Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10017)
| Target Name | Glutathione-specific gamma-glutamylcyclotransferase 1 (CHAC1) | ||||
|---|---|---|---|---|---|
| Synonyms |
Blocks Notch protein ; Cation transport regulator-like protein 1
Click to Show/Hide
|
||||
| Gene Name | CHAC1 | ||||
| Sequence |
MKQESAAPNTPPTSQSPTPSAQFPRNDGDPQALWIFGYGSLVWRPDFAYSDSRVGFVRGY
SRRFWQGDTFHRGSDKMPGRVVTLLEDHEGCTWGVAYQVQGEQVSKALKYLNVREAVLGG YDTKEVTFYPQDAPDQPLKALAYVATPQNPGYLGPAPEEAIATQILACRGFSGHNLEYLL RLADFMQLCGPQAQDEHLAAIVDAVGTMLPCFCPTEQALALV Click to Show/Hide
|
||||
| Family | Gamma-glutamylcyclotransferase family | ||||
| Function |
Catalyzes the cleavage of glutathione into 5-oxo-L-proline and a Cys-Gly dipeptide. Acts specifically on glutathione, but not on other gamma-glutamyl peptides. Glutathione depletion is an important factor for apoptosis initiation and execution. Acts as a pro-apoptotic component of the unfolded protein response pathway by mediating the pro-apoptotic effects of the ATF4-ATF3-DDIT3/CHOP cascade. Negative regulator of Notch signaling pathway involved in embryonic neurogenesis: acts by inhibiting Notch cleavage by furin, maintaining Notch in an immature inactive form, thereby promoting neurogenesis in embryos.
Click to Show/Hide
|
||||
| Gene ID | 79094 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
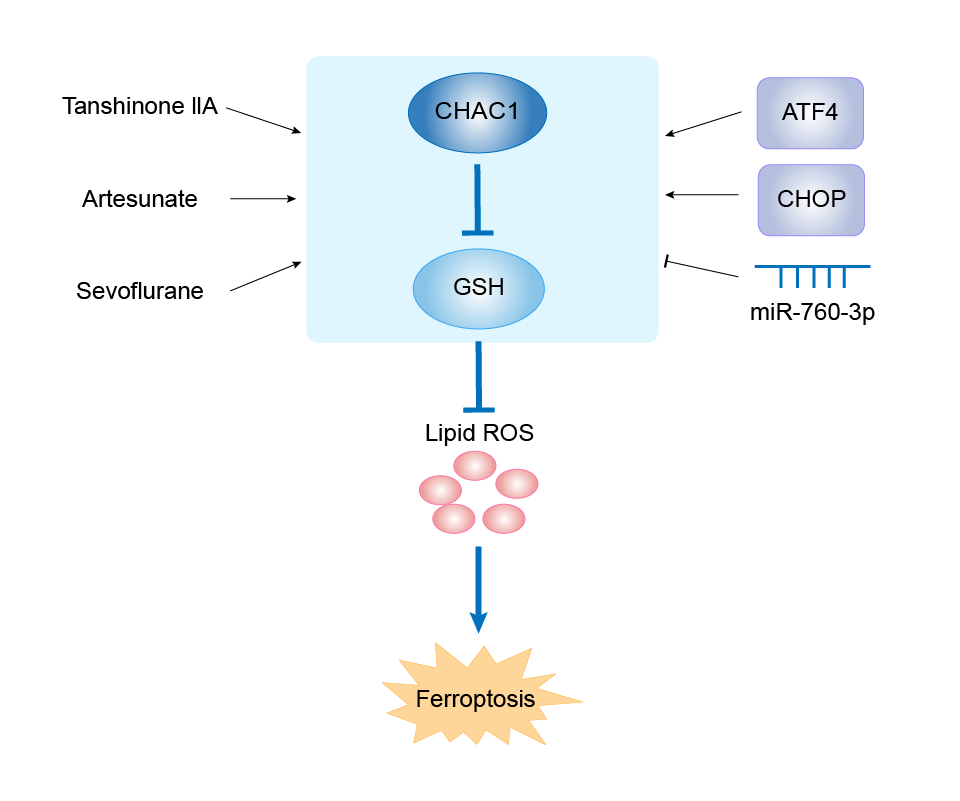
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
CHAC1 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
Cyclic AMP-dependent transcription factor ATF-4 (ATF4)
Burkitt lymphoma [ICD-11: 2A85]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Artesunate | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Daudi cells | Burkitt lymphoma | Homo sapiens | CVCL_0008 | |
| CA46 cells | Burkitt lymphoma | Homo sapiens | CVCL_1101 | ||
| In Vivo Model |
Four -week-old NOD/SCID mice were purchased from Chu Shang Technology (Kunming, China). CA-46 cells were collected and re-suspended in PBS at a concentration of 1-5 x 107 cells/mL. Totally, 0.2 mL cells were inoculated subcutaneously in the middle and posterior armpits of mice. When the transplanted tumor was established, the mice were injected the artesunate solution intraperitoneally in accordance with the body weight (200 mg/kg) daily.
