Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0100)
| Name |
Sevoflurane
|
||||
|---|---|---|---|---|---|
| Synonyms |
sevoflurane; 28523-86-6; 1,1,1,3,3,3-Hexafluoro-2-(fluoromethoxy)propane; Ultane; Sevofluran; Sevorane; Sojourn; MR6S4; sevoflo; Sevofluranum; Sevoflurano; Sevofluranum [INN-Latin]; Sevoflurano [INN-Spanish]; Fluoromethyl 1,1,1,3,3,3-Hexafluoroisopropyl Ether; Sevocalm; Propane, 1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)-; MR-6S4; Fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl)ethyl ether; NSC-760367; 38LVP0K73A; CHEBI:9130; DTXSID8046614; NCGC00167421-01; Sevofrane; Bax 3084; 1173021-96-9; Petrem; fluoromethyl hexafluoroisopropyl ether; Sevoflurane-d3, Fluoromethyl 1,1,1,3,3,3-hexafluoro-2-propyl ether-d3, Fluoromethyl 2,2,2-trifluoro-1-(trifluoromethyl)ethyl ether-d3; Ultane (TN); BRN 2041023; UNII-38LVP0K73A; HSDB 8059; MFCD00153189; Propane,1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)-; Sevoflurane [USAN:USP:INN:BAN:JAN]; SEVOFLURANE [MI]; SEVOFLURANE [INN]; SEVOFLURANE [JAN]; F0691; SEVOFLURANE [USAN]; SEVOFLURANE [VANDF]; (CF3)2CHOCH2F; SEVOFLURANE [MART.]; SCHEMBL61918; SEVOFLURANE [USP-RS]; SEVOFLURANE [WHO-DD]; GTPL7296; CHEMBL1200694; DTXCID6026614; SEVOFLURANE [GREEN BOOK]; Sevoflurane (JP17/USAN/INN); SEVOFLURANE [EP IMPURITY]; SEVOFLURANE [ORANGE BOOK]; HMS3264N21; Pharmakon1600-01503680; SEVOFLURANE [EP MONOGRAPH]; SEVOFLURANE [USP IMPURITY]; SEVOFLURANE [USP MONOGRAPH]; Tox21_112425; ETHER, FLUOROMETHYL 2,2,2-TRIFLUORO-1-(TRIFLUOROMETHYL)ETHYL-; Fluoromethyl 1,1,1,3,3,3-hexafluoroisopropyl ether (Sevoflurane); NSC760367; AKOS007930500; CCG-213707; DB01236; NSC 760367; NCGC00167421-02; SEVOFLURANE [EMA EPAR VETERINARY]; AC-15484; AS-13261; Fluoromethyl 2H-hexafluoroprop-2-yl ether; CAS-28523-86-6; FT-0605909; S2464; 6-CHLOROBENZIMIDAZOLE-4-CARBOXYLICACID; C07520; D00547; D78401; EN300-123016; AB01563174_01; A819479; Q419394; SR-01000944968; J-524240; SR-01000944968-1; Z1269113624; 1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)propane;Sevoflurane
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
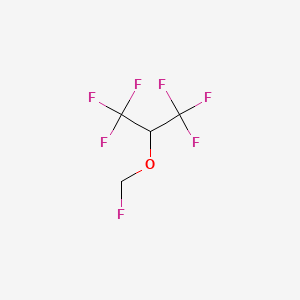 |
||||
| Formula |
C4H3F7O
|
||||
| IUPAC Name |
1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy)propane
|
||||
| Canonical SMILES |
C(OC(C(F)(F)F)C(F)(F)F)F
|
||||
| InChI |
InChI=1S/C4H3F7O/c5-1-12-2(3(6,7)8)4(9,10)11/h2H,1H2
|
||||
| InChIKey |
DFEYYRMXOJXZRJ-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cognition disorder | ICD-11: MB21 | |||
| Responsed Regulator | E3 ubiquitin-protein ligase MIB2 (MIB2) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Ubiquitin mediated proteolysis | hsa04120 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| In Vitro Model | mPRs (Mouse primary neurons) | ||||
| In Vivo Model |
Male C57BL/6 mice were obtained from Beijing HFK Bioscience Co., Ltd., China. The mice were then randomly separated into sham and sevoflurane administrated (SEV) groups, with each group containing 20 animals. In SEV groups, mice were placed in an anesthetizing chamber and exposed to 2.5% sevoflurane (CAS No. 28523-86-6, no. S2464, Selleck, Shanghai, China) with complete oxygen for 2 h, and sham group mice were conducted with the same procedure without sevoflurane exposure.
