Ferroptosis Target Information
General Information of the Ferroptosis Target (ID: TAR10011)
| Target Name | Polyunsaturated fatty acid 5-lipoxygenase (ALOX5) | ||||
|---|---|---|---|---|---|
| Synonyms |
Arachidonate 5-lipoxygenase
Click to Show/Hide
|
||||
| Gene Name | ALOX5 | ||||
| Sequence |
MPSYTVTVATGSQWFAGTDDYIYLSLVGSAGCSEKHLLDKPFYNDFERGAVDSYDVTVDE
ELGEIQLVRIEKRKYWLNDDWYLKYITLKTPHGDYIEFPCYRWITGDVEVVLRDGRAKLA RDDQIHILKQHRRKELETRQKQYRWMEWNPGFPLSIDAKCHKDLPRDIQFDSEKGVDFVL NYSKAMENLFINRFMHMFQSSWNDFADFEKIFVKISNTISERVMNHWQEDLMFGYQFLNG CNPVLIRRCTELPEKLPVTTEMVECSLERQLSLEQEVQQGNIFIVDFELLDGIDANKTDP CTLQFLAAPICLLYKNLANKIVPIAIQLNQIPGDENPIFLPSDAKYDWLLAKIWVRSSDF HVHQTITHLLRTHLVSEVFGIAMYRQLPAVHPIFKLLVAHVRFTIAINTKAREQLICECG LFDKANATGGGGHVQMVQRAMKDLTYASLCFPEAIKARGMESKEDIPYYFYRDDGLLVWE AIRTFTAEVVDIYYEGDQVVEEDPELQDFVNDVYVYGMRGRKSSGFPKSVKSREQLSEYL TVVIFTASAQHAAVNFGQYDWCSWIPNAPPTMRAPPPTAKGVVTIEQIVDTLPDRGRSCW HLGAVWALSQFQENELFLGMYPEEHFIEKPVKEAMARFRKNLEAIVSVIAERNKKKQLPY YYLSPDRIPNSVAI Click to Show/Hide
|
||||
| Family | Lipoxygenase family | ||||
| Function |
Catalyzes the oxygenation of arachidonate ((5Z,8Z,11Z,14Z)- eicosatetraenoate) to 5-hydroperoxyeicosatetraenoate (5-HPETE) followed by the dehydration to 5,6- epoxyeicosatetraenoate (Leukotriene A4/LTA4), the first two steps in the biosynthesis of leukotrienes, which are potent mediators of inflammation. Also catalyzes the oxygenation of arachidonate into 8- hydroperoxyicosatetraenoate (8-HPETE) and 12- hydroperoxyicosatetraenoate (12-HPETE). Displays lipoxin synthase activity being able to convert (15S)-HETE into a conjugate tetraene. Although arachidonate is the preferred substrate, this enzyme can also metabolize oxidized fatty acids derived from arachidonate such as (15S)-HETE, eicosapentaenoate (EPA) such as (18R)- and (18S)-HEPE or docosahexaenoate (DHA) which lead to the formation of specialized pro-resolving mediators (SPM) lipoxin and resolvins E and D respectively, therefore it participates in anti-inflammatory responses. Oxidation of DHA directly inhibits endothelial cell proliferation and sprouting angiogenesis via peroxisome proliferator-activated receptor gamma (PPARgamma). It does not catalyze the oxygenation of linoleic acid and does not convert (5S)-HETE to lipoxin isomers. In addition to inflammatory processes, it participates in dendritic cell migration, wound healing through an antioxidant mechanism based on heme oxygenase-1 (HO-1) regulation expression, monocyte adhesion to the endothelium via ITGAM expression on monocytes. Moreover, it helps establish an adaptive humoral immunity by regulating primary resting B cells and follicular helper T cells and participates in the CD40-induced production of reactive oxygen species (ROS) after CD40 ligation in B cells through interaction with PIK3R1 that bridges ALOX5 with CD40. May also play a role in glucose homeostasis, regulation of insulin secretion and palmitic acid-induced insulin resistance via AMPK. Can regulate bone mineralization and fat cell differentiation increases in induced pluripotent stem cells.
