Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0060)
| Name |
Dihydroquercetin
|
||||
|---|---|---|---|---|---|
| Synonyms |
TAXIFOLIN; (+)-Taxifolin; 480-18-2; dihydroquercetin; Distylin; Taxifoliol; (+)-Dihydroquercetin; (2R,3R)-Dihydroquercetin; 24198-97-8; Lavitol; Diquertin; Lariksin; 2,3-Dihydroquercetin; Flamena D; (+/-)-Taxifolin; TAXIFOLIN-(+); 17654-26-1; (2R,3R)-3,3',4',5,7-Pentahydroxyflavanone; trans-Dihydroquercetin; (2R,3R)-(+)-Taxifolin; 3,5,7,3',4'-Pentahydroxyflavanone; (2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychroman-4-one; taxifolin (dihydroquercetin); (2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one; UNII-9SOB9E3987; FLAMENA; CCRIS 9292; (2R,3R)-2-(3,4-DIHYDROXYPHENYL)-3,5,7-TRIHYDROXY-2,3-DIHYDRO-4H-CHROMEN-4-ONE; 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-, (2R,3R)-; CHEBI:17948; Flavanone, 3,3',4',5,7-pentahydroxy-; (+)-(2R,3R)-dihydroquercetin; (+/-)-dihydroquercetin; 9SOB9E3987; (2R-trans)-2-(3,4-Dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-4-benzopyrone; Taxifolin, (+/-)-; EINECS 207-543-4; EAS93SC1VS; BRN 0093548; Dihydroquercetin, (+/-)-; DTXSID8022450; 5-18-05-00451 (Beilstein Handbook Reference); 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-; Flavone, 2,3-dihydro-3,3',4',5,7-pentahydroxy-; DIHYDROQUERCETIN, (+)-(2R,3R)-; trans dihydroquercetin; (2R,3R)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one; 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-, (2R-trans)-; (?)-Taxifolin; (2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-chroman-4-one; rel-(2R,3R)-2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxychroman-4-one; 4H-1-Benzopyran-4-one, 2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-, trans-(+/-)-; DQH; SMR000466389; (+)-trans-Taxifolin; a cis or trans dihydroquercetin; (+/-)-Taxifolin hydrate; (+)-Dihydroquercetin; (+)-Taxifolin; Taxifolin (Tax); ()-Taxifolin; MFCD00006845; (+)-trans Taxifolin; UNII-EAS93SC1VS; SCHEMBL39786; MLS000759539; MLS001066341; MLS001074712; MLS001424044; MLS002153142; BIDD:ER0483; Taxifolin, analytical standard; DIHYDROQUERCETIN [INCI]; DTXCID502450; MEGxp0_000741; ACon1_000239; DIHYDROQUERCETIN [WHO-DD]; rel-(2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one; BDBM212435; DTXSID301017215; HMS2051M22; HMS2234L14; HY-N0136; AC-935; s2366; AKOS015965399; CCG-100909; CS-3365; DB02224; KS-1312; NC00159; NCGC00016024-08; NCGC00180750-01; 20254-28-8; AS-17723; '(2R,3R)-TRANS-DIHYDROQUERCETIN'; SW197539-2; T3666; C01617; (2R,3R)-3,5,7,3',4'-Pentahydroxyflavanone; Q412191; SR-01000759385; (+)-TAXIFOLIN (CONSTITUENT OF MILK THISTLE); Flavanone, 3,3',4',5,7-pentahydroxy-, (R,R)-; J-011205; SR-01000759385-6; Taxifolin, primary pharmaceutical reference standard; (+)-TAXIFOLIN (CONSTITUENT OF MARITIME PINE); F40AB773-26FA-4112-A46D-DC970AF64BC1; Flavanone, 3,3',4',5,7-pentahydroxy-, (2R)-trans-; Flavanone, 3,3',4',5,7-pentahydroxy-, trans-(+)-; (+)-TAXIFOLIN (CONSTITUENT OF MARITIME PINE) [DSC]; (+)-TAXIFOLIN (CONSTITUENT OF MILK THISTLE) [DSC]; Flavanone, 3,3',4',5,7-pentahydroxy-, (2R,3R)-(+)-; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one, (2R-trans)-; 4H-1-Benzopyran-4-one,2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-,(2R,3R)-rel-
Click to Show/Hide
|
||||
| Status |
Preclinical
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
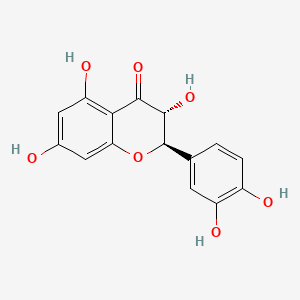 |
||||
| Formula |
C15H12O7
|
||||
| IUPAC Name |
(2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydrochromen-4-one
|
||||
| Canonical SMILES |
C1=CC(=C(C=C1C2C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O
|
||||
| InChI |
InChI=1S/C15H12O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,14-19,21H/t14-,15+/m0/s1
|
||||
| InChIKey |
CXQWRCVTCMQVQX-LSDHHAIUSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear receptor coactivator 4 (NCOA4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Pulmonary fibrosis | ICD-11: CB03 | |||
| Responsed Regulator | Microtubule-associated proteins 1A/1B light chain 3A (MAP1LC3A) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hBEs (Human bronchial epithelial cells) | ||||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| In Vivo Model |
Eight-week-old male C57BL/6 mice were purchased from Hubei University of Medicine (Shiyan, China). The SiO2-induced mouse pulmonary fibrosis model was performed. In brief, each group of mice was anesthetized with 1% pentobarbital sodium intraperitoneally at 40 mg/kg body weight and their tracheae had been surgically exposed. In addition, SiO2 suspension (20 mg in 50 ul saline) was instilled in the mice. The vehicle control groups were given an equivalent amount of 0.9% sterile saline. After one week of acclimation, mice were divided randomly into four groups (n = 8 per group). Control group, SiO2 group, SiO2 and low dose of DHQ group (DHQ-L, 10 mg/kg) as well as large dose of DHQ group (DHQ-H, 50 mg/kg).
