Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10035)
| Target Name | Nuclear receptor coactivator 4 (NCOA4) | ||||
|---|---|---|---|---|---|
| Synonyms |
Androgen receptor coactivator 70 kDa protein; Androgen receptor-associated protein of 70 kDa; Ret-activating protein ELE1
Click to Show/Hide
|
||||
| Gene Name | NCOA4 | ||||
| Sequence |
MNTFQDQSGSSSNREPLLRCSDARRDLELAIGGVLRAEQQIKDNLREVKAQIHSCISRHL
ECLRSREVWLYEQVDLIYQLKEETLQQQAQQLYSLLGQFNCLTHQLECTQNKDLANQVSV CLERLGSLTLKPEDSTVLLFEADTITLRQTITTFGSLKTIQIPEHLMAHASSANIGPFLE KRGCISMPEQKSASGIVAVPFSEWLLGSKPASGYQAPYIPSTDPQDWLTQKQTLENSQTS SRACNFFNNVGGNLKGLENWLLKSEKSSYQKCNSHSTTSSFSIEMEKVGDQELPDQDEMD LSDWLVTPQESHKLRKPENGSRETSEKFKLLFQSYNVNDWLVKTDSCTNCQGNQPKGVEI ENLGNLKCLNDHLEAKKPLSTPSMVTEDWLVQNHQDPCKVEEVCRANEPCTSFAECVCDE NCEKEALYKWLLKKEGKDKNGMPVEPKPEPEKHKDSLNMWLCPRKEVIEQTKAPKAMTPS RIADSFQVIKNSPLSEWLIRPPYKEGSPKEVPGTEDRAGKQKFKSPMNTSWCSFNTADWV LPGKKMGNLSQLSSGEDKWLLRKKAQEVLLNSPLQEEHNFPPDHYGLPAVCDLFACMQLK VDKEKWLYRTPLQM Click to Show/Hide
|
||||
| Function |
Enhances the androgen receptor transcriptional activity in prostate cancer cells. Ligand-independent coactivator of the peroxisome proliferator-activated receptor (PPAR) gamma.
Click to Show/Hide
|
||||
| Gene ID | 8031 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
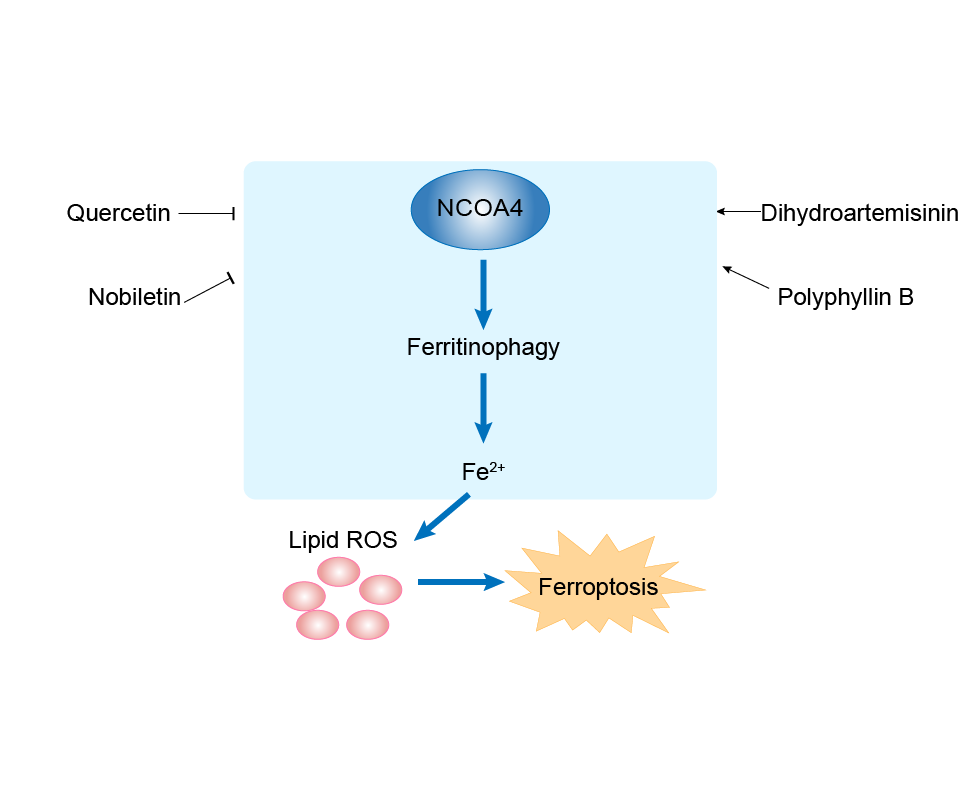
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
NCOA4 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
mRNA decay activator protein ZFP36 (ZFP36)
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
Fifty-six 8-week-old male C57BL/6 mice were obtained from the Experimental Animal Center of Yangzhou University (Yangzhou, China). Controls underwent a sham operation that consisted of exposure, but not ligation, of the common bile duct. Erastin (30 mg/kg, once every other day) and sorafenib (10 mg/kg, once every other day) were suspended in sterile phosphate-buffered saline (PBS; Sigma, P5368) and given by intraperitoneal injection for 2 weeks after the BDL operation. VA-Lip-control-vector and VA-Lip-Zfp36-plasmid (0.75 mg/kg) were administered intravenously 3 times a week for 2 weeks after the BDL operation.
Click to Show/Hide
|
||||
| Response Description | Sorafenib monotherapy led to ZFP36 downregulation, ferritinophagy activation, and ferroptosis induction in human HSCs. ZFP36 plasmid markedly upregulated, whereas FBXW7 plasmid apparently downregulaed, ferritin and NCOA4 expression in sorafenib-treated HSC-LX2 cells. The study identified ZFP36-autophagy-dependent ferroptosis as a potential target for the treatment of liver fibrosis. | ||||
Mitogen-activated protein kinase 14 (MAPK14)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Artesunate | Investigative | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
In Vitro Model |
U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response Description | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 ( MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
Microtubule-associated proteins 1A/1B light chain 3A (MAP1LC3A)
Pulmonary fibrosis [ICD-11: CB03]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Dihydroquercetin | Preclinical | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hBEs (Human bronchial epithelial cells) | ||||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| In Vivo Model |
Eight-week-old male C57BL/6 mice were purchased from Hubei University of Medicine (Shiyan, China). The SiO2-induced mouse pulmonary fibrosis model was performed. In brief, each group of mice was anesthetized with 1% pentobarbital sodium intraperitoneally at 40 mg/kg body weight and their tracheae had been surgically exposed. In addition, SiO2 suspension (20 mg in 50 ul saline) was instilled in the mice. The vehicle control groups were given an equivalent amount of 0.9% sterile saline. After one week of acclimation, mice were divided randomly into four groups (n = 8 per group). Control group, SiO2 group, SiO2 and low dose of DHQ group (DHQ-L, 10 mg/kg) as well as large dose of DHQ group (DHQ-H, 50 mg/kg).