Click to Show/Hide
|
||||
| Response regulation | Postoperative cognitive dysfunction (POCD) is a complication of the central nervous system (CNS) often occurred after surgery or anesthesia in the elder patients. Downregulation of MIB2 could alleviate the sevoflurane-anesthesia-induced cognitive dysfunction and neuron injury through reducing ferroptosis via GPX4. | ||||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Cerebral ischemia | ICD-11: 8B10 | |||
| Responsed Regulator | Transcription factor Sp1 (SP1) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell apoptosis | |||||
| Cell proliferation | |||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 | |
| In Vivo Model |
Adult male SD rats (250-300 g) were purchased from Charies River (Beijing, China). The animals were placed in laboratory cages, kept on a 12-h light-dark cycle, and had free access to food and water throughout the study. The rats were randomly assigned to the sham (only the left neck was exposed without ligation) group, MACO group, and sevo + MACO (2.5% sevoflurane before refusion) group. The MCAO model was made by a modified nylon suture method. After 1 h of ischemia, the suture was gently pulled to the beginning of the external carotid artery and re-perfused for 24 h. For sevoflurane postconditioning, rats were stabilized in a gas-tight anesthesia chamber with sevoflurane inhalation for 1 h at the onset of blood refusion. Sevoflurane (AbbVie, Japan) was delivered at a concentration of 2.5% through a vaporizer (Vapor 2000, Germany). In the sham or MCAO group, rats were only exposed to the mixed gas (95% O2 and 5% CO2).
Click to Show/Hide
|
||||
| Response regulation | Sevoflurane treatment inhibits ferroptosis and increases apoptosis events by inhibiting the SP1/ASCL4 axis, thereby reducing cerebral ischemia-reperfusion injury damage. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Sixty-four male Sprague-Dawley rats (2 weeks), weighing 20-30 g. All animals were brought from the Institute of Medical Laboratory Animals at the Chinese Academy of Medical Sciences and were kept in the same unit in a temperature-controlled environment [(22 ± 1) ]. The rats were fasted for 12 h before the experiment and drank water freely. After being numbered according to body weight, the rats were randomly divided into four groups using the random number table. The number of rats in each group was 16. The experimental groups were as follows: sham-operated group (S group, n = 16), the model group receiving HIR (HIR group, n = 16), sevoflurane group treated (HIR + Sev group, n = 16), and desferrioxamine treated group [deferoxamine (HIR + Sev + DFO) group, n = 16]. In HIR+Sev and HIR + Sev + DFO groups, rats were placed in an anesthetizing chamber and exposed to 3.6% sevoflurane (Cayman, 23996, USA) with complete oxygen for 2 h, and sham and HIR group rats were conducted with the same procedure without sevoflurane exposure. DFO (100 mg/kg, MCE, HY-B0988, China) was administered continuously daily for 6 days before surgery in the HIR + Sev + DFO group. Other groups were given equal amounts of saline.
Click to Show/Hide
|
||||
| Response regulation | TFRC levels and ACSL4 levels were elevated after sevoflurane administration, suggesting that ferroptosis occurs in whole-brain regions of young rats after HIR and that sevoflurane aggravates the extent of ferroptosis. The results suggest that ferroptosis may mediate sevoflurane-aggravated young rats' brain injury induced by liver transplantation. | ||||
Diamine acetyltransferase 1 (SAT1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Neurotoxicity | ICD-11: NE61 | |||
| Responsed Regulator | Cellular tumor antigen p53 (TP53) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Pregnant rats were placed in a dedicated plastic chamber with ambient gas at a flow rate of 2L/min. Fer-1 solubilized in saline and 1% dimethyl sulfoxide (DMSO) and PD146176 (a specific 15LOX inhibitor) dissolved in corn oil containing 1% DMSO were administered intraperitoneally to rats at a dose of 5 mg/kg 1 h before each exposure, respectively. Similarly, 0.5 mg/kg Ku55933 (an ATM inhibitor), which is diluted in saline containing 1% DMSO, was intraperitoneally administered 2 h previously.
