Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0311)
| Name |
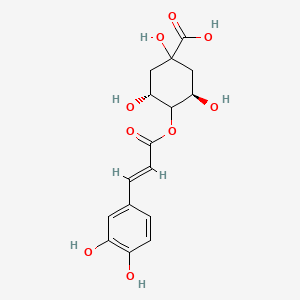
Cryptochlorogenic acid
|
||||
|---|---|---|---|---|---|
| Synonyms |
Cryptochlorogenic acid; 905-99-7; 4-Caffeoylquinic acid; 4-O-Caffeoylquinic acid; 4-Cqa; 4-o-Caffeoyl quinic acid; Quinic acid 4-O-caffeate; 4-O-(3,4-Dihydroxycinnamoyl)-D-quinic acid; F23DJ84IZ9; (3R,5R)-4-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,3,5-trihydroxycyclohexane-1-carboxylic acid; CHEMBL4203706; 4-(3,4-Dihydroxycinnamoyl)quinic acid; (1alpha,3R,4alpha,5R)-4-[[3-(3,4-Dihydroxyphenyl)-1-oxo-2-propen-1-yl]oxy]-1,3,5-trihydroxycyclohexanecarboxylic acid; 82638-23-1; 87099-73-8; rel-(1S,3R,4S,5R)-4-(((E)-3-(3,4-Dihydroxyphenyl)acryloyl)oxy)-1,3,5-trihydroxycyclohexanecarboxylic acid; 4-O-trans-caffeoylquinic acid; MFCD10566638; Cryptochlorogenic-acid; 4-Caffeoylquinic acid;4-O-Caffeoylquinic acid; Quinic acid, 4-caffeoyl-; UNII-F23DJ84IZ9; 4-O-(E)-caffeoylquinic acid; CHEMBL3092676; Cinnamic acid, 3,4-dihydroxy-, 4-carboxy-2,4,6-trihydroxycyclohexyl ester; Quinic acid, 4-caffeoyl-, E-; SCHEMBL18180782; SCHEMBL20883249; ACon1_000120; CHEBI:75491; HY-N0787; BDBM50455380; s9319; 4-O-Caffeoylquinic acid, >=98.0%; AKOS037514601; CCG-268076; CS-3767; NCGC00180861-01; (1alpha,3alpha,4alpha,5beta)-4-(3-(3,4-(Dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-1,3,5-trihydroxycyclohexanecarboxylic acid; (3R,5R)-4-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,3,5-trihydroxy-cyclohexanecarboxylic acid; BS-17821; PD118874; Cryptochlorogenic acid, analytical standard; 4-Caffeoylquinic acid/ Cryptochlorogenic acid; Q-100886; B0B55D52-5101-4E6D-AA67-72D1CECBAA5A; Q27145347; 49B68A4C-6EC9-4441-B7D7-E3B740A8CDEC; (1S,3R,4S,5R)-4-{[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}-1,3,5-trihydroxycyclohexanecarboxylic acid; CYCLOHEXANECARBOXYLIC ACID, 4-(((2E)-3-(3,4-DIHYDROXYPHENYL)-1-OXO-2-PROPEN-1-YL)OXY)-1,3,5-TRIHYDROXY-, (1.ALPHA.,3R,4.ALPHA.,5R)-; Cyclohexanecarboxylic acid, 4-(((2E)-3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl)oxy)-1,3,5-trihydroxy-, (1alpha,3R,4alpha,5R)-; Cyclohexanecarboxylic acid, 4-((3-(3,4-dihydroxyphenyl)-1-oxo-2-1R-propenyl)oxy)-1,3,5-trihydroxy-, (1R-(1alpha,3alpha,4alpha,5beta))-; CYCLOHEXANECARBOXYLIC ACID, 4-((3-(3,4-DIHYDROXYPHENYL)-1-OXO-2-PROPEN-1-YL)OXY)-1,3,5-TRIHYDROXY-, (1.ALPHA.,3R,4.ALPHA.,5R)-; Cyclohexanecarboxylic acid, 4-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propen-1-yl)oxy)-1,3,5-trihydroxy-, (1alpha,3R,4alpha,5R)-; Cyclohexanecarboxylic acid, 4-((3-(3,4-dihydroxyphenyl)-1-oxo-2-propenyl)oxy)-1,3,5-trihydroxy-, (1alpha,3R,4alpha,5R)-
Click to Show/Hide
|
||||
| Structure |
 |
||||
| Formula |
C16H18O9
|
||||
| IUPAC Name |
(3R,5R)-4-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-1,3,5-trihydroxycyclohexane-1-carboxylic acid
|
||||
| Canonical SMILES |
C1C(C(C(CC1(C(=O)O)O)O)OC(=O)C=CC2=CC(=C(C=C2)O)O)O
|
||||
| InChI |
InChI=1S/C16H18O9/c17-9-3-1-8(5-10(9)18)2-4-13(21)25-14-11(19)6-16(24,15(22)23)7-12(14)20/h1-5,11-12,14,17-20,24H,6-7H2,(H,22,23)/b4-2+/t11-,12-,14?,16?/m1/s1
|
||||
| InChIKey |
GYFFKZTYYAFCTR-AVXJPILUSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear receptor coactivator 4 (NCOA4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Diabetes mellitus | ICD-11: 5A10 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | INS-1 cells | Insulinoma | Rattus norvegicus | CVCL_0352 | |
| In Vivo Model |