Click to Show/Hide
|
||||
| Response Description | Dihydroquercetin suppressed ferritinophagy by down-regulation of microtubule-associated protein 1A/ 1B-light chain 3 (LC3), and up-regulation of ferritin heavy chain 1 (FTH1), nuclear receptor co-activator 4 (NCOA4) in activated HBE cells. Research revealed that inhibition of ferritinophagy-mediated HBE cells ferroptosis was responsible for DHQ to ameliorate SiO2-induced lung fibrosis. | ||||
F-box/WD repeat-containing protein 7 (FBXW7)
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
Fifty-six 8-week-old male C57BL/6 mice were obtained from the Experimental Animal Center of Yangzhou University (Yangzhou, China). Controls underwent a sham operation that consisted of exposure, but not ligation, of the common bile duct. Erastin (30 mg/kg, once every other day) and sorafenib (10 mg/kg, once every other day) were suspended in sterile phosphate-buffered saline (PBS; Sigma, P5368) and given by intraperitoneal injection for 2 weeks after the BDL operation. VA-Lip-control-vector and VA-Lip-Zfp36-plasmid (0.75 mg/kg) were administered intravenously 3 times a week for 2 weeks after the BDL operation.
Click to Show/Hide
|
||||
| Response Description | Sorafenib monotherapy led to ZFP36 downregulation, ferritinophagy activation, and ferroptosis induction in human HSCs. ZFP36 plasmid markedly upregulated, whereas FBXW7 plasmid apparently downregulaed, ferritin and NCOA4 expression in sorafenib-treated HSC-LX2 cells. The study identified ZFP36-autophagy-dependent ferroptosis as a potential target for the treatment of liver fibrosis. | ||||
ELAV-like protein 1 (ELAVL1)
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
Eight-week-old male C57BL/6 mice were purchased from the Experimental Animal Center of Yangzhou University (Yangzhou, China). Sorafenib (10 mg/kg, once every other day) was suspended in sterile phosphate-buffered saline (PBS; Sigma, P5368) and given by intraperitoneal injection for 2 weeks after the BDL operation. The livers were collected 2 weeks after surgery under general anesthesia.
Click to Show/Hide
|
||||
| Response Description | Sorafenib treatment remarkably upregulated NCOA4 expression, and 3 critical events including ELAVL1 upregulation, ferritinophagy activation, and ferroptosis induction occur in primary human HSCs from fibrotic patients receiving sorafenib monotherapy. ELAVL1 is a potential target for the treatment of liver fibrosis. | ||||
Autophagy-related protein 16-1
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Sorafenib | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
Fifty-six 8-week-old male C57BL/6 mice were obtained from the Experimental Animal Center of Yangzhou University (Yangzhou, China). Controls underwent a sham operation that consisted of exposure, but not ligation, of the common bile duct. Erastin (30 mg/kg, once every other day) and sorafenib (10 mg/kg, once every other day) were suspended in sterile phosphate-buffered saline (PBS; Sigma, P5368) and given by intraperitoneal injection for 2 weeks after the BDL operation. VA-Lip-control-vector and VA-Lip-Zfp36-plasmid (0.75 mg/kg) were administered intravenously 3 times a week for 2 weeks after the BDL operation.
Click to Show/Hide
|
||||
| Response Description | Sorafenib monotherapy led to ZFP36 downregulation, ferritinophagy activation, and ferroptosis induction in human HSCs. ATG16L1 plasmid eliminated the inhibitory action of ZFP36 plasmid on ferroptosis, and FBXW7 plasmid enhanced the effect of ATG16L1 plasmid on autophagy. ZFP36 plasmid markedly upregulated, whereas FBXW7 plasmid apparently downregulaed, ferritin and NCOA4 expression in sorafenib-treated HSC-LX2 cells. The study identified ZFP36-autophagy-dependent ferroptosis as a potential target for the treatment of liver fibrosis. | ||||
Ubiquitin-like modifier-activating enzyme ATG7 (ATG7)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
mEFs (Mouse embryonic fibroblasts) | |||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Panc 02.03 cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1633 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Response Description | Autophagy contributes to ferroptosis by degradation of ferritin in fibroblasts and pancreatic cancer cells. Knockout or knockdown of Atg5 (autophagy-related 5) and Atg7 limited erastin-induced ferroptosis with decreased intracellular ferrous iron levels, and lipid peroxidation. Remarkably, NCOA4 was a selective cargo receptor for the selective autophagic turnover of ferritin (namely ferritinophagy) in ferroptosis. | |||
Transcription factor Jun (JUN)
Degenerative arthritis [ICD-11: FA05]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [6] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hCDs (Chondrocytes) | ||||
| In Vivo Model |
Adult male C57BL/6 mice (eight weeks of age) were used for in vivo experiments. We randomly divided mice into four groups with 9 mice per group: SHAM + AAV9-GFP, SHAM + AAV9-NCOA4, DMM + AAV9-GFP, and DMM + AAV9-NCOA4 groups. For in vivo experiment of SP600125 administration, we randomly divided mice into three groups with 6 mice per group: DMM + AAV9-GFP, DMM + AAV9-GFP + SP600125, and DMM + AAV9-NCOA4 + SP600125. 10 ul of SP600125 (1 mg/kg) or vehicle solution was injected articularly once per week for 7 weeks.
Click to Show/Hide
|
||||
| Response Description | NCOA4 was upregulated in a JNK- JUN signaling-dependent manner in which JUN could directly bind to the promoter of Ncoa4 and initial the transcription of Ncoa4. This work highlights the role of JNK- JUN-NCOA4 axis and ferritinophagy in chondrocyte ferroptosis and osteoarthritis (OA) pathogenesis, suggesting this axis as a potential target for OA treatment. | ||||
Polypyrimidine tract-binding protein 1 (PTBP1)
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [7] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
Huh-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| HEK-293T cells | Normal | Homo sapiens | CVCL_0063 | ||
| In Vivo Model |
A total of eight male BALB/c nude mice (4-5 weeks old; weight, 13-18 g) were obtained from Beijing Vital River Laboratories. The mice were randomly divided into the following two groups: The NC + SF and sh-PTBP1 + SF groups. Hep3B cells (2 x 106) were subcutaneously injected into the right flank of each mouse. The mice were injected intraperitoneally with SF (10 mg/kg) every 2 days for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | PTBP1 mediates ferroptosis in liver cancer cells by regulating NCOA4 translation.In vivoexperiments reconfirmed the role of the PTBP1NCOA4axis in a xenograft transplantation model. It was observed that the mean tumor weight increased afterPTBP1knockout. | ||||
NAD-dependent protein deacylase sirtuin-6 (SIRT6)
Thyroid cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [8] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
CAL62 cells | Thyroid gland anaplastic carcinoma | Homo sapiens | CVCL_1112 | |
| BHT101 cells | Anaplastic thyroid carcinoma | Homo sapiens | CVCL_1085 | ||
| In Vivo Model |
Six-week-old male BALB/c-nu mice were provided by Beijing Vital River Laboratory Animal Technology Co. Ltd. All mice were randomly divided into 6 equal groups (CAL62-NC-Blank, CAL62-NC-sulfasalazine, CAL62-NC-sulfasalazine + CQ; CAL62-SIRT6-Blank, CAL62-SIRT6-sulfasalazine, CAL62-SIRT6-sulfasalazine + CQ). CAL62-NC or CAL62-SIRT6 cells (5 x 106) suspended in 100 ul PBS were injected subcutaneously into the axilla of each nude mouse. After 5 days, the mice were treated with different reagents: solvent (100 ul 0.1 M NaOH and 100 ul saline), sulfasalazine (200 mg/kg, i.p., dissolved in 100 ul 0.1 M NaOH), CQ (50 mg/kg, i.p., dissolved in 100 ul saline).
