Ferroptosis-centered Drug Response Information
General Information of the Drug (ID: ferrodrug0035)
| Name |
Melatonin
|
||||
|---|---|---|---|---|---|
| Synonyms |
Melatonin; 73-31-4; Melatonine; N-Acetyl-5-methoxytryptamine; Circadin; N-[2-(5-Methoxy-1H-indol-3-yl)ethyl]acetamide; 5-Methoxy-N-acetyltryptamine; Melatol; N-(2-(5-Methoxy-1H-indol-3-yl)ethyl)acetamide; Melovine; Melatonex; Acetamide, N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-; N-[2-(5-methoxyindol-3-yl)ethyl]acetamide; 8041-44-9; N-acetyl-5-methoxy-tryptamine; N-(2-(5-Methoxyindol-3-yl)ethyl)acetamide; CCRIS 3472; NSC 113928; Acetamide, N-(2-(5-methoxyindol-3-yl)ethyl)-; UNII-JL5DK93RCL; JL5DK93RCL; Acetamide, N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-; EINECS 200-797-7; NSC-56423; 5-methoxy n-acetyl-tryptamine; NSC-113928; BRN 0205542; DTXSID1022421; CHEBI:16796; HSDB 7509; BCI-049; CHEMBL45; MFCD00005655; NSC113928; J5.258B; 3-(n-acetyl-2-aminoethyl)-5-methoxyindole; DTXCID002421; Acetamide, N-[2-(5-methoxyindol-3-yl)ethyl]-; Melatonin (JAN); 5-22-12-00042 (Beilstein Handbook Reference); NSC56423; N-[2-(5-Methoxy-1H-indol-3-yl)-ethyl]-acetamide; MT6; CAS-73-31-4; NCGC00015680-11; MELATONIN [JAN]; MELATONIN (MART.); MELATONIN [MART.]; MELATONIN (USP-RS); MELATONIN [USP-RS]; Melatonina; Melapure; Posidorm; Vivitas; Primex; Sleep Right; WLN: T56 BMJ D2MV1 GO1; Night Rest; Rx Balance; Revital Melatonin; Nature'S Harmony; [3H]melatonin; Melatonin, Powder; Melatonina (TN); Mela-T; [3H]-melatonin; ML1; SMR000326666; N-(2-(5-methoxyindol-3-yl)ethyl)-Acetamide; N-[2-(5-methoxyindol-3-yl)ethyl]-Acetamide; N-[2-(5-Methoxy-1H-indol-3-yl)ethyl)acetamide; SR-01000075559; [3H]MLT; Melatobel; Melaxen; Melatonine;; Guna-dermo; Melatobel (TN); TNP00300; Prestwick_312; IN1244; Therapeutic Melatonin; Spectrum_000185; NMR/14327425; MELATONIN [DSC]; Guna-dermo (Salt/Mix); MELATONIN [MI]; MELATONIN [HSDB]; MELATONIN [INCI]; Prestwick0_000458; Prestwick1_000458; Prestwick2_000458; Prestwick3_000458; Spectrum2_001344; Spectrum3_001393; Spectrum4_000066; Spectrum5_001745; Lopac-M-5250; MELATONIN [VANDF]; M1105; ChemDiv2_003916; M 5250; Melatonin, >=99.5%; MELATONIN [WHO-DD]; Lopac0_000787; Oprea1_104553; Oprea1_814234; SCHEMBL19018; BSPBio_000536; BSPBio_003006; GTPL224; KBioGR_000591; KBioSS_000665; MELATONIN [EMA EPAR]; Acetamide, {N-[2-(5-methoxyindol-3-yl)ethyl]-}; MLS000859594; MLS001055382; MLS001240204; BIDD:ER0618; DivK1c_000353; SPECTRUM1500690; SPBio_001527; SPBio_002475; Melatonin (synth.) ultra-pure; MELATONIN [GREEN BOOK]; BDBM9019; BPBio1_000590; GTPL1357; Acetamide, {N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-}; HMS501B15; KBio1_000353; KBio2_000665; KBio2_003233; KBio2_005801; KBio3_002226; Melatonin 1.0 mg/ml in Methanol; NINDS_000353; 3-N-Acetyl-5-methoxyl tryptamine; GLXC-25215; HMS1380B22; HMS1569K18; HMS1921E04; HMS2089F09; HMS2096K18; HMS2233D23; HMS3262M16; HMS3370J20; HMS3413P14; HMS3654A22; HMS3677P14; HMS3713K18; HMS3884M05; Melatonin (synth.) standard-grade; AMY33320; BCP28154; HY-B0075; Tox21_110195; Tox21_201527; Tox21_302926; Tox21_500787; 4-ACETAMIDO-4'-ISOTHIO-CYANATOSTILBENE-2,2'-DISULFONIC