Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0009)
| Name |
Quercetin
|
||||
|---|---|---|---|---|---|
| Synonyms |
quercetin; 117-39-5; Meletin; Sophoretin; Quercetine; Xanthaurine; Quercetol; Quertine; Quercitin; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one; 3,3',4',5,7-Pentahydroxyflavone; Cyanidelonon 1522; Flavin meletin; 3,5,7,3',4'-Pentahydroxyflavone; Quertin; T-Gelb bzw. grun 1; C.I. Natural Yellow 10; Quercetin content; Kvercetin; C.I. 75670; C.I. Natural red 1; Cyanidenolon 1522; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one; CI Natural Yellow 10; Korvitin; Lipoflavon; 3',4',5,7-Tetrahydroxyflavan-3-ol; C.I. Natural yellow 10 & 13; Flavone, 3,3',4',5,7-pentahydroxy-; NSC 9219; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one; CCRIS 1639; 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-; HSDB 3529; NCI-C60106; 3'-hydroxykaempferol; Corvitin; CHEBI:16243; NSC9219; 3,5,7-Trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-on; AI3-26018; UNII-9IKM0I5T1E; NSC-9219; EINECS 204-187-1; 9IKM0I5T1E; Quercetin (GMP); 3',4',5,7-tetrahydroxyflavon-3-ol; BRN 0317313; CI 75670; DTXSID4021218; 3,3',4,5,7-Pentahydroxyflavone; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromen-4-one; CHEMBL50; Ci-75670; MFCD00006828; NSC-57655; LDN-0052529; Flavone, 3,4',5,5',7-pentahydroxy-; DTXCID001218; 2-(3,4-Dihydroxy-phenyl)-3,5,7-trihydroxy-chromen-4-one; 74893-81-5; 3,5,7-trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-one; Quercetin (constituent of ginkgo); 5-18-05-00494 (Beilstein Handbook Reference); 3,5,7,3',4'-Pentahydroxyflavon; Kvercetin [Czech]; Natural Yellow 10; QUERCETIN (IARC); QUERCETIN [IARC]; 7255-55-2; QUE; BRD9794; Dikvertin; BRD-9794; 2-(3,4-DIHYDROXYPHENYL)-3,5,7-TRIHYDROXY-4H-BENZOPYRAN-4-ONE; CAS-117-39-5; 3',4',5,7-tetrahydroxyflavonol; 3,5,7,3',4'-pentahydroflavone; NSC57655; NSC58588; SR-01000076098; MixCom3_000183; Ritacetin; Quer; 4dfu; 4mra; Quercetin2H2O; Meletin;Sophoretin; KUC104418N; KUC107684N; LIM-5662; LNS-5662; TNP00070; TNP00089; CI Natural Red 1; KSC-23-76; Quercetin_sathishkumar; KSC-10-126; Quercetin (Sophoretin); Quercetin - Sophoretin; Spectrum_000124; Tocris-1125; 3cf8; QUERCETIN [DSC]; QUERCETIN [MI]; BiomolKI_000062; QUERCETIN [HSDB]; QUERCETIN [INCI]; Maybridge1_008992; Prestwick0_000507; Prestwick1_000507; Prestwick2_000507; Prestwick3_000507; Spectrum2_000059; Spectrum3_000642; Spectrum4_000807; Spectrum5_001389; Lopac-Q-0125; QUERCETIN [VANDF]; P0042; C.I. natural yellow 13; BiomolKI2_000068; Enicostemma Littorale Blume; UPCMLD-DP081; Q 0125; QUERCETIN [USP-RS]; QUERCETIN [WHO-DD]; NCIOpen2_007628; NCIOpen2_007882; BIDD:PXR0007; Lopac0_000999; SCHEMBL19723; BSPBio_000433; BSPBio_001068; BSPBio_002243; KBioGR_000408; KBioGR_001293; KBioSS_000408; KBioSS_000584; MLS006011766; BIDD:ER0315; DivK1c_000485; SCHEMBL219729; SPECTRUM1500672; T-GELB BZW, GRUN 1; CU-01000012502-3; SPBio_000217; SPBio_002354; BDBM7460; BPBio1_000477; GTPL5346; MEGxp0_000381; SGCUT00001; 3,4',5,7-Pentahydroxyflavone; CI Natural Yellow 10 & 13; NIOSH/LK8760000; UPCMLD-DP081:001; ACon1_000560; HMS501I07; KBio1_000485; KBio2_000408; KBio2_000584; KBio2_002976; KBio2_003152; KBio2_005544; KBio2_005720; KBio3_000775; KBio3_000776; KBio3_001463; 3,7,3',4'-Pentahydroxyflavone; NINDS_000485; 3',5,7-Tetrahydroxyflavan-3-ol; Bio1_000369; Bio1_000858; Bio1_001347; Bio2_000374; Bio2_000854; HMS1362F09; HMS1792F09; HMS1923O19; HMS1990F09; HMS3263G19; HMS3267M12; HMS3414J21; HMS3649D04; HMS3656C15; HMS3678J19; to_000078; 