Click to Show/Hide
|
||||
| Response Description | SIRT6-driven sensitivity to ferroptosis via NCOA4-dependent autophagy and proposed ferroptosis inducers as promising therapeutic agents for anaplastic thyroid cancer patients. The clinically used ferroptosis inducer sulfasalazine showed promising therapeutic effects on SIRT6-upregulated thyroid cancer cells in vivo. | ||||
hsa-miR-6862-5p (miRNA)
Fibrosarcoma [ICD-11: 2B53]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [9] | |||
| Regulator for Ferroptosis | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 |
| Response Description | Reduced NCOA4 expression resulted from a lower rate of hypoxic NCOA4 transcription combined with a micro RNA 6862-5p-dependent degradation of NCOA4 mRNA, the latter being regulated by c-jun N-terminal kinase (JNK). Pharmacological inhibition of JNK under hypoxia increased NCOA4 and prevented FTMT induction in Fibrosarcoma. | |||
Coatomer subunit zeta-1 (COPZ1)
Glioblastoma [ICD-11: 2A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [10] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell proliferation | |||||
In Vitro Model |
U-87MG cells | Glioblastoma | Homo sapiens | CVCL_GP63 | |
| U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | ||
| A-172 cells | Glioblastoma | Homo sapiens | CVCL_0131 | ||
| LN-229 cells | Glioblastoma | Homo sapiens | CVCL_0393 | ||
| T98 cells | Glioblastoma | Homo sapiens | CVCL_B368 | ||
| In Vivo Model |
Mice were divided into two groups (10 mice per group) and anesthetized with an intraperitoneal injection (80 uL) containing ketamine HCl (25 mg/mL), xylazine (2.5 mg/mL), and 14.25% ethyl alcohol (diluted 1:3 in 0.9% NaCl). U87MG-NC and U87MG-sh-COPZ1#1 glioma cells (106 cells diluted in 10 uL PBS per animal) were injected into the right frontal lobes of each mouse using the following coordinates: 1 mm anterior and 2.5 mm lateral to the bregma, at a depth of 2 mm.
Click to Show/Hide
|
||||
| Response Description | COPZ1 knockdown also led to the increase in nuclear receptor coactivator 4 (NCOA4), resulting in the degradation of ferritin, and a subsequent increase in the intracellular levels of ferrous iron and ultimately ferroptosis.The COPZ1/NCOA4/FTH1 axis is therefore a novel therapeutic target for the treatment of human glioblastoma. | ||||
Autophagy protein 5 (ATG5)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [5] | |||
| Regulator for Ferroptosis | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
mEFs (Mouse embryonic fibroblasts) | |||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Panc 02.03 cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1633 | |
| HT-1080 cells | Fibrosarcoma | Homo sapiens | CVCL_0317 | |
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Response Description | Autophagy contributes to ferroptosis by degradation of ferritin in fibroblasts and pancreatic cancer cells. Knockout or knockdown of Atg5 (autophagy-related 5) and Atg7 limited erastin-induced ferroptosis with decreased intracellular ferrous iron levels, and lipid peroxidation. Remarkably, NCOA4 was a selective cargo receptor for the selective autophagic turnover of ferritin (namely ferritinophagy) in ferroptosis. | |||
Alpha-ketoglutarate-dependent dioxygenase FTO (FTO)
Liver fibrosis [ICD-11: DB93]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [11] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
ICR mice (8-week-old, 18-22 g) were obtained from Yangzhou University (Yangzhou, China). There were 8 mice in each group and they were randomly divided into 6 groups. Mice were treated with Vehicle, CCl4, VA-Lip-control-vector+CCl4+Erastin, VA-Lip-Mettl4-shRNA+CCl4+Erastin, VA-Lip-Fto-plasmid+CCl4+Erastin, VA-Lip- Ythdf1-shRNA+CCl4+Erastin, respectively. A mixture of olive oil and carbon tetrachloride (CCl4) (9:1 (v/v)) was used to trigger liver fibrosis in mouse model by intraperitoneal injection (0.1 ml/20 g body weight), according to our previous reports.
Click to Show/Hide
|
||||
| Response Description | m6A reader YTHDF1 promoted BECN1 mRNA stability via recognizing the m6A binding site, thus triggering autophagy activation, and eventually leading to HSC ferroptosis. FTO plasmid and METTL4 shRNA markedly impaired erastin-induced upregulation of NCOA4 and downregulation of FTH1 in HSC-LX2 cells. Overall, m6A modification-dependent ferroptosis as a potential target for the treatment of liver fibrosis. | ||||
Unspecific Regulator
Oesophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [12] | ||||
| Responsed Drug | Allicin | Investigative | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| HET-1A cells | Normal | Homo sapiens | CVCL_3702 | ||
| In Vivo Model |
All mice were housed in a specific pathogen-free environment under a standard 12 h light-dark cycle at 25 and had ad libitum access to food and water. Approximately 4 x 106 KYSE510 cells in 100 uL of normal saline were subcutaneously injected into the right flank of mice (n = 20 in total). All mice were allocated to a control or 10 mg/kg allicin group (n = 10 per group), as previously described (Suddek 2014). The mice were orally administered allicin or normal saline once daily for 28 days.
Click to Show/Hide
|
||||
| Response Description | In summary, allicin may induce cell death in esophageal squamous cell carcinoma (ESCC) cells by activating AMPK/mTOR-mediated autophagy and ferroptosis. Furthermore, ATG5 and ATG7 expression increased in tumors after allicin treatment. In contrast, NCOA4 expression increased, but the protein level of FTH1 and TfR1 decreased in tumors after allicin treatment. | ||||
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [13] | ||||
| Responsed Drug | Polyphyllin B | Investigative | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
In Vitro Model |
NUGC-3 cells | Gastric carcinoma | Homo sapiens | CVCL_1612 | |
| MKN-1 cells | Gastric carcinoma | Homo sapiens | CVCL_1415 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| In Vivo Model |
The nude mice were raised in our laboratory for a week before the experiment. Then, 5 x 106 MKN-1 cells were subcutaneously injected to establish the subcutaneous xenograft tumour model in nude mice. When the maximum diameter of the xenograft tumours grew steadily to 1 cm, they were dissected completely and cut into 1 mm3 tissue fragments. Then, the tissue fragment was inserted into the surface of the serosa on the greater curvature of the stomach. Different doses of PB (2.5 mg/kg or 5.0 mg/kg) were given by intraperitoneal injection once a day for 3 weeks. The control group was given the same volume of vehicle. The positive control group was given 5-Fu at the dose of 10 mg/kg. The body weight and tumour size of nude mice were recorded. Mice were administered fluorescein substrate (150 mg/kg) intraperitoneally for in vivo imaging twice a week on a Xenogen IVIS 200 imaging system (Caliper Life Sciences, USA). The tumour inhibition rate was analysed using LT Living Image 4.3 Software.
