KTCTL-26 cells | Clear cell renal cell carcinoma | Homo sapiens | CVCL_5872 | ||
| A-498 cells | Renal cell carcinoma | Homo sapiens | CVCL_1056 | ||
| Response regulation | Artesunate (ART) significantly increased cytotoxicity and inhibited proliferation and clonogenic growth in both parental and sunitinib-resistant renal cell carcinoma (RCC) cells. P53 exclusively appeared in the KTCTL-26 cells, indicating that p53 might be predictive for ART-dependent ferroptosis. Thus, ART may hold promise for treating selected patients with advanced and even therapy-resistant RCC. | ||||
| Experiment 5 Reporting the Ferroptosis-centered Drug Act on This Target | [8] | ||||
| Responsed Disease | Liver fibrosis | ICD-11: DB93 | |||
| Responsed Regulator | Microtubule-associated proteins 1A/1B light chain 3A (MAP1LC3A) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hHSCs (Human hepatic stellate cells) | ||||
| hHSCs (Human hepatic stellate cells) | |||||
| In Vivo Model |
6-8-week-old, 20 ± 2 g, male ICR mice, obtained from Nanjing Medical University (Nanjing, China), were randomly divided into five groups (n = 8 per group). Mouse model of chronic liver fibrosis was established by 10% carbon tetrachloride (CCl4, 0.5 ml/100 g body weight) injection. Groups are follows: (1) Control group was intraperitoneally (i.p.) injected with olive oil; (2) Model group was i.p. injected with 10% CCl4 every other day a week for 8 weeks; (3) Low-dose artesunate treatment groups were i.p. injected by CCl4 every other day a week for 8 weeks and daily i.p. injected by 50 mg/kg artesunate for last 4 weeks; (4) Middle-dose artesunate treatment groups were i.p. injected by CCl4 every other day a week for 8 weeks and daily i.p. injected by 100 mg/kg artesunate for last 4 weeks; (5) High-dose artesunate treatment groups were i.p. injected by CCl4 every other day a week for 8 weeks and daily i.p. injected by 200 mg/kg artesunate for last 4 weeks.
Click to Show/Hide
|
||||
| Response regulation | Artesunate evidently triggered ferritinophagy accompanied by up-regulation of LC3 (microtubule-associated protein light chain 3), Atg3, Atg5, Atg6/beclin1, Atg12 (autophagy related genes) and down-regulation of p62, FTH1 (ferritin heavy chain), NCOA4 (nuclear receptor co-activator 4) in activated HSCs. These results suggested that ferritinophagy-mediated HSC ferroptosis was responsible for artesunate-induced anti-fibrosis efficacy in liver fibrosis. | ||||
| Experiment 6 Reporting the Ferroptosis-centered Drug Act on This Target | [10] | ||||
| Responsed Disease | Merkel cell carcinoma | ICD-11: 2C34 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | MKL-1 cells | Merkel cell carcinoma | Homo sapiens | CVCL_2600 | |
| MKL-2 cells | Merkel cell carcinoma | Homo sapiens | CVCL_D027 | ||
| MS-1 cells | Lung small cell carcinoma | Homo sapiens | CVCL_1429 | ||
| WaGa cells | Merkel cell carcinoma | Homo sapiens | CVCL_E998 | ||
| PeTa cells | Merkel cell carcinoma | Homo sapiens | CVCL_LC73 | ||
| In Vivo Model |
Five-week-old female NOD.CB17/Prkdcscid mice (Charles River) were used for the xenotransplantation experiments. They were housed under specific pathogen-free conditions. Each mouse was injected subcutaneously with a suspension of 5 x 106 MKL-1 or WaGa tumor cells mixed with an equal volume of Matrigel (Corning) in a total volume of 100 uL. The tumor size was measured daily using a vernier calipers.
Click to Show/Hide
|
||||
| Response regulation | Artesunate predominantly induces ferroptosis in MCPyV-positive merkel cell carcinoma (MCC) cells since known ferroptosis-inhibitors like DFO, BAF-A1, Fer-1 and -mercaptoethanol reduced artesunate-induced death. Rosiglitazone (Rosi), an inhibitor of the Acyl-CoA synthetase long-chain family member 4 (ACSL4). Indeed, Rosi exerted a protective effect on all three tested artesunate-treated MCC cell lines. | ||||
| Experiment 7 Reporting the Ferroptosis-centered Drug Act on This Target | [11] | ||||
| Responsed Disease | Osteoporosis | ICD-11: FB83 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | RAW 264.7 cells | Leukemia | Mus musculus | CVCL_0493 | |
| In Vivo Model |
The mice were fed a standard pellet diet (mice maintenance diet; Tengxin Biotechnology Co., Ltd) and distilled water ad libitum and kept at a 12-h lightdark cycle, a temperature of 23 to 25 and a relative humidity of 50% ± 5%. Mice were divided into four groups: control (Ctrl), ferric ammonium citrate (FAC), ART and combined treatment (FAC + ART). FAC (Sigma-Aldrich; 40 mg/kg) were injected intraperitoneally every 3 days for 8 weeks. ART group mice were administered 50 mg/kg by oral gavage every other day for 8 weeks.
Click to Show/Hide
|
||||
| Response regulation | ART inhibits iron-uptake stimulated osteoclast differentiation by inducing ferroptosis. Artemisinin compounds are potential drugs for treating iron overload-induced osteoporosis. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [9] | |||
| Target for Ferroptosis | Marker/Suppressor | |||
| Responsed Disease | Extraocular muscles disorder | ICD-11: 9C82 | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hOFs (Human ocular fibroblasts) | |||
| Response regulation | Expression of mitochondrial GPX4 but no other forms of GPX4 was decreased after artesunate treatment and that mitochondrial GPX4 overexpression rescued artesunate-induced lipid peroxidation and ferroptosis. Other cellular ferroptosis defense mechanisms, including cellular FSP1 and Nrf2, were also inhibited by artesunate. In conclusion, our study demonstrated that artesunate protects against fibrosis through abrogation of fibroblast activation and induction of mitochondria-dependent ferroptosis in ocular fibrosis, which may offer a potential treatment for ocular fibrosis. | |||
Ferroptosis suppressor protein 1 (AIFM2)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [9] | |||
| Target for Ferroptosis | Suppressor | |||
| Responsed Disease | Extraocular muscles disorder | ICD-11: 9C82 | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | hOFs (Human ocular fibroblasts) | |||
| Response regulation | Expression of mitochondrial GPX4 but no other forms of GPX4 was decreased after artesunate treatment and that mitochondrial GPX4 overexpression rescued artesunate-induced lipid peroxidation and ferroptosis. Other cellular ferroptosis defense mechanisms, including cellular FSP1 and Nrf2, were also inhibited by artesunate. In conclusion, our study demonstrated that artesunate protects against fibrosis through abrogation of fibroblast activation and induction of mitochondria-dependent ferroptosis in ocular fibrosis, which may offer a potential treatment for ocular fibrosis. | |||
References