Click to Show/Hide
|
||||
| Response Description | Artesunate induced ferroptosis in different types of Burkitt's lymphoma cells, and caused a significant ERS response in tumor cells. The activation of the ATF4-CHOP-CHAC1 pathway up-regulated the expression of CHAC1 and degraded intracellular GSH, thus weakening the ability of lymphoma cells to resist ferroptosis. | ||||
Cytoplasmic polyadenylation element-binding protein 1 (CPEB1)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
AGS cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| SNU-1 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0099 | ||
| Hs746T cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0333 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| GES-1 cells | Normal | Homo sapiens | CVCL_EQ22 | ||
| In Vivo Model |
Healthy male nude mice for 5 weeks were randomly divided into four groups, namely: A: Lv-NC + Vehicle group, B: Lv-NC + Erastin group, C: Lv-exCPEB1 + Vehicle group, and D: Lv-exCPEB1 + Erastin group. The living environment of nude mice in each group was 12 h light and 12 h dark, the temperature was 22 ± 1, the humidity was 45-55%. The model was established 1 week after adaptive feeding. GC cells (1 x 106) with stable overexpression of CPEB1 at the logarithmic growth stage were selected and injected subcutaneously in nude mice. When the tumor volume reached 100 mm3, nude mice in groups B and D were injected intraperitoneally with Erastin (30 mg/kg) twice a day (morning and night, respectively), and the tumor tissue diameter and volume were measured and calculated every 3 days after administration.
Click to Show/Hide
|
||||
| Response Description | CPEB1 overexpression reduced the expression of twist1, an inhibitor of activating transcription factor 4 (ATF4), thereby activating the ATF4/ChaC Glutathione Specific Gamma-Glutamylcyclotransferase 1 (CHAC1) pathway (CHAC1, a molecule known to induce GSH degradation). Furthermore, re-expression of twist1 in gastric cancer cells impaired the effects of CPEB1 overexpression in presence of erastin. | ||||
Unspecific Regulator
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Responsed Drug | Tanshinone IIA | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| NCI-N87 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1603 | ||
| In Vivo Model |
All mice were housed under a setting of 12-h light/dark cycle at 22 ± 1, 55% humidity and fed with water and food provided at regular time. During the entire maintenance period, all mice were permitted free cage activity without joint immobilization. The initial body weights of the mice were between 20 and 23 grams. After subcutaneous injection of 2 x 106 BGC-823 gastric cancer cells into the back of NOD-SCID mice, the mice were treated with or without Tan IIA (50 mg/kg) or Tan IIA in combination with Fer-1 (50 mg/kg). Tan IIA was diluted in DMSO:Methanol:Hydroxypropyl-b-cydodextrin (HP-b-CD) = 1:1:1. Fer-1 was also dissolved in DMSO:Methanol:HP-b-CD. Seven days after BGC-823 gastric cancer cells injection, intraperitoneal injection with Tan IIA was carried out every other day followed by killing at day 22 of tumor cell inoculation. All mice were killed by dislocation of the cervical vertebrae. Before killing, the tumor volume was measured every 3 days. All experiments were carried out using six mice each group in three independent experiments of a time-dependent manner with three time points.