Click to Show/Hide
|
||||
| Response Description | We identified a novel GPx4 inhibitor, polyphyllin B (PB), which can induce ferroptosis by down-regulating GPx4 expression in gastric cancer cells. It has also been shown to inhibit cell proliferation, suppress invasion and migration, induce apoptosis, and block the cell cycle progression in GC cellsin vitro. Then, immunofluorescence and western blotting assay confirmed that PB can regulate the expression of LC3B, TFR1, NOCA4 and FTH1in vitro, which suggested that suggest that PB may increase the level of Fe2+by transporting Fe3+into the cell by TFR1 and promoting NCOA4-dependent iron autophagy. | ||||
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [14] | ||||
| Responsed Drug | Itaconic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 cells in 100 ul PBS were injected subcutaneously into the right of the dorsal midline in 6- to 8-week-old femaleathymic nude mice(n = 6 mice/group). After the tumor reached 60-80 mm3 on day 7, the mice were randomly grouped and then given intraperitoneal injections with itaconic acid (50 mg/kg, once every other day) at day 7 for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Itaconic acid-induced expression and activation of NFE2L2 serves as a defense mechanism to limit ferroptosis by producing antioxidant genes. Consequently, impaired NCOA4 expression prevented, whereas a disrupted NFE2L2 pathway enhanced, sensitivity to itaconic acid-induced ferroptosis in pancreatic cancer cells. | ||||
Hepatocellular carcinoma [ICD-11: 2C12]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [15] | |||
| Responsed Drug | Formosanin C | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response Description | The saponin formosanin C (FC) is a novel natural ferroptosis inducer, which triggered a stronger ferroptosis in human hepatocellular carcinoma HepG2 cells containing a higher level of NCOA4 and a lower level of FTH1 compared to Hep3B cells. | |||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [16] | |||
| Responsed Drug | 2-pyridylhydrazone dithiocarbamate s-acetic acid | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| Cell apoptosis | ||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Response Description | 2-pyridylhydrazone dithiocarbamate s-acetic acid (PdtaA) induced both apoptosis and cell cycle arrest. Notably, PdtaA also induced ferroptosis via downregulation of GPx4 and xCT in liver cancer cells. Autophagy inhibitor 3-methyladenin or genetic knockdown of NCOA4 was employed to inhibit ferritinophagy, which significantly neutralized the action of PdtaA in both apoptosis and ferroptosis. | |||
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [18] | |||
| Responsed Drug | Dihydroartemisinin | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SiHa cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | |
| Response Description | Dihydroartemisinin (DHA) treatment initiated ferroptosis, as evidenced by the accumulation of reactive oxygen species (ROS), malondialdehyde (MDA) and liquid peroxidation (LPO) levels and simultaneously depletion of glutathione peroxidase 4 (GPX4) and glutathione (GSH). Moreover, nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy was also induced by DHA leading to subsequent increases of intracellular labile iron pool (LIP), exacerbated the Fenton reaction resulting in excessive ROS production, and enhanced cervical cancer ferroptosis. | |||
Diabetes mellitus [ICD-11: 5A10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [19] | ||||
| Responsed Drug | Cryptochlorogenic acid | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
INS-1 cells | Insulinoma | Rattus norvegicus | CVCL_0352 | |
| In Vivo Model |
Sixty Sprague-Dawley (SD) rats with weights ranging from 250-270 g were obtained from experimental animal center of Xiamen university. For diabetes model group, fasting was performed for 12 h before experiment. The rats (ten rats per group) were assigned into Control group, Model (DM) treated with 50 mg/kg streptozotocin (STZ) via abdominal injection, positive control group and experimental groups. The blood glucose level, which is served as the indicator for the diabetes, was monitored herein. The glucose level after modeling is above 16.7 mmol/l, supporting that the modeling is successful.
Click to Show/Hide
|
||||
| Response Description | Cryptochlorogenic acid (CCA) functions via inhibition of ferroptosis by activation of cystine/glutamate transporter system (XC)/glutathione peroxidase 4(GPX4)/Nrf2 and inhibition of nuclear receptor coactivator 4 (NCOA4) in diabetes. System xc- which is composed of SLC7A11 and SLC3A2, served as the provider of GSH synthesis. | ||||
Parkinson disease [ICD-11: 8A00]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [20] | ||||
| Responsed Drug | Paraquat | Investigative | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| Ferroptosis | hsa04216 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
In Vitro Model |
SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Twenty male C57BL/6 mice at 12 weeks old were purchased from Hebei Medical University Experimental Animal Center. 10 mice of the experimental group were intraperitoneally injected with PQ (10 mg PQ (salt)/kg/dose) three times a week for 3 weeks according to the previous report. Ten mice of the control group were intraperitoneally injected with the same dose of normal saline. Once the experimental schedule was completed, firstly, the animals were used for behavioral tests. Then, the mice were anesthetized with 0.4% pentobarbital sodium (1 mL/100 g) solution and perfused. The substantia nigra tissue was exfoliated for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Paraquat (PQ) significantly caused the iron accumulation in cytoplasm and mitochondria through ferritinophagy pathway induced by NCOA4. Iron overload initiated lipid peroxidation through 12Lox, further inducing ferroptosis by producing lipid ROS. PQ downregulated SLC7A11 and GPX4 expression and upregulated Cox2 expression. Bcl2/Bax and P-p38/p38 pathways mediated the cross-talk between ferroptosis and apoptosis induced by PQ. These data further demonstrated the complexity of Parkinson's disease occurrence. | ||||
Periodontitis [ICD-11: DA0C]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [21] | |||
| Responsed Drug | Butyrate | Investigative | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
In Vitro Model |
hPDLFs (Human periodontal ligament fibroblasts) | |||
| Response Description | Periodontitis-level butyrate disrupted iron homeostasis by activation of NCOA4-mediated ferritinophagy, leading to ferroptosis in PDLFs. Butyrate-induced iron accumulation, reactive oxygen species (ROS) generation, glutathione depletion and lipid peroxidation in PDLFs, and the butyrate-induced ferroptosis can be blocked by the lipid peroxide scavenger ferrostatin-1. | |||
Ischemia/reperfusion injury [ICD-11: DB98]
| In total 3 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [22] | ||||
| Responsed Drug | Nobiletin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague-Dawley (SD) rats (4 weeks old, 90-110 g) were obtained from the Vital River Biological company. Rats were kept in a specific-pathogen-free (SPF) environment, with access to food and tap water at an ambient temperature of 20-22 . All institutional and national guidelines for the care and use of laboratory animals were followed. The protocols were reviewed and approved by the Institution of Animal Care and Use Committee of Renmin Hospital of Wuhan University (IACUC, license no. 20200303).
Click to Show/Hide
|
||||
| Response Description | Both ferrostain-1 and nobiletin decreased the expression of ferroptosis-related proteins including Acyl-CoA synthetase long chain family member 4 (ACSL4) and nuclear receptor coactivator 4 (NCOA4) but not glutathione peroxidase 4 (GPX4) in rats with mature T2DM and cells with HFHG and H/R injury. Nobiletin has therapeutic potential for alleviating myocardial ischemia-reperfusion injury associated with ACSL4- and NCOA4-related ferroptosis. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Disease Response of This Regulator | [23] | ||||
| Responsed Drug | DNA (cytosine-5)-methyltransferase 1 | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Fifty specific pathogen-free male SpragueDawley rats (weighing 210-240 g) were purchased from Beijing Huakang Biotechnology Co., Ltd (Beijing, China). The DS model was established by injecting 1% streptozotocin into the tail vein at 60 mg/kg dose. After 3 days, if the fasting blood glucose level was higher than 16.7 mmol/L, the DS model was successfully built. The NS and the I/R group were given 0.9% sodium chloride injection. Thereafter, the general conditions for normal and DM rats are showed in Table Table2.2. After 8 weeks, all the rats were intraperitoneally injected with 1.5% sodium pentobarbital at a dose of 0.005 mL/g. They were given electrocardiogram (ECG) monitoring management.
