Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0106)
| Name |
Penicillamine
|
||||
|---|---|---|---|---|---|
| Synonyms |
D-Penicillamine; penicillamine; 52-67-5; Cuprimine; D-(-)-Penicillamine; 3-Mercapto-D-valine; Depen; Cuprenil; D-Penamine; (-)-Penicillamine; (2S)-2-Amino-3-methyl-3-sulfanylbutanoic acid; Artamine; D-Valine, 3-mercapto-; D-Mercaptovaline; Mercaptovaline; Cupripen; Depamine; Kuprenil; Mercaptyl; Pendramine; Penicillamin; Perdolat; Trolovol; D-3-Mercaptovaline; (S)-3,3-Dimethylcysteine; Penicilamina; Penicillaminum; Sufirtan; beta-Thiovaline; D-beta,beta-Dimethylcysteine; beta,beta-Dimethylcysteine; 3-sulfanyl-D-valine; D-Penicilamine; Penicillamina [DCIT]; Penicilamina [INN-Spanish]; Penicillaminum [INN-Latin]; Dimethylcysteine; Metalcaptase; (D)-PENICILLAMINE; (S)-2-amino-3-mercapto-3-methylbutanoic acid; Distamine; CHEBI:7959; (S)-Penicillamine; Penicillamine (Cuprimine); Reduced penicillamine; GNN1DV99GX; (2S)-2-amino-3-methyl-3-sulfanyl-butanoic acid; CHEMBL1430; Penicilllamine; Reduced D-penicillamine; D-Penicyllamine; 3,3-Dimethyl-D-cysteine; DTXSID6037069; D-(-)-2-Amino-3-mercapto-3-methylbutanoic acid; NSC-81549; (S)-Penicillamin; d,3-Mercaptovaline; NCGC00024359-04; Penicillamina; MFCD00064302; Sufortan; Cuprimine (TN); Valine, 3-mercapto-, D-; CCRIS 2904; D-beta-Mercaptovaline; Depen (TN); HSDB 3378; D(-)PENICILLAMINE HYDROCHLORIDE; SR-01000000262; EINECS 200-148-8; UNII-GNN1DV99GX; NSC 81549; alpha-Amino-beta-methyl-beta-mercaptobutyric acid; Distamine (*Hydrochloride*); d-penicillamin; Penicillamine (JAN/USP/INN); dimethyl cysteine; Metalcaptase (*Hydrochloride*); penicillamine-(d); 3-Thio-D-valine; Penicillamine,(S); NSC81549; penicillamine-(racemic); Spectrum_000283; Penicillamine [USAN:USP:INN:BAN:JAN]; Spectrum2_001029; Spectrum3_000541; Spectrum4_000470; Spectrum5_001196; PENICILLAMINE [MI]; Epitope ID:113237; P-1280; PENICILLAMINE [INN]; PENICILLAMINE [JAN]; SCHEMBL4343; PENICILLAMINE [HSDB]; PENICILLAMINE [USAN]; BSPBio_002181; KBioGR_000920; KBioSS_000763; PENICILLAMINE [VANDF]; cid_92173; DivK1c_000314; PENICILLAMINE [MART.]; SPBio_001217; D-Penicillamine, 98-101%; PENICILLAMINE [USP-RS]; PENICILLAMINE [WHO-DD]; GTPL7264; DTXCID4017069; BDBM39346; KBio1_000314; KBio2_000763; KBio2_003331; KBio2_005899; KBio3_001681; NINDS_000314; VVNCNSJFMMFHPL-VKHMYHEASA-N; PENICILLAMINE [EP IMPURITY]; PENICILLAMINE [ORANGE BOOK]; BCP17247; HY-B0300; STR02534; PENICILLAMINE [EP MONOGRAPH]; Tox21_110899; BDBM50217941; PENICILLAMINE [USP MONOGRAPH]; s1853; AKOS006237201; AM83710; CCG-266197; DB00859; CAS-52-67-5; IDI1_000314; SMP1_000042; NCGC00018283-01; NCGC00024359-05; NCGC00024359-06; P0147; EN300-52608; C07418; D00496; M06142; P15236; Q421239; SR-01000000262-3; SR-01000000262-4; Z234896485; (2S)-2-amino-3-mercapto-3-methyl-butyric acid;hydrochloride; (2S)-2-amino-3-mercapto-3-methylbutanoic acid;hydrochloride; (2S)-2-azanyl-3-methyl-3-sulfanyl-butanoic acid;hydrochloride; Penicillamine, European Pharmacopoeia (EP) Reference Standard; (2S)-2-amino-3-methyl-3-sulfanylbutanoic acid3-sulfanyl-D-valine; Penicillamine, United States Pharmacopeia (USP) Reference Standard
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
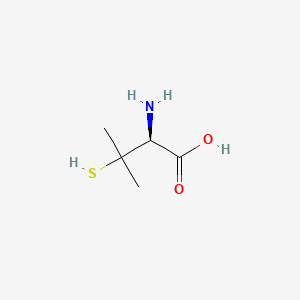 |
||||
| Formula |
C5H11NO2S
|
||||
| IUPAC Name |