Click to Show/Hide
|
||||
| Gene ID | 240 | ||||
| Uniprot ID | |||||
| Target Type | Driver Suppressor Marker | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
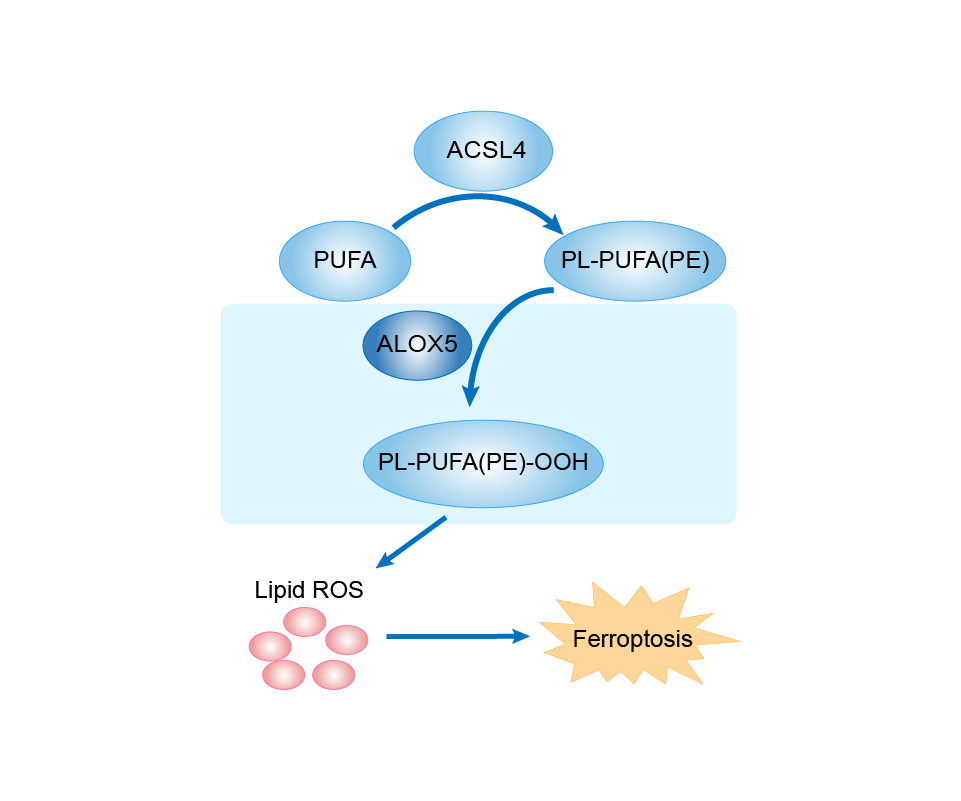
|
|||||
Tissue Relative Abundances of This Target
Full List of Regulator(s) of This Ferroptosis Target and Corresponding Disease/Drug Response(s)
ALOX5 can be involved in and affect the ferroptosis by the following regulators, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulting from the regulation of certain regulators.
Browse Regulator related Disease
Browse Regulator related Drug
mmu-miR-351-5p (miRNA)
Traumatic brain injury [ICD-11: NA07]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Drug | Melatonin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Male C57BL/6 mice aged 6-8 weeks were purchased from the Chongqing Medical University Animal Experiment Center (Chongqing, China). Mice were randomly allocated into three groups including sham group, TBI group, and melatonin treatment group. 30 mice were used for MRI and cerebral blood flow (CBF) monitoring at the 3rd and 7th day, with 5 in each group; 15 mice were used for EEG detection at the 14th and 30th days, training and testing inwater mazeduring the 25th-30th days, with 5 in each group. 15 mice were used for brain water content determination on the 3rd day, with 5 in each group. 9 mice were used for RNA sequencing, with 3 in each group; 75 mice were used for brain tissue acquisition, with 5 in each group with 5 time points (1st, 3rd, 7th, 14th, and 30th day after the operation).
Click to Show/Hide
|
||||
| Response Description | Melatonin administration reduced the level of circPtpn14 (mmu_circ_0000130), which functioned by acting as a miR-351-5p sponge to positively regulate the expression of the ferroptosis-related 5-lipoxygenase (5-LOX). In addition, melatonin alleviated longterm sleep disorders and improved neurological function in Traumatic brain injury (TBI) mice. Thus, these findings suggested that melatonin might potentially protect the injured brain by attenuating ferroptosis and ER stress. | ||||
CircPtpn14 (circRNA)
Traumatic brain injury [ICD-11: NA07]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Drug | Melatonin | Investigative | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Male C57BL/6 mice aged 6-8 weeks were purchased from the Chongqing Medical University Animal Experiment Center (Chongqing, China). Mice were randomly allocated into three groups including sham group, TBI group, and melatonin treatment group. 30 mice were used for MRI and cerebral blood flow (CBF) monitoring at the 3rd and 7th day, with 5 in each group; 15 mice were used for EEG detection at the 14th and 30th days, training and testing inwater mazeduring the 25th-30th days, with 5 in each group. 15 mice were used for brain water content determination on the 3rd day, with 5 in each group. 9 mice were used for RNA sequencing, with 3 in each group; 75 mice were used for brain tissue acquisition, with 5 in each group with 5 time points (1st, 3rd, 7th, 14th, and 30th day after the operation).
