Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0095)
| Name |
Edaravone
|
||||
|---|---|---|---|---|---|
| Synonyms |
edaravone; 89-25-8; 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE; Radicut; Norphenazone; 1-Phenyl-3-methyl-5-pyrazolone; MCI-186; Developer Z; Methylphenylpyrazolone; C.I. Developer 1; Norantipyrine; Phenyl methyl pyrazolone; Phenylmethylpyrazolone; Radicava; 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one; 3-Methyl-1-phenyl-1H-pyrazol-5(4H)-one; 1-Phenyl-3-methyl-5-oxo-2-pyrazoline; 3H-Pyrazol-3-one, 2,4-dihydro-5-methyl-2-phenyl-; 1-Phenyl-3-methylpyrazolone; 5-methyl-2-phenyl-4H-pyrazol-3-one; 1-Phenyl-3-methylpyrazolone-5; Edaravone (MCI-186); 3-Methyl-1-phenylpyrazol-5-one; 3-Methyl-1-phenyl-2-pyrazoline-5-one; NCI-C03952; 2-Pyrazolin-5-one, 3-methyl-1-phenyl-; 5-Pyrazolone, 3-methyl-1-phenyl-; 2,4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one; Colorex pmp; Jarocol pmp; NSC-2629; 1-Fenyl-3-methyl-2-pyrazolin-5-on; NSC-26139; CHEBI:31530; 3-methyl-1-phenyl-4,5-dihydro-1H-pyrazol-5-one; S798V6YJRP; MLS000069602; 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE (MCI-186); DTXSID9021130; CI Developer 1; NCGC00164015-01; SMR000059020; edaravone(jan); Edaravone [INN]; DTXCID201130; Monopyrazolone; WLN: T5NMV DHJ BR& E1; CAS-89-25-8; CCRIS 512; Radicut (TN); HSDB 4102; 3H-Pyrazol-3-one,4-dihydro-5-methyl-2-phenyl-; SR-01000000135; 1-Fenyl-3-methyl-2-pyrazolin-5-on [Czech]; EINECS 201-891-0; MFCD00003138; UNII-S798V6YJRP; BRN 0609575; AI3-03557; MCI186; (Edaravone); Radicava (TN); (MCI-186); IN1263; CDS1_000986; Spectrum_000267; Tocris-0786; EDARAVONE [JAN]; MCI-186; Edaravone; Edaravone [USAN:INN]; EDARAVONE [HSDB]; EDARAVONE [USAN]; Maybridge1_005738; Opera_ID_1057; Spectrum2_001574; Spectrum3_000971; Spectrum4_001091; Spectrum5_001217; NORPHENAZONE [MI]; M0687; EDARAVONE [MART.]; EC 201-891-0; EDARAVONE [WHO-DD]; SCHEMBL4704; BSPBio_001235; BSPBio_002601; KBioGR_000575; KBioGR_001502; KBioSS_000575; KBioSS_000747; AE-641/00371017; MLS001146878; MLS002415675; MLS006011753; DivK1c_001018; DivK1c_002026; SPECTRUM1503635; SPBio_001508; CHEMBL290916; Edaravone (USAN/JP17/INN); EDARAVONE [ORANGE BOOK]; BCBcMAP01_000127; GTPL11994; HMS503K17; HMS557M18; KBio1_001018; KBio2_000575; KBio2_000747; KBio2_003143; KBio2_003315; KBio2_005711; KBio2_005883; KBio3_001029; KBio3_001030; KBio3_001821; NSC2629; NINDS_001018; BCPP000246; Bio1_000438; Bio1_000927; Bio1_001416; Bio2_000448; Bio2_000928; HMS1362M17; HMS1792M17; HMS1990M17; HMS2234M19; HMS3266F04; HMS3403M17; HMS3411L05; HMS3654L15; HMS3675L05; HMS3884A11; Pharmakon1600-01503635; BCP26336; HY-B0099; NSC26139; Tox21_112077; Tox21_201747; Tox21_302819; BDBM50200541; CCG-39352; NSC758622; s1326; STK201315; 3-methyl-1-phenyl-2-pyrazolin-5one; AKOS000313817; Tox21_112077_1; AC-4745; BCP9000635; CS-1832; DB12243; NSC-758622; PHENYL METHYL PYRAZOLONE [INCI]; SB19128; IDI1_001018; IDI1_002203; NCGC00018218-01; NCGC00018218-02; NCGC00018218-03; NCGC00018218-04; NCGC00018218-05; NCGC00018218-06; NCGC00018218-07; NCGC00018218-08; NCGC00018218-10; NCGC00018218-17; NCGC00022665-02; NCGC00022665-04; NCGC00022665-05; NCGC00022665-06; NCGC00256515-01; NCGC00259296-01; PHENAZONE IMPURITY A [EP IMPURITY]; SBI-0051836.P002; AM20060748; FT-0608243; SW148216-2; 5-methyl-2-phenyl-2,4-dihydro-3-pyrazolone; EN300-16234; 3-Methyl-1-phenyl-2-pyrazoline-5-one, 99%; 4E-901; 5-methyl-2-phenyl-2,4-dihydro-pyrazol-3-one; D01552; D86209; 