Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0172)
| Name |
Allicin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Allicin; 539-86-6; Diallyl thiosulfinate; S-allyl prop-2-ene-1-sulfinothioate; Diallyldisulfid-S-oxid; Thio-2-propene-1-sulfinic acid S-allyl ester; Allylthiosulphinic acid allyl ester; DADSO; S-Allyl acrylo-1-sulphinothioate; 2-Propene-1-sulfinothioic acid, S-2-propenyl ester; C6H10OS2; CCRIS 9053; S-allyl 2-propene-1-sulfinothioate; EINECS 208-727-7; UNII-3C39BY17Y6; BRN 1752823; 3-prop-2-enylsulfinylsulfanylprop-1-ene; 2-Propene-1-sulfinothioic acid S-2-propenyl ester; CHEBI:28411; 3C39BY17Y6; 2-Propene-1-sulfinic acid, thio-, S-allyl ester; CHEMBL359965; DTXSID6043707; 3-[(prop-2-ene-1-sulfinyl)sulfanyl]prop-1-ene; 4-04-00-00007 (Beilstein Handbook Reference); S-prop-2-en-1-yl prop-2-ene-1-sulfinothioate; S-2-PROPEN-1-YL 2-PROPENE-1-SULFINOTHIOATE; allimin; diallyl disulfide-oxide; allylthiosulfinate; Allicin kit; 3-allylsulfinylsulfanylprop-1-ene; Allicin, tech. grade; Diallyldisulfid-S-oxide; Allicin (not validated); Dianyctrsnlgide;Allisatin; ALLICIN [MI]; ALLICIN [WHO-DD]; ALLICIN(TECH GRADE); SCHEMBL2920; GTPL2419; 2-Propene-1-sulfinothioic Acid S-2-Propen-1-yl Ester; DTXCID4023707; JDLKFOPOAOFWQN-UHFFFAOYSA-N; 3-allylsulfinylsulfanyl-prop-1-ene; BCP08391; HY-N0315; BDBM50240948; MFCD00468100; NSC707388; s3860; AKOS006282482; ALLICIN (CONSTITUENT OF GARLIC); CCG-266298; DB11780; FD10435; NSC-707388; 3-(prop-2-enylsulfinylthio)-1-propene; AC-34150; XA162045; XA176305; CS-0008813; FT-0621982; ALLICIN (CONSTITUENT OF GARLIC) [DSC]; C07600; EN300-217463; S-2-Propenyl 2-propene-1-sulfinothioate, 9CI; A829889; Q409641; Q-200609; 2-Propene-1-sulfinothioic acid, S-2-propenyl ester (9CI); Diallyl thiosulfinate and Allylthiosulphinic acid allyl ester; Diallyldisulfid-S-oxid, 3-prop-2-enylsulfinylsulfanylprop-1-ene
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
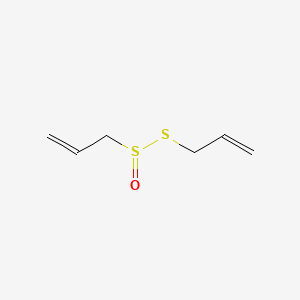 |
||||
| Formula |
C6H10OS2
|
||||
| IUPAC Name |
3-prop-2-enylsulfinylsulfanylprop-1-ene
|
||||
| Canonical SMILES |
C=CCSS(=O)CC=C
|
||||
| InChI |
InChI=1S/C6H10OS2/c1-3-5-8-9(7)6-4-2/h3-4H,1-2,5-6H2
|
||||
| InChIKey |
JDLKFOPOAOFWQN-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Unspecific Target
| In total 3 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Oesophageal cancer | ICD-11: 2B70 | |||
| Responsed Regulator | Ubiquitin-like modifier-activating enzyme ATG7 (ATG7) | Driver | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| HET-1A cells | Normal | Homo sapiens | CVCL_3702 | ||
| In Vivo Model |
All mice were housed in a specific pathogen-free environment under a standard 12 h light-dark cycle at 25 and had ad libitum access to food and water. Approximately 4 x 106 KYSE510 cells in 100 uL of normal saline were subcutaneously injected into the right flank of mice (n = 20 in total). All mice were allocated to a control or 10 mg/kg allicin group (n = 10 per group), as previously described (Suddek 2014). The mice were orally administered allicin or normal saline once daily for 28 days.