Click to Show/Hide
|
||||
| Response Description | Inhibition of DNA (cytosine-5)-methyltransferase 1 (DNMT-1) could reduce ferroptosis during diabetes myocardial ischemia/reperfusion injury and the NCOA4-mediated ferritinophagy may participate in the process. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Disease Response of This Regulator | [24] | ||||
| Responsed Drug | Cyanidin-3-glucoside | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
In Vitro Model |
CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Adult male Sprague Dawley (SD) rats weighing 260-280 g were purchased from Qinglongshan Animal Farm (Nanjing, China). After a week of adaptation, the rats were randomly assigned into five groups (n = 8): (1) sham group, rats receiving saline gavage and sham surgery were used as control group; (2) I/R model group, rats receiving saline gavage and left anterior descending (LAD) ligation surgery were used as the model group; (3) C3G-10 group, I/R model plus intraperitoneal injection of 10 mg/kg C3G; (4) C3G-20 group, I/R model plus intraperitoneal injection of 20 mg/kg C3G; and (5) DIL group, I/R model plus oral administration of 20 mg/kg diltiazem. C3G and DIL were dissolved in DMSO and then diluted with saline so that the DMSO concentration was less than 0.1% (v/v).
Click to Show/Hide
|
||||
| Response Description | The administration of Cyanidin-3-glucoside (C3G) reduced the infarction area, mitigated pathological alterations, inhibited ST segment elevation, and attenuated oxidative stress and ferroptosis-related protein expression. C3G also suppressed the expressions of USP19, Beclin1, NCOA4, and LC3II/LC3I. In addition, treatment with C3G relieved oxidative stress, downregulated LC3II/LC3I, reduced autophagosome number, downregulated TfR1 expression, and upregulated the expressions of FTH1 and GPX4 in OGD/R-induced H9c2 cells. Taken together, C3G could be a potential agent to protect myocardium from myocardial ischemia-reperfusion (IR) injury. | ||||
Acute kidney failure [ICD-11: GB60]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [25] | ||||
| Responsed Drug | Isoliquiritigenin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (aged 6-8 weeks and weighing 22-25g) were obtained from the Experimental Animal Center, Sichuan Provincial Peoples Hospital, and were fed a standard laboratory diet. LPS and ISL were dissolved in normal saline and 0.5% Tween-20/saline, respectively. AKI mice were developed by intraperitoneal (i.p.) LPS injection. A total of 30 mice were randomly divided into six groups (n = 5): control, ISL, Fer, LPS, LPS plus ISL, and LPS plus Fer. An intraperitoneal injection of LPS (10 mg/kg) was made to induce septic AKI. ISL was administered via gavage at 50 mg/kg 30 min before LPS injection. Mice were dosed intraperitoneally with Fer (Ferrostatin-1, SML0583, Sigma-Aldrich, St. Louis, MO) at 5 mg/kg. Mice were sacrificed by cervical dislocation 8 h after LPS injection. Kidney tissue and serum samples were collected concurrently.
Click to Show/Hide
|
||||
| Response Description | Isoliquiritigenin attenuates septic acute kidney injury by regulating ferritinophagy-mediated ferroptosis. ISL inhibited Fe2+ and lipid peroxidation accumulation in LPS-stimulated HK2 cells. It also increased the expression of GPX4 and xCT, reduced the expression of HMGB1 and NCOA4 then attenuated mitochondria injury in renal tubular following LPS stimulation. | ||||
Kidney calculus [ICD-11: GB70]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [26] | ||||
| Responsed Drug | Oxalate | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Five-week-old male SD (Sprague-Dawley) rats (130-180 g) were used as experimental subjects. The control group had a normal diet, and the stone model group drank water containing 0.75% ethylene glycol. After feeding for one month, rats were sacrificed, and their kidneys were removed and subjected to silver nitrate staining, immunohistochemistry, and western blotting on the specimens of the normal control group and the kidney stone model group to explore the expression of NCOA4 in kidney stone model rats.
Click to Show/Hide
|
||||
| Response Description | Oxalate activates autophagy to induce ferroptosis of renal tubular epithelial cells and participates in the formation of kidney stones. Moreover, after oxalate treatment, overexpression of the BENC1 gene increased cell oxidative damage and ferroptosis. In addition, knockdown of NCOA4 reversed the effect of oxalate-induced ferroptosis in HK-2 cells. | ||||
Injury of intra-abdominal organs [ICD-11: NB91]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [27] | ||||
| Responsed Drug | Quercetin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
In Vitro Model |
Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| In Vivo Model |
Six-week-old male C57BL/6J mice (18-20 g) were obtained from Zhejiang Vital River Laboratory (Zhejiang, China). 32 mice were divided randomly into 4 groups: Saline group (CONT), 25 mg/kg/day ACR group (ACR), 25 mg/kg/day ACR with a low dose of 25 mg/kg/day QCT group (ACR + QCT (L)), and 25 mg/kg/day ACR with a high dose of 50 mg/kg/day QCT group (ACR + QCT (H)), 8 animals in each group.
Click to Show/Hide
|
||||
| Response Description | Quercetin (QCT) specifically reacted with autophagic cargo receptor NCOA4, blocked the degradation of iron storage protein FTH1, and eventually downregulated the intracellular iron levels and the consequent ferroptosis. Collectively, our results presented a unique approach to alleviate ACR-induced liver injury by targeting ferroptosis with QCT. | ||||
mRNA decay activator protein ZFP36 (ZFP36)
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Liver fibrosis [ICD-11: DB93] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
Fifty-six 8-week-old male C57BL/6 mice were obtained from the Experimental Animal Center of Yangzhou University (Yangzhou, China). Controls underwent a sham operation that consisted of exposure, but not ligation, of the common bile duct. Erastin (30 mg/kg, once every other day) and sorafenib (10 mg/kg, once every other day) were suspended in sterile phosphate-buffered saline (PBS; Sigma, P5368) and given by intraperitoneal injection for 2 weeks after the BDL operation. VA-Lip-control-vector and VA-Lip-Zfp36-plasmid (0.75 mg/kg) were administered intravenously 3 times a week for 2 weeks after the BDL operation.
Click to Show/Hide
|
||||
| Response Description | Sorafenib monotherapy led to ZFP36 downregulation, ferritinophagy activation, and ferroptosis induction in human HSCs. ZFP36 plasmid markedly upregulated, whereas FBXW7 plasmid apparently downregulaed, ferritin and NCOA4 expression in sorafenib-treated HSC-LX2 cells. The study identified ZFP36-autophagy-dependent ferroptosis as a potential target for the treatment of liver fibrosis. | ||||
Mitogen-activated protein kinase 14 (MAPK14)
Artesunate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Glioblastoma [ICD-11: 2A00] | ||||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | U-251MG cells | Astrocytoma | Homo sapiens | CVCL_0021 | |
| In Vivo Model |
The xenografts were established via the subcutaneous inoculation of U251 cells (1 x 107 cells/per mouse) into the armpit of one mouse. After two weeks of growth, the cancer tissues were cut into pieces with the dimensions of 1.5 x 1.5 x 1.5 mm3 and inoculated subcutaneously into the right armpit of the mice with a puncture needle. When tumor volume reached approximately 80 mm3, mice were randomly divided into four groups (n = 5): Vehicle control, ART (20 mg/kg), ART (40 mg/kg), and TMZ (40 mg/kg). TMZ was used as the positive control. Drugs and vehicle were given by intraperitoneal injection daily for 21 days. Tumor volume and body weight were measured every three days.
