Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0181)
| Name |
Ruxolitinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Ruxolitinib; 941678-49-5; INCB018424; Ruxolitinib (INCB018424); INCB-018424; (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile; INCB 018424; R-Ruxolitinib; INC424; UNII-82S8X8XX8H; 82S8X8XX8H; CHEBI:66919; (R)-ruxolitinib; INCB18424; INC 424; INC-424; (3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile; INCB-18424; HSDB 8259; DTXSID10240930; (3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propanenitrile; (betaR)-beta-Cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazole-1-propanenitrile; (3R)-3-cyclopentyl-3-(4-{7H-pyrrolo[2,3-d]pyrimidin-4-yl}-1H-pyrazol-1-yl)propanenitrile; (3R)-3-CYCLOPENTYL-3-(4-(7H-PYRROLO(2,3-D)PYRIMIDIN-4-YL)PYRAZOL-1-YL)PROPANENITRILE; 1H-Pyrazole-1-propanenitrile, beta-cyclopentyl-4-(7H-pyrrolo(2,3-d)pyrimidin-4-yl)-,(betaR)-; Ruxolitinib [INN]; Ruxolitinib [USAN:INN]; ruxolitinibum; INCB 18424; (3R)-3-cyclopentyl-3-(4-(7H-pyrrolo(2,3-d)pyrimidin-4-yl)-1H-pyrazol-1-yl)propanenitrile; RXT; RUXOLITINIB [MI]; 1092939-15-5; Ruxolitinib (USAN/INN); INCB18424,Ruxolitinib; RUXOLITINIB [USAN]; RUXOLITINIB [VANDF]; RUXOLITINIB [WHO-DD]; SCHEMBL171319; GTPL5688; INCB018424 - Ruxolitinib; CHEMBL1789941; SCHEMBL16546708; DTXCID40163421; L01XE18; HFNKQEVNSGCOJV-OAHLLOKOSA-N; Oral JAK Inhibitor INCB18424; AMY24152; EX-A1670; BDBM50355501; MFCD12031592; NSC763371; NSC800874; s1378; AKOS016842401; BCP9000784; CCG-264889; CS-0864; DB08877; NSC-763371; NSC-800874; NCGC00244253-01; NCGC00244253-02; NCGC00244253-11; NCGC00244253-12; AC-24280; AS-18619; HY-50856; A14955; A23578; D09959; AB01565782_02; EN300-18772174; Q7383611; BRD-K53972329-001-02-1; BRD-K53972329-001-03-9; (3R)-3-cyclopentyl-3-[4-(1H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propanenitrile; (R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H -pyrazol-1-yl)-3-cyclopentylpropanenitrile; 3-Cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-(beta-R)-1H-pyrazole-1-propanenitrile; 3R)-3-cyclopentyl-3-(4-(7H-pyrrolo(2,3-d)pyrimidin-4-yl)pyrazol-1-yl)propanenitrile; A-Cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-(AR)-1H-pyrazole-1-propanenitrile; (?R)-?-Cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazole-1-propanenitrile; (3R)-3-Cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile; (?R)-?-Cyclopentyl-4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazole-1-propanenitrile; INC 424; INCB 018424; Ruxolitinib; (3R)-3-(4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl)-3-cyclopentylpropanenitrile;Ruxolitinib; 1H-PYRAZOLE-1-PROPANENITRILE, .BETA.-CYCLOPENTYL-4-(7H-PYRROLO(2,3-D)PYRIMIDIN-4-YL)-, (.BETA.R)-; 1H-PYRAZOLE-1-PROPANENITRILE, beta-CYCLOPENTYL-4-(7H-PYRROLO(2,3-D)PYRIMIDIN-4-YL)-, (betaR)-
Click to Show/Hide
|
||||
| Structure |
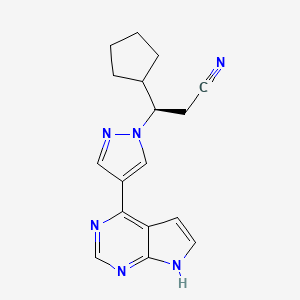 |
||||
| Formula |
C17H18N6
|
||||
| IUPAC Name |
(3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propanenitrile
|
||||
| Canonical SMILES |
C1CCC(C1)C(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3
|
||||
| InChI |
InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1
|
||||
| InChIKey |
HFNKQEVNSGCOJV-OAHLLOKOSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Transferrin receptor protein 1 (TFRC)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor/Driver | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Adult male C57BL/6J mice (6-8 weeks, weighting 20-25 g) were used for all experiments. Mice were housed in pairs in a cage with access to food and water ad libitum. Mice were anesthetized with 4% chloral hydrate (0.4 mg/g) and mounted in a stereotaxic system (David Kopf Instruments, Tujunga, California). A midline skin incision was performed on the scalp to expose the skull, and a 5-mm craniotomy was made lateral to the sagittal suture and centered between bregma and lambda. The skull cap was then removed carefully to avoid destroying the dura mater.
Click to Show/Hide
|
||||
| Response regulation | Ruxolitinib exerts neuroprotection via repressing ferroptosis in a mouse model of traumatic brain injury. Ruxo significantly inhibited the expressions of COX2 and TfR1. In addition, Ruxo also reversed the lower expression of GPX4 caused by Traumatic brain injury. | ||||
Prostaglandin G/H synthase 2 (PTGS2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Adult male C57BL/6J mice (6-8 weeks, weighting 20-25 g) were used for all experiments. Mice were housed in pairs in a cage with access to food and water ad libitum. Mice were anesthetized with 4% chloral hydrate (0.4 mg/g) and mounted in a stereotaxic system (David Kopf Instruments, Tujunga, California). A midline skin incision was performed on the scalp to expose the skull, and a 5-mm craniotomy was made lateral to the sagittal suture and centered between bregma and lambda. The skull cap was then removed carefully to avoid destroying the dura mater.
Click to Show/Hide
|
||||
| Response regulation | Ruxolitinib exerts neuroprotection via repressing ferroptosis in a mouse model of traumatic brain injury. Ruxo significantly inhibited the expressions of COX2 and TfR1. In addition, Ruxo also reversed the lower expression of GPX4 caused by Traumatic brain injury. | ||||