Click to Show/Hide
|
||||
| Response regulation | Sevoflurane could enhance 15LO2-PEBP1 interaction and activate ATM and its downstream P53/SAT1 pathway, which might be attributed to excessive p-ATM nuclear translocation, indicating a potential therapeutic target for ameliorating sevoflurane-induced neurotoxicity. | ||||
Unspecific Target
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Responsed Disease | Neurotoxicity | ICD-11: NE61 | |||
| Responsed Regulator | Serine-protein kinase ATM (ATM) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Pregnant rats were placed in a dedicated plastic chamber with ambient gas at a flow rate of 2L/min. Fer-1 solubilized in saline and 1% dimethyl sulfoxide (DMSO) and PD146176 (a specific 15LOX inhibitor) dissolved in corn oil containing 1% DMSO were administered intraperitoneally to rats at a dose of 5 mg/kg 1 h before each exposure, respectively. Similarly, 0.5 mg/kg Ku55933 (an ATM inhibitor), which is diluted in saline containing 1% DMSO, was intraperitoneally administered 2 h previously.
Click to Show/Hide
|
||||
| Response regulation | Sevoflurane could enhance 15LO2-PEBP1 interaction and activate ATM and its downstream P53/SAT1 pathway, which might be attributed to excessive p-ATM nuclear translocation, indicating a potential therapeutic target for ameliorating sevoflurane-induced neurotoxicity. | ||||
Transferrin receptor protein 1 (TFRC)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Marker/Suppressor/Driver | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Sixty-four male Sprague-Dawley rats (2 weeks), weighing 20-30 g. All animals were brought from the Institute of Medical Laboratory Animals at the Chinese Academy of Medical Sciences and were kept in the same unit in a temperature-controlled environment [(22 ± 1) ]. The rats were fasted for 12 h before the experiment and drank water freely. After being numbered according to body weight, the rats were randomly divided into four groups using the random number table. The number of rats in each group was 16. The experimental groups were as follows: sham-operated group (S group, n = 16), the model group receiving HIR (HIR group, n = 16), sevoflurane group treated (HIR + Sev group, n = 16), and desferrioxamine treated group [deferoxamine (HIR + Sev + DFO) group, n = 16]. In HIR+Sev and HIR + Sev + DFO groups, rats were placed in an anesthetizing chamber and exposed to 3.6% sevoflurane (Cayman, 23996, USA) with complete oxygen for 2 h, and sham and HIR group rats were conducted with the same procedure without sevoflurane exposure. DFO (100 mg/kg, MCE, HY-B0988, China) was administered continuously daily for 6 days before surgery in the HIR + Sev + DFO group. Other groups were given equal amounts of saline.
Click to Show/Hide
|
||||
| Response regulation | TFRC levels and ACSL4 levels were elevated after sevoflurane administration, suggesting that ferroptosis occurs in whole-brain regions of young rats after HIR and that sevoflurane aggravates the extent of ferroptosis. The results suggest that ferroptosis may mediate sevoflurane-aggravated young rats' brain injury induced by liver transplantation. | ||||
Heme oxygenase 1 (HMOX1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [5] | ||||
| Target for Ferroptosis | Driver/Suppressor | ||||
| Responsed Disease | Lung injury | ICD-11: NB32 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | BEAS-2B cells | Normal | Homo sapiens | CVCL_0168 | |
| In Vivo Model |
Male C57BL/6 mice (8 weeks, 23-25 g) were obtained from Beijing Hua Fu Kang Biotechnology Co. LTD (Beijing, China). A total of 96 mice were randomly divided into 6 groups (n = 16 per group): Sham group, LPS group, Fer-1 group, Fe group, Sev group, and Fe + Sev group. Mice were given 5 mg/kg LPS intranasally to construct LPS-induced ALI model. To study the role of ferroptosis in ALI, the mice in the Fer-1 or Fe groups were administered with Fer-1 (0.8 mg/kg; Ferrostatin-1, the inhibitor of ferroptosis) or Fe (8 mg/kg; Fe-citrate (III), ferroptosis inducer) via tail vein injection once a day for 3 consecutive days before treatment with LPS, respectively. To study the effect of Sev on ALI mice, the mice in Sev group were treated with LPS for 2 h, and then Sev, delivered by gaseous admixture (oxygen) at a concentration of 3% via a calibrated vaporizer, was administered via an endotracheal tube for 4 h. In order to study the effect of Sev on ferroptosis, the mice in Fe + Sev group were administered with Fe via tail vein injection once a day for 3 consecutive days, and then treated with LPS and Sev. Sham group was given 0.9% NaCl (containing 0.1% DMSO).
Click to Show/Hide
|
||||
| Response regulation | Sevoflurane (Sev) could eliminate the worsening effects of ferroptosis inducer Fe-citrate on LPS-induced acute lung injury (ALI) to a certain extent. Sev inhibited ferroptosis by up-regulating HO-1 expression to reduce LPS-induced ALI, which may provide a possible mechanism for the application of Sev in clinical anesthesia. | ||||
References