(2S)-2-amino-3-methyl-3-sulfanylbutanoic acid
|
||||
| Canonical SMILES |
CC(C)(C(C(=O)O)N)S
|
||||
| InChI |
InChI=1S/C5H11NO2S/c1-5(2,9)3(6)4(7)8/h3,9H,6H2,1-2H3,(H,7,8)/t3-/m0/s1
|
||||
| InChIKey |
VVNCNSJFMMFHPL-VKHMYHEASA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Prostaglandin G/H synthase 2 (PTGS2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Responsed Regulator | Aquaporin-11 (AQP11) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were provided by at the Centre for Animals of Central South University (Changsha, China). To prepare the seizure mouse model, the mice underwent the intrahippocampal injection of KA as described in our previous investigation. For short, mice were anesthetized with sodium phenobarbital (50 mg/kg, i.p.) and carefully placed on a stereotaxic apparatus. Then, KA (1 uL, 250 ng/uL dissolved in saline) was stereotactically injected into the hippocampus according to the following coordinates: anteroposterior -2.0 mm; lateral -1.3 mm; dorsoventral -1.2 mm. After injection, the infusion needle was kept in place for 5-10 min to avoid liquid reflux. Mice in the control group underwent the same surgical procedure but received injection with an equal volume of phosphate buffered saline (PBS) instead of KA.
Click to Show/Hide
|
||||
| Response regulation | D-penicillamine (DPA) can be repurposed to cure seizure disorders such as epilepsy. Furthermore, ferroptosis-associated indices including acyl-coA synthetase long chain family member 4 (ACSL4), prostaglandin-endoperoxide synthase 2 (Ptgs2) gene and lipid peroxide (LPO) level were significantly decreased in KA mouse model after DPA treatment. The effects of DPA on ferroptosis process are dependent upon Aqp11. | ||||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Status epilepticus | ICD-11: 8A66 | |||
| Responsed Regulator | Aquaporin-11 (AQP11) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Male C57BL/6J mice (6-8 weeks of age, weighing 18-22 g) were provided by at the Centre for Animals of Central South University (Changsha, China). To prepare the seizure mouse model, the mice underwent the intrahippocampal injection of KA as described in our previous investigation. For short, mice were anesthetized with sodium phenobarbital (50 mg/kg, i.p.) and carefully placed on a stereotaxic apparatus. Then, KA (1 uL, 250 ng/uL dissolved in saline) was stereotactically injected into the hippocampus according to the following coordinates: anteroposterior -2.0 mm; lateral -1.3 mm; dorsoventral -1.2 mm. After injection, the infusion needle was kept in place for 5-10 min to avoid liquid reflux. Mice in the control group underwent the same surgical procedure but received injection with an equal volume of phosphate buffered saline (PBS) instead of KA.
Click to Show/Hide
|
||||
| Response regulation | D-penicillamine can be repurposed to cure seizure disorders such as epilepsy. D-penicillamine reveals the amelioration of seizure-induced neuronal injury via inhibiting Aqp11-dependent ferroptosis. Furthermore, ferroptosis-associated indices including acyl-coA synthetase long chain family member 4 (ACSL4), prostaglandin-endoperoxide synthase 2 (Ptgs2) gene and lipid peroxide (LPO) level were significantly decreased in KA mouse model after DPA treatment. | ||||