Click to Show/Hide
|
||||
| Response regulation | In summary, allicin may induce cell death in esophageal squamous cell carcinoma (ESCC) cells by activating AMPK/mTOR-mediated autophagy and ferroptosis. Furthermore, ATG5 and ATG7 expression increased in tumors after allicin treatment. In contrast, NCOA4 expression increased, but the protein level of FTH1 and TfR1 decreased in tumors after allicin treatment. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Oesophageal cancer | ICD-11: 2B70 | |||
| Responsed Regulator | Serine/threonine-protein kinase mTOR (MTOR) | Suppressor | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| HET-1A cells | Normal | Homo sapiens | CVCL_3702 | ||
| In Vivo Model |
All mice were housed in a specific pathogen-free environment under a standard 12 h light-dark cycle at 25 and had ad libitum access to food and water. Approximately 4 x 106 KYSE510 cells in 100 uL of normal saline were subcutaneously injected into the right flank of mice (n = 20 in total). All mice were allocated to a control or 10 mg/kg allicin group (n = 10 per group), as previously described (Suddek 2014). The mice were orally administered allicin or normal saline once daily for 28 days.
Click to Show/Hide
|
||||
| Response regulation | In summary, allicin may induce cell death in esophageal squamous cell carcinoma (ESCC) cells by activating AMPK/mTOR-mediated autophagy and ferroptosis. Furthermore, ATG5 and ATG7 expression increased in tumors after allicin treatment. In contrast, NCOA4 expression increased, but the protein level of FTH1 and TfR1 decreased in tumors after allicin treatment. | ||||
| Experiment 3 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Responsed Disease | Oesophageal cancer | ICD-11: 2B70 | |||
| Responsed Regulator | Autophagy protein 5 (ATG5) | Driver | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| HET-1A cells | Normal | Homo sapiens | CVCL_3702 | ||
| In Vivo Model |
All mice were housed in a specific pathogen-free environment under a standard 12 h light-dark cycle at 25 and had ad libitum access to food and water. Approximately 4 x 106 KYSE510 cells in 100 uL of normal saline were subcutaneously injected into the right flank of mice (n = 20 in total). All mice were allocated to a control or 10 mg/kg allicin group (n = 10 per group), as previously described (Suddek 2014). The mice were orally administered allicin or normal saline once daily for 28 days.
Click to Show/Hide
|
||||
| Response regulation | In summary, allicin may induce cell death in esophageal squamous cell carcinoma (ESCC) cells by activating AMPK/mTOR-mediated autophagy and ferroptosis. Furthermore, ATG5 and ATG7 expression increased in tumors after allicin treatment. In contrast, NCOA4 expression increased, but the protein level of FTH1 and TfR1 decreased in tumors after allicin treatment. | ||||
Nuclear receptor coactivator 4 (NCOA4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Oesophageal cancer | ICD-11: 2B70 | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | TE-1 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1759 | |
| KYSE-510 cells | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| HET-1A cells | Normal | Homo sapiens | CVCL_3702 | ||
| In Vivo Model |
All mice were housed in a specific pathogen-free environment under a standard 12 h light-dark cycle at 25 and had ad libitum access to food and water. Approximately 4 x 106 KYSE510 cells in 100 uL of normal saline were subcutaneously injected into the right flank of mice (n = 20 in total). All mice were allocated to a control or 10 mg/kg allicin group (n = 10 per group), as previously described (Suddek 2014). The mice were orally administered allicin or normal saline once daily for 28 days.
Click to Show/Hide
|
||||
| Response regulation | In summary, allicin may induce cell death in esophageal squamous cell carcinoma (ESCC) cells by activating AMPK/mTOR-mediated autophagy and ferroptosis. Furthermore, ATG5 and ATG7 expression increased in tumors after allicin treatment. In contrast, NCOA4 expression increased, but the protein level of FTH1 and TfR1 decreased in tumors after allicin treatment. | ||||