Sixty Sprague-Dawley (SD) rats with weights ranging from 250-270 g were obtained from experimental animal center of Xiamen university. For diabetes model group, fasting was performed for 12 h before experiment. The rats (ten rats per group) were assigned into Control group, Model (DM) treated with 50 mg/kg streptozotocin (STZ) via abdominal injection, positive control group and experimental groups. The blood glucose level, which is served as the indicator for the diabetes, was monitored herein. The glucose level after modeling is above 16.7 mmol/l, supporting that the modeling is successful.
Click to Show/Hide
|
||||
| Response regulation | Cryptochlorogenic acid (CCA) functions via inhibition of ferroptosis by activation of cystine/glutamate transporter system (XC)/glutathione peroxidase 4(GPX4)/Nrf2 and inhibition of nuclear receptor coactivator 4 (NCOA4) in diabetes. System xc- which is composed of SLC7A11 and SLC3A2, served as the provider of GSH synthesis. | ||||
Cystine/glutamate transporter (SLC7A11)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Diabetes mellitus | ICD-11: 5A10 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | INS-1 cells | Insulinoma | Rattus norvegicus | CVCL_0352 | |
| In Vivo Model |
Sixty Sprague-Dawley (SD) rats with weights ranging from 250-270 g were obtained from experimental animal center of Xiamen university. For diabetes model group, fasting was performed for 12 h before experiment. The rats (ten rats per group) were assigned into Control group, Model (DM) treated with 50 mg/kg streptozotocin (STZ) via abdominal injection, positive control group and experimental groups. The blood glucose level, which is served as the indicator for the diabetes, was monitored herein. The glucose level after modeling is above 16.7 mmol/l, supporting that the modeling is successful.
Click to Show/Hide
|
||||
| Response regulation | Cryptochlorogenic acid (CCA) functions via inhibition of ferroptosis by activation of cystine/glutamate transporter system (XC)/glutathione peroxidase 4(GPX4)/Nrf2 and inhibition of nuclear receptor coactivator 4 (NCOA4) in diabetes. System xc- which is composed of SLC7A11 and SLC3A2, served as the provider of GSH synthesis. | ||||
4F2 cell-surface antigen heavy chain (SLC3A2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Diabetes mellitus | ICD-11: 5A10 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | INS-1 cells | Insulinoma | Rattus norvegicus | CVCL_0352 | |
| In Vivo Model |
Sixty Sprague-Dawley (SD) rats with weights ranging from 250-270 g were obtained from experimental animal center of Xiamen university. For diabetes model group, fasting was performed for 12 h before experiment. The rats (ten rats per group) were assigned into Control group, Model (DM) treated with 50 mg/kg streptozotocin (STZ) via abdominal injection, positive control group and experimental groups. The blood glucose level, which is served as the indicator for the diabetes, was monitored herein. The glucose level after modeling is above 16.7 mmol/l, supporting that the modeling is successful.
Click to Show/Hide
|
||||
| Response regulation | Cryptochlorogenic acid (CCA) functions via inhibition of ferroptosis by activation of cystine/glutamate transporter system (XC)/glutathione peroxidase 4(GPX4)/Nrf2 and inhibition of nuclear receptor coactivator 4 (NCOA4) in diabetes. System xc- which is composed of SLC7A11 and SLC3A2, served as the provider of GSH synthesis. | ||||