Click to Show/Hide
|
||||
| Response Description | Tanshinone IIA increased lipid peroxidation and up-regulated Ptgs2 and Chac1 expression, two markers of ferroptosis. In addition, Tan IIA also up-regulated p53 expression and down-regulated xCT expression. Therefore, Tan IIA could suppress the proliferation of gastric cancer via inducing p53 upregulation-mediated ferroptosis. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Responsed Drug | Dihydroartemisinin | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| In Vivo Model |
A total of 32 male BALB/c nude mice (age, 6-8 weeks; weight, 18-20 g) were obtained from HFK Bioscience Co. Ltd. and housed in a specific pathogen-free facility. The mice were randomly divided into four groups (n = 8/group) and PLC cells were subcutaneously injected into the nude mice. When the xenografted tumors had grown to 80-100 mm3, half of the mice in each group were administered with 100 mg/kg DHA for 5 days/week by gavage.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein responseinduced upregulation of CHAC1 expression. DHA also promoted the transcription of CHAC1. | ||||
Cyclic AMP-dependent transcription factor ATF-4 (ATF4)
Artesunate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Burkitt lymphoma [ICD-11: 2A85] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Daudi cells | Burkitt lymphoma | Homo sapiens | CVCL_0008 | |
| CA46 cells | Burkitt lymphoma | Homo sapiens | CVCL_1101 | ||
| In Vivo Model |
Four -week-old NOD/SCID mice were purchased from Chu Shang Technology (Kunming, China). CA-46 cells were collected and re-suspended in PBS at a concentration of 1-5 x 107 cells/mL. Totally, 0.2 mL cells were inoculated subcutaneously in the middle and posterior armpits of mice. When the transplanted tumor was established, the mice were injected the artesunate solution intraperitoneally in accordance with the body weight (200 mg/kg) daily.
Click to Show/Hide
|
||||
| Response Description | Artesunate induced ferroptosis in different types of Burkitt's lymphoma cells, and caused a significant ERS response in tumor cells. The activation of the ATF4-CHOP-CHAC1 pathway up-regulated the expression of CHAC1 and degraded intracellular GSH, thus weakening the ability of lymphoma cells to resist ferroptosis. | ||||
Unspecific Regulator
Tanshinone IIA
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | BGC-823 cells | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| NCI-N87 cells | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1603 | ||
| In Vivo Model |
All mice were housed under a setting of 12-h light/dark cycle at 22 ± 1, 55% humidity and fed with water and food provided at regular time. During the entire maintenance period, all mice were permitted free cage activity without joint immobilization. The initial body weights of the mice were between 20 and 23 grams. After subcutaneous injection of 2 x 106 BGC-823 gastric cancer cells into the back of NOD-SCID mice, the mice were treated with or without Tan IIA (50 mg/kg) or Tan IIA in combination with Fer-1 (50 mg/kg). Tan IIA was diluted in DMSO:Methanol:Hydroxypropyl-b-cydodextrin (HP-b-CD) = 1:1:1. Fer-1 was also dissolved in DMSO:Methanol:HP-b-CD. Seven days after BGC-823 gastric cancer cells injection, intraperitoneal injection with Tan IIA was carried out every other day followed by killing at day 22 of tumor cell inoculation. All mice were killed by dislocation of the cervical vertebrae. Before killing, the tumor volume was measured every 3 days. All experiments were carried out using six mice each group in three independent experiments of a time-dependent manner with three time points.
Click to Show/Hide
|
||||
| Response Description | Tanshinone IIA increased lipid peroxidation and up-regulated Ptgs2 and Chac1 expression, two markers of ferroptosis. In addition, Tan IIA also up-regulated p53 expression and down-regulated xCT expression. Therefore, Tan IIA could suppress the proliferation of gastric cancer via inducing p53 upregulation-mediated ferroptosis. | ||||
Dihydroartemisinin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| PLC/PRF/5 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0485 | ||
| In Vivo Model |
A total of 32 male BALB/c nude mice (age, 6-8 weeks; weight, 18-20 g) were obtained from HFK Bioscience Co. Ltd. and housed in a specific pathogen-free facility. The mice were randomly divided into four groups (n = 8/group) and PLC cells were subcutaneously injected into the nude mice. When the xenografted tumors had grown to 80-100 mm3, half of the mice in each group were administered with 100 mg/kg DHA for 5 days/week by gavage.
Click to Show/Hide
|
||||
| Response Description | Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein responseinduced upregulation of CHAC1 expression. DHA also promoted the transcription of CHAC1. | ||||
References
