Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0088)
| Name |
Zileuton
|
||||
|---|---|---|---|---|---|
| Synonyms |
ZILEUTON; 111406-87-2; Zyflo; Leutrol; 1-(1-(Benzo[b]thiophen-2-yl)ethyl)-1-hydroxyurea; Zyflo CR; Zileutonum; Abbott 64077; Zileutonum [INN-Latin]; 1-[1-(1-benzothiophen-2-yl)ethyl]-1-hydroxyurea; A-64077; N-(1-Benzo(b)thien-2-ylethyl)-N-hydroxyurea; A 64077; Zyflo (TN); ABBOTT-64077; Urea, N-(1-benzo[b]thien-2-ylethyl)-N-hydroxy-; CHEMBL93; 1-[1-(1-benzothien-2-yl)ethyl]-1-hydroxyurea; NSC-730712; NSC-759277; N-[1-(benzo[b]thiophen-2-yl)ethyl]-N-hydroxyurea; V1L22WVE2S; (+-)-1-(1-Benzo(b)thien-2-ylethyl)-1-hydroxyurea; (+/-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea; DTXSID9023752; CHEBI:10112; n-(1-benzo[b]thien-2-ylethyl)-n-hydroxyurea; (+-)-1-(1-Benzo[b]thien-2-ylethyl)-1-hydroxyurea; N-(1-BENZO(B)THIEN-2-YL-ETHYL)-N-HYDROXYUREA; NCGC00159453-02; Urea, N-(1-benzo(b)thien-2-ylethyl)-N-hydroxy-; DTXCID003752; Ziluton; Zyflo Filmtab; SMR000466377; CAS-111406-87-2; Zileuton (USP/INN); SR-01000759349; (+/-)-1-(1-benzo(b)thien-2-ylethyl)-1-hydroxyurea; UNII-V1L22WVE2S; CTI-02; ( inverted exclamation markA)-1(1-Benzo[b]thien-2-ylethyl)-1-hydroxyure; ABT-077; Zileuton [USAN:USP:INN:BAN]; Zileuton- Bio-X; MFCD00866097; starbld0016861; ZILEUTON [USAN]; ZILEUTON [INN]; ZILEUTON [MI]; ZILEUTON [VANDF]; Prestwick0_001090; ZILEUTON [MART.]; ZILEUTON [USP-RS]; ZILEUTON [WHO-DD]; SCHEMBL4209; MLS000759510; MLS001424079; MLS006011971; ZILEUTON [ORANGE BOOK]; GTPL5297; Zileuton, >=98% (HPLC); SCHEMBL18251470; ZILEUTON [USP MONOGRAPH]; Urea, N-(1-benzo(b)thien-2-ylethyl)-N-hydroxy-, (+-)-; MWLSOWXNZPKENC-UHFFFAOYSA-N; HMS2051M20; HMS2089J12; HMS2093H06; HMS2235O04; HMS3369I17; HMS3393M20; HMS3654J08; HMS3714E07; HMS3872F13; Pharmakon1600-01505906; AMY12540; BCP16199; Tox21_111680; Tox21_301148; BBL029070; BDBM50000541; NSC730712; NSC759277; s1443; STL373010; AKOS000280127; AKOS016340558; Tox21_111680_1; CCG-100901; CCG-213571; CS-1563; DB00744; KS-1195; NC00151; NSC 730712; NSC 759277; SB19083; MRF-0000030; NCGC00159453-03; NCGC00159453-04; NCGC00159453-05; NCGC00159453-06; NCGC00255046-01; AC-13198; AC-31490; BZ164573; HY-14164; SBI-0206869.P001; BB 0261152; FT-0601582; FT-0675903; SW197531-3; D00414; EN300-123022; N-(1-benzo[b]thien-2-ylethyl)-N-hydroxy-urea; ()-1(1-Benzo[b]thien-2-ylethyl)-1-hydroxyure; AB00639921-06; AB00639921-08; AB00639921-09; AB00639921_10; AB00639921_11; A802357; Q202998; J-002574; J-525169; SR-01000759349-4; SR-01000759349-5; SR-01000759349-6; Zileuton, commercially available as 600 mg tablets; BRD-A56359832-001-04-6; Z1546610483; Zileuton, United States Pharmacopeia (USP) Reference Standard; UREA, N-(1-BENZO(B)THIEN-2-YLETHYL)-N-HYDROXY-, (+/-)-
Click to Show/Hide
|
||||
| Status |
Approved
|
||||
| Drug Type |
Small molecular drug
|
||||
| Structure |
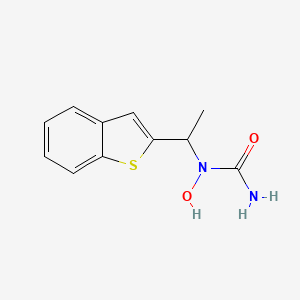 |
||||
| Formula |
C11H12N2O2S
|
||||
| IUPAC Name |
1-[1-(1-benzothiophen-2-yl)ethyl]-1-hydroxyurea
|
||||
| Canonical SMILES |
CC(C1=CC2=CC=CC=C2S1)N(C(=O)N)O
|
||||
| InChI |
InChI=1S/C11H12N2O2S/c1-7(13(15)11(12)14)10-6-8-4-2-3-5-9(8)16-10/h2-7,15H,1H3,(H2,12,14)
|
||||
| InChIKey |
MWLSOWXNZPKENC-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Full List of Ferroptosis Target Related to This Drug
Polyunsaturated fatty acid 5-lipoxygenase (ALOX5)
| In total 1 item(s) under this Target | ||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | |||
| Target for Ferroptosis | Driver | |||
| Responsed Disease | Health | ICD-11: N.A. | ||
| Pathway Response | Fatty acid metabolism | hsa01212 | ||
| Ferroptosis | hsa04216 | |||
| Cell Process | Cell ferroptosis | |||
| In Vitro Model | HT22 cells | Normal | Mus musculus | CVCL_0321 |
| Response regulation | Both the 5-LOX inhibitor zileuton and the ferropotosis inhibitor ferrostatin-1 acted through the same cascade to protect against glutamate oxidative toxicity. In conclusion, zileuton protected neurons from glutamate-induced oxidative stress at least in part by inhibiting ferroptosis. | |||