ACID; CCG-38837; HSCI1_000400; Melatonin, powder, >=98% (TLC); STK386880; AKOS000276269; Tox21_110195_1; CS-1769; DB01065; KS-1454; LP00787; SDCCGMLS-0065812.P001; SDCCGMLS-0065812.P002; SDCCGSBI-0050765.P003; IDI1_000353; IDI1_002631; SMP2_000309; N-acetyl-5-methoxy-tryptamine Melatonine; NCGC00015680-01; NCGC00015680-02; NCGC00015680-03; NCGC00015680-04; NCGC00015680-05; NCGC00015680-06; NCGC00015680-07; NCGC00015680-08; NCGC00015680-09; NCGC00015680-10; NCGC00015680-12; NCGC00015680-13; NCGC00015680-14; NCGC00015680-15; NCGC00015680-16; NCGC00015680-18; NCGC00015680-35; NCGC00090727-01; NCGC00090727-02; NCGC00090727-03; NCGC00090727-04; NCGC00090727-05; NCGC00090727-06; NCGC00090727-07; NCGC00090727-08; NCGC00090727-09; NCGC00256404-01; NCGC00259077-01; NCGC00261472-01; AC-10019; BA164660; NCI60_004378; SY051401; AB00053279; EU-0100787; FT-0628191; FT-0658928; FT-0670984; S1204; SW196607-4; C01598; D08170; M02088; AB00053279-10; AB00053279_12; EN300-6486827; {N-[2-(5-methoxyindol-3-yl)ethyl]-} Acetamide; A929721; L001261; N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-Acetamide; Q180912; SR-01000075559-1; SR-01000075559-6; SR-01000075559-7; SR-01000075559-8; {N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-} Acetamide; BRD-K97530723-001-07-6; BRD-K97530723-001-11-8; F1929-1777; Melatonin, British Pharmacopoeia (BP) Reference Standard; Z1191880499; 0E2B08C1-B325-45B1-8939-6F9081EFDFA4; Acetamide, N-[2-(5-methoxyindol-3-yl)ethyl]- (6CI,8CI); Acetamide, N-[2-(5-methoxy-1H-indol-3-yl)ethyl]- (9CI); Melatonin, United States Pharmacopeia (USP) Reference Standard; Melatonin, Pharmaceutical Secondary Standard; Certified Reference Material
Click to Show/Hide
|
||||
| Structure |
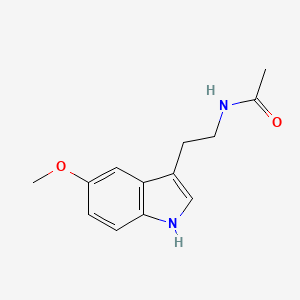 |
||||
| Formula |
C13H16N2O2
|
||||
| IUPAC Name |
N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide
|
||||
| Canonical SMILES |
CC(=O)NCCC1=CNC2=C1C=C(C=C2)OC
|
||||
| InChI |
InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16)
|
||||
| InChIKey |
DRLFMBDRBRZALE-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
Full List of Ferroptosis Target Related to This Drug
Polyunsaturated fatty acid 5-lipoxygenase (ALOX5)
| In total 2 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Responsed Regulator | CircPtpn14 (circRNA) | Driver | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Male C57BL/6 mice aged 6-8 weeks were purchased from the Chongqing Medical University Animal Experiment Center (Chongqing, China). Mice were randomly allocated into three groups including sham group, TBI group, and melatonin treatment group. 30 mice were used for MRI and cerebral blood flow (CBF) monitoring at the 3rd and 7th day, with 5 in each group; 15 mice were used for EEG detection at the 14th and 30th days, training and testing inwater mazeduring the 25th-30th days, with 5 in each group. 15 mice were used for brain water content determination on the 3rd day, with 5 in each group. 9 mice were used for RNA sequencing, with 3 in each group; 75 mice were used for brain tissue acquisition, with 5 in each group with 5 time points (1st, 3rd, 7th, 14th, and 30th day after the operation).