3,4',5,5',7-pentahydroxyflavone; Tox21_202308; Tox21_300285; Tox21_500999; BBL005513; CCG-40054; Flavone,3',4',5,7-pentahydroxy-; HB0542; HY-18085G; LMPK12110004; NSC 57655; NSC324608; NSC756660; s2391; STK365650; Quercetin, >=95% (HPLC), solid; 3,4',5,5',7-pentahydroxy-Flavone; AKOS000511724; Quercetin 1000 microg/mL in Acetone; CS-3981; DB04216; DS-3416; LP00999; NSC-756660; SDCCGSBI-0050972.P003; IDI1_000485; IDI1_002129; LDN 0052529; SMP1_000252; NCGC00015870-01; NCGC00015870-02; NCGC00015870-03; NCGC00015870-04; NCGC00015870-05; NCGC00015870-06; NCGC00015870-07; NCGC00015870-08; NCGC00015870-09; NCGC00015870-10; NCGC00015870-11; NCGC00015870-12; NCGC00015870-13; NCGC00015870-14; NCGC00015870-15; NCGC00015870-16; NCGC00015870-17; NCGC00015870-18; NCGC00015870-19; NCGC00015870-21; NCGC00015870-22; NCGC00015870-23; NCGC00015870-24; NCGC00015870-25; NCGC00015870-28; NCGC00015870-48; NCGC00015870-50; NCGC00025016-01; NCGC00025016-02; NCGC00025016-03; NCGC00025016-04; NCGC00025016-05; NCGC00025016-06; NCGC00025016-07; NCGC00025016-08; NCGC00168962-01; NCGC00168962-02; NCGC00168962-03; NCGC00168962-04; NCGC00254218-01; NCGC00259857-01; NCGC00261684-01; Quercetin 100 microg/mL in Acetonitrile; AC-19596; AC-29756; HY-18085; NCI60_042036; SMR000112559; SY057722; (+)-3,3',4',5,7-Pentahydroxyflavone; Quercetin, Sophoretin, Meletin, Quercetine; CS-0638666; EU-0100999; FT-0603318; FT-0655108; LK87600000; Q0025; SW148203-4; Quercetin; 3,3',4',5,7-Pentahydroxyflavone; C00389; EN300-199773; K00029; S00057; QUERCETIN (CONSTITUENT OF GINKGO) [DSC]; WLN: T66 BO EVJ CR CQ DQ & DQ GQ IQ; 2-(3,4-Dihydroxyphenyl)-4H-1-benzopyran-4-one; Flavone, 3,3',4',5,7-pentahydroxy-, (+)-; Q409478; Q-200333; SR-01000076098-1; SR-01000076098-3; SR-01000076098-7; SR-01000076098-8; BRD-K97399794-001-02-1; BRD-K97399794-001-07-0; BRD-K97399794-001-09-6; BRD-K97399794-001-11-2; BRD-K97399794-335-03-1; SR-01000076098-11; Z57176222; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chromone;hydrate; QUERCETIN (CONSTITUENT OF HAWTHORN LEAF WITH FLOWER); 49643640-FD4C-4B93-BD28-0D7C2021CC52; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one #; (+)-4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-; 4H-1-Benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-,zirconium(2+)salt(1:1); 4H-1-Benzopyran-4-one,2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-, zirconium(2+) salt (1:1)
Click to Show/Hide
|
||||
| Structure |
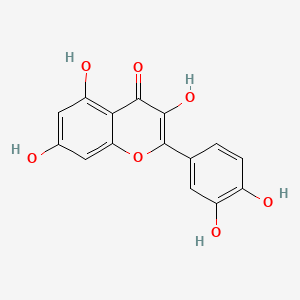 |
||||
| Formula |
C15H10O7
|
||||
| IUPAC Name |
2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one
|
||||
| Canonical SMILES |
C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O
|
||||
| InChI |
InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H
|
||||
| InChIKey |
REFJWTPEDVJJIY-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from Gempharmatech Co., Ltd (Changzhou, China). All mice were housed in cages with standard laboratory conditions: a consistent temperature of 24 , a 12 h light/dark cycle, and free access to water and food. The mice were randomized into four groups: 1) the KA group (n = 6), injected intraperitoneally with 20 mg/kg KA, as described in a previous study; while 2) the control group (n = 6), injected intraperitoneally with an equal volume of PBS; 3) the KA + QCT group (n = 6): this group was givenintragastric administrationof 50 mg/kg of QCT once daily for 21 days before KA injection based on the literature; and 4) the KA+ferrostatin1 (Fer-1) group (n = 6), injected intraperitoneally with a well-known ferroptosis inhibitor (3 mg/kg Fer-1) for 21 days before KA administration, as described in a previous study.