3-?Methyl-?1-?phenyl-?2-?pyrazolin-?5-?one; AB00375776_14; AB00375776_15; 2 4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one; 2,4-dihydro-2-phenyl-5-methyl-3H-pyrazol-3-one; A843105; Q335099; Q-200386; SR-01000000135-2; SR-01000000135-3; SR-01000000135-5; 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one #; BRD-K35458079-001-04-2; BRD-K35458079-001-12-5; BRD-K35458079-001-23-2; Z50145861; F0391-0021; 3-Methyl-1-phenyl-2-pyrazoline-5-one, SAJ special grade; 3-Methyl-1-phenyl-2-pyrazoline-5-one, purum, >=98.0% (NT); 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (Edaravone); Phenazone impurity A, European Pharmacopoeia (EP) Reference Standard; 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one;3-Methyl-1-phenyl-2-pyrazolin-5-one; InChI=1/C10H10N2O/c1-8-7-10(13)12(11-8)9-5-3-2-4-6-9/h2-6H,7H2,1H
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
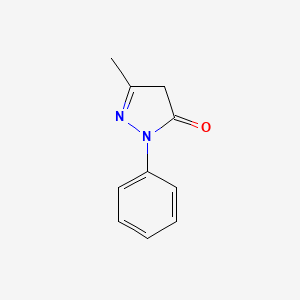 |
||||
| Formula |
C10H10N2O
|
||||
| IUPAC Name |
5-methyl-2-phenyl-4H-pyrazol-3-one
|
||||
| Canonical SMILES |
CC1=NN(C(=O)C1)C2=CC=CC=C2
|
||||
| InChI |
InChI=1S/C10H10N2O/c1-8-7-10(13)12(11-8)9-5-3-2-4-6-9/h2-6H,7H2,1H3
|
||||
| InChIKey |
QELUYTUMUWHWMC-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Depressive disorder | ICD-11: 6A70 | |||
| Responsed Regulator | NAD-dependent protein deacetylase sirtuin-1 (SIRT1) | Suppressor | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Fatty acid metabolism | hsa01212 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell proliferation | |||||
| In Vitro Model | hBCs (Brain cells) | ||||
| In Vivo Model |
Male C57BL/6J mice (aged 7-8 weeks) and retired male CD-1 mice (aged 16-20 weeks) were obtained from the Experimental Animal Centre of Chongqing Medical University (Chongqing, China). The experimental animals were housed in cages under a 12 h light/12 h dark cycle (lights on at 8:00 a.m.), 60 ± 5% humidity, and a temperature of 23 ± 1 with access to water and food freely. All experimental procedures were conducted in accordance with the Ethics Committee of Chongqing Medical University. EDA was purchased from Sigma-Aldrich (St. Louis, USA) and was dissolved in Vehicle (NaCl, 0.9%) at a dosage of 10 mg/kg. EX527 (a Sirt1 inhibitor) and ML385 (a Nrf2 inhibitor) were obtained from MedChemExpress (New Jersey, USA).
Click to Show/Hide
|
||||
| Response regulation | The inflammation and oxidative stress (OS) have been considered crucial components of the pathogenesis of depression. Edaravone possesses potent antidepressant and anxiolytic properties through Sirt1/Nrf2/HO-1/Gpx4 axis and Gpx4-mediated ferroptosis may play a key role in this effect. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Cerebral ischemia | ICD-11: 8B10 | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
Seventy-three specific-pathogen-free (SPF)grade healthy male Sprague Dawley (SD) rats, weighing 240 ± 20 g, were purchased from Hunan Slake Jingda Experimental Animal Co., Ltd., China (animal certificate number SCXK (Xiang) 2013-0004). The animals were reared in an SPF animal laboratory, and the ambient temperature was maintained at 23 ± 1 . All protocols followed the ARRIVE guidelines in terms of study design, sample size, randomization, outcome measures, data analysis, experimental procedures, and reporting of results. This study was approved by the Animal Ethics Committee of the Hunan University of Chinese Medicine.