Click to Show/Hide
|
||||
| Response Description | Artesunate triggers ferroptosis in glioblastoma in vitro and in vivo through regulation of iron metabolism and p38 ( MAPK14) and ERK signaling pathways. Meanwhile, ART reduced the protein level of GPX4 and FPN1, increased the protein level of DMT1, TfR, ferritin and NCOA4. | ||||
Microtubule-associated proteins 1A/1B light chain 3A (MAP1LC3A)
Dihydroquercetin
[Preclinical]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Pulmonary fibrosis [ICD-11: CB03] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hBEs (Human bronchial epithelial cells) | ||||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| In Vivo Model |
Eight-week-old male C57BL/6 mice were purchased from Hubei University of Medicine (Shiyan, China). The SiO2-induced mouse pulmonary fibrosis model was performed. In brief, each group of mice was anesthetized with 1% pentobarbital sodium intraperitoneally at 40 mg/kg body weight and their tracheae had been surgically exposed. In addition, SiO2 suspension (20 mg in 50 ul saline) was instilled in the mice. The vehicle control groups were given an equivalent amount of 0.9% sterile saline. After one week of acclimation, mice were divided randomly into four groups (n = 8 per group). Control group, SiO2 group, SiO2 and low dose of DHQ group (DHQ-L, 10 mg/kg) as well as large dose of DHQ group (DHQ-H, 50 mg/kg).
Click to Show/Hide
|
||||
| Response Description | Dihydroquercetin suppressed ferritinophagy by down-regulation of microtubule-associated protein 1A/ 1B-light chain 3 (LC3), and up-regulation of ferritin heavy chain 1 (FTH1), nuclear receptor co-activator 4 (NCOA4) in activated HBE cells. Research revealed that inhibition of ferritinophagy-mediated HBE cells ferroptosis was responsible for DHQ to ameliorate SiO2-induced lung fibrosis. | ||||
F-box/WD repeat-containing protein 7 (FBXW7)
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Liver fibrosis [ICD-11: DB93] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
Fifty-six 8-week-old male C57BL/6 mice were obtained from the Experimental Animal Center of Yangzhou University (Yangzhou, China). Controls underwent a sham operation that consisted of exposure, but not ligation, of the common bile duct. Erastin (30 mg/kg, once every other day) and sorafenib (10 mg/kg, once every other day) were suspended in sterile phosphate-buffered saline (PBS; Sigma, P5368) and given by intraperitoneal injection for 2 weeks after the BDL operation. VA-Lip-control-vector and VA-Lip-Zfp36-plasmid (0.75 mg/kg) were administered intravenously 3 times a week for 2 weeks after the BDL operation.
Click to Show/Hide
|
||||
| Response Description | Sorafenib monotherapy led to ZFP36 downregulation, ferritinophagy activation, and ferroptosis induction in human HSCs. ZFP36 plasmid markedly upregulated, whereas FBXW7 plasmid apparently downregulaed, ferritin and NCOA4 expression in sorafenib-treated HSC-LX2 cells. The study identified ZFP36-autophagy-dependent ferroptosis as a potential target for the treatment of liver fibrosis. | ||||
ELAV-like protein 1 (ELAVL1)
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Liver fibrosis [ICD-11: DB93] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
Eight-week-old male C57BL/6 mice were purchased from the Experimental Animal Center of Yangzhou University (Yangzhou, China). Sorafenib (10 mg/kg, once every other day) was suspended in sterile phosphate-buffered saline (PBS; Sigma, P5368) and given by intraperitoneal injection for 2 weeks after the BDL operation. The livers were collected 2 weeks after surgery under general anesthesia.
Click to Show/Hide
|
||||
| Response Description | Sorafenib treatment remarkably upregulated NCOA4 expression, and 3 critical events including ELAVL1 upregulation, ferritinophagy activation, and ferroptosis induction occur in primary human HSCs from fibrotic patients receiving sorafenib monotherapy. ELAVL1 is a potential target for the treatment of liver fibrosis. | ||||
Autophagy-related protein 16-1
Sorafenib
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Liver fibrosis [ICD-11: DB93] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hHSCs (Human hepatic stellate cells) | ||||
| In Vivo Model |
Fifty-six 8-week-old male C57BL/6 mice were obtained from the Experimental Animal Center of Yangzhou University (Yangzhou, China). Controls underwent a sham operation that consisted of exposure, but not ligation, of the common bile duct. Erastin (30 mg/kg, once every other day) and sorafenib (10 mg/kg, once every other day) were suspended in sterile phosphate-buffered saline (PBS; Sigma, P5368) and given by intraperitoneal injection for 2 weeks after the BDL operation. VA-Lip-control-vector and VA-Lip-Zfp36-plasmid (0.75 mg/kg) were administered intravenously 3 times a week for 2 weeks after the BDL operation.
Click to Show/Hide
|
||||
| Response Description | Sorafenib monotherapy led to ZFP36 downregulation, ferritinophagy activation, and ferroptosis induction in human HSCs. ATG16L1 plasmid eliminated the inhibitory action of ZFP36 plasmid on ferroptosis, and FBXW7 plasmid enhanced the effect of ATG16L1 plasmid on autophagy. ZFP36 plasmid markedly upregulated, whereas FBXW7 plasmid apparently downregulaed, ferritin and NCOA4 expression in sorafenib-treated HSC-LX2 cells. The study identified ZFP36-autophagy-dependent ferroptosis as a potential target for the treatment of liver fibrosis. | ||||
Unspecific Regulator
Allicin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [12] | ||||
| Responsed Disease | Oesophageal cancer [ICD-11: 2B70] | ||||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| HET-1A cells | Normal | Homo sapiens | CVCL_3702 | ||
| In Vivo Model |
All mice were housed in a specific pathogen-free environment under a standard 12 h light-dark cycle at 25 and had ad libitum access to food and water. Approximately 4 x 106 KYSE510 cells in 100 uL of normal saline were subcutaneously injected into the right flank of mice (n = 20 in total). All mice were allocated to a control or 10 mg/kg allicin group (n = 10 per group), as previously described (Suddek 2014). The mice were orally administered allicin or normal saline once daily for 28 days.
Click to Show/Hide
|
||||
| Response Description | In summary, allicin may induce cell death in esophageal squamous cell carcinoma (ESCC) cells by activating AMPK/mTOR-mediated autophagy and ferroptosis. Furthermore, ATG5 and ATG7 expression increased in tumors after allicin treatment. In contrast, NCOA4 expression increased, but the protein level of FTH1 and TfR1 decreased in tumors after allicin treatment. | ||||
Polyphyllin B
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [13] | ||||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | ||||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | NUGC-3 cells | Gastric carcinoma | Homo sapiens | CVCL_1612 | |
| MKN-1 cells | Gastric carcinoma | Homo sapiens | CVCL_1415 | ||
| MKN45 cells | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
| HGC-27 cells | Gastric carcinoma | Homo sapiens | CVCL_1279 | ||
| NUGC-4 cells | Gastric signet ring cell adenocarcinoma | Homo sapiens | CVCL_3082 | ||
| In Vivo Model |
The nude mice were raised in our laboratory for a week before the experiment. Then, 5 x 106 MKN-1 cells were subcutaneously injected to establish the subcutaneous xenograft tumour model in nude mice. When the maximum diameter of the xenograft tumours grew steadily to 1 cm, they were dissected completely and cut into 1 mm3 tissue fragments. Then, the tissue fragment was inserted into the surface of the serosa on the greater curvature of the stomach. Different doses of PB (2.5 mg/kg or 5.0 mg/kg) were given by intraperitoneal injection once a day for 3 weeks. The control group was given the same volume of vehicle. The positive control group was given 5-Fu at the dose of 10 mg/kg. The body weight and tumour size of nude mice were recorded. Mice were administered fluorescein substrate (150 mg/kg) intraperitoneally for in vivo imaging twice a week on a Xenogen IVIS 200 imaging system (Caliper Life Sciences, USA). The tumour inhibition rate was analysed using LT Living Image 4.3 Software.