Click to Show/Hide
|
||||
| Response regulation | Melatonin administration reduced the level of circPtpn14 (mmu_circ_0000130), which functioned by acting as a miR-351-5p sponge to positively regulate the expression of the ferroptosis-related 5-lipoxygenase (5-LOX). In addition, melatonin alleviated longterm sleep disorders and improved neurological function in Traumatic brain injury (TBI) mice. Thus, these findings suggested that melatonin might potentially protect the injured brain by attenuating ferroptosis and ER stress. | ||||
| Experiment 2 Reporting the Ferroptosis-centered Drug Act on This Target | [1] | ||||
| Target for Ferroptosis | Driver | ||||
| Responsed Disease | Traumatic brain injury | ICD-11: NA07 | |||
| Responsed Regulator | mmu-miR-351-5p (miRNA) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | bEnd.3 cells | Normal | Mus musculus | CVCL_0170 | |
| In Vivo Model |
Male C57BL/6 mice aged 6-8 weeks were purchased from the Chongqing Medical University Animal Experiment Center (Chongqing, China). Mice were randomly allocated into three groups including sham group, TBI group, and melatonin treatment group. 30 mice were used for MRI and cerebral blood flow (CBF) monitoring at the 3rd and 7th day, with 5 in each group; 15 mice were used for EEG detection at the 14th and 30th days, training and testing inwater mazeduring the 25th-30th days, with 5 in each group. 15 mice were used for brain water content determination on the 3rd day, with 5 in each group. 9 mice were used for RNA sequencing, with 3 in each group; 75 mice were used for brain tissue acquisition, with 5 in each group with 5 time points (1st, 3rd, 7th, 14th, and 30th day after the operation).
Click to Show/Hide
|
||||
| Response regulation | Melatonin administration reduced the level of circPtpn14 (mmu_circ_0000130), which functioned by acting as a miR-351-5p sponge to positively regulate the expression of the ferroptosis-related 5-lipoxygenase (5-LOX). In addition, melatonin alleviated longterm sleep disorders and improved neurological function in Traumatic brain injury (TBI) mice. Thus, these findings suggested that melatonin might potentially protect the injured brain by attenuating ferroptosis and ER stress. | ||||
Phospholipid hydroperoxide glutathione peroxidase (GPX4)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Suppressor | ||||
| Responsed Disease | Cataract | ICD-11: 9B10 | |||
| Responsed Regulator | NAD-dependent protein deacylase sirtuin-6 (SIRT6) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | B-3 cells | Normal | Homo sapiens | CVCL_6367 | |
| In Vivo Model |
Six-week-old albino Sprague Dawley (SD) male rats were provided by the Experimental Animal Centre of the Second Affiliated Hospitalof Harbin Medical University. Fifteen minutes before exposure, the rats were anaesthetized by intraperitoneal injection of a mixture of 90 mg/kg ketamine and 15 mg/kg xylazine. Then, tropicamide phenylephrine was dropped in both eyes; at the same time, the rats that received drug treatment were injected subconjunctivally (5 ul/eye) with 500 mM Fer-1, 200 mM MT or the same dose of DMSO used to dissolve the drug using a 28-gauge needle and a Hamilton microinjector. After another 5 min, a single eye of every experimental group rat was exposed to UVB (312 nm) 5 W/m2 for 30 min. Every time, UVB exposure was synchronized with the drug injection, and the frequency was every other day until it was stopped 9 weeks later.