Click to Show/Hide
|
||||
| Response regulation | Edaravone inhibits ferroptosis to attenuate cerebral ischemia-reperfusion injury, probably through the activation of the Nrf2/FPN pathway. | ||||
Unspecific Target
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | |||
| Responsed Disease | Neuroinflammation | ICD-11: 8C1Z | ||
| Responsed Regulator | Toll-like receptor 4 (TLR4) | Driver | ||
| Pathway Response | Ferroptosis | hsa04216 | ||
| Fatty acid metabolism | hsa01212 | |||
| Cell Process | Cell ferroptosis | |||
| Cell apoptosis | ||||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response regulation | Edaravone could significantly reduce A1-42-induced apoptosis of HT22 cells and formation of pro-inflammatory factors TNF-, IL-1 and IL-6, prevent the activation of TLR4/NF-kB /NLRP3 signaling pathway, and inhibit ferroptosis and lipid peroxidation. Taken together, EDA contributes to inhibiting neuroinflammatory injury and ferroptosis in A 1-42-induced HT22 cells, and thus may be a potential candidate for the treatment of AD. | |||
Solute carrier family 40 member 1 (SLC40A1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cerebral ischemia | ICD-11: 8B10 | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vivo Model |
Seventy-three specific-pathogen-free (SPF)grade healthy male Sprague Dawley (SD) rats, weighing 240 ± 20 g, were purchased from Hunan Slake Jingda Experimental Animal Co., Ltd., China (animal certificate number SCXK (Xiang) 2013-0004). The animals were reared in an SPF animal laboratory, and the ambient temperature was maintained at 23 ± 1 . All protocols followed the ARRIVE guidelines in terms of study design, sample size, randomization, outcome measures, data analysis, experimental procedures, and reporting of results. This study was approved by the Animal Ethics Committee of the Hunan University of Chinese Medicine.
Click to Show/Hide
|
||||
| Response regulation | Edaravone inhibits ferroptosis to attenuate cerebral ischemia-reperfusion injury, probably through the activation of the Nrf2/FPN pathway. | ||||
Polyunsaturated fatty acid 5-lipoxygenase (ALOX5)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Spinal cord injury | ICD-11: ND51 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rSCTs (Rat spinal cord tissues) | ||||
| In Vivo Model |
The rats were initially anesthetized with 5% isoflurane (RWD life science, Shenzhen, China) and then maintained with 22.5% isoflurane. A 1-cm midline incision was made over the thoracic vertebrae, and laminectomy on T10 and the caudal half of T9 vertebrae was performed. Spinal cord contusion injury was conducted by NYU Impactor Model III (W.M. Keck Center for Collaborative Neuroscience Rutgers, The State University of New Jersey, United States) using a 10-g node dropping freely from a height of 2.5 cm and muscles and skin sutured in layers. Sham controls underwent laminectomy without the contusion. To prevent infection at the incision, cefuroxime sodium was applied for 3 days after injury. The bladders were emptied manually twice daily in the first week after injury.
Click to Show/Hide
|
||||
| Response regulation | Edaravone not only rescues the ferroptosis negative regulators, xCT and GPX4, but also downregulates those pro-ferroptosis factors, ACSL4 and 5-LOX. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. And in the acute phase of spinal cord injury (SCI), edaravone reduced neuronal cell death and neuroinflammation. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Spinal cord injury | ICD-11: ND51 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rSCTs (Rat spinal cord tissues) | ||||
| In Vivo Model |
The rats were initially anesthetized with 5% isoflurane (RWD life science, Shenzhen, China) and then maintained with 22.5% isoflurane. A 1-cm midline incision was made over the thoracic vertebrae, and laminectomy on T10 and the caudal half of T9 vertebrae was performed. Spinal cord contusion injury was conducted by NYU Impactor Model III (W.M. Keck Center for Collaborative Neuroscience Rutgers, The State University of New Jersey, United States) using a 10-g node dropping freely from a height of 2.5 cm and muscles and skin sutured in layers. Sham controls underwent laminectomy without the contusion. To prevent infection at the incision, cefuroxime sodium was applied for 3 days after injury. The bladders were emptied manually twice daily in the first week after injury.
Click to Show/Hide
|
||||
| Response regulation | Edaravone not only rescues the ferroptosis negative regulators, xCT and GPX4, but also downregulates those pro-ferroptosis factors, ACSL4 and 5-LOX. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. And in the acute phase of spinal cord injury (SCI), edaravone reduced neuronal cell death and neuroinflammation. | ||||
Long-chain-fatty-acid--CoA ligase 4 (ACSL4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [4] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Spinal cord injury | ICD-11: ND51 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rSCTs (Rat spinal cord tissues) | ||||
| In Vivo Model |
The rats were initially anesthetized with 5% isoflurane (RWD life science, Shenzhen, China) and then maintained with 22.5% isoflurane. A 1-cm midline incision was made over the thoracic vertebrae, and laminectomy on T10 and the caudal half of T9 vertebrae was performed. Spinal cord contusion injury was conducted by NYU Impactor Model III (W.M. Keck Center for Collaborative Neuroscience Rutgers, The State University of New Jersey, United States) using a 10-g node dropping freely from a height of 2.5 cm and muscles and skin sutured in layers. Sham controls underwent laminectomy without the contusion. To prevent infection at the incision, cefuroxime sodium was applied for 3 days after injury. The bladders were emptied manually twice daily in the first week after injury.
Click to Show/Hide
|
||||
| Response regulation | Edaravone not only rescues the ferroptosis negative regulators, xCT and GPX4, but also downregulates those pro-ferroptosis factors, ACSL4 and 5-LOX. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. And in the acute phase of spinal cord injury (SCI), edaravone reduced neuronal cell death and neuroinflammation. | ||||
References