Click to Show/Hide
|
||||
| Response Description | We identified a novel GPx4 inhibitor, polyphyllin B (PB), which can induce ferroptosis by down-regulating GPx4 expression in gastric cancer cells. It has also been shown to inhibit cell proliferation, suppress invasion and migration, induce apoptosis, and block the cell cycle progression in GC cellsin vitro. Then, immunofluorescence and western blotting assay confirmed that PB can regulate the expression of LC3B, TFR1, NOCA4 and FTH1in vitro, which suggested that suggest that PB may increase the level of Fe2+by transporting Fe3+into the cell by TFR1 and promoting NCOA4-dependent iron autophagy. | ||||
Itaconic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [14] | ||||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| THP-1 cells | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 PANC1 cells in 100 ul PBS were injected subcutaneously into the right of the dorsal midline in 6- to 8-week-old femaleathymic nude mice(n = 6 mice/group). After the tumor reached 60-80 mm3 on day 7, the mice were randomly grouped and then given intraperitoneal injections with itaconic acid (50 mg/kg, once every other day) at day 7 for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | Itaconic acid-induced expression and activation of NFE2L2 serves as a defense mechanism to limit ferroptosis by producing antioxidant genes. Consequently, impaired NCOA4 expression prevented, whereas a disrupted NFE2L2 pathway enhanced, sensitivity to itaconic acid-induced ferroptosis in pancreatic cancer cells. | ||||
Formosanin C
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [15] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Autophagy | hsa04140 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | Hep 3B2.1-7 cells | Hepatocellular carcinoma | Homo sapiens | CVCL_0326 |
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Response Description | The saponin formosanin C (FC) is a novel natural ferroptosis inducer, which triggered a stronger ferroptosis in human hepatocellular carcinoma HepG2 cells containing a higher level of NCOA4 and a lower level of FTH1 compared to Hep3B cells. | |||
2-pyridylhydrazone dithiocarbamate s-acetic acid
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [16] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| Cell apoptosis | ||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Response Description | 2-pyridylhydrazone dithiocarbamate s-acetic acid (PdtaA) induced both apoptosis and cell cycle arrest. Notably, PdtaA also induced ferroptosis via downregulation of GPx4 and xCT in liver cancer cells. Autophagy inhibitor 3-methyladenin or genetic knockdown of NCOA4 was employed to inhibit ferritinophagy, which significantly neutralized the action of PdtaA in both apoptosis and ferroptosis. | |||
Dihydroartemisinin
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [18] | |||
| Responsed Disease | Cervical cancer [ICD-11: 2C77] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HeLa cells | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| SiHa cells | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | |
| Response Description | Dihydroartemisinin (DHA) treatment initiated ferroptosis, as evidenced by the accumulation of reactive oxygen species (ROS), malondialdehyde (MDA) and liquid peroxidation (LPO) levels and simultaneously depletion of glutathione peroxidase 4 (GPX4) and glutathione (GSH). Moreover, nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy was also induced by DHA leading to subsequent increases of intracellular labile iron pool (LIP), exacerbated the Fenton reaction resulting in excessive ROS production, and enhanced cervical cancer ferroptosis. | |||
Cryptochlorogenic acid
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [19] | ||||
| Responsed Disease | Diabetes mellitus [ICD-11: 5A10] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | INS-1 cells | Insulinoma | Rattus norvegicus | CVCL_0352 | |
| In Vivo Model |
Sixty Sprague-Dawley (SD) rats with weights ranging from 250-270 g were obtained from experimental animal center of Xiamen university. For diabetes model group, fasting was performed for 12 h before experiment. The rats (ten rats per group) were assigned into Control group, Model (DM) treated with 50 mg/kg streptozotocin (STZ) via abdominal injection, positive control group and experimental groups. The blood glucose level, which is served as the indicator for the diabetes, was monitored herein. The glucose level after modeling is above 16.7 mmol/l, supporting that the modeling is successful.
Click to Show/Hide
|
||||
| Response Description | Cryptochlorogenic acid (CCA) functions via inhibition of ferroptosis by activation of cystine/glutamate transporter system (XC)/glutathione peroxidase 4(GPX4)/Nrf2 and inhibition of nuclear receptor coactivator 4 (NCOA4) in diabetes. System xc- which is composed of SLC7A11 and SLC3A2, served as the provider of GSH synthesis. | ||||
Paraquat
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [20] | ||||
| Responsed Disease | Parkinson disease [ICD-11: 8A00] | ||||
| Pathway Response | Apoptosis | hsa04210 | |||
| Ferroptosis | hsa04216 | ||||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| Cell apoptosis | |||||
| In Vitro Model | SH-SY5Y cells | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In Vivo Model |
Twenty male C57BL/6 mice at 12 weeks old were purchased from Hebei Medical University Experimental Animal Center. 10 mice of the experimental group were intraperitoneally injected with PQ (10 mg PQ (salt)/kg/dose) three times a week for 3 weeks according to the previous report. Ten mice of the control group were intraperitoneally injected with the same dose of normal saline. Once the experimental schedule was completed, firstly, the animals were used for behavioral tests. Then, the mice were anesthetized with 0.4% pentobarbital sodium (1 mL/100 g) solution and perfused. The substantia nigra tissue was exfoliated for subsequent experiments.
Click to Show/Hide
|
||||
| Response Description | Paraquat (PQ) significantly caused the iron accumulation in cytoplasm and mitochondria through ferritinophagy pathway induced by NCOA4. Iron overload initiated lipid peroxidation through 12Lox, further inducing ferroptosis by producing lipid ROS. PQ downregulated SLC7A11 and GPX4 expression and upregulated Cox2 expression. Bcl2/Bax and P-p38/p38 pathways mediated the cross-talk between ferroptosis and apoptosis induced by PQ. These data further demonstrated the complexity of Parkinson's disease occurrence. | ||||
Butyrate
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [21] | |||
| Responsed Disease | Periodontitis [ICD-11: DA0C] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| Cell autophagy | ||||
| In Vitro Model | hPDLFs (Human periodontal ligament fibroblasts) | |||
| Response Description | Periodontitis-level butyrate disrupted iron homeostasis by activation of NCOA4-mediated ferritinophagy, leading to ferroptosis in PDLFs. Butyrate-induced iron accumulation, reactive oxygen species (ROS) generation, glutathione depletion and lipid peroxidation in PDLFs, and the butyrate-induced ferroptosis can be blocked by the lipid peroxide scavenger ferrostatin-1. | |||
Nobiletin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [22] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Male Sprague-Dawley (SD) rats (4 weeks old, 90-110 g) were obtained from the Vital River Biological company. Rats were kept in a specific-pathogen-free (SPF) environment, with access to food and tap water at an ambient temperature of 20-22 . All institutional and national guidelines for the care and use of laboratory animals were followed. The protocols were reviewed and approved by the Institution of Animal Care and Use Committee of Renmin Hospital of Wuhan University (IACUC, license no. 20200303).