Click to Show/Hide
|
||||
| Response regulation | Dihydroquercetin suppressed ferritinophagy by down-regulation of microtubule-associated protein 1A/ 1B-light chain 3 (LC3), and up-regulation of ferritin heavy chain 1 (FTH1), nuclear receptor co-activator 4 (NCOA4) in activated HBE cells. Research revealed that inhibition of ferritinophagy-mediated HBE cells ferroptosis was responsible for DHQ to ameliorate SiO2-induced lung fibrosis. | ||||
Ferritin heavy chain (FTH1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Pulmonary fibrosis | ICD-11: CB03 | |||
| Responsed Regulator | Microtubule-associated proteins 1A/1B light chain 3A (MAP1LC3A) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Autophagy | hsa04140 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | hBEs (Human bronchial epithelial cells) | ||||
| MRC-5 cells | Normal | Homo sapiens | CVCL_0440 | ||
| In Vivo Model |
Eight-week-old male C57BL/6 mice were purchased from Hubei University of Medicine (Shiyan, China). The SiO2-induced mouse pulmonary fibrosis model was performed. In brief, each group of mice was anesthetized with 1% pentobarbital sodium intraperitoneally at 40 mg/kg body weight and their tracheae had been surgically exposed. In addition, SiO2 suspension (20 mg in 50 ul saline) was instilled in the mice. The vehicle control groups were given an equivalent amount of 0.9% sterile saline. After one week of acclimation, mice were divided randomly into four groups (n = 8 per group). Control group, SiO2 group, SiO2 and low dose of DHQ group (DHQ-L, 10 mg/kg) as well as large dose of DHQ group (DHQ-H, 50 mg/kg).
Click to Show/Hide
|
||||
| Response regulation | Dihydroquercetin suppressed ferritinophagy by down-regulation of microtubule-associated protein 1A/ 1B-light chain 3 (LC3), and up-regulation of ferritin heavy chain 1 (FTH1), nuclear receptor co-activator 4 (NCOA4) in activated HBE cells. Research revealed that inhibition of ferritinophagy-mediated HBE cells ferroptosis was responsible for DHQ to ameliorate SiO2-induced lung fibrosis. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Chronic obstructive pulmonary disease | ICD-11: CA22 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HBE1 cells | Normal | Homo sapiens | CVCL_0287 | |
| In Vivo Model |
Thirty-two male BALB/c mice (21-25 g, 6-8 weeks) were purchased from Hunan Slyke Jingda Laboratory Animal Co., Ltd. and kept in a clean unit at 23 ± 2 , 50% ± 10% relative humidity and 12 h rhythm of light and dark. Mice were randomly divided into four groups (n = 8 for each group): the control group, cigarette smoke-inducedCOPDgroup, COPD + low dose (50mg/kg/d)DHQgroup, and COPD + high dose (100 mg/kg/d) DHQ group. The mice in the control group were maintained in fresh air and given anintraperitoneal injectionof 0.3 ml/20 g phosphate-buffered saline (PBS) on Days 0, 11, and 23. The COPD mouse model was established as previously described. Mice in this group were exposed to cigarette smoke for 2 cycles per day (1 h per cycle), 6 days per week for 4 consecutive weeks in a sealed box with ventilation holes except for Days 0, 11, and 22, and over these 3 days, the mice were intraperitoneally injected with 0.3 ml/20g 100% CSE. Mice in the COPD+low-dose DHQ group and COPD+high-dose DHQ group were treated with cigarette smoke and 100% CSE as mentioned above and intraperitoneally injected with DHQ for 25 consecutive days except for Days 0, 11, and 22, while mice in the control and COPD groups were intraperitoneally injected with an equal volume of PBS except for Days 0, 11, and 22. All mice were sacrificed by intraperitoneal injection of 0.5 ml 3% chloral hydrateon the 29 th day of the experiment.
Click to Show/Hide
|
||||
| Response regulation | Treatment with DHQ (Taxifolin) significantly reverses the ferroptosis induced by cigarette smoke both in vivo and in vitro via a Nrf2-dependent signaling pathway. These findings may provide novel therapeutic options for the treatment of chronic obstructive pulmonary disease (COPD) patients. | ||||
References