Click to Show/Hide
|
||||
| Response regulation | Melatonin inhibited ferroptosis through the SIRT6/p-Nrf2/GPX4 and SIRT6/COA4/FTH1 pathways to neutralize lipid peroxidation toxicity, which protected cells against ferroptotic stress in vitro and delayed cataract formation caused by UVB exposure in rats. | ||||
Ferritin heavy chain (FTH1)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [2] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Cataract | ICD-11: 9B10 | |||
| Responsed Regulator | NAD-dependent protein deacylase sirtuin-6 (SIRT6) | Suppressor | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| Cell autophagy | |||||
| In Vitro Model | B-3 cells | Normal | Homo sapiens | CVCL_6367 | |
| In Vivo Model |
Six-week-old albino Sprague Dawley (SD) male rats were provided by the Experimental Animal Centre of the Second Affiliated Hospitalof Harbin Medical University. Fifteen minutes before exposure, the rats were anaesthetized by intraperitoneal injection of a mixture of 90 mg/kg ketamine and 15 mg/kg xylazine. Then, tropicamide phenylephrine was dropped in both eyes; at the same time, the rats that received drug treatment were injected subconjunctivally (5 ul/eye) with 500 mM Fer-1, 200 mM MT or the same dose of DMSO used to dissolve the drug using a 28-gauge needle and a Hamilton microinjector. After another 5 min, a single eye of every experimental group rat was exposed to UVB (312 nm) 5 W/m2 for 30 min. Every time, UVB exposure was synchronized with the drug injection, and the frequency was every other day until it was stopped 9 weeks later.
Click to Show/Hide
|
||||
| Response regulation | Melatonin inhibited ferroptosis through the SIRT6/p-Nrf2/GPX4 and SIRT6/COA4/FTH1 pathways to neutralize lipid peroxidation toxicity, which protected cells against ferroptotic stress in vitro and delayed cataract formation caused by UVB exposure in rats. | ||||
Nuclear factor erythroid 2-related factor 2 (NFE2L2)
| In total 1 item(s) under this Target | |||||
| Experiment 1 Reporting the Ferroptosis-centered Drug Act on This Target | [3] | ||||
| Target for Ferroptosis | Marker/Suppressor | ||||
| Responsed Disease | Osteoporosis | ICD-11: FB83 | |||
| Pathway Response | Fatty acid metabolism | hsa01212 | |||
| Ferroptosis | hsa04216 | ||||
| Cell Process | Cell ferroptosis | ||||
| In Vitro Model | MC3T3-E1 cells | Normal | Mus musculus | CVCL_0409 | |
| In Vivo Model |
Eight-week-old specific-pathogen-free Sprague Dawley rats weighing 220 ± 20 g were purchased from China Medical University, Department of Experimental Animals. A total of 60 rats were used to determine the targets of bone histomorphometry; 45 rats were used to establish a diabetic model, and remaining 15 rats were divided into a control group. The diabetic rats were divided into three groups (n = 15 each) treated with intraperitoneal injection of high-dose melatonin (50 mg/kg, HMT group), intraperitoneal injection of low-dose melatonin (10 mg/kg, LMT group), and a control T2DM group.
Click to Show/Hide
|
||||
| Response regulation | High glucose induces ferroptosis via increased ROS/lipid peroxidation/glutathione depletion in type 2 diabetic osteoporosis. More importantly, melatonin significantly reduced the level of ferroptosis and improved the osteogenic capacity of MC3T3-E1 through activating the Nrf2/HO-1 pathway in vivo and in vitro. | ||||
References