Click to Show/Hide
|
||||
| Response Description | Melatonin administration reduced the level of circPtpn14 (mmu_circ_0000130), which functioned by acting as a miR-351-5p sponge to positively regulate the expression of the ferroptosis-related 5-lipoxygenase (5-LOX). In addition, melatonin alleviated longterm sleep disorders and improved neurological function in Traumatic brain injury (TBI) mice. Thus, these findings suggested that melatonin might potentially protect the injured brain by attenuating ferroptosis and ER stress. | ||||
Microsomal glutathione S-transferase 1 (MGST1)
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [2] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Apoptosis | hsa04210 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
CFPAC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_1119 | |
| Panc 02.03 cells | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1633 | ||
| PANC-1 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | ||
| MIA PaCa-2 cells | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | ||
| A-549 cells | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| Hep-G2 cells | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| In Vivo Model |
To generate murine subcutaneous tumors, 5 x 106 CFPAC1 cells in 100 ul PBS were injected subcutaneously to the right of the dorsal midline in 6- to 8-week-old athymic nude female mice. Once the tumors reached around 70-80 mm3 at day 7, mice were randomly allocated into groups and then treated with imidazole ketone erastin (IKE; 40 mg/kg, i.p., once every other day) in the absence or presence of liproxstatin-1 (10 mg/kg, i.p., once every other day) for 2 weeks.
Click to Show/Hide
|
||||
| Response Description | MGST1 inhibits ferroptotic cancer cell death partly by binding to ALOX5, resulting in reduced lipid peroxidation. The expression of MGST1 is positively correlated with NFE2L2 expression in pancreatic tumors, which is implicated in the poor prognosis of patients with pancreatic ductal adenocarcinoma (PDAC). | ||||
Unspecific Regulator
Spinal cord injury [ICD-11: ND51]
| In total 1 item(s) under this disease | |||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [3] | ||||
| Responsed Drug | Edaravone | Approved | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
In Vitro Model |
rSCTs (Rat spinal cord tissues) | ||||
| In Vivo Model |
The rats were initially anesthetized with 5% isoflurane (RWD life science, Shenzhen, China) and then maintained with 22.5% isoflurane. A 1-cm midline incision was made over the thoracic vertebrae, and laminectomy on T10 and the caudal half of T9 vertebrae was performed. Spinal cord contusion injury was conducted by NYU Impactor Model III (W.M. Keck Center for Collaborative Neuroscience Rutgers, The State University of New Jersey, United States) using a 10-g node dropping freely from a height of 2.5 cm and muscles and skin sutured in layers. Sham controls underwent laminectomy without the contusion. To prevent infection at the incision, cefuroxime sodium was applied for 3 days after injury. The bladders were emptied manually twice daily in the first week after injury.
Click to Show/Hide
|
||||
| Response Description | Edaravone not only rescues the ferroptosis negative regulators, xCT and GPX4, but also downregulates those pro-ferroptosis factors, ACSL4 and 5-LOX. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. And in the acute phase of spinal cord injury (SCI), edaravone reduced neuronal cell death and neuroinflammation. | ||||
Health [ICD-11: N.A.]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the Ferroptosis-centered Disease Response of This Regulator | [4] | |||
| Responsed Drug | Zileuton | Approved | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
In Vitro Model |
HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Both the 5-LOX inhibitor zileuton and the ferropotosis inhibitor ferrostatin-1 acted through the same cascade to protect against glutamate oxidative toxicity. In conclusion, zileuton protected neurons from glutamate-induced oxidative stress at least in part by inhibiting ferroptosis. | |||
mmu-miR-351-5p (miRNA)
Melatonin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Suppressor | ||||
| Responsed Disease | Traumatic brain injury [ICD-11: NA07] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Male C57BL/6 mice aged 6-8 weeks were purchased from the Chongqing Medical University Animal Experiment Center (Chongqing, China). Mice were randomly allocated into three groups including sham group, TBI group, and melatonin treatment group. 30 mice were used for MRI and cerebral blood flow (CBF) monitoring at the 3rd and 7th day, with 5 in each group; 15 mice were used for EEG detection at the 14th and 30th days, training and testing inwater mazeduring the 25th-30th days, with 5 in each group. 15 mice were used for brain water content determination on the 3rd day, with 5 in each group. 9 mice were used for RNA sequencing, with 3 in each group; 75 mice were used for brain tissue acquisition, with 5 in each group with 5 time points (1st, 3rd, 7th, 14th, and 30th day after the operation).