Click to Show/Hide
|
||||
| Response regulation | The association between the Nrf2-mediated ferroptosis pathway and seizures in a clinical setting. Quercetin effectively protects against seizure-induced neuron death in vivo and in vitro and alleviates cognitive function impairment via the SIRT1/Nrf2/SLC7A11/GPX4 pathway. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from Gempharmatech Co., Ltd (Changzhou, China). All mice were housed in cages with standard laboratory conditions: a consistent temperature of 24 , a 12 h light/dark cycle, and free access to water and food. The mice were randomized into four groups: 1) the KA group (n = 6), injected intraperitoneally with 20 mg/kg KA, as described in a previous study; while 2) the control group (n = 6), injected intraperitoneally with an equal volume of PBS; 3) the KA + QCT group (n = 6): this group was givenintragastric administrationof 50 mg/kg of QCT once daily for 21 days before KA injection based on the literature; and 4) the KA+ferrostatin1 (Fer-1) group (n = 6), injected intraperitoneally with a well-known ferroptosis inhibitor (3 mg/kg Fer-1) for 21 days before KA administration, as described in a previous study.
Click to Show/Hide
|
||||
| Response regulation | The association between the Nrf2-mediated ferroptosis pathway and seizures in a clinical setting. Quercetin effectively protects against seizure-induced neuron death in vivo and in vitro and alleviates cognitive function impairment via the SIRT1/Nrf2/SLC7A11/GPX4 pathway. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were obtained from Gempharmatech Co., Ltd (Changzhou, China). All mice were housed in cages with standard laboratory conditions: a consistent temperature of 24 , a 12 h light/dark cycle, and free access to water and food. The mice were randomized into four groups: 1) the KA group (n = 6), injected intraperitoneally with 20 mg/kg KA, as described in a previous study; while 2) the control group (n = 6), injected intraperitoneally with an equal volume of PBS; 3) the KA + QCT group (n = 6): this group was givenintragastric administrationof 50 mg/kg of QCT once daily for 21 days before KA injection based on the literature; and 4) the KA+ferrostatin1 (Fer-1) group (n = 6), injected intraperitoneally with a well-known ferroptosis inhibitor (3 mg/kg Fer-1) for 21 days before KA administration, as described in a previous study.
Click to Show/Hide
|
||||
| Response regulation | The association between the Nrf2-mediated ferroptosis pathway and seizures in a clinical setting. Quercetin effectively protects against seizure-induced neuron death in vivo and in vitro and alleviates cognitive function impairment via the SIRT1/Nrf2/SLC7A11/GPX4 pathway. | ||||
Nuclear receptor coactivator 4 (NCOA4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| In Vivo Model |
Six-week-old male C57BL/6J mice (18-20 g) were obtained from Zhejiang Vital River Laboratory (Zhejiang, China). 32 mice were divided randomly into 4 groups: Saline group (CONT), 25 mg/kg/day ACR group (ACR), 25 mg/kg/day ACR with a low dose of 25 mg/kg/day QCT group (ACR + QCT (L)), and 25 mg/kg/day ACR with a high dose of 50 mg/kg/day QCT group (ACR + QCT (H)), 8 animals in each group.
Click to Show/Hide
|
||||
| Response regulation | Quercetin (QCT) specifically reacted with autophagic cargo receptor NCOA4, blocked the degradation of iron storage protein FTH1, and eventually downregulated the intracellular iron levels and the consequent ferroptosis. Collectively, our results presented a unique approach to alleviate ACR-induced liver injury by targeting ferroptosis with QCT. | ||||
Ferritin heavy chain (FTH1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Injury of intra-abdominal organs | ICD-11: NB91 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| In Vivo Model |
Six-week-old male C57BL/6J mice (18-20 g) were obtained from Zhejiang Vital River Laboratory (Zhejiang, China). 32 mice were divided randomly into 4 groups: Saline group (CONT), 25 mg/kg/day ACR group (ACR), 25 mg/kg/day ACR with a low dose of 25 mg/kg/day QCT group (ACR + QCT (L)), and 25 mg/kg/day ACR with a high dose of 50 mg/kg/day QCT group (ACR + QCT (H)), 8 animals in each group.
Click to Show/Hide
|
||||
| Response regulation | Quercetin (QCT) specifically reacted with autophagic cargo receptor NCOA4, blocked the degradation of iron storage protein FTH1, and eventually downregulated the intracellular iron levels and the consequent ferroptosis. Collectively, our results presented a unique approach to alleviate ACR-induced liver injury by targeting ferroptosis with QCT. | ||||
References