Click to Show/Hide
|
||||
| Response Description | Both ferrostain-1 and nobiletin decreased the expression of ferroptosis-related proteins including Acyl-CoA synthetase long chain family member 4 (ACSL4) and nuclear receptor coactivator 4 (NCOA4) but not glutathione peroxidase 4 (GPX4) in rats with mature T2DM and cells with HFHG and H/R injury. Nobiletin has therapeutic potential for alleviating myocardial ischemia-reperfusion injury associated with ACSL4- and NCOA4-related ferroptosis. | ||||
DNA (cytosine-5)-methyltransferase 1
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [23] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Fifty specific pathogen-free male SpragueDawley rats (weighing 210-240 g) were purchased from Beijing Huakang Biotechnology Co., Ltd (Beijing, China). The DS model was established by injecting 1% streptozotocin into the tail vein at 60 mg/kg dose. After 3 days, if the fasting blood glucose level was higher than 16.7 mmol/L, the DS model was successfully built. The NS and the I/R group were given 0.9% sodium chloride injection. Thereafter, the general conditions for normal and DM rats are showed in Table Table2.2. After 8 weeks, all the rats were intraperitoneally injected with 1.5% sodium pentobarbital at a dose of 0.005 mL/g. They were given electrocardiogram (ECG) monitoring management.
Click to Show/Hide
|
||||
| Response Description | Inhibition of DNA (cytosine-5)-methyltransferase 1 (DNMT-1) could reduce ferroptosis during diabetes myocardial ischemia/reperfusion injury and the NCOA4-mediated ferritinophagy may participate in the process. | ||||
Cyanidin-3-glucoside
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [24] | ||||
| Responsed Disease | Ischemia/reperfusion injury [ICD-11: DB98] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| Cell autophagy | |||||
| In Vitro Model | CHO-S/H9C2 cells | Normal | Cricetulus griseus | CVCL_A0TS | |
| In Vivo Model |
Adult male Sprague Dawley (SD) rats weighing 260-280 g were purchased from Qinglongshan Animal Farm (Nanjing, China). After a week of adaptation, the rats were randomly assigned into five groups (n = 8): (1) sham group, rats receiving saline gavage and sham surgery were used as control group; (2) I/R model group, rats receiving saline gavage and left anterior descending (LAD) ligation surgery were used as the model group; (3) C3G-10 group, I/R model plus intraperitoneal injection of 10 mg/kg C3G; (4) C3G-20 group, I/R model plus intraperitoneal injection of 20 mg/kg C3G; and (5) DIL group, I/R model plus oral administration of 20 mg/kg diltiazem. C3G and DIL were dissolved in DMSO and then diluted with saline so that the DMSO concentration was less than 0.1% (v/v).
Click to Show/Hide
|
||||
| Response Description | The administration of Cyanidin-3-glucoside (C3G) reduced the infarction area, mitigated pathological alterations, inhibited ST segment elevation, and attenuated oxidative stress and ferroptosis-related protein expression. C3G also suppressed the expressions of USP19, Beclin1, NCOA4, and LC3II/LC3I. In addition, treatment with C3G relieved oxidative stress, downregulated LC3II/LC3I, reduced autophagosome number, downregulated TfR1 expression, and upregulated the expressions of FTH1 and GPX4 in OGD/R-induced H9c2 cells. Taken together, C3G could be a potential agent to protect myocardium from myocardial ischemia-reperfusion (IR) injury. | ||||
Isoliquiritigenin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [25] | ||||
| Responsed Disease | Acute kidney failure [ICD-11: GB60] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Male C57BL/6 mice (aged 6-8 weeks and weighing 22-25g) were obtained from the Experimental Animal Center, Sichuan Provincial Peoples Hospital, and were fed a standard laboratory diet. LPS and ISL were dissolved in normal saline and 0.5% Tween-20/saline, respectively. AKI mice were developed by intraperitoneal (i.p.) LPS injection. A total of 30 mice were randomly divided into six groups (n = 5): control, ISL, Fer, LPS, LPS plus ISL, and LPS plus Fer. An intraperitoneal injection of LPS (10 mg/kg) was made to induce septic AKI. ISL was administered via gavage at 50 mg/kg 30 min before LPS injection. Mice were dosed intraperitoneally with Fer (Ferrostatin-1, SML0583, Sigma-Aldrich, St. Louis, MO) at 5 mg/kg. Mice were sacrificed by cervical dislocation 8 h after LPS injection. Kidney tissue and serum samples were collected concurrently.
Click to Show/Hide
|
||||
| Response Description | Isoliquiritigenin attenuates septic acute kidney injury by regulating ferritinophagy-mediated ferroptosis. ISL inhibited Fe2+ and lipid peroxidation accumulation in LPS-stimulated HK2 cells. It also increased the expression of GPX4 and xCT, reduced the expression of HMGB1 and NCOA4 then attenuated mitochondria injury in renal tubular following LPS stimulation. | ||||
Oxalate
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [26] | ||||
| Responsed Disease | Kidney calculus [ICD-11: GB70] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | HK-2 cells | Normal | Homo sapiens | CVCL_0302 | |
| In Vivo Model |
Five-week-old male SD (Sprague-Dawley) rats (130-180 g) were used as experimental subjects. The control group had a normal diet, and the stone model group drank water containing 0.75% ethylene glycol. After feeding for one month, rats were sacrificed, and their kidneys were removed and subjected to silver nitrate staining, immunohistochemistry, and western blotting on the specimens of the normal control group and the kidney stone model group to explore the expression of NCOA4 in kidney stone model rats.
Click to Show/Hide
|
||||
| Response Description | Oxalate activates autophagy to induce ferroptosis of renal tubular epithelial cells and participates in the formation of kidney stones. Moreover, after oxalate treatment, overexpression of the BENC1 gene increased cell oxidative damage and ferroptosis. In addition, knockdown of NCOA4 reversed the effect of oxalate-induced ferroptosis in HK-2 cells. | ||||
Quercetin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [27] | ||||
| Responsed Disease | Injury of intra-abdominal organs [ICD-11: NB91] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| In Vivo Model |
Six-week-old male C57BL/6J mice (18-20 g) were obtained from Zhejiang Vital River Laboratory (Zhejiang, China). 32 mice were divided randomly into 4 groups: Saline group (CONT), 25 mg/kg/day ACR group (ACR), 25 mg/kg/day ACR with a low dose of 25 mg/kg/day QCT group (ACR + QCT (L)), and 25 mg/kg/day ACR with a high dose of 50 mg/kg/day QCT group (ACR + QCT (H)), 8 animals in each group.
Click to Show/Hide
|
||||
| Response Description | Quercetin (QCT) specifically reacted with autophagic cargo receptor NCOA4, blocked the degradation of iron storage protein FTH1, and eventually downregulated the intracellular iron levels and the consequent ferroptosis. Collectively, our results presented a unique approach to alleviate ACR-induced liver injury by targeting ferroptosis with QCT. | ||||
References