Click to Show/Hide
|
||||
| Response Description | Melatonin administration reduced the level of circPtpn14 (mmu_circ_0000130), which functioned by acting as a miR-351-5p sponge to positively regulate the expression of the ferroptosis-related 5-lipoxygenase (5-LOX). In addition, melatonin alleviated longterm sleep disorders and improved neurological function in Traumatic brain injury (TBI) mice. Thus, these findings suggested that melatonin might potentially protect the injured brain by attenuating ferroptosis and ER stress. | ||||
CircPtpn14 (circRNA)
Melatonin
[Investigative]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [1] | ||||
| Regulator for Ferroptosis | Driver | ||||
| Responsed Disease | Traumatic brain injury [ICD-11: NA07] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Male C57BL/6 mice aged 6-8 weeks were purchased from the Chongqing Medical University Animal Experiment Center (Chongqing, China). Mice were randomly allocated into three groups including sham group, TBI group, and melatonin treatment group. 30 mice were used for MRI and cerebral blood flow (CBF) monitoring at the 3rd and 7th day, with 5 in each group; 15 mice were used for EEG detection at the 14th and 30th days, training and testing inwater mazeduring the 25th-30th days, with 5 in each group. 15 mice were used for brain water content determination on the 3rd day, with 5 in each group. 9 mice were used for RNA sequencing, with 3 in each group; 75 mice were used for brain tissue acquisition, with 5 in each group with 5 time points (1st, 3rd, 7th, 14th, and 30th day after the operation).
Click to Show/Hide
|
||||
| Response Description | Melatonin administration reduced the level of circPtpn14 (mmu_circ_0000130), which functioned by acting as a miR-351-5p sponge to positively regulate the expression of the ferroptosis-related 5-lipoxygenase (5-LOX). In addition, melatonin alleviated longterm sleep disorders and improved neurological function in Traumatic brain injury (TBI) mice. Thus, these findings suggested that melatonin might potentially protect the injured brain by attenuating ferroptosis and ER stress. | ||||
Unspecific Regulator
Edaravone
[Approved]
| In total 1 item(s) under this drug | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [3] | ||||
| Responsed Disease | Spinal cord injury [ICD-11: ND51] | ||||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | rSCTs (Rat spinal cord tissues) | ||||
| In Vivo Model |
The rats were initially anesthetized with 5% isoflurane (RWD life science, Shenzhen, China) and then maintained with 22.5% isoflurane. A 1-cm midline incision was made over the thoracic vertebrae, and laminectomy on T10 and the caudal half of T9 vertebrae was performed. Spinal cord contusion injury was conducted by NYU Impactor Model III (W.M. Keck Center for Collaborative Neuroscience Rutgers, The State University of New Jersey, United States) using a 10-g node dropping freely from a height of 2.5 cm and muscles and skin sutured in layers. Sham controls underwent laminectomy without the contusion. To prevent infection at the incision, cefuroxime sodium was applied for 3 days after injury. The bladders were emptied manually twice daily in the first week after injury.
Click to Show/Hide
|
||||
| Response Description | Edaravone not only rescues the ferroptosis negative regulators, xCT and GPX4, but also downregulates those pro-ferroptosis factors, ACSL4 and 5-LOX. Therefore, secondary injury below the lesion site is reversed by edaravone via ferroptosis inhibition. And in the acute phase of spinal cord injury (SCI), edaravone reduced neuronal cell death and neuroinflammation. | ||||
Zileuton
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Response of This Regulator | [4] | |||
| Responsed Disease | Health [ICD-11: N.A.] | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response Description | Both the 5-LOX inhibitor zileuton and the ferropotosis inhibitor ferrostatin-1 acted through the same cascade to protect against glutamate oxidative toxicity. In conclusion, zileuton protected neurons from glutamate-induced oxidative stress at least in part by inhibiting ferroptosis. | |||
References